Flesh Quality, Shelf Life, and Freshness Assessment of Sea Bream Reared in a Coastal Mediterranean Integrated Multi-Trophic Aquaculture System
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing System
2.2. Analytical Determinations
2.2.1. pH, Colour, and Textural Parameters of Sea Bream Fillets
2.2.2. Chemical and Fatty Acid Analysis and Lipid Oxidation of Sea Bream Fillets
2.2.3. Freshness Assessment of Sea Bream by QIM and Torry Scheme
2.3. Statistical Analysis
3. Results
3.1. Growth Parameters
3.1.1. pH, Colour, and Textural Parameters and Lipid Oxidation of Sea Bream Fillets
3.1.2. Chemical and Fatty Acid Analysis of Sea Bream Fillets
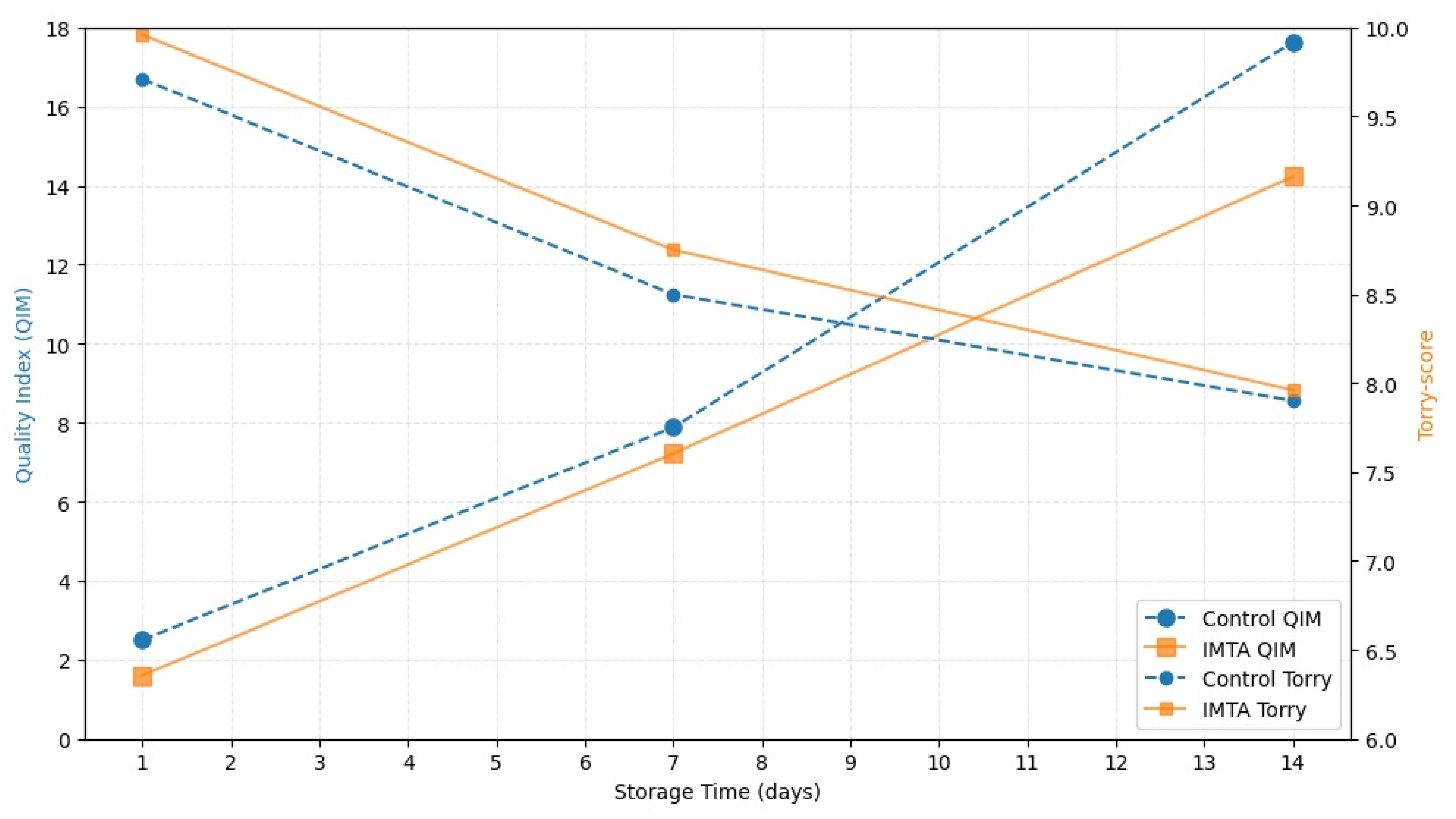
3.1.3. Sensorial Analysis of Sea Bream Fillets
4. Discussion
5. Conclusions and Future Direction
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chopin, F.; Schulmann, K.; Skrzypek, E.; Lehmann, J.; Dujardin, J.R.; Martelat, J.E.; Lexa, O.; Corsini, M.; Edel, J.B.; Štípská, P.; et al. Crustal Influx, Indentation, Ductile Thinning and Gravity Redistribution in a Continental Wedge: Building a Moldanubian Mantled Gneiss Dome with Underthrust Saxothuringian Material (European Variscan Belt). Tectonics 2012, 31, 1–27. [Google Scholar] [CrossRef]
- Reid, G.K.; Lefebvre, S.; Filgueira, R.; Robinson, S.M.C.; Broch, O.J.; Dumas, A.; Chopin, T.B.R. Performance Measures and Models for Open-water Integrated Multi-trophic Aquaculture. Rev. Aquac. 2020, 12, 47–75. [Google Scholar] [CrossRef]
- Azhar, M.; Memiş, D. Application of the IMTA (Integrated Multi-Trophic Aquaculture) System in Freshwater, Brackish and Marine Aquaculture. Aquat. Sci. Eng. 2023, 38, 106–121. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Zahedi, S.; Mohammadi, A. Integrated Multitrophic Aquaculture (IMTA) as an Environmentally Friendly System for Sustainable Aquaculture: Functionality, Species, and Application of Biofloc Technology (BFT). Environ. Sci. Pollut. Res. 2022, 29, 67513–67531. [Google Scholar] [CrossRef]
- Rosati, S.; Maiuro, L.; Lombardi, S.J.; Iaffaldano, N.; Di Iorio, M.; Cariglia, M.; Lopez, F.; Cofelice, M.; Tremonte, P.; Sorrentino, E. Integrated Biotechnological Strategies for the Sustainability and Quality of Mediterranean Sea Bass (Dicentrarchus labrax) and Sea Bream (Sparus aurata). Foods 2025, 14, 1020. [Google Scholar] [CrossRef] [PubMed]
- Turlybek, N.; Nurbekova, Z.; Mukhamejanova, A.; Baimurzina, B.; Kulatayeva, M.; Aubakirova, K.M.; Alikulov, Z. Sustainable Aquaculture Systems and Their Impact on Fish Nutritional Quality. Fishes 2025, 10, 206. [Google Scholar] [CrossRef]
- Toledo-Guedes, K.; Atalah, J.; Izquierdo-Gomez, D.; Fernandez-Jover, D.; Uglem, I.; Sanchez-Jerez, P.; Arechavala-Lopez, P.; Dempster, T. Domesticating the Wild through Escapees of Two Iconic Mediterranean Farmed Fish Species. Sci. Rep. 2024, 14, 23772. [Google Scholar] [CrossRef] [PubMed]
- Batır, E.; Metin, Ö.; Yıldız, M.; Özel, O.T.; Fidan, D. Sustainable Land-Based IMTA: Holistic Management of Finfish, Mussel, and Macroalgae Interactions, Emphasizing Water Quality and Nutrient Dynamics. J. Environ. Manag. 2024, 372, 123411. [Google Scholar] [CrossRef] [PubMed]
- Mhalhel, K.; Levanti, M.; Abbate, F.; Laurà, R.; Guerrera, M.C.; Aragona, M.; Porcino, C.; Briglia, M.; Germanà, A.; Montalbano, G. Review on Gilthead Seabream (Sparus aurata) Aquaculture: Life Cycle, Growth, Aquaculture Practices and Challenges. J. Mar. Sci. Eng. 2023, 11, 2008. [Google Scholar] [CrossRef]
- Marhuenda-Egea, F.C.; Sánchez-Jerez, P. Metabolomic Insights into Wild and Farmed Gilthead Seabream (Sparus aurata): Lipid Composition, Freshness Indicators, and Environmental Adaptations. Molecules 2025, 30, 770. [Google Scholar] [CrossRef]
- Rusco, G.; Roncarati, A.; Di Iorio, M.; Cariglia, M.; Longo, C.; Iaffaldano, N. Can IMTA System Improve the Productivity and Quality Traits of Aquatic Organisms Produced at Different Trophic Levels? The Benefits of IMTA—Not Only for the Ecosystem. Biology 2024, 13, 946. [Google Scholar] [CrossRef] [PubMed]
- Piper, L.; De Cosmo, L.M.; Sestino, A.; Giangrande, A.; Stabili, L.; Longo, C.; Guido, G. Perceived Social Welfare as a Driver of Green Products Consumption: Evidences from an Integrated Multi-Trophic Aquaculture Production. Curr. Res. Environ. Sustain. 2021, 3, 100081. [Google Scholar] [CrossRef]
- Cangiano, T.; DellaGreca, M.; Fiorentino, A.; Isidori, M.; Monaco, P.; Zarrelli, A. Lactone Diterpenes from the Aquatic Plant Potamogeton Natans. Phytochemistry 2001, 56, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Cangiano, T.; Dellagreca, M.; Fiorentino, A.; Isidori, M.; Monaco, P.; Zarrelli, A. Effect of Ent-Labdane Diterpenes from Potamogetonaceae on Selenastrum Capricornutum and Other Aquatic Organisms. J. Chem. Ecol. 2002, 28, 1091–1102. [Google Scholar] [CrossRef]
- Burić, M.; Bavčević, L.; Grgurić, S.; Vresnik, F.; Križan, J.; Antonić, O. Modelling the Environmental Footprint of Sea Bream Cage Aquaculture in Relation to Spatial Stocking Design. J. Environ. Manag. 2020, 270, 110811. [Google Scholar] [CrossRef] [PubMed]
- Shpigel, M.; Ari, T.B.; Shauli, L.; Odintsov, V.; Ben-Ezra, D. Nutrient Recovery and Sludge Management in Seabream and Grey Mullet Co-Culture in Integrated Multi-Trophic Aquaculture (IMTA). Aquaculture 2016, 464, 316–322. [Google Scholar] [CrossRef]
- Ferreira, J.G.; Saurel, C.; Ferreira, J.M. Cultivation of Gilthead Bream in Monoculture and Integrated Multi-Trophic Aquaculture. Analysis of Production and Environmental Effects by Means of the FARM Model. Aquaculture 2012, 358, 23–34. [Google Scholar] [CrossRef]
- Trani, R.; Pierri, C.; Schiavo, A.; Lazic, T.; Mercurio, M.; Coccia, I.; Giangrande, A.; Longo, C. Response of Hard-Bottom Macro-Zoobenthos to the Transition of a Mediterranean Mariculture Fish Plant (Mar Grande of Taranto, Ionian Sea) into an Integrated Multi-Trophic Aquaculture (IMTA) System. J. Mar. Sci. Eng. 2025, 13, 143. [Google Scholar] [CrossRef]
- Giangrande, A.; Pierri, C.; Arduini, D.; Borghese, J.; Licciano, M.; Trani, R.; Corriero, G.; Basile, G.; Cecere, E.; Petrocelli, A.; et al. An Innovative IMTA System: Polychaetes, Sponges and Macroalgae Co-Cultured in a Southern Italian In-Shore Mariculture Plant (Ionian Sea). J. Mar. Sci. Eng. 2020, 8, 733. [Google Scholar] [CrossRef]
- Aguilo-Arce, J.; Ferriol, P.; Puthod, P.; Trani, R.; Longo, C. The remedia life integrated multitrophic aquaculture system as a powerful sponge biomass supply. Biol. Mar. Mediterr. 2024, 28, 87–89. [Google Scholar]
- Stabili, L.; Giangrande, A.; Arduini, D.; Borghese, J.; Petrocelli, A.; Alabiso, G.; Ricci, P.; Cavallo, R.A.; Acquaviva, M.I.; Narracci, M.; et al. Environmental Quality Improvement of a Mariculture Plant after Its Conversion into a Multi-Trophic System. Sci. Total Environ. 2023, 884, 163846. [Google Scholar] [CrossRef] [PubMed]
- Borghese, J.; Giangrande, A.; Arduini, D.; Trani, R.; Doria, L.; Anglano, M.; Aguilo-Arce, J.; Toso, A.; Putignano, M.; Rizzo, L.; et al. Exploring the Potential Effects of IMTA on Water Column Seston through Intensive Short-Time Cycles Approach. Mar. Pollut. Bull. 2025, 212, 117580. [Google Scholar] [CrossRef]
- Giangrande, A.; Licciano, M.; Arduini, D.; Borghese, J.; Pierri, C.; Trani, R.; Longo, C.; Petrocelli, A.; Ricci, P.; Alabiso, G.; et al. An Integrated Monitoring Approach to the Evaluation of the Environmental Impact of an Inshore Mariculture Plant (Mar Grande of Taranto, Ionian Sea). Biology 2022, 11, 617. [Google Scholar] [CrossRef]
- Arduini, D.; Borghese, J.; Gravina, M.F.; Trani, R.; Longo, C.; Pierri, C.; Giangrande, A. Biofouling Role in Mariculture Environment Restoration: An Example in the Mar Grande of Taranto (Mediterranean Sea). Front. Mar. Sci. 2022, 9, 842616. [Google Scholar] [CrossRef]
- Protection of Animals Used for Scientific purposesText with EEA Relevance. The Directive 2010/63/EU of the European Parliament and of the Council; Council of the European Parliament: Strasbourg, France, 2010. [Google Scholar]
- Metcalfe, J.D.; Craig, J.F. Ethical Justification for the Use and Treatment of Fishes in Research: An Update. J. Fish Biol. 2011, 78, 393–394. [Google Scholar] [CrossRef]
- No. 1099/2009 Protection of Animals at the Time of Killing Text with EEA Relevance. In Council of the European Parliament Council Regulation (EC). 2009. Available online: https://eur-lex.europa.eu/eli/reg/2009/1099/oj/eng (accessed on 12 June 2025).
- Tarricone, S.; Ragni, M.; Carbonara, C.; Giannico, F.; Bozzo, F.; Petrontino, A.; Caputi Jambrenghi, A.; Colonna, M.A. Growth Performance and Flesh Quality of Sea Bass (Dicentrarchus labrax) Fed with Diets Containing Olive Oil in Partial Replacement of Fish Oil—With or Without Supplementation with Rosmarinus officinalis L. Essential Oil. Animals 2024, 14, 3237. [Google Scholar] [CrossRef]
- Tarricone, S.; Caputi Jambrenghi, A.; Cagnetta, P.; Ragni, M. Wild and Farmed Sea Bass (Dicentrarchus labrax): Comparison of Biometry Traits, Chemical and Fatty Acid Composition of Fillets. Fishes 2022, 7, 45. [Google Scholar] [CrossRef]
- Sim, Y.J.; Cho, S.H. Effect of Partial or Complete Substitution of Fish Meal by Meat Meal in the Feed of Red Sea Bream (Pagrus Major) on the Growth Performance and Feed Utilization. Aquac. Nutr. 2025, 2025, 9589317. [Google Scholar] [CrossRef]
- Tarricone, S.; Iaffaldano, N.; Colonna, M.A.; Giannico, F.; Selvaggi, M.; Caputi Jambrenghi, A.; Cariglia, M.; Ragni, M. Effects of Dietary Red Grape Extract on the Quality Traits in Juvenile European Sea Bass (Dicentrarchus labrax L.). Animals 2023, 13, 254. [Google Scholar] [CrossRef]
- Wang, Z.; Qiao, F.; Zhang, W.; Parisi, G.; Du, Z.; Zhang, M. The Flesh Texture of Teleost Fish: Characteristics and Interventional Strategies. Rev. Aquac. 2024, 16, 508–535. [Google Scholar] [CrossRef]
- Agulheiro-Santos, A.C.; Machado, G.; Eusébio, T.; Lança, M.J. Textural Analysis of Sea Lamprey Muscle From Guadiana and Mondego Rivers (Portugal) Using the Warner-Bratzler Shear Method. J. Texture Stud. 2025, 56, e70002. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemistry. Official Methods of Analysis of the AOAC, 17th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2000. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Christie, W.W. Lipid Analysis: Isolation, Separation, Identification and Structural Analysis of Lipids; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-1-4831-8717-4. [Google Scholar]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Dambrosio, A.; Quaglia, N.C.; Colonna, M.A.; Capuozzo, F.; Giannico, F.; Tarricone, S.; Caputi Jambrenghi, A.; Ragni, M. Shelf-Life and Quality of Anchovies (Engraulis encrasicolus) Refrigerated Using Different Packaging Materials. Fishes 2023, 8, 268. [Google Scholar] [CrossRef]
- Martinsdottir, E.; Schelvis, R.; Hylding, G.; Sveinsdottir, K. Fishery Products, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Nguyen, M.V.; Karnue, S.; Kakooza, D. Effect of Packaging Method and Storage Temperature on the Sensory Quality and Lipid Stability of Fresh Snakehead Fish (Channa striata) Fillets. Food Sci. Technol. 2023, 43, e116222. [Google Scholar] [CrossRef]
- Shewan, J.M.; Macintosh, R.G.; Tucker, C.G.; Ehrenberg, A.S.C. The Development of a Numerical Scoring System for the Sensory Assessment of the Spoilage of Wet White Fish Stored in Ice. J. Sci. Food Agric. 1953, 4, 283–298. [Google Scholar] [CrossRef]
- Python The Python Language Reference. Available online: https://docs.python.org/3/reference/index.html (accessed on 12 June 2025).
- Ghosh, A.K.; Hasanuzzaman, A.F.; Islam, S.S.; Sarower, M.G.; Mistry, S.K.; Arafat, S.T.; Huq, K.A. Integrated Multi-Trophic Aquaculture (IMTA): Enhancing Growth, Production, Immunological Responses, and Environmental Management in Aquaculture. Aquac. Int. 2025, 33, 336. [Google Scholar] [CrossRef]
- Estévez, A.; Vasilaki, P. Organic Production of Gilthead Sea Bream (Sparus aurata) Using Organic Certified Green Pea Protein and Seaweed. Effects on Growth, Feed Conversion and Final Product Quality. Aquaculture 2023, 571, 739490. [Google Scholar] [CrossRef]
- Latremouille, D.N. Fin Erosion in Aquaculture and Natural Environments. Rev. Fish. Sci. 2003, 11, 315–335. [Google Scholar] [CrossRef]
- Bordignon, F.; Ferrarese, L.; Solimeo, A.; Di Leva, V.; Trocino, A. Animal-Based Measures for Operational Welfare Indicators at Wholesale Level in Gilthead Seabream (Sparus aurata) Reared in the Mediterranean Sea. Aquaculture 2025, 603, 742417. [Google Scholar] [CrossRef]
- Filipa-Silva, A.; Monteiro, M.; Costa, R.S.; Sá, T.; Marques, A.; Valente, L.M.P.; Figueiredo-Silva, C. Comparative Study of Dietary Selenium Sources on Gilthead Seabream (Sparus aurata): Growth, Nutrient Utilization, Stress Response and Final Product Quality. Aquaculture 2025, 595, 741508. [Google Scholar] [CrossRef]
- Orban, E.; Sinesio, F.; Paoletti, F. The Functional Properties of the Proteins, Texture and the Sensory Characteristics of Frozen Sea Bream Fillets (Sparus aurata) from Different Farming Systems. LWT-Food Sci. Technol. 1997, 30, 214–217. [Google Scholar] [CrossRef]
- Ayala, M.D.; Santaella, M.; Martínez, C.; Periago, M.J.; Blanco, A.; Vázquez, J.M.; Albors, O.L. Muscle Tissue Structure and Flesh Texture in Gilthead Sea Bream, Sparus aurata L., Fillets Preserved by Refrigeration and by Vacuum Packaging. LWT-Food Sci. Technol. 2011, 44, 1098–1106. [Google Scholar] [CrossRef]
- Attouchi, M.; Sadok, S. The Effects of Essential Oils Addition on the Quality of Wild and Farmed Sea Bream (Sparus aurata) Stored in Ice. Food Bioprocess Technol. 2012, 5, 1803–1816. [Google Scholar] [CrossRef]
- Sáez, M.I.; Sabio, J.; Galafat, A.; Vizcaíno, A.J.; Alarcón-López, F.J.; Moya, T.F.M. Evaluation of White Grape Marc Extract as an Additive to Extend the Shelf-Life of Fish Fillets. Foods 2025, 14, 1438. [Google Scholar] [CrossRef]
- Abbas, K.A.; Mohamed, A.; Jamilah, B.; Ebrahimian, M. A Review on Correlations between Fish Freshness and pH during Cold Storage. Am. J. Biochem. Biotechnol. 2008, 4, 416–421. [Google Scholar] [CrossRef]
- Yildiz, M.; Şener, E.; Timur, M. The Effects of Seasons and Different Feeds on Fatty Acid Composition in Fillets of Cultured Gilthead Sea Bream (Sparus aurata L.) and European Sea Bass (Dicentrarchus labrax L.) in Turkey. Turk. J. Vet. Anim. Sci. 2006, 30, 133–141. [Google Scholar]
- Yildiz, M.; Şener, E.; Timur, M. Effects of Differences in Diet and Seasonal Changes on the Fatty Acid Composition in Fillets from Farmed and Wild Sea Bream (Sparus aurata L.) and Sea Bass (Dicentrarchus labrax L.). Int. J. Food Sci. Technol. 2008, 43, 853–858. [Google Scholar] [CrossRef]
- Vasconi, M.; Caprino, F.; Bellagamba, F.; Moretti, V.M. Fatty Acid Composition of Gilthead Sea Bream (Sparus aurata) Fillets as Affected by Current Changes in Aquafeed Formulation. Turk. J. Fish. Aquat. Sci. 2017, 17, 451–459. [Google Scholar] [CrossRef]
- Meinam, M.; Deepti, M.; Madhulika; Ngasotter, S. Emerging Aquaculture Technologies for Food and Nutritional Security. In Food Security, Nutrition and Sustainability Through Aquaculture Technologies; Sundaray, J.K., Rather, M.A., Ahmad, I., Amin, A., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 19–41. ISBN 978-3-031-75830-0. [Google Scholar]
- Calanche, J.; Pedrós, S.; Roncalés, P.; Beltrán, J.A. Design of Predictive Tools to Estimate Freshness Index in Farmed Sea Bream (Sparus aurata) Stored in Ice. Foods 2020, 9, 69. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Boziaris, I.S.; Drosinos, E.H. Detection of Fish Spoilage. In Handbook of Seafood and Seafood Products Analysis; CRC: Boca Raton, FL, USA, 2024; ISBN 978-1-003-28940-1. [Google Scholar]
- Alasalvar, C.; Taylor, K.D.A.; Öksüz, A.; Garthwaite, T.; Alexis, M.N.; Grigorakis, K. Freshness Assessment of Cultured Sea Bream (Sparus aurata) by Chemical, Physical and Sensory Methods. Food Chem. 2001, 72, 33–40. [Google Scholar] [CrossRef]
- Šimat, V.; Bogdanović, T.; Krželj, M.; Soldo, A.; Maršić-Lučić, J. Differences in Chemical, Physical and Sensory Properties during Shelf Life Assessment of Wild and Farmed Gilthead Sea Bream (Sparus aurata L.). J. Appl. Ichthyol. 2012, 28, 95–101. [Google Scholar] [CrossRef]
- Lougovois, V.P.; Kyranas, E.R.; Kyrana, V.R. Comparison of Selected Methods of Assessing Freshness Quality and Remaining Storage Life of Iced Gilthead Sea Bream (Sparus aurata). Food Res. Int. 2003, 36, 551–560. [Google Scholar] [CrossRef]

| % | |
|---|---|
| Chemical composition (% on DM basis) | |
| Moisture | 6.20 |
| Crude Protein | 44.58 |
| Total Lipid | 16.05 |
| Total N-free extracts | 13.03 |
| Fiber | 1.90 |
| Ash | 9.13 |
| Gross Energy (MJ/kg) | 20.21 |
| Fatty Acid Profile (% FA methyl esters) | |
| C14:0 | 5.3 |
| C15:0 | 0.45 |
| C16:0 | 15.7 |
| C17:0 | 5.2 |
| C18:0 | 3.6 |
| C20:0 | 1.7 |
| C16:1 n-7 | 7.6 |
| C162 n-4 | 1.0 |
| C16:3 n-4 | 1.0 |
| C18:1 n-9 | 13.7 |
| C18:1 n-7 | 4.5 |
| C20:1 n-9 | 3.3 |
| C18:2 n-6 | 5.0 |
| C20:2 n-6 | 0.5 |
| C18:3 n-6 | 1.1 |
| C18:3 n-3 | 1.8 |
| C18:4 n-3 | 1.6 |
| C20:4 n-3 | 0.7 |
| C20:5 n-3 | 11.2 |
| C22:5 n-3 | 0.85 |
| C22:6 n-3 | 11.3 |
| Control | IMTA | SEM 1 | p-Value | |
|---|---|---|---|---|
| Total body weight (g) | 277.59 b | 331.44 a | 0.863 | 0.046 |
| Total body length (cm) | 26.74 | 26.25 | 0.273 | 0.082 |
| Fork length (cm) | 24.95 | 24.46 | 0.277 | 0.091 |
| Tail length (cm) | 5.24 b | 6.03 a | 0.074 | 0.027 |
| Relative profile (%) | 36.45 | 36.19 | 0.146 | 0.857 |
| Cranial index (%) | 20.99 B | 24.56 A | 0.863 | 0.001 |
| Condition factor (%) | 1.45 | 1.83 | 0.032 | 0.145 |
| Edible yield (%) | 38.15 A | 32.70 B | 0.155 | 0.001 |
| Viscerosomatic Index (%) | 6.01 | 5.19 | 0.107 | 0.451 |
| Hepatosomatic index (%) | 1.13 a | 0.96 b | 0.081 | 0.015 |
| Parameters | Control | IMTA | SEM 1 | Effects 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 | 14 | 1 | 7 | 14 | R | S | R × S | ||
| pH | 6.26 | 6.33 | 6.34 | 5.96 | 6.08 | 6.18 | 0.079 | 0.076 | 0.156 | 0.159 |
| Colour indices of sea bream dorsal skin area | ||||||||||
| L* | 59.09 X | 52.49 XY | 49.16 Y | 48.39 X | 46.03 XY | 43.09 Y | 0.985 | 0.231 | 0.006 | 0.061 |
| a* | −2.14 Y | −2.40 XY | −2.72 X | −1.90 Y | −2.12 XY | −2.40 X | 0.209 | 0.084 | 0.002 | 0.074 |
| b* | 4.15 Y | 4.49 Y | 5.52 X | 4.14 Y | 4.21 Y | 4.98 X | 0.319 | 0.091 | 0.001 | 0.043 |
| Colour indices of sea bream fillet | ||||||||||
| L* | 59.33 Y | 62.03 XY | 65.08 X | 58.14 Y | 60.38 XY | 62.09 X | 0.985 | 0.231 | 0.006 | 0.081 |
| a* | −1.14 Y | −1.40 XY | −1.52 X | −1.09 Y | −1.23 XY | −1.38 Y | 0.209 | 0.084 | 0.002 | 0.054 |
| b* | −0.15 Y | −0.09 XY | 0.20 X | −0.24 Y | −0.19 XY | 0.58 X | 0.319 | 0.091 | 0.001 | 0.073 |
| Hardness (N) | 12.36 X | 10.10 XY | 6.38 Y | 12.90 X | 9.98 XY | 9.12 Y | 0.937 | 0.353 | 0.001 | 0.026 |
| Gumminess (N) | 5.78 X | 4.45 XY | 3.25 Y | 5.55 X | 4.18 XY | 2.64 Y | 0.232 | 0.294 | 0.001 | 0.034 |
| Springiness (mm) | 2.36 | 1.94 | 1.64 | 2.29 | 1.61 | 1.35 | 0.064 | 0.077 | 0.091 | 0.121 |
| Cohesion Force Resilience | 0.79 X | 0.54 XY | 0.49 Y | 0.76 X | 0.60 XY | 0.57 Y | 0.064 | 0.072 | 0.008 | 0.031 |
| Chewiness (N × mm) | 13.56 X | 11.11 XY | 8.56 Y | 12.68 X | 11.67 XY | 8.57 Y | 0.557 | 0.221 | 0.001 | 0.021 |
| MDA (mg/kg) | 0.18 aY | 0.22 Y | 0.50 aX | 0.12 bY | 0.18 Y | 0.32 bX | 0.018 | 0.043 | 0.002 | 0.001 |
| Parameters | Control | IMTA | SEM 1 | Effects 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 | 14 | 1 | 7 | 14 | R | S | R × S | ||
| Moisture | 72.59 x | 71.23 bxy | 70.11 y | 72.86 x | 72.31 ax | 70.08 y | 0.340 | 0.017 | 0.025 | 0.010 |
| Protein | 19.38 | 19.50 | 19.72 | 19.20 | 19.27 | 19.88 | 0.284 | 0.155 | 0.156 | 0.077 |
| Lipid | 5.61 | 5.99 | 6.35 | 5.50 | 5.81 | 6.36 | 0.264 | 0.082 | 0.096 | 0.101 |
| Ash | 1.34 | 1.72 | 1.82 | 1.34 | 1.65 | 1.97 | 0.121 | 0.065 | 0.071 | 0.059 |
| N-free extract | 1.08 | 1.52 | 1.70 | 1.10 | 1.41 | 1.71 | 0.208 | 0.743 | 0.124 | 0.069 |
| Parameters | Control | IMTA | SEM 1 | Effects 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 | 14 | 1 | 7 | 14 | R | S | R × S | ||
| C10:0 | 0.02 | 0.03 | 0.03 | 0.04 | 0.04 | 0.05 | 0.002 | 0.236 | 0.804 | 0.166 |
| C12:0 | 0.07 | 0.07 | 0.08 | 0.05 | 0.06 | 0.07 | 0.002 | 0.229 | 0.062 | 0.113 |
| C14:0 (Myristic) | 2.45 A | 2.51 A | 2.58 A | 1.91 B | 2.11 B | 2.16 B | 0.047 | 0.002 | 0.372 | 0.032 |
| C15:0 (Pentadecylic) | 0.27 | 0.29 | 0.31 | 0.24 | 0.25 | 0.28 | 0.005 | 0.361 | 0.060 | 0.372 |
| C16:0 (Palmitic) | 14.88 Ay | 16.16 Ax | 17.96 Ax | 13.72 By | 14.52 Bx | 14.89 Bx | 0.093 | 0.003 | 0.013 | 0.043 |
| C17:0 | 0.24 | 0.24 | 0.26 | 0.25 | 0.29 | 0.30 | 0.009 | 0.144 | 0.062 | 0.086 |
| C18:0 (Stearic) | 4.12 y | 4.28 x | 4.64 x | 3.81 y | 4.24 x | 4.28 x | 0.054 | 0.337 | 0.041 | 0.117 |
| C20:0 | 0.12 b | 0.13 b | 0.16 b | 0.43 a | 0.44 a | 0.47 a | 0.023 | 0.023 | 0.061 | 0.084 |
| C21:0 | 0.57 | 0.68 | 0.68 | 0.59 | 0.68 | 0.69 | 0.024 | 0.808 | 0.149 | 0.561 |
| C22:0 | 0.19 by | 0.22 bxy | 0.25 bx | 0.29 ay | 0.32 axy | 0.35 ax | 0.009 | 0.036 | 0.027 | 0.014 |
| C23:0 | 1.21 BY | 1.52 XY | 1.78 AY | 1.46 AY | 1.58 XY | 1.74 AX | 0.037 | 0.008 | 0.006 | 0.016 |
| Total SFA 3 | 24.19 aY | 26.07 aX | 28.78 aX | 22.81 bY | 24.57 bX | 25.11 bX | 0.109 | 0.018 | 0.001 | 0.022 |
| C16:1 n-9s | 4.16 a | 3.69 | 3.66 a | 3.51 b | 3.68 | 3.41 b | 0.053 | 0.035 | 0.320 | 0.088 |
| C17:1 | 0.16 | 0.14 | 0.14 | 0.19 | 0.17 | 0.17 | 0.004 | 0.074 | 0.063 | 0.083 |
| C18:l n-9 (Oleic) | 34.12 bx | 33.96 bxy | 32.58 by | 35.37 a | 34.41 a | 34.34 a | 0.188 | 0.032 | 0.045 | 0.057 |
| C20:l n-9 (Eicosanoic) | 0.36 | 0.34 | 0.29 | 0.33 | 0.32 | 0.30 | 0.006 | 0.155 | 0.195 | 0.411 |
| C24:1 n-9 | 0.53 | 0.50 | 0.48 | 0.48 | 0.48 | 0.47 | 0.009 | 0.190 | 0.529 | 0.394 |
| Total MUFA 4 | 39.41 | 38.71 | 37.22 | 39.96 | 39.14 | 38.76 | 0.220 | 0.097 | 0.057 | 0.067 |
| C18:2 n6 (linoleic) | 21.92 | 21.70 | 21.52 | 22.74 | 22.18 | 22.43 | 0.166 | 0.735 | 0.151 | 0.754 |
| C18:3 n-3 (α-linolenic) | 1.94 | 1.73 | 1.62 | 1.67 | 1.75 | 1.75 | 0.072 | 0.071 | 0.056 | 0.131 |
| C18:3 n-6 (γ -linolenic) | 4.24 a,x | 3.56 y | 3.33 y | 3.57 b | 3.53 | 3.48 | 0.039 | 0.038 | 0.043 | 0.148 |
| C20:3 n-6 | 0.34 bx | 0.27 by | 0.25 by | 0.41 ax | 0.37 ay | 0.34 ay | 0.025 | 0.018 | 0.047 | 0.053 |
| C20:3 n-3 | 0.20 | 0.18 | 0.17 | 0.18 | 0.18 | 0.16 | 0.004 | 0.202 | 0.055 | 0.486 |
| C20:4 n-6 ARA | 0.41 x | 0.35 y | 0.34 y | 0.45 x | 0.38 y | 0.35 y | 0.029 | 0.075 | 0.032 | 0.082 |
| C20:5 n-3 (eicosapentaenoic, EPA) | 0.35 | 0.33 | 0.33 | 0.32 | 0.32 | 0.32 | 0.009 | 0.077 | 0.069 | 0.066 |
| C22:5 n-3 (docosapentaenoic, DPA) | 1.32 b | 1.32 b | 1.31 b | 1.53 a | 1.50 a | 1.42 a | 0.030 | 0.023 | 0.072 | 0.334 |
| C22:6 n-3 (docosahexaenoic, DHA) | 4.81 b | 4.62 b | 4.39 b | 5.47 a | 5.30 a | 5.17 a | 0.152 | 0.043 | 0.061 | 0.074 |
| Total PUFA 5 | 35.71 bx | 34.23 by | 33.39 by | 37.00 ax | 35.63 ay | 35.54 ay | 0.255 | 0.039 | 0.047 | 0.048 |
| Total n-6 6 | 27.20 x | 26.10 y | 25.61 y | 27.81 x | 26.96 y | 26.77 y | 0.142 | 0.073 | 0.041 | 0.098 |
| Total n-3 7 | 8.62 bx | 8.17 bxy | 7.83 by | 9.25 a | 9.09 a | 8.92 a | 0.084 | 0.041 | 0.037 | 0.038 |
| n-3/n-6 | 0.32 | 0.31 | 0.31 | 0.33 | 0.34 | 0.33 | 0.003 | 0.069 | 0.081 | 0.132 |
| AI (Atherogenic Index) | 0.33 ay | 0.36 axy | 0.40 ax | 0.28 b | 0.31 b | 0.32 b | 0.094 | 0.039 | 0.042 | 0.084 |
| TI (Thrombogenic Index) | 0.36 ay | 0.42 ax | 0.45 ax | 0.29 by | 0.35 bx | 0.36 bx | 0.074 | 0.027 | 0.034 | 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarricone, S.; Colonna, M.A.; Ragni, M.; Trani, R.; Giangrande, A.; Basile, G.; Stabili, L.; Carbonara, C.; Giannico, F.; Longo, C. Flesh Quality, Shelf Life, and Freshness Assessment of Sea Bream Reared in a Coastal Mediterranean Integrated Multi-Trophic Aquaculture System. Animals 2025, 15, 2425. https://doi.org/10.3390/ani15162425
Tarricone S, Colonna MA, Ragni M, Trani R, Giangrande A, Basile G, Stabili L, Carbonara C, Giannico F, Longo C. Flesh Quality, Shelf Life, and Freshness Assessment of Sea Bream Reared in a Coastal Mediterranean Integrated Multi-Trophic Aquaculture System. Animals. 2025; 15(16):2425. https://doi.org/10.3390/ani15162425
Chicago/Turabian StyleTarricone, Simona, Maria Antonietta Colonna, Marco Ragni, Roberta Trani, Adriana Giangrande, Grazia Basile, Loredana Stabili, Claudia Carbonara, Francesco Giannico, and Caterina Longo. 2025. "Flesh Quality, Shelf Life, and Freshness Assessment of Sea Bream Reared in a Coastal Mediterranean Integrated Multi-Trophic Aquaculture System" Animals 15, no. 16: 2425. https://doi.org/10.3390/ani15162425
APA StyleTarricone, S., Colonna, M. A., Ragni, M., Trani, R., Giangrande, A., Basile, G., Stabili, L., Carbonara, C., Giannico, F., & Longo, C. (2025). Flesh Quality, Shelf Life, and Freshness Assessment of Sea Bream Reared in a Coastal Mediterranean Integrated Multi-Trophic Aquaculture System. Animals, 15(16), 2425. https://doi.org/10.3390/ani15162425











