Genetic Parameters of the Growth Rate, Survival Rate and Feed Efficiency Ratio of Turbot (Scophthalmus maximus) at an Early Growth Stage
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Broodstock and Experimental Family
2.2. Larval and Juvenile Rearing
2.3. Data Collection
2.4. Statistical and Genetic Analysis
3. Results
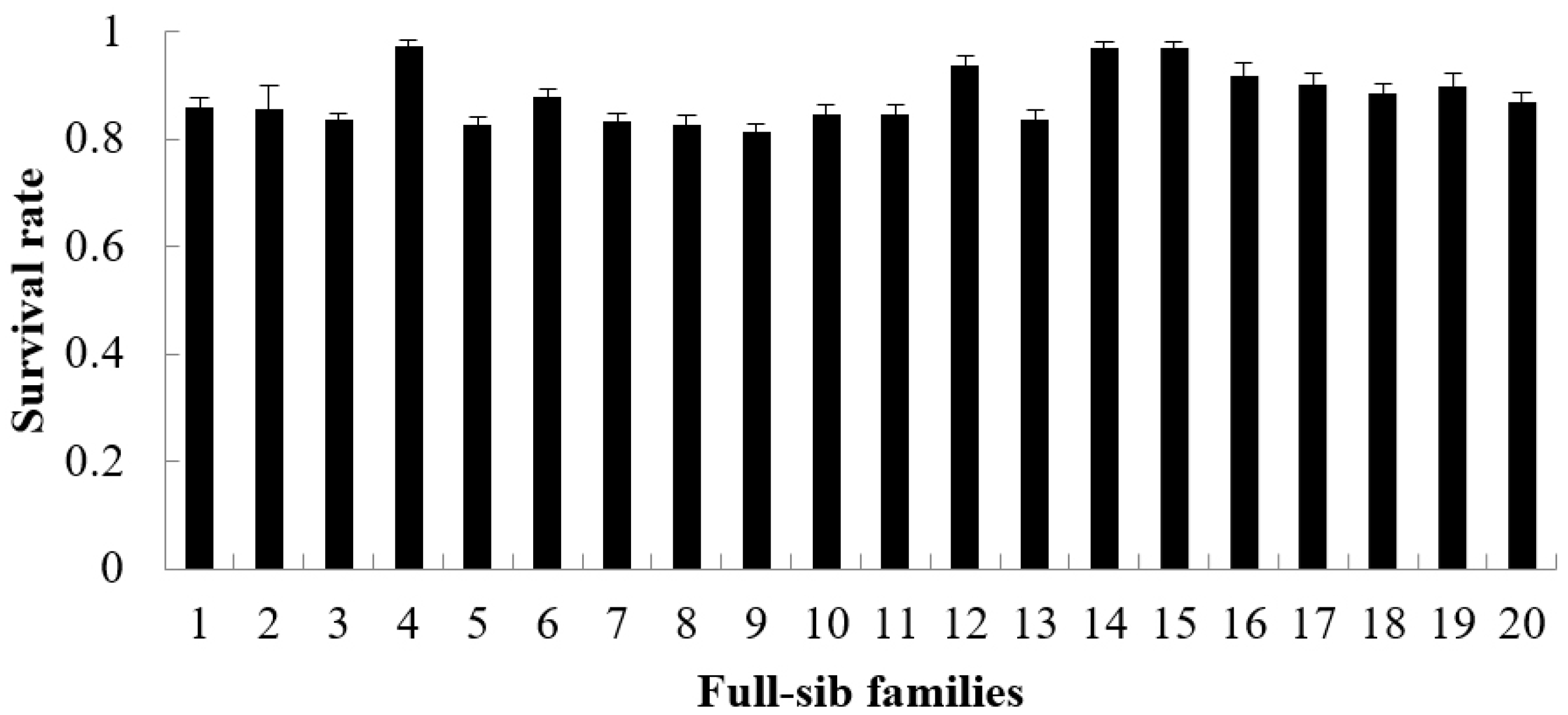
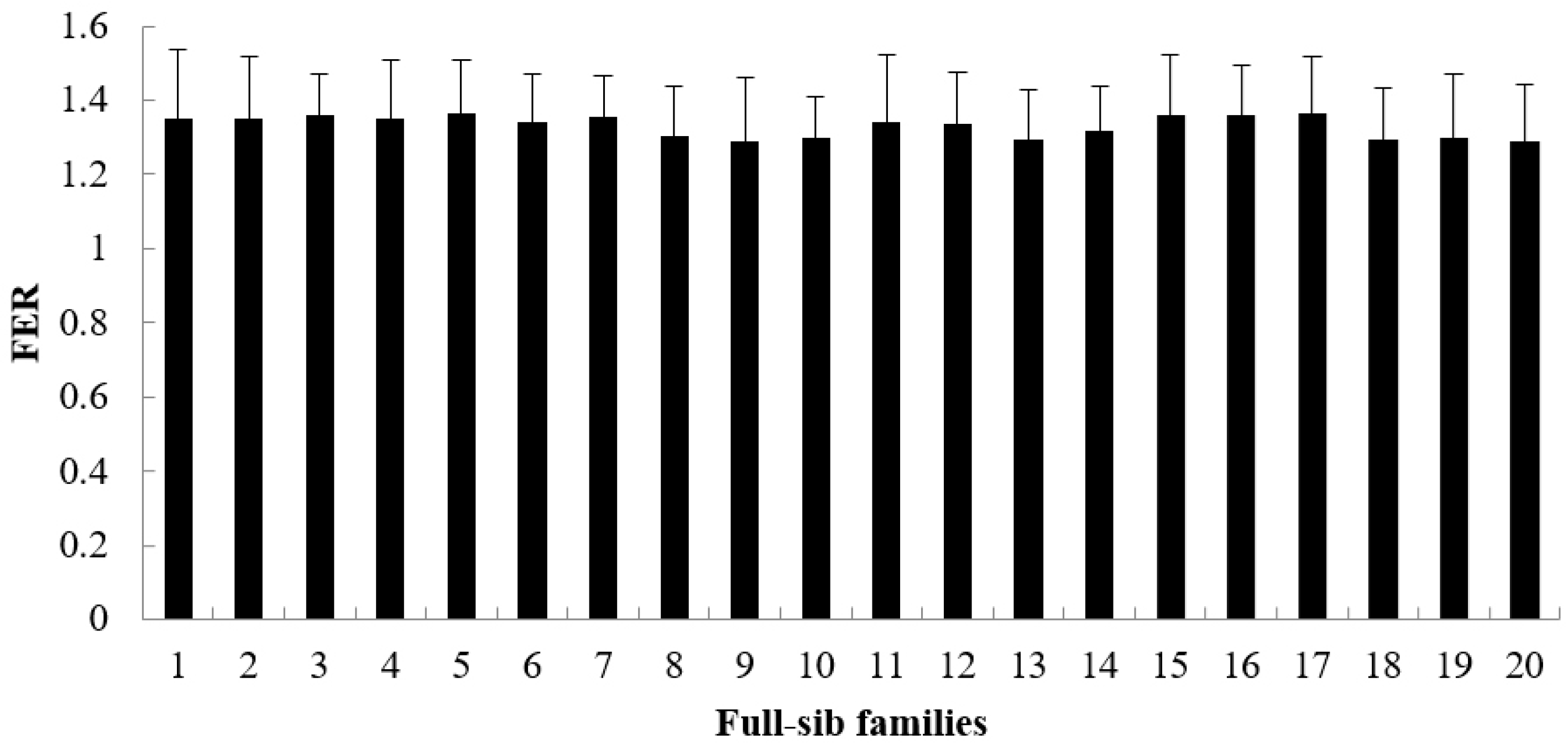
3.1. Descriptive Summary of the Growth Rate, Survival Rate and FER
3.2. Genetic Evaluation for the Growth Rate, Survival Rate and FER
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.A.; Ma, A.; Huang, Z.; Sun, Z.; Cui, W.; Qu, J.; Yu, H. Estimation of genetic parameters for upper thermal tolerances and growth-related traits in turbot Scophthalmus maximus. J. Oceanol. Limnol. 2019, 37, 1736–1745. [Google Scholar] [CrossRef]
- Wang, X.A.; Ma, A.J.; Huang, Z.H.; Zhou, Z. Heritability and genetic correlation of survival in turbot (Scophthalmus maximus). Chin. J. Oceanol. Limnol. 2010, 28, 1200–1205. [Google Scholar] [CrossRef]
- Chavanne, H.; Janssen, K.; Hofherr, J.; Contini, F.; Haffray, P.; Consortium, A.; Komen, H.; Nielsen, E.E.; Bargelloni, L. A comprehensive survey on selective breeding programs and seed market in the European aquaculture fish industry. Aquac. Int. 2016, 24, 1287–1307. [Google Scholar] [CrossRef]
- de Verdal, H.; Komen, H.; Quillet, E.; Chatain, B.; Allal, F.; Benzie, J.A.H.; Vandeputte, M. Improving feed efficiency in fish using selective breeding: A review. Rev. Aquac. 2018, 10, 833–851. [Google Scholar] [CrossRef]
- Folke, C.; Kautsky, N.; Troell, M. The costs of eutrophication from salmon farming: Implications for policy. J. Environ. Manag. 1994, 40, 173–182. [Google Scholar] [CrossRef]
- Mathieu, B.; Hans, K.; Gus, R.; Marc, V. The genetic correlation between feed efficiency ratio and growth rate affects the design of a breeding program for more sustainable fish production. Genet. Sel. Evol. 2020, 52, 5. [Google Scholar]
- Besson, M.; Aubin, J.; Komen, H.; Poelman, M.; Quillet, E.; Vandeputte, M.; van Arendonk, J.A.M.; de Boer, I.J.M. Environmental impacts of genetic improvement of growth rate and feed efficiency ratio in fish farming under rearing density and nitrogen output limitations. J. Clean. Prod. 2016, 116, 100–109. [Google Scholar] [CrossRef]
- Besson, M.; de Boer, I.J.M.; Vandeputte, M.; van Arendonk, J.A.M.; Quillet, E.; Komen, H.; Aubin, J. Effect of production quotas on economic and environmental values of growth rate and feed efficiency in sea cage fish farming. PLoS ONE 2017, 12, e0173131. [Google Scholar] [CrossRef] [PubMed]
- de Verdal, H.; Haffray, P.; Douchet, V.; Vandeputte, M. Impact of a divergent selective breeding programme on individual feed conversion ratio in Nile tilapia Oreochromis niloticus measured in groups by video-recording. Aquaculture 2022, 548, 737572. [Google Scholar] [CrossRef]
- Henryon, M.; Jokumsen, A.; Berg, P.; Lund, I.; Pedersen, P.B.; Olesen, N.J.; Slierendrecht, W.J. Genetic variation for growth rate, feed conversion efficiency, and disease resistance exists within a farmed population of rainbow trout. Aquaculture 2002, 209, 59–76. [Google Scholar] [CrossRef]
- Taylor, R.S.; Kube, P.D.; Muller, W.J.; Elliott, N.G. Genetic variation of gross gill pathology and survival of Atlantic salmon (Salmo salar L.) during natural amoebic gill disease challenge. Aquaculture 2009, 294, 172–179. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Goto, T.; Nishino, J.; Nakaoka, H.; Tanave, A.; Takano-Shimizu, T.; Mott, R.F.; Koide, T. Selective breeding and selection mapping using a novel wild-derived heterogeneous stock of mice revealed two closely-linked loci for tameness. Sci. Rep. 2017, 7, 4607. [Google Scholar] [CrossRef]
- Wang, X.A.; Ma, A.J. Genetic parameters for resistance against Vibrio anguillarum in turbot Scophthalmus maximus. J. Fish Dis. 2019, 42, 713–720. [Google Scholar] [CrossRef]
- Fu, J.J.; Shen, Y.B.; Xu, X.Y.; Liu, C.C.; Li, J.L. Genetic parameter estimates and genotype by environment interaction analyses for early growth traits in grass carp (Ctenopharyngodon idella). Aquac. Int. 2015, 23, 1427–1441. [Google Scholar] [CrossRef]
- Sun, M.M.; Huang, J.H.; Jiang, S.G.; Yang, Q.B.; Zhou, F.L.; Zhu, C.Y.; Yang, L.S.; Su, T.F. Estimates of heritability and genetic correlations for growth-related traits in the tiger prawn Penaeus monodon. Aquac. Res. 2015, 46, 1363–1368. [Google Scholar] [CrossRef]
- Thodesen, J.; Gjerde, B.; Grisdale-Helland, B.; Storebakken, T. Genetic variation in feed intake, growth and feed utilization in Atlantic salmon (Salmo salar). Aquaculture 2001, 194, 273–281. [Google Scholar] [CrossRef]
- Mambrini, M.; Sanchez, M.-P.; Chevassus, B.; Labbé, L.; Quillet, E.; Boujard, T. Selection for growth increase feed intake and affects feeding behavior of brown trout. Livest. Prod. Sci. 2004, 88, 85–98. [Google Scholar] [CrossRef]
- Li, M.H.; Robinson, E.H.; Bosworth, B.G. Effects of periodic feed deprivation on growth, feed efficiency, processing yield, and body composition of channel catfish Ictalurus punctatus. J. World. Aquac. Soc. 2005, 36, 444–453. [Google Scholar] [CrossRef]
- Albrektsen, S.; Mundheim, H.; Aksnes, A. Growth, feed efficiency, digestibility and nutrient distribution in Atlantic cod (Gadus morhua) fed two diffrent fish meal qualities at three dietary levels of vegetable protein sources. Aquaculture 2007, 261, 626–640. [Google Scholar] [CrossRef]
- Aknes, A.; Hope, B.; Jönsson, E.; Björnsson, B.T.; Albrektsen, S. Size-fractionated fish hydrolysate as feed ingredient for rainbow trout (Oncorhynchus mykiss) fed high plan protein diets. I: Growth, growth regulation and feed utilization. Aquaculture 2006, 261, 305–317. [Google Scholar] [CrossRef]
- Kolstad, K.; Grisdale-Helland, B.; Gjerde, B. Family differences in feed efficiency in Atlantic salmon (Salmo salar). Aquaculture 2004, 241, 169–177. [Google Scholar] [CrossRef]
- Doupé, R.G.; Lymbery, A.J. Toward the genetic improvement of feed conversion efficiency in fish. J. World. Aquac. Soc. 2003, 34, 245–254. [Google Scholar] [CrossRef]
- Kause, A.; Tobin, D.; Dobly, A.; Houlihan, D.; Martin, S.; Mäntysaari, E.A.; Ritola, O.; Ruohonen, K. Recording strategies and selection potential of feed intake measured using the X-ray method in rainbow trout. Genet. Sel. Evol. 2006, 38, 389–409. [Google Scholar] [CrossRef]
- McComish, T.S.T. Laboratory Experiments on Growth and Food Conversion by the Bluegill; University of Missouri: Columbia, MO, USA, 1971; p. 203. [Google Scholar]
- Hayward, R.S.; Wang, N.; Noltie, D.B. Group holding impedes compensatory growth of hybrid sunfish. Aquaculture 2000, 183, 299–305. [Google Scholar] [CrossRef]
- Silverstein, J.T. Relationship among feed intake, feed efficiency, and growth in juvenile rainbow trout. N. Am. J. Aquac. 2006, 68, 168–175. [Google Scholar] [CrossRef]
- Wang, X.A.; Ma, A.J. Dynamic genetic analysis for body weight and main length ratio in turbot Scophthalmus maximus. Acta Oceanol. Sin. 2020, 39, 22–27. [Google Scholar] [CrossRef]
- Boldman, K.G.; Kriese, L.A.; Van Vleck, L.D.; Van Tassell, C.P.; Kachman, S.D. A Manual for Use of MTDFREML—A Set of Programs to Obtain Estimates of Variance and Covariances (Draft); U.S. Department of Agriculture-Agricultural Research Service: Clay Center, NE, USA, 1995.
- Ahmad, A.; Sonesson, A.K.; Hatlen, B.; Bæverfjord, G.; Berg, P.; Norris, A.; Difford, G.F. Genetic analysis of individual feed intake and efffciency in Atlantic salmon smolts using X-ray imaging. Aquaculture 2025, 608, 742715. [Google Scholar] [CrossRef]
- Kause, A.; Tobin, D.; Houlihan, D.; Martin, S.A.M.; Mäntysaari, E.A.; Ritola, O.; Ruohonen, K. Feed efficiency of rainbow trout can be improved through selection: Different genetic potential on alternative diets. J. Anim. Sci. 2006, 84, 807–817. [Google Scholar] [CrossRef]
- Kinghorn, B. Genetic variation in food conversion efficiency and growth in rainbow trout. Aquaculture 1983, 32, 141–155. [Google Scholar] [CrossRef]
- Quinton, C.D.; Kause, A.; Ruohonen, K.; Koskela, J. Genetic relationships of body composition and feed utilization traits in European whitefish (Coregonus lavaretus L.) and implications for selective breeding in fishmeal- and soybean meal-based diet environments. J. Anim. Sci. 2007, 85, 3198–3208. [Google Scholar] [CrossRef]
- Quinton, C.D.; Kause, A.; Koskela, J.; Ritola, O. Breeding salmonids for feed efficiency in current fishmeal and future plant-based diet environments. Genet. Sel. Evol. 2007, 39, 431–446. [Google Scholar] [CrossRef]
- Daulé, S.; Vandeputte, M.; Vergnet, A.; Guinand, B.; Grima, L.; Chatain, B. Effect of selection for fasting tolerance on feed intake, growth and feed efficiency in the European sea bass Dicentrarchus labrax. Aquaculture 2014, 250, S42–S49. [Google Scholar] [CrossRef]
- Moran, D.; Schleyken, J.; Flammensbeck, C.; Fantham, W.; Ashton, D.; Wellenreuther, M. Enhanced survival and growth in the selectively bred Chrysophrys auratus (Australasian snapper, tamure). Aquaculture 2023, 563, 738970. [Google Scholar] [CrossRef]
- Vandeputte, M.; Corraze, G.; Doerffinger, J.; Enez, F.; Clota, F.; Terrier, F.; Horat, M.; Larroquet, L.; Petit, V.; Haffray, P.; et al. Realised genetic gains on growth, survival, feed conversion ratio and quality traits after ten generations of multi-trait selection in rainbow trout, fed a standard diet or a “future” fish-free and soy-free diet. Aquac. Rep. 2022, 27, 101363. [Google Scholar] [CrossRef]
- Elvy, J.E.; Symonds, J.E.; Hilton, Z.; Walker, S.P.; Tremblay, L.A.; Casanovas, P.; Herbert, N.A. The relationship of feed intake, growth, nutrient retention, and oxygen consumption to feed conversion ratio of farmed saltwater Chinook salmon (Oncorhynchus tshawytscha). Aquaculture 2022, 554, 738184. [Google Scholar] [CrossRef]
- Kajbaf, K.; Overturf, K.; Kumar, V. Integrated alternative approaches to select feed-efcient rainbow trout families to enhance the plant protein utilization. Sci. Rep. 2024, 14, 3869. [Google Scholar] [CrossRef]
- Perry, G.M.; Martyniuk, C.M.; Ferguson, M.M.; Danzmannc, R.G. Genetic parameters for upper thermal tolerance and growth-related traits in rainbow trout (Oncorhynchus mykiss). Aquaculture 2005, 250, 120–128. [Google Scholar] [CrossRef]
- Liu, Z.F.; Chang, H.W.; Xu, F.; Zhao, H.C.; Zhu, L.G.; Sun, Z.B.; Yang, M.C.; Wang, X.A.; Ma, A.J. Genome-wide association study of feed conversion ratio in turbot (Scophthalmus maximus) based on genome resequencing. Aquac. Rep. 2023, 33, 101804. [Google Scholar] [CrossRef]
- Chang, H.W.; Liu, Z.F.; Sun, Z.B.; Ma, A.J.; Wang, X.A.; Yang, J.K.; Xu, R.J. Screening of microsatellite markers associated with feed conversion ratio in Scophthalmus maximus. J. Fish. China 2023, 47, 133–143. [Google Scholar]

| Economic Traits | Variance Components | |||
|---|---|---|---|---|
| h2 | ||||
| Growth rate | 0.000411 | 0.000016 | 0.001342 | 0.232335 |
| Survival rate | 0.000310 | 0.000047 | 0.002487 | 0.109001 |
| FER | 0.000131 | 0.000018 | 0.001137 | 0.101866 |
| Economic Traits | Growth Rate | Survival Rate | FER |
|---|---|---|---|
| Growth rate | 0.0919 ± 0.02344 | 0.4609 ± 0.0470 ** | |
| Survival rate | 0.0984 ± 0.0133 | 0.2472 ± 0.0133 ** | |
| FER | 0.5654 ± 0.0896 ** | 0.3732 ± 0.0755 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gou, D.; Wang, Y.; Wang, X.; Sun, Z.; Ma, A. Genetic Parameters of the Growth Rate, Survival Rate and Feed Efficiency Ratio of Turbot (Scophthalmus maximus) at an Early Growth Stage. Animals 2025, 15, 2424. https://doi.org/10.3390/ani15162424
Gou D, Wang Y, Wang X, Sun Z, Ma A. Genetic Parameters of the Growth Rate, Survival Rate and Feed Efficiency Ratio of Turbot (Scophthalmus maximus) at an Early Growth Stage. Animals. 2025; 15(16):2424. https://doi.org/10.3390/ani15162424
Chicago/Turabian StyleGou, Donghui, Yilin Wang, Xinan Wang, Zhibin Sun, and Aijun Ma. 2025. "Genetic Parameters of the Growth Rate, Survival Rate and Feed Efficiency Ratio of Turbot (Scophthalmus maximus) at an Early Growth Stage" Animals 15, no. 16: 2424. https://doi.org/10.3390/ani15162424
APA StyleGou, D., Wang, Y., Wang, X., Sun, Z., & Ma, A. (2025). Genetic Parameters of the Growth Rate, Survival Rate and Feed Efficiency Ratio of Turbot (Scophthalmus maximus) at an Early Growth Stage. Animals, 15(16), 2424. https://doi.org/10.3390/ani15162424






