Serum TNF-Alpha and IL-10 Predict Reduced Sensitivity to Fear- and Anxiety-Related Traits in Healthy Older Dogs: Preliminary Evidence for Immune–Personality Signatures in Later Life
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Personality and Cognitive Scales
2.2.1. Dog Impulsivity Assessment Scale (DIAS)
2.2.2. Reinforcement Sensitivity Theory Personality Questionnaire-Dog (RSTPQ-D)
2.2.3. Canine Cognitive Assessment Scale (CCAS)
2.3. Cytokine and Immunoglobulin Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montoya, M.; Morrison, J.A.; Arrignon, F.; Spofford, N.; Charles, H.; Hours, M.-A.; Biourge, V. Life Expectancy Tables for Dogs and Cats Derived from Clinical Data. Front. Vet. Sci. 2023, 10, 1082102. [Google Scholar] [CrossRef] [PubMed]
- Butterwick, R.F. Impact of Nutrition on Ageing the Process. Bridging the Gap: The Animal Perspective. Br. J. Nutr. 2015, 113, S23–S25. [Google Scholar] [CrossRef]
- Guglielmini, C. Cardiovascular Diseases in the Ageing Dog: Diagnostic and Therapeutic Problems. Vet. Res. Commun. 2003, 27, 555–560. [Google Scholar] [CrossRef]
- Sutter, N.B.; Bustamante, C.D.; Chase, K.; Gray, M.M.; Zhao, K.; Zhu, L.; Padhukasahasram, B.; Karlins, E.; Davis, S.; Jones, P.G.; et al. A Single IGF1 Allele Is a Major Determinant of Small Size in Dogs. Science 2007, 316, 112–115. [Google Scholar] [CrossRef]
- Vajányi, D.; Skurková, L.; Peťková, B.; Kottferová, L.; Kasičová, Z.; Simanová, V.; Kottferová, J. Ageing Canine Companions: Most Common Manifestations and the Impact of Selected Factors. Appl. Anim. Behav. Sci. 2024, 271, 106164. [Google Scholar] [CrossRef]
- Blanchard, T.; Mugnier, A.; Déjean, S.; Priymenko, N.; Meynadier, A. Exploring Frailty in Apparently Healthy Senior Dogs: A Cross-Sectional Study. BMC Vet. Res. 2024, 20, 436. [Google Scholar] [CrossRef]
- Chen, F.L.; Ullal, T.V.; Graves, J.L.; Ratcliff, E.R.; Naka, A.; McKenzie, B.; Carttar, T.A.; Super, K.M.; Austriaco, J.; Weber, S.Y.; et al. Evaluating Instruments for Assessing Healthspan: A Multi-Center Cross-Sectional Study on Health-Related Quality of Life (HRQL) and Frailty in the Companion Dog. GeroScience 2023, 45, 2089–2108. [Google Scholar] [CrossRef]
- Hajek, A.; Kretzler, B.; König, H.-H. Relationship between Personality Factors and Frailty. A Systematic Review. Arch. Gerontol. Geriatr. 2021, 97, 104508. [Google Scholar] [CrossRef]
- Fali, T.; Vallet, H.; Sauce, D. Impact of Stress on Aged Immune System Compartments: Overview from Fundamental to Clinical Data. Exp. Gerontol. 2018, 105, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and Anti-Inflammaging: A Systemic Perspective on Aging and Longevity Emerged from Studies in Humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Day, M.J. Ageing, Immunosenescence and Inflammageing in the Dog and Cat. J. Comp. Pathol. 2010, 142, S60–S69. [Google Scholar] [CrossRef]
- Piotti, P.; Pierantoni, L.; Albertini, M.; Pirrone, F. Inflammation and Behavior Changes in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2024, 54, 1–16. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B. Inflammaging Decreases Adaptive and Innate Immune Responses in Mice and Humans. Biogerontology 2016, 17, 7–19. [Google Scholar] [CrossRef]
- Jiménez, A.G. Inflammaging in Domestic Dogs: Basal Level Concentrations of IL-6, IL-1β, and TNF-α in Serum of Healthy Dogs of Different Body Sizes and Ages. Biogerontology 2023, 24, 593–602. [Google Scholar] [CrossRef]
- Schmid, S.M.; Hoffman, J.M.; Prescott, J.; Ernst, H.; Promislow, D.E.L.; Dog Aging Project Consortium; Akey, J.M.; Benton, B.; Borenstein, E.; Castelhano, M.G.; et al. The Companion Dog as a Model for Inflammaging: A Cross-Sectional Pilot Study. GeroScience 2024, 46, 5395–5407. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.E.; Colyer, A.; Haydock, R.M.; Hayek, M.G.; Park, J. Understanding How Dogs Age: Longitudinal Analysis of Markers of Inflammation, Immune Function, and Oxidative Stress. J. Gerontol. Ser. A 2018, 73, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Stamouli, E.C.; Politis, A.M. Pro-Inflammatory Cytokines in Alzheimer’s Disease. Psychiatriki 2016, 27, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Chapagain, D.; Wallis, L.J.; Range, F.; Affenzeller, N.; Serra, J.; Virányi, Z. Behavioural and Cognitive Changes in Aged Pet Dogs: No Effects of an Enriched Diet and Lifelong Training. PLoS ONE 2020, 15, e0238517. [Google Scholar] [CrossRef]
- Rosado, B.; González-Martínez, Á.; Pesini, P.; García-Belenguer, S.; Palacio, J.; Villegas, A.; Suárez, M.-L.; Santamarina, G.; Sarasa, M. Effect of Age and Severity of Cognitive Dysfunction on Spontaneous Activity in Pet Dogs—Part 1: Locomotor and Exploratory Behaviour. Vet. J. 2012, 194, 189–195. [Google Scholar] [CrossRef]
- Tapp, P.D.; Siwak, C.T.; Estrada, J.; Head, E.; Muggenburg, B.A.; Cotman, C.W.; Milgram, N.W. Size and Reversal Learning in the Beagle Dog as a Measure of Executive Function and Inhibitory Control in Aging. Learn. Mem. 2003, 10, 64–73. [Google Scholar] [CrossRef]
- Landsberg, G.M.; Nichol, J.; Araujo, J.A. Cognitive Dysfunction Syndrome. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 749–768. [Google Scholar] [CrossRef]
- Head, E. A Canine Model of Human Aging and Alzheimer’s Disease. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2013, 1832, 1384–1389. [Google Scholar] [CrossRef]
- Salvin, H.E.; McGreevy, P.D.; Sachdev, P.S.; Valenzuela, M.J. Under Diagnosis of Canine Cognitive Dysfunction: A Cross-Sectional Survey of Older Companion Dogs. Vet. J. 2010, 184, 277–281. [Google Scholar] [CrossRef]
- Rosado, B.; González-Martínez, Á.; Pesini, P.; García-Belenguer, S.; Palacio, J.; Villegas, A.; Suárez, M.-L.; Santamarina, G.; Sarasa, M. Effect of Age and Severity of Cognitive Dysfunction on Spontaneous Activity in Pet Dogs—Part 2: Social Responsiveness. Vet. J. 2012, 194, 196–201. [Google Scholar] [CrossRef]
- Wallis, L.J.; Virányi, Z.; Müller, C.A.; Serisier, S.; Huber, L.; Range, F. Aging Effects on Discrimination Learning, Logical Reasoning and Memory in Pet Dogs. AGE 2016, 38, 6. [Google Scholar] [CrossRef] [PubMed]
- Carere, C.; Maestripieri, D. (Eds.) Animal Personalities: Behavior, Physiology, and Evolution; The University of Chicago Press: Chicago, IL, USA, 2013; ISBN 978-0-226-92197-6. [Google Scholar]
- McCrae, R.R.; John, O.P. An Introduction to the Five-Factor Model and Its Applications. J. Personal. 1992, 60, 175–215. [Google Scholar] [CrossRef] [PubMed]
- Salonen, M.; Mikkola, S.; Hakanen, E.; Sulkama, S.; Puurunen, J.; Lohi, H. Reliability and Validity of a Dog Personality and Unwanted Behavior Survey. Animals 2021, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Barnard, S.; Wells, D.L.; Milligan, A.D.S.; Arnott, G.; Hepper, P.G. Personality Traits Affecting Judgement Bias Task Performance in Dogs (Canis familiaris). Sci. Rep. 2018, 8, 6660. [Google Scholar] [CrossRef]
- Wallis, L.J.; Szabó, D.; Kubinyi, E. Cross-Sectional Age Differences in Canine Personality Traits; Influence of Breed, Sex, Previous Trauma, and Dog Obedience Tasks. Front. Vet. Sci. 2020, 6, 493. [Google Scholar] [CrossRef]
- Kogan, J.N.; Edelstein, B.A.; McKee, D.R. Assessment of Anxiety in Older Adults. J. Anxiety Disord. 2000, 14, 109–132. [Google Scholar] [CrossRef]
- Meyza, K.Z.; Boguszewski, P.M.; Nikolaev, E.; Zagrodzka, J. Age Increases Anxiety and Reactivity of the Fear/Anxiety Circuit in Lewis Rats. Behav. Brain Res. 2011, 225, 192–200. [Google Scholar] [CrossRef]
- Grandin, T. (Ed.) Genetics and the Behavior of Domestic Animals; Academic Press: San Diego, CA, USA, 1998; ISBN 978-0-12-295130-5. [Google Scholar]
- Masini, C.V.; Day, H.E.W.; Campeau, S. Long-Term Habituation to Repeated Loud Noise Is Impaired by Relatively Short Interstressor Intervals in Rats. Behav. Neurosci. 2008, 122, 210–223. [Google Scholar] [CrossRef]
- Careau, V.; Réale, D.; Humphries, M.M.; Thomas, D.W. The Pace of Life under Artificial Selection: Personality, Energy Expenditure, and Longevity Are Correlated in Domestic Dogs. Am. Nat. 2010, 175, 753–758. [Google Scholar] [CrossRef]
- Turcsán, B.; Wallis, L.; Berczik, J.; Range, F.; Kubinyi, E.; Virányi, Z. Individual and Group Level Personality Change across the Lifespan in Dogs. Sci. Rep. 2020, 10, 17276. [Google Scholar] [CrossRef]
- Bellows, J.; Colitz, C.M.H.; Daristotle, L.; Ingram, D.K.; Lepine, A.; Marks, S.L.; Sanderson, S.L.; Tomlinson, J.; Zhang, J. Defining Healthy Aging in Older Dogs and Differentiating Healthy Aging from Disease. J. Am. Vet. Med. Assoc. 2015, 246, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Piotti, P.; Karagiannis, C.; Satchell, L.; Michelazzi, M.; Albertini, M.; Alleva, E.; Pirrone, F. Use of the Milan Pet Quality of Life Instrument (MPQL) to Measure Pets’ Quality of Life during COVID-19. Animals 2021, 11, 1336. [Google Scholar] [CrossRef]
- Piotti, P.; Satchell, L.P.; Lockhart, T.S. Impulsivity and Behaviour Problems in Dogs: A Reinforcement Sensitivity Theory Perspective. Behav. Process. 2018, 151, 104–110. [Google Scholar] [CrossRef]
- Sheppard, G.; Mills, D.S. The Development of a Psychometric Scale for the Evaluation of the Emotional Predispositions of Pet Dogs. Int. J. Comp. Psychol. 2002, 15, 201–222. [Google Scholar] [CrossRef]
- Le Brech, S.; Amat, M.; Temple, D.; Manteca, X. Evaluation of Two Practical Tools to Assess Cognitive Impairment in Aged Dogs. Animals 2022, 12, 3538. [Google Scholar] [CrossRef] [PubMed]
- Wrightson, R.; Albertini, M.; Pirrone, F.; McPeake, K.; Piotti, P. The Relationship between Signs of Medical Conditions and Cognitive Decline in Senior Dogs. Animals 2023, 13, 2203. [Google Scholar] [CrossRef]
- Gianella, P.; Cagnasso, F.; Giordano, A.; Borrelli, A.; Bottero, E.; Bruno, B.; Ferriani, R.; Borella, F.; Meazzi, S.; Scavone, D.; et al. Comparative Evaluation of Lipid Profile, C-Reactive Protein and Paraoxonase-1 Activity in Dogs with Inflammatory Protein-Losing Enteropathy and Healthy Dogs. Animals 2024, 14, 3119. [Google Scholar] [CrossRef]
- Rossi, G.; Giordano, A.; Pezzia, F.; Kjelgaard-Hansen, M.; Paltrinieri, S. Serum Paraoxonase 1 Activity in Dogs: Preanalytical and Analytical Factors and Correlation with C-reactive Protein and Alpha-2-globulin. Vet. Clin. Pathol. 2013, 42, 329–341. [Google Scholar] [CrossRef]
- Salvin, H.E.; McGreevy, P.D.; Sachdev, P.S.; Valenzuela, M.J. Growing Old Gracefully—Behavioral Changes Associated with “Successful Aging” in the Dog, Canis Familiaris. J. Vet. Behav. 2011, 6, 313–320. [Google Scholar] [CrossRef]
- Studzinski, C.; Christie, L.; Araujo, J.; Burnham, W.; Head, E.; Cotman, C.; Milgram, N. Visuospatial Function in the Beagle Dog: An Early Marker of Cognitive Decline in a Model of Human Aging and Dementia. Neurobiol. Learn. Mem. 2006, 86, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Wallis, L.J.; Range, F.; Müller, C.A.; Serisier, S.; Huber, L.; Zsó, V. Lifespan Development of Attentiveness in Domestic Dogs: Drawing Parallels with Humans. Front. Psychol. 2014, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Salt, C.; Morris, P.J.; German, A.J.; Wilson, D.; Lund, E.M.; Cole, T.J.; Butterwick, R.F. Growth Standard Charts for Monitoring Bodyweight in Dogs of Different Sizes. PLoS ONE 2017, 12, e0182064. [Google Scholar] [CrossRef] [PubMed]
- Radakovich, L.B.; Pannone, S.C.; Truelove, M.P.; Olver, C.S.; Santangelo, K.S. Hematology and Biochemistry of Aging-Evidence of “Anemia of the Elderly” in Old Dogs. Vet. Clin. Pathol. 2017, 46, 34–45. [Google Scholar] [CrossRef]
- McMahon, J.E.; Graves, J.L.; Tovar, A.P.; Peloquin, M.; Greenwood, K.; Chen, F.L.; Nelson, M.; McCandless, E.E.; Halioua-Haubold, C.-L.; Juarez-Salinas, D. Translational Immune and Metabolic Markers of Aging in Dogs. Sci. Rep. 2025, 15, 14460. [Google Scholar] [CrossRef]
- Wright, H.F.; Mills, D.S.; Pollux, P.M.J. Development and Validation of a Psychometric Tool forAssessing Impulsivity in the Domestic Dog (Canis familiaris). Int. J. Comp. Psychol. 2011, 24, 210–225. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Lopes, P.C. Why Are Behavioral and Immune Traits Linked? Horm. Behav. 2017, 88, 52–59. [Google Scholar] [CrossRef]
- Wagner, E.-Y.N.; Ajdacic-Gross, V.; Strippoli, M.-P.F.; Gholam-Rezaee, M.; Glaus, J.; Vandeleur, C.; Castelao, E.; Vollenweider, P.; Preisig, M.; Von Känel, R. Associations of Personality Traits With Chronic Low-Grade Inflammation in a Swiss Community Sample. Front. Psychiatry 2019, 10, 819. [Google Scholar] [CrossRef]
- Corr, P.J.J.A. Gray’s Reinforcement Sensitivity Theory: Tests of the Joint Subsystems Hypothesis of Anxiety and Impulsivity. Personal. Individ. Differ. 2002, 33, 511–532. [Google Scholar] [CrossRef]
- Kennis, M.; Rademaker, A.R.; Geuze, E. Neural Correlates of Personality: An Integrative Review. Neurosci. Biobehav. Rev. 2013, 37, 73–95. [Google Scholar] [CrossRef]
- Tovote, P.; Esposito, M.S.; Botta, P.; Chaudun, F.; Fadok, J.P.; Markovic, M.; Wolff, S.B.E.; Ramakrishnan, C.; Fenno, L.; Deisseroth, K.; et al. Midbrain Circuits for Defensive Behaviour. Nature 2016, 534, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Corr, P.J.; McNaughton, N. Reinforcement Sensitivity Theory and Personality. In The Reinforcement Sensitivity Theory of Personality; Corr, P.J., Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 155–187. ISBN 978-0-521-85179-4. [Google Scholar]
- Blanchard, D.C.; Blanchard, R.J. Ethoexperimental Approaches to the Biology of Emotion. Annu. Rev. Psychol. 1988, 39, 43–68. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, N.; Corr, P.J. Survival Circuits and Risk Assessment. Curr. Opin. Behav. Sci. 2018, 24, 14–20. [Google Scholar] [CrossRef]
- Roelofs, K. Freeze for Action: Neurobiological Mechanisms in Animal and Human Freezing. Phil. Trans. R. Soc. B 2017, 372, 20160206. [Google Scholar] [CrossRef]
- Wulferding, D.; Kim, G.; Kim, H.; Yang, I.; Bauer, E.D.; Ronning, F.; Movshovich, R.; Kim, J. Local Characterization of a Heavy-Fermion Superconductor via Sub-Kelvin Magnetic Force Microscopy. Appl. Phys. Lett. 2020, 117, 252601. [Google Scholar] [CrossRef]
- Fanselow, M.S. Neural Organization of the Defensive Behavior System Responsible for Fear. Psychon. Bull. Rev. 1994, 1, 429–438. [Google Scholar] [CrossRef]
- Schulkin, J.; Sterling, P. Allostasis: A Brain-Centered, Predictive Mode of Physiological Regulation. Trends Neurosci. 2019, 42, 740–752. [Google Scholar] [CrossRef] [PubMed]
- Armon, G.; Melamed, S.; Shirom, A.; Berliner, S.; Shapira, I. The Associations of the Five Factor Model of Personality with Inflammatory Biomarkers: A Four-Year Prospective Study. Personal. Individ. Differ. 2013, 54, 750–755. [Google Scholar] [CrossRef]
- Chapman, B.P.; Khan, A.; Harper, M.; Stockman, D.; Fiscella, K.; Walton, J.; Duberstein, P.; Talbot, N.; Lyness, J.M.; Moynihan, J. Gender, Race/Ethnicity, Personality, and Interleukin-6 in Urban Primary Care Patients. Brain Behav. Immun. 2009, 23, 636–642. [Google Scholar] [CrossRef]
- Mõttus, R.; Luciano, M.; Starr, J.M.; Pollard, M.C.; Deary, I.J. Personality Traits and Inflammation in Men and Women in Their Early 70s: The Lothian Birth Cohort 1936 Study of Healthy Aging. Psychosom. Med. 2013, 75, 11–19. [Google Scholar] [CrossRef]
- Michopoulos, V.; Powers, A.; Gillespie, C.F.; Ressler, K.J.; Jovanovic, T. Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology 2017, 42, 254–270. [Google Scholar] [CrossRef]
- Turiano, N.A.; Mroczek, D.K.; Moynihan, J.; Chapman, B.P. Big 5 Personality Traits and Interleukin-6: Evidence for “Healthy Neuroticism” in a US Population Sample. Brain Behav. Immun. 2013, 28, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.; Murray, D.R. Pathogens, Personality, and Culture: Disease Prevalence Predicts Worldwide Variability in Sociosexuality, Extraversion, and Openness to Experience. J. Personal. Soc. Psychol. 2008, 95, 212–221. [Google Scholar] [CrossRef]
- Schaller, M. The Behavioural Immune System and the Psychology of Human Sociality. Phil. Trans. R. Soc. B 2011, 366, 3418–3426. [Google Scholar] [CrossRef]
- Vodovotz, Y.; Arciero, J.; Verschure, P.F.M.J.; Katz, D.L. A Multiscale Inflammatory Map: Linking Individual Stress to Societal Dysfunction. Front. Sci. 2024, 1, 1239462. [Google Scholar] [CrossRef]
- Chiang, J.J.; Lam, P.H.; Chen, E.; Miller, G.E. Psychological Stress during Childhood and Adolescence and Its Association with Inflammation across the Lifespan: A Critical Review and Meta-Analysis. Psychol. Bull. 2022, 148, 27–66. [Google Scholar] [CrossRef]
- De La Fuente, M. Role of the Immune System in Aging. Inmunología 2008, 27, 176–191. [Google Scholar] [CrossRef]
- Luchetti, M.; Barkley, J.M.; Stephan, Y.; Terracciano, A.; Sutin, A.R. Five-Factor Model Personality Traits and Inflammatory Markers: New Data and a Meta-Analysis. Psychoneuroendocrinology 2014, 50, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Widiger, T.A.; Oltmanns, J.R. Neuroticism Is a Fundamental Domain of Personality with Enormous Public Health Implications. World Psychiatry 2017, 16, 144–145. [Google Scholar] [CrossRef]
- Piotti, P.; Albertini, M.; Trabucco, L.P.; Ripari, L.; Karagiannis, C.; Bandi, C.; Pirrone, F. Personality and Cognitive Profiles of Animal-Assisted Intervention Dogs and Pet Dogs in an Unsolvable Task. Animals 2021, 11, 2144. [Google Scholar] [CrossRef]
- Kiesecker, J.M.; Skelly, D.K.; Beard, K.H.; Preisser, E. Behavioral Reduction of Infection Risk. Proc. Natl. Acad. Sci. USA 1999, 96, 9165–9168. [Google Scholar] [CrossRef]
- Seignourel, P.J.; Kunik, M.E.; Snow, L.; Wilson, N.; Stanley, M. Anxiety in Dementia: A Critical Review. Clin. Psychol. Rev. 2008, 28, 1071–1082. [Google Scholar] [CrossRef]
- Lopes Fagundes, A.L.; Hewison, L.; McPeake, K.J.; Zulch, H.; Mills, D.S. Noise Sensitivities in Dogs: An Exploration of Signs in Dogs with and without Musculoskeletal Pain Using Qualitative Content Analysis. Front. Vet. Sci. 2018, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Labaka, A.; Gómez-Lázaro, E.; Vegas, O.; Pérez-Tejada, J.; Arregi, A.; Garmendia, L. Reduced Hippocampal IL-10 Expression, Altered Monoaminergic Activity and Anxiety and Depressive-like Behavior in Female Mice Subjected to Chronic Social Instability Stress. Behav. Brain Res. 2017, 335, 8–18. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The Regulation of IL-10 Production by Immune Cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Ying, Z.-J.; Huang, Y.-Y.; Shao, M.-M.; Chi, C.-H.; Jiang, M.-X.; Chen, Y.-H.; Chen, Y.; Sun, M.-X.; Zhu, Y.-Y.; Li, X. Relationships of Low Serum Levels of Interleukin-10 With Poststroke Anxiety and Cognitive Impairment in Patients With Clinical Acute Stroke. J. Clin. Neurol. 2023, 19, 242–250. [Google Scholar] [CrossRef]
- Munshi, S.; Parrilli, V.; Rosenkranz, J.A. Peripheral Anti-Inflammatory Cytokine Interleukin-10 Treatment Mitigates Interleukin-1β—Induced Anxiety and Sickness Behaviors in Adult Male Rats. Behav. Brain Res. 2019, 372, 112024. [Google Scholar] [CrossRef]
- Mesquita, A.R.; Correia-Neves, M.; Roque, S.; Castro, A.G.; Vieira, P.; Pedrosa, J.; Palha, J.A.; Sousa, N. IL-10 Modulates Depressive-like Behavior. J. Psychiatr. Res. 2008, 43, 89–97. [Google Scholar] [CrossRef]
- Trifunović, J.; Miller, L.; Debeljak, Ž.; Horvat, V. Pathologic Patterns of Interleukin 10 Expression—A Review. Biochem. Med. 2015, 25, 36–48. [Google Scholar] [CrossRef]
- Arroube, A.; Pereira, A.F. Dog Neuter, Yes or No? A Summary of the Motivations, Benefits, and Harms, with Special Emphasis on the Behavioral Aspect. Animals 2025, 15, 1063. [Google Scholar] [CrossRef]
- Kolkmeyer, C.A.; Zambrano Cardona, A.M.; Gansloßer, U. Personality Unleashed: Surveying Correlation of Neuter Status and Social Behaviour in Mixed-Breed Male Dogs across Weight Classes. Animals 2024, 14, 2445. [Google Scholar] [CrossRef]
- Hsu, Y.; Serpell, J.A. Development and Validation of a Questionnaire for Measuring Behavior and Temperament Traits in Pet Dogs. J. Am. Vet. Med. Assoc. 2003, 223, 1293–1300. [Google Scholar] [CrossRef]
- Sundburg, C.R.; Belanger, J.M.; Bannasch, D.L.; Famula, T.R.; Oberbauer, A.M. Gonadectomy Effects on the Risk of Immune Disorders in the Dog: A Retrospective Study. BMC Vet. Res. 2016, 12, 278. [Google Scholar] [CrossRef]
| Age Group (N) | Median Age (min–max) (Years) | Sex | Desexed (%) | Purebred (%) | Age at Acquisition | Main Sources |
|---|---|---|---|---|---|---|
| F/M | 0–2 months/ 3–5 months/ 6–12 months/>12 months | Homebred/friends-relative/pet shop-online/registered breeder/shelter-rescue/stray | ||||
| 1–4 years old (22) | 3 (1–4) | 11/11 | 41 | 41 | 11/5/3/3 | 6/6/0/4/5/1 |
| ≥11 years old (22) | 11 (11–18) | 9/13 | 86 * | 50 | 7/6/1/8 | 0/6/3/7/5/1 |
| Age Group | DIAS Factor 1 | DIAS Factor 2 | DIAS Factor 3 | DIAS Total Score | RST FFFS | RST BIS | RST BAS | |
|---|---|---|---|---|---|---|---|---|
| 1–4 years old | Median | 0.42 | 0.36 | 0.76 | 0.61 | 2.57 | 2.64 | 4.07 |
| Minimum | 0.24 | 0.20 | 0.48 | 0.47 | 1.00 | 1.00 | 1.57 | |
| Maximum | 0.64 | 0.60 | 1.00 | 0.77 | 4.43 | 4.57 | 5.00 | |
| ≥11 years old | Median | 0.40 | 0.52 | 0.64 | 0.54 | 2.00 | 3.00 | 3.21 |
| Minimum | 0.22 | 0.20 | 0.36 | 0.42 | 1.00 | 1.00 | 1.00 | |
| Maximum | 0.64 | 0.76 | 0.88 | 0.66 | 3.57 | 4.71 | 5.00 | |
| Group | ||
|---|---|---|
| Cytokine | Young | Senior |
| Median | ||
| IQR (25th–75th percentile) | ||
| TNF (pg/mL) | 14.40 52.07 (2.10–54.17) | 43.303 103.31 (9.06–112.37) |
| IL10 (pg/mL) | 24.27 26.12 (8.38–34.50) | 16.28 24.08 (6.37–30.45) |
| IL6 (pg/mL) | 0.74 167.21 (0.10–167.31) | 9.44 3027.22 (0.10–3037.32) |
| Parameter | B | Std. Error | Sig. | Exp(B) | 95% Wald Confidence Interval for Exp(B) | |
|---|---|---|---|---|---|---|
| Upper | Lower | |||||
| Dependent Variable: BIS | ||||||
| [Age group = LS-G] * IL-10 | −0.022 | 0.005 | 0.001 | 0.978 | 0.968 | 0.989 |
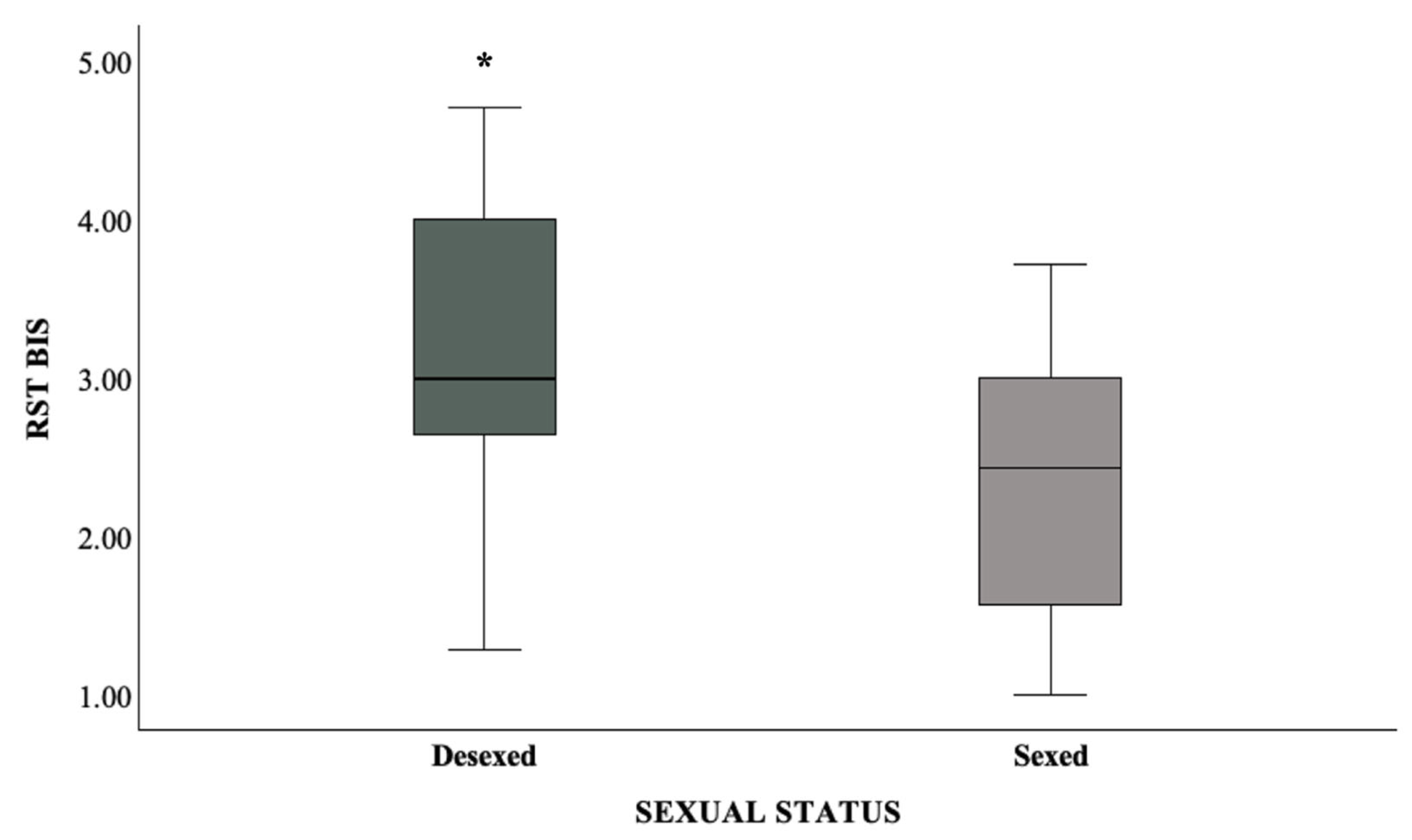
| [Sex status = Desexed] | 0.249 | 0.114 | 0.028 | 1.283 | 1.027 | 1.603 |
| Dependent Variable: FFSS | ||||||
| [Age group = LS-G] * TNF-α | −0.001 | 0.002 | 0.001 | 0.999 | 0.999 | 1.000 |
| [Age group = LS-G] * BIS | 0.215 | 0.068 | 0.002 | 1.240 | 0.980 | 1.332 |
| Cytokine | Reinforcement Sensitivity Theory (RST) Trait | Observed Association | Adaptive Interpretation | Maladaptive Interpretation | Note |
|---|---|---|---|---|---|
| TNF-α | FFFS | ↓ FFFS ↑ TNF-α | Protective downregulation of threat responses to reduce metabolic cost and emotional burden in older, vulnerable individuals, possibly reflecting a compensatory recalibration during inflammaging | Neuroinflammation or chronic immune activation impairs functional defensive circuits, leading to emotional disengagement or reduced vigilance | The co-variation may be either casual or reflect concomitant components of broader adaptive or maladaptive profiles associated with aging |
| IL-10 | BIS | ↓ BIS ↑ IL-10 | Modulation of emotional responses by suppressing excessive anxiety, promoting resilience to stress and enhancing regulatory balance | Overregulation may dampen necessary caution, possibly reflecting early signs of immune–behavioral dysregulation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirrone, F.; Bettoni, V.; Albertini, M.; Giordano, A.; Melzi, S.; Naji, A.K.T.; Nonnis, S.; Piotti, P.; Schifino, L.L.M.; Paltrinieri, S. Serum TNF-Alpha and IL-10 Predict Reduced Sensitivity to Fear- and Anxiety-Related Traits in Healthy Older Dogs: Preliminary Evidence for Immune–Personality Signatures in Later Life. Animals 2025, 15, 2418. https://doi.org/10.3390/ani15162418
Pirrone F, Bettoni V, Albertini M, Giordano A, Melzi S, Naji AKT, Nonnis S, Piotti P, Schifino LLM, Paltrinieri S. Serum TNF-Alpha and IL-10 Predict Reduced Sensitivity to Fear- and Anxiety-Related Traits in Healthy Older Dogs: Preliminary Evidence for Immune–Personality Signatures in Later Life. Animals. 2025; 15(16):2418. https://doi.org/10.3390/ani15162418
Chicago/Turabian StylePirrone, Federica, Virginia Bettoni, Mariangela Albertini, Alessia Giordano, Stefania Melzi, Amna K. T. Naji, Simona Nonnis, Patrizia Piotti, Letizia L. M. Schifino, and Saverio Paltrinieri. 2025. "Serum TNF-Alpha and IL-10 Predict Reduced Sensitivity to Fear- and Anxiety-Related Traits in Healthy Older Dogs: Preliminary Evidence for Immune–Personality Signatures in Later Life" Animals 15, no. 16: 2418. https://doi.org/10.3390/ani15162418
APA StylePirrone, F., Bettoni, V., Albertini, M., Giordano, A., Melzi, S., Naji, A. K. T., Nonnis, S., Piotti, P., Schifino, L. L. M., & Paltrinieri, S. (2025). Serum TNF-Alpha and IL-10 Predict Reduced Sensitivity to Fear- and Anxiety-Related Traits in Healthy Older Dogs: Preliminary Evidence for Immune–Personality Signatures in Later Life. Animals, 15(16), 2418. https://doi.org/10.3390/ani15162418






