Simple Summary
This study investigated the genotypes of an X-linked single-nucleotide variant in the bovine FOXP3 gene in herds of Bali cattle, Jaliteng cattle, and water buffaloes. This study also investigated the relationship between genotypes and serum concentrations of anti-Müllerian hormone in parous and non-parous Holstein Friesian cows. The G allele frequency was markedly high in Bali (0.944) and Jaliteng cattle (0.714) and water buffaloes (1) and moderately high in Holstein Friesian cattle (0.514). Anti-Müllerian hormone levels were significantly lower (p < 0.05) in parous Holstein Friesian cows carrying the G allele than in parous cows carrying the A/A genotype. Our results suggest that the G allele of the FOXP3 gene variant may be originally a wild-type variant in cattle breeds and water buffalo and that the G allele may be associated with infertility in cattle and other bovid species.
Abstract
Reproductive failure in cattle production is a global concern and is influenced by various factors, including genetic alterations. This study explored the relationship between an X-linked single-nucleotide variant (NC_037357.1: g.87298881A>G, rs135720414) in the upstream of the bovine forkhead box P3 (FOXP3) gene and infertility. To this end, we examined the genotypes of the variant in old Asian cattle breeds, including 48 Bali and 5 Jaliteng cattle, and 20 water buffaloes, which have recently shown subclinical signs of infertility and repeated breeding problems among populations in Indonesia. We also examined the genotypes in 69 parous and 39 non-parous Holstein Friesian (HF) cows and investigated the relationship between the genotypes and serum concentration of anti-Müllerian hormone (AMH). The G allele frequency was markedly high in Bali (0.944) and Jaliteng cattle (0.714), and water buffaloes (1), suggesting that the G allele may be originally a wild-type variant in old Asian cattle and buffaloes. In HF cows, the G allele frequency was moderately high, and the AMH concentration was significantly lower (p < 0.05) in parous cows carrying the G allele (A/G and G/G genotypes) than in parous cows with the A/A genotype. In contrast, there were no significant differences in AMH concentrations among the three genotypes of non-parous HF cows. This suggests that both G allele and aging are associated with infertility in HF cows. In conclusion, the G allele of the FOXP3 gene variant may potentially be associated with infertility in different bovine breeds and species. Therefore, special attention should be paid to this variant, and infertility in bovine herds may be improved by selection and/or introduction of the A allele.
1. Introduction
Reproductive failure in cattle is a significant challenge in dairy and beef cattle industries worldwide [1,2]. This stems from a complex combination of managerial, environmental, physiological, and genetic factors. These factors influence various aspects, including selective breeding, postpartum conditions, calving intervals, and the occurrence of healthy calf births [3]. Maternal factors, including age, oocyte defects, endocrinological dysfunction, nutritional abnormalities, genital tract infections, and genetic alterations, are recognized as important factors contributing to infertility [4]. Repeat breeding is a major issue affecting fertility as it leads to reduced fertility in lactating dairy cows on a global scale [5]. Understanding the genetic foundation of reproductive failure is crucial for enhancing herd fertility, rather than individual fertility [4]. Recently, both human and animal studies have increasingly focused on exploring the roles of maternal immunoregulatory cells and their controlling genes to determine their potential involvement in infertility [6,7].
The FOXP3 gene encodes a forkhead box P3 protein that plays a critical role in regulating the development and function of regulatory T cells as a master regulator, which is important for regulating the immune system and suppressing excessive immune responses [6,7]. Dysregulation of the immune system or an excessive immune response can lead to embryo rejection or disruption of normal reproductive processes, which in turn contribute to infertility [8]. In humans, mutations in the FOXP3 gene are associated with poor embryonic development, abnormal ovarian follicle development, and poor oocyte quality, all of which contribute to reduced fertility in females [9]; furthermore, in males, mutations in the FOXP3 gene are associated with testicular inflammation [10].
In cattle, the FOXP3 gene plays an important role in regulating immune responses and immune system development and is believed to be associated with infertility in bovine species [11,12]. Recently, a genome-wide association study (GWAS) found an association between recurrent infertility, such as repeat breeders, and an X-linked maternal single-nucleotide variant located 2175 base pairs upstream of the start codon of the bovine FOXP3 gene (NC_037357.1: g.87298881A>G, rs135720414) in Japanese Black (JB; Bos taurus) cows with lower FOXP3 transcript levels [11].
Accordingly, this variant may be a useful target for initiatives to increase cattle herd fertility because of the relationship between a higher frequency of the G allele variant of the FOXP3 gene and recurrent infertility in JB cows [11]. Our research team preliminarily investigated frequencies of the G allele in various cattle breeds including JB, Holstein Friesian (HF; B. taurus), Korean Hanwoo (KH; B. taurus coreanae), and Indonesian Madura (IM; a crossbreed between B. indicus and B. javanicus) cattle and found a higher frequency of G allele in the local Asian cattle breed IM (0.700), a moderate frequency in the dairy cattle HF (0.466), and low frequencies in the beef cattle breeds JB (0.250) and KH (0.112) [12].
Thus, it would be crucial to evaluate the associations between high frequencies of the G allele and infertility in different breeds and bovid species. Old Asian cattle breeds and water buffaloes (Bubalus bubalis) in Indonesia have long suffered from subclinical infertility and repeat-breeding problems. These old Asian cattle breeds include Bali cattle (B. javanicus domesticus) and Jaliteng cattle, a crossbreed between Bali and banteng cattle (B. javanicus), which is a wild bovine species found in Southeast Asia. We hypothesized that the frequency of the FOXP3 gene variant may be associated with infertility in these cattle breeds and species because one of the Asian local cattle, IM, has a high frequency (0.700) of the G allele variant [12]. Therefore, we performed genotyping of the FOXP3 gene variants in herds of these old Asian breeds and species.
Furthermore, we investigated how the FOXP3 gene variant is associated with the peripheral concentration of anti-Müllerian hormone (AMH), a biomarker of fertility that indicates the size of the ovarian reserve [13,14]. To this end, we also performed genotyping of the variant in a herd of HF cows in which AMH concentration was measured and investigated the relationship between the genotypes and AMH concentration in parous and non-parous HF cows.
2. Materials and Methods
2.1. Animals and Blood Collection
In 2023, blood samples were collected via jugular or caudal venipuncture from 48 Bali cattle (7 males, 41 females; 1–12 years old and over, mean 6.7 years), 5 Jaliteng cattle (3 males, 2 females; 2–10 years old, mean age 6.6 years), and 20 water buffaloes (5 males, 15 females; 3–12 years old, mean 7.2 years). Blood was collected to genotype FOXP3 variants. The parity of females was 1–16 (mean 4.3) times in Bali cattle, 6–8 (mean 7.0) times in Jaliteng cattle, and 1–5 (mean 3.3) times in water buffalo. Bali cattle were housed in South Sulawesi and East Java Provinces, Indonesia. Jaliteng cattle were housed in East Java Province, Indonesia. Water buffaloes were housed in South Sulawesi Province, Indonesia. Representative images of the Bali and Jaliteng cattle, and water buffaloes, are shown in Figure 1.
Figure 1.
Representative appearances of Bali (Bos javanicus domesticus; (A), male; (B), female) and Jaliteng ((C), male; (D), female) cattle, a crossbreed between Bali and banteng cattle (B. javanicus), and water buffaloes (Bubalus bubalis, (E)), which were used in this study.
In 2019, blood samples were collected via jugular or caudal venipuncture from 69 parous and 39 non-parous HF cows housed in Fukuoka Prefecture, Japan. Blood was collected to measure AMH concentration and to genotype the FOXP3 variant in parous cows 4 weeks after delivery and in non-parous cows during pregnancy. The parity of the parous cows ranged from 1 to 7 (average 3.2) times. For the measurement of AMH concentration, serum was obtained by centrifugation immediately after blood collection and was stored at −30 °C until measurement.
2.2. DNA Extraction and Genotyping
The collected blood was spotted onto Flinders Technology Associates filter paper (FTA card) (QIAcard FTA Classic; Qiagen, Hilden, Germany) and stored at room temperature or in a refrigerator (4 °C) until DNA extraction. DNA was extracted from discs punched out of the blood-spotted FTA cards following appropriate treatment as previously described [15].
Genotyping of the bovine FOXP3 gene variant (NC_037357.1: g.87298881A>G, rs135720414) was performed as previously described [12] using the extracted DNA as a template. PCR amplifications were performed using a StepOne Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Specific primer pairs (forward, CCATGTGGCTTCTGAGAAATAGTCA and reverse, TACCTGGAGGGCCAGACT) and TaqMan minor groove binder probes (A-allele, TCTTCCTGCATTGTCTG and G-allele, TCTTCCTGCACTGTCTG), linked to each fluorescent reporter dye (6-carboxyrhodamine and 6-carboxyfluorescein, respectively) at the 5′ end and a non-fluorescent quencher dye at the 3′ end, were used. Real-time PCR amplifications were carried out in a final volume of 10 µL consisting of 2× PCR master mix (TaqMan GTXpress Master Mix; Applied Biosystems) and 80× genotyping assay mix (TaqMan SNP Genotyping Assays; Applied Biosystems) containing the specific primers at 450 nM, TaqMan probes at 100 nM, and template DNA. A negative control containing nuclease-free water instead of the template DNA was included in each run. The cycling conditions were 20 s at 95 °C, followed by 50 cycles of 3 s at 95 °C and 20 s at 60 °C, with a subsequent holding stage at 25 °C for 30 s. The data obtained were analyzed using StepOne version 2.3 (Applied Biosystems).
Female animals were categorized into three genotypes (A/A, A/G, and G/G), and male animals were categorized into two types of hemizygotes (A/- and G/-). G allele frequency was calculated based on the number of G alleles among the total number of X chromosomes in each herd.
DNA samples with three genotypes (A/A, A/G, and G/G or G/-) from 1 HF (1 female), 5 Bali (2 males, 3 females), and 3 Jaliteng cattle (1 male, 2 females) and 2 water buffaloes (1 male, 1 female) were used to validate the genotyping assay, following genotype confirmation based on Sanger sequencing (Kazusa Genome Technologies Ltd., Kisarazu, Japan). For Sanger sequencing, PCR was performed using a forward primer (AGGGCTCAGATGCAGAC) and a reverse primer (GGATATGGTCTGTCTGGT), which produced a 166 base pair amplicon [12]. Genotyping using the real-time PCR assay was performed in 48 Bali cattle (7 males, 41 females), 5 Jaliteng cattle (3 males, 2 females), 20 water buffaloes (5 males, 15 females), and 108 HF cows.
2.3. Measurement of AMH
AMH concentration was measured using a bovine AMH ELISA kit (AnshLabs, Webster, TX, USA) according to the manufacturer’s instructions [16]. Undiluted serum (50 µL) was used in the assay. The assay’s limit of detection was 11 pg/mL, and the coefficient of variation was 2.9% according to the manufacturer.
2.4. Statistical Analysis
Statistical analyses were performed using R version 4.5.0. Differences in AMH concentrations among the three genotypes were analyzed using the Friedman rank sum test and Wilcoxon rank sum exact test with Bonferroni correction. Differences at p < 0.05 were considered statistically significant.
3. Results
3.1. Allele Frequencies of the FOXP3 Gene Variant
We surveyed 48 Bali and 5 Jaliteng cattle and 20 water buffaloes in Indonesia and 108 HF cows in Japan, using real-time PCR genotyping. The survey results are summarized in Table 1. Among the 48 Bali cattle, only 5 cows were heterozygous (A/G) for the A and G alleles; however, 36 cows and 7 males had only the G allele (G/G and G/-, respectively), resulting in a very high G allele frequency (0.944). Among the 5 Jaliteng cattle, 2 cows were heterozygous (A/G), resulting in a high G allele frequency (0.714). All 20 water buffaloes had only the G allele (G/G or G/-), resulting in a maximal G allele frequency (1) in this herd. In contrast, the A and G alleles were almost equally present in HF cows, resulting in a moderately high G allele frequency (0.514).

Table 1.
The numbers of cattle and water buffaloes genotyped and the frequencies of the bovine FOXP3 gene variant.
We evaluated the genotyping results of one to five individuals from each of the four assessed herds using Sanger sequencing. The genotyping results obtained by real-time PCR were consistent with the Sanger sequencing results from all herds used in this study (Figure 2). The representative real-time PCR amplification plots of the A/A, A/G, and G/G or G/- genotypes are shown in Figure S1.
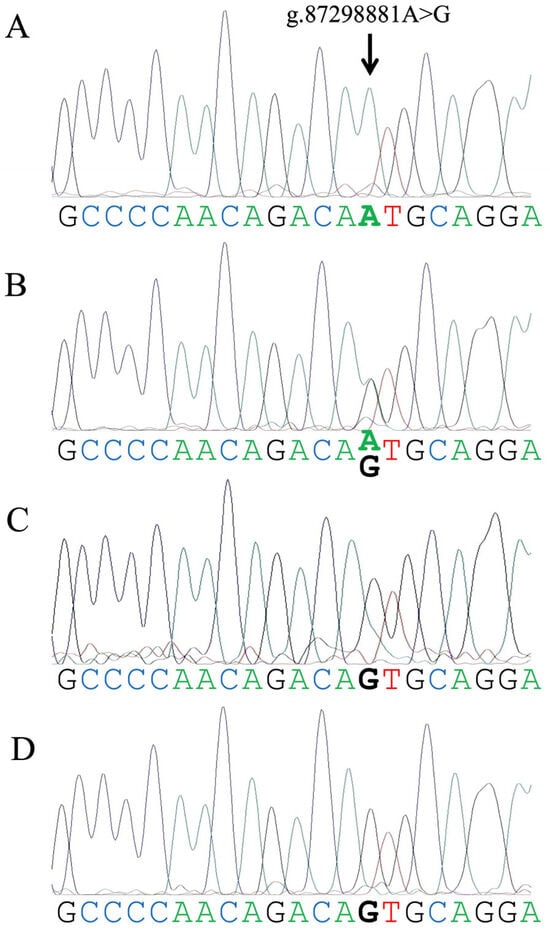
Figure 2.
Representative Sanger sequencing electropherograms illustrating the A/A ((A), a Holstein Friesian cow), A/G ((B), a Bali cow), G/- ((C), a Jaliteng bull), and G/G ((D), a female water buffalo) genotypes associated with a single-nucleotide variant (arrow, g.87298881A>G) in the upstream of the bovine FOXP3 gene.
3.2. AMH Concentration in Parous and Non-Parous HF Cows
We measured the serum AMH concentration in 108 HF cows, which comprised 69 parous and 39 non-parous cows. The AMH concentration was 477 ± 295 (mean ± standard deviation) and 506 ± 389 pg/mL in parous and non-parous cows, respectively. Based on the genotyping results, parous cows were further divided into 23 with genotype A/A, 27 with A/G, and 19 with G/G and non-parous cows into 7 with genotype A/A, 18 with A/G, and 14 with G/G. The AMH concentration was 629 ± 304, 424 ± 273, and 372 ± 254 pg/mL in parous cows with A/A, A/G, and G/G genotypes, respectively, and 585 ± 402, 422 ± 265, and 574 ± 507 pg/mL in non-parous cows with A/A, A/G, and G/G genotypes, respectively. The AMH concentrations are shown as box-and-whisker plots in parous (Figure 3A) and non-parous cows (Figure 3B).
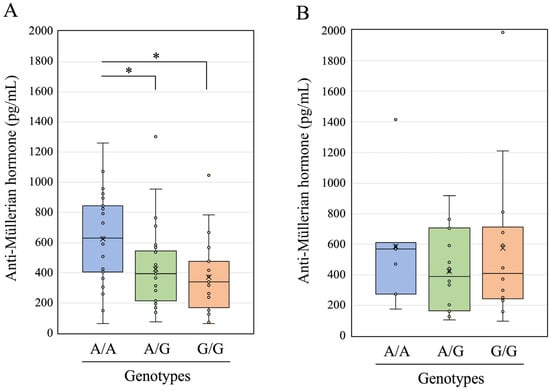
Figure 3.
Anti-Müllerian hormone concentration in parous cows (A) and non-parous cows (B) with three different genotypes (A/A, A/G, and G/G) of a single-nucleotide variant (g.87298881A>G) upstream of the bovine FOXP3 gene. * Differences in data between different groups were statistically significant (p < 0.05) using the Wilcoxon rank sum exact test with Bonferroni correction.
The AMH levels differed significantly between the three genotypes of parous HF cows (p < 0.01; Friedman rank sum test). We thus further analyzed the differences in AMH concentrations in parous cows among the three genotypes using the Wilcoxon rank sum exact test with Bonferroni correction. The AMH concentrations in the A/G and G/G genotypes were significantly (p < 0.05) lower than those in the A/A genotype in parous cows (Figure 3A). In contrast, no significant differences were observed among the three genotypes of non-parous HF cows using either the Friedman rank sum test or the Wilcoxon rank sum exact test with Bonferroni correction (Figure 3B).
4. Discussion
Bali cattle (Figure 1A,B), a domestic bovine breed from Bali Island, Indonesia, is known for its adaptability and valuable contributions to regional agriculture [17]. Owing to their compact bodies, strong muscles, and upward-curving horns, they serve as draught animals and provide sought-after meat [18]. Thriving on limited resources and displaying disease resistance, these cattle play a vital role in Bali’s agriculture [19]. However, issues such as infertility and repeat breeding impede reproductive efficiency and productivity [20]. Implementing effective management strategies encompassing nutrition, healthcare, and genetic selection is considered essential to address these concerns and ensure the long-term sustainability of Bali cattle breeding programs.
Jaliteng cattle (Figure 1C,D), a subspecies of banteng native to Southeast Asia, are prized for their adaptability, genetic heritage, and cultural value [21,22,23]. Originating from crossbreeding Bali cattle with Javanese banteng bulls, these medium-to-large-sized animals possess distinctively curved horns [24]. They inherit favorable traits from their parent species, including meat quality and disease resistance, thereby promoting livestock productivity and genetic diversity. However, they are endangered because of habitat loss, hunting, and reproductive challenges [25,26]. Infertility and breeding issues among Jaliteng cattle have long been major concerns and require urgent attention for their conservation and management [27]. Preserving these unique animals is crucial for sustainable agriculture and the livelihoods of local farmers in Indonesia.
Water buffalo (Figure 1E) is a widespread bovine species in Asia [28]. Regarding their agricultural uses, water buffaloes are used for plowing fields, transporting goods, and supporting rural economies [29]. Water buffaloes provide milk with high fat and protein content, contributing to dairy production. They play important roles in meat production, food security, conservation, cultural symbolism, and environmental balance [30]. Despite their numerous benefits, buffalo breeding challenges persist because of factors such as low conception rates, infertility, and limited access to quality breeding bulls [31]. Addressing these issues requires improved reproductive techniques, veterinary care, and scientific investigations to optimize buffalo reproduction and increase herd sizes.
A previous GWAS identified a FOXP3 gene variant (NC_037357.1: g.87298881A>G, rs135720414) associated with repeat breeding in JB cattle, indicating that the G allele is a variant associated with infertility [11]. In the present study, we examined herds of Bali and Jaliteng cattle, and water buffaloes, using real-time PCR genotyping targeting this variant. We demonstrated that the frequency of the risk-type G allele was markedly high in Bali (0.944) and Jaliteng (0.714) cattle and water buffaloes (1) and moderately high in HF cattle (0.514), as shown in Table 1. Our previous study reported that the G allele frequency ranged widely from 0.112 to 0.700 among several cattle breeds, including KH (0.112), JB (0.250), HF (0.466), and IM (0.700) cattle [12]. Among the breeds examined previously, IM, with the highest G allele frequency, is an Indonesian native bovine breed developed by crossing zebu (B. indicus) and banteng cattle, the herds of which are predominantly reared by small-scale farmers on Madura Island, East Java, Indonesia [32,33,34]. In our previous study, we speculated that the high G allele frequency in IM cattle may be attributed to the genetic contribution of banteng cattle [12]. In the present study, Bali and Jaliteng cattle native to banteng had a very high G frequency, which could also be attributed to the genetic contribution of banteng. Indonesian cattle and buffaloes are primarily bred through natural mating with local breeding bulls, which can result in inbreeding and the spread of single mutant genes within limited breeding areas [34]. These factors contribute to a reduction in genetic variation and an increase in the prevalence of specific undesirable alleles. Based on these data from banteng-related cattle breeds and water buffaloes, the G allele of the FOXP3 gene may originally be a wild-type variant in cattle breeds and buffaloes, which is an undesirable trait for domestic animals. In contrast, modern taurine cattle, including beef (JB and KH) and dairy (HF) cattle, may have been unintentionally selected for the non-risk A allele during domestication and development [12], resulting in low-and-moderate G allele frequencies. Further studies are required to confirm these hypotheses by increasing the sample size of various bovine breeds, especially Asian old cattle breeds and water buffaloes.
Water buffaloes and cattle belong to the family Bovidae and subfamily Bovinae, with the genera Bubalus (buffalo) and Bos (cattle) belonging to the tribe Bovini [35]. The evolutionary history of buffaloes and cattle involves ancient hybridization events within their genomes [36]. Given that major and local Indonesian cattle breeds, including Bali, Jaliteng, and IM cattle, exhibit high frequencies of the G allele, the high G allele frequency observed in Indonesian water buffaloes may be influenced by their common ancestral origin and genetic hybridization, as observed between banteng and zebu cattle [37]. However, in the current study, we preliminarily examined a small herd of water buffaloes and obtained data based on a small population. Further research is required to confirm this hypothesis.
Among the taurine cattle breeds examined, HF dairy cattle had a moderate frequency of the G allele: 0.466 in our previous study and 0.514 in the current study, which was higher than those in beef cattle, including JB (0.250) and KH (0.112) [12]. The higher frequency of the G allele in HF dairy cattle may be because breeding programs for this breed have focused mainly on milk yield and quality rather than fertility. Previously, we investigated the relationship between the genotype of this variant and the reproductive performance in a small HF herd (19 cows) [38]. This study revealed that there are significant differences (p = 0.017) in the formation of follicular cysts (A/A: 0%, A/G: 61.5%, and G/G: 100%) among the three genotypes, and it confirmed a significant difference (p = 0.046) in the postpartum days open between cows with follicular cysts and those without cysts. These findings suggest that the G allele is associated with infertility in HF dairy cows; however, the limited number of cows in that study prevented this observation from being fully generalizable [38].
Therefore, in the present study, we measured serum AMH concentrations in a moderately sized HF herd (108 cows) and compared the results among the three genotypes after dividing them into 69 parous and 39 non-parous cows. The AMH concentration in parous cows with the A/A genotype was significantly higher than that in parous cows with the A/G and G/G genotypes (Figure 3A). Peripheral AMH concentrations are indicative of the size of the ovarian reserve and are promising biomarkers of fertility that could be utilized to improve breeding schemes for the reproductive performance of dairy cows [13]. Inflammation during the early postpartum period may decrease peripheral AMH levels, and subsequently affect the reproductive prognosis of postpartum HF cows [14]. Therefore, decreased AMH concentrations are associated with infertility in dairy cows. Based on our findings, HF cows carrying the G allele may be disadvantaged in terms of reproductive performance. However, in the present study, there were no significant differences among the three genotypes of non-parous HF cows (Figure 3B). This suggests that the combination of the G allele and aging may affect the fertility of dairy cows through multiple mechanisms, including decreased AMH concentrations as indicated in this study, increased number of follicular cysts [38], and postpartum inflammation [14]. Further studies are needed to resolve these issues by increasing the sample size from various bovine breeds and analyzing multivariable parameters such as age, parity number, and postpartum period that can influence AMH levels.
5. Conclusions
Our study found that the G allele frequency of the bovine FOXP3 gene was markedly high in banteng-related cattle breeds, water buffaloes, and HF cattle. These data suggest that the G allele may be a wild-type variant in bovine species. We also found that the AMH concentration in the A/G and G/G genotypes was significantly lower than that in the A/A genotype in parous HF cows, suggesting that the G allele, combined with aging, is associated with infertility. Therefore, infertility in bovine herds may be improved by the selection and/or introduction of the A allele of the bovine FOXP3 gene. In particular, the high frequency of the G allele in Bali, Jaliteng, and HF cattle suggests the need for focused efforts to address and reduce infertility issues in this population by using individuals carrying the A allele for breeding. However, the relationship among genetic background, aging, and reproductive disorders differs for each cattle breed and water buffalo; therefore, careful examinations are required for each individual breed. Further studies are necessary to advance breeding strategies and management practices for these valuable bovids.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ani15162407/s1; Figure S1: Representative real-time PCR amplification plots of the A/A, A/G, and G/G or G/- genotypes associated with an X-linked single-nucleotide variant (NC_037357.1: g.87298881A>G, rs135720414) in the upstream of the FOXP3 gene. Amplification is graphed as fluorescence intensity (ΔRn values) versus cycle number. ΔRn values denote the reporter dye signal, normalized to the internal reference dye, and adjusted for the baseline signal determined in the initial cycles of PCR. The blue and red lines signify amplification in the presence of A- and G-alleles, respectively.
Author Contributions
Conceptualization, A.A.F., O.S.W., M.T. and O.Y.; data curation, A.A.F., O.S.W., M.T. and O.Y.; formal analysis, M.S.I., S.M., T.M.R. and A.Y.; investigation, A.A.F., O.S.W., T.D.L., M.F.M., N.T.L., H.O., M.S.I., S.M., T.M.R. and A.Y.; methodology, M.T. and O.Y.; resources, T.D.L., M.F.M., N.T.L. and H.O.; project administration, M.T. and O.Y.; supervision, O.Y.; validation, O.S.W., T.D.L., M.F.M., N.T.L., H.O., M.S.I., S.M., T.M.R. and A.Y.; visualization, O.S.W. and O.Y.; writing—original draft, A.A.F.; writing—review and editing, O.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by grants from the Japan Racing Association (grant numbers R3-no. 18 and R6-no. 10; Mitsuhiro Takagi, and Osamu Yamato) and the Ministry of Education, Culture, Sports, Science, and Technology of Japan (grant number 17H03927; Osamu Yamato).
Institutional Review Board Statement
The experiments conducted in this study were performed in accordance with the guidelines regulating animal use and ethics of Kagoshima University (no. VM15041; approval date: 29 September 2015) and Yamaguchi University, Japan (no. 40, 1995; approval date: 27 March 2017), and oral informed consent was obtained from cooperating farmers.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank owners and breeders for providing blood samples of their animals.
Conflicts of Interest
Author Hiroaki Okawa was employed by the company Guardian Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FOXP3 | forkhead box P |
| GWAS | genome-wide association study |
| JB | Japanese Black |
| HF | Holstein Friesian |
| KH | Korean Hanwoo |
| IM | Indonesian Madura |
| AMH | anti-Müllerian hormone |
| FTA | Flinders Technology Associates |
References
- McDougall, S. Reproduction performance and management of dairy cattle. J. Reprod. Dev. 2006, 52, 185–194. [Google Scholar] [CrossRef]
- Yoo, H.S. Infectious causes of reproductive disorders in cattle. J. Reprod. Dev. 2010, 56, 53–60. [Google Scholar] [CrossRef]
- Deka, R.P.; Magnusson, U.; Grace, D.; Randolph, T.F.; Shome, R.; Lindahl, J.F. Estimates of the economic cost caused by five major reproductive problems in dairy animals in Assam and Bihar, India. Animals 2021, 11, 3116. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; Williams, E.; Evans, A. A review of the causes of poor fertility in high milk producing dairy cows. Anim. Reprod. Sci. 2011, 123, 127–138. [Google Scholar] [CrossRef]
- Yaginuma, H.; Funeshima, N.; Tanikawa, N.; Miyamura, M.; Tsuchiya, H.; Noguchi, T.; Iwata, H.; Kuwayama, T.; Shirasuna, K.; Hamano, S. Improvement of fertility in repeat breeder dairy cattle by embryo transfer following artificial insemination: Possibility of interferon tau replenishment effect. J. Reprod. Dev. 2019, 65, 223–229. [Google Scholar] [CrossRef]
- Li, Z.; Li, D.; Tsun, A.; Li, B. FOXP3+ regulatory T cells and their functional regulation. Cell Mol. Immunol. 2015, 12, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S.; Nakashima, A.; Shima, T.; Saito, S. New paradigm in the role of regulatory T cells during pregnancy. Front. Immunol. 2019, 10, 573. [Google Scholar] [CrossRef]
- Oliveira, L.D.J.; Barreto, R.D.S.N.; Perecin, F.; Mansouri-Attia, N.; Pereira, F.T.V.; Meirelles, F.V. Modulation of maternal immune system during pregnancy in the cow. Reprod. Domest. Anim. 2012, 47, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Patnam, A.K.; Vinu, R.; Vijayalakshmi, J.; Venkatachalam, P.; Rani, G.U. Association of ESR and FOXP3 gene polymorphisms with outcome of ovarian stimulation in infertile females undergoing IVF. Mol. Cytogenet. 2014, 7, P61. [Google Scholar] [CrossRef]
- Jacobo, P.; Guazzone, V.A.; Jarazo-Dietrich, S.; Theas, M.S.; Lustig, L. Differential changes in CD4+ and CD8+ effector and regulatory T lymphocyte subsets in the testis of rats undergoing autoimmune orchitis. J. Reprod. Immunol. 2009, 81, 44–54. [Google Scholar] [CrossRef]
- Arishima, T.; Sasaki, S.; Isobe, T.; Ikebata, Y.; Shimbara, S.; Ikeda, S.; Kawashima, K.; Suzuki, Y.; Watanabe, M.; Sugano, S. Maternal variant in the upstream of FOXP3 gene on the X chromosome is associated with recurrent infertility in Japanese Black cattle. BMC Genet. 2017, 18, 103. [Google Scholar] [CrossRef]
- Islam, M.S.; Takagi, M.; Lee, K.-W.; Chang, H.-S.; Okawa, H.; Yunus, M.; Lestari, T.D.; Tacharina, M.R.; Pervin, S.; Rakib, T.M. Frequency of an X-linked maternal variant of the bovine FOXP3 gene associated with infertility in different cattle breeds: A pilot study. Animals 2022, 12, 1044. [Google Scholar] [CrossRef]
- Mossa, F.; Ireland, J.J. Physiology and endocrinology symposium: Anti-Müllerian hormone: A biomarker for the ovarian reserve, ovarian function, and fertility in dairy cows. J. Anim. Sci. 2019, 97, 1446–1455. [Google Scholar] [CrossRef]
- Okawa, H.; Monniaux, D.; Mizokami, C.; Fujikura, A.; Takano, T.; Sato, S.; Shinya, U.; Kawashima, C.; Yamato, O.; Fushimi, Y.; et al. Association between anti-Müllerian hormone concentration and inflammation markers in serum during the peripartum period in dairy cows. Animals 2021, 11, 1241. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, K.; Chang, H.-S.; Yabuki, A.; Kawamichi, T.; Kawahara, N.; Hayashi, D.; Hossain, M.A.; Rahman, M.M.; Uddin, M.M.; Yamato, O. Novel rapid genotyping assays for neuronal ceroid lipofuscinosis in Border Collie dogs and high frequency of the mutant allele in Japan. J. Vet. Diagn. Investig. 2011, 23, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Fushimi, Y.; Monniaux, D.; Takagi, M. Efficacy of a single measurement of plasma anti-Müllerian hormone concentration for ovum pick-up donor selection of Japanese Black heifers in herd breeding programs. J. Reprod. Dev. 2019, 65, 369–374. [Google Scholar] [CrossRef]
- Astaman, P.; Siregar, A.; Munizu, M. Risk identification of Bali cattle on traditional farming: A review. IOP Conf. Ser. Earth Environ. Sci. 2021, 807, 032089. [Google Scholar] [CrossRef]
- Hayanti, S.Y.; Handiwirawan, E.; Susilawati, E. Diversity of qualitative characteristics and their use to distinguish the origin of the Bali cattle population. Ind. J. Anim. Res. 2022, 56, 1041–1046. [Google Scholar] [CrossRef]
- Martojo, H. Indigenous Bali cattle is most suitable for sustainable small farming in Indonesia. Reprod. Domest. Anim. 2012, 47, 10–14. [Google Scholar] [CrossRef]
- Gunawan, A.; Sari, R.; Parwoto, Y.; Uddin, M. Non genetic factors effect on reproductive performance and preweaning mortality from artificially and naturally bred in Bali cattle. J. Indones. Trop. Anim. Agric. 2011, 36, 83–90. [Google Scholar] [CrossRef][Green Version]
- Pedrono, M.; Tuan, H.M.; Chouteau, P.; Vallejo, F. Status and distribution of the Endangered banteng Bos javanicus birmanicus in Vietnam: A conservation tragedy. Oryx 2009, 43, 618–625. [Google Scholar] [CrossRef]
- Matsubayashi, H.; Hanzawa, K.; Kono, T.; Ishige, T.; Gakuhari, T.; Lagan, P.; Sunjoto, I.; Sukor, J.R.A.; Sinun, W.; Ahmad, A.H. First molecular data on Bornean banteng Bos javanicus lowi (Cetartiodactyla, Bovidae) from Sabah, Malaysian Borneo. Mammalia 2014, 78, 523–531. [Google Scholar] [CrossRef]
- Ishige, T.; Gakuhari, T.; Hanzawa, K.; Kono, T.; Sunjoto, I.; Sukor, J.R.A.; Ahmad, A.H.; Matsubayashi, H. Complete mitochondrial genomes of the tooth of a poached Bornean banteng (Bos javanicus lowi; Cetartiodactyla, Bovidae). Mitochondrial DNA A DNA Mapp. Seq. Anal. 2016, 27, 2453–2454. [Google Scholar] [CrossRef]
- Purwantara, B.; Noor, R.; Andersson, G.; Rodriguez-Martinez, H. Banteng and Bali cattle in Indonesia: Status and forecasts. Reprod. Domest. Anim. 2021, 47, 2–6. [Google Scholar] [CrossRef]
- Sansinena, M.; Hylan, D.; Hebert, K.; Denniston, R.; Godke, R. Banteng (Bos javanicus) embryos and pregnancies produced by interspecies nuclear transfer. Theriogenology 2005, 63, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Rahman, D.A.; Herliansyah, R.; Rianti, P.; Rahmat, U.M.; Firdaus, A.Y.; Syamsudin, M. Ecology and conservation of the Endangered banteng (Bos javanicus) in Indonesia tropical lowland forest. Hayati J. Biosci. 2019, 26, 68–80. [Google Scholar] [CrossRef]
- Lenstra, J.; Bradley, D. Systematics and phylogeny of cattle. In The Genetics of Cattle; Fries, R., Ruvinsky, A., Eds.; CAB International: Wallingford, UK, 1999; pp. 1–14. ISBN 0851992587. [Google Scholar]
- Hasan, M.M.; Sarker, M.T.; Sri, A. Effect of spatial distribution on meat quality traits of buffalo in Bangladesh. Biol. Sci. 2017, 1, 1–15. [Google Scholar] [CrossRef]
- Bilal, M.; Suleman, M.; Raziq, A. Buffalo: Black gold of Pakistan. Livestock Res. Rural Dev. 2006, 18, 140–151. [Google Scholar]
- Karli, B.; Gül, M.; Akpinar, M.G.; Tascioğlu, Y.; Bozkurt, Y. Problems of water buffalo breeding in Turkey and suggestions for its development. R. Bras. Zootec. 2018, 47, e20170230. [Google Scholar] [CrossRef]
- Purohit, G.N. Recent developments in the diagnosis and therapy of repeat breeding cows and buffaloes. CABI Rev. 2008, 3, 1–34. [Google Scholar] [CrossRef]
- Popescu, C.P.; Smith, W.G. A cytogenetic investigation of Madura cattle. Reprod. Domest. Anim. 1988, 23, 145–148. [Google Scholar] [CrossRef]
- Mohamad, K.; Olsson, M.; Andersson, G.; Purwantara, B.; van Tol, H.; Rodriguez-Martinez, H.; Colenbrander, B.; Lenstra, J. The origin of Indonesian cattle and conservation genetics of the Bali cattle breed. Reprod. Domest. Anim. 2012, 47, 18–20. [Google Scholar] [CrossRef]
- Riszqina, M.; Isbandi, M.; Rianto, E.; Santoso, S. Income of Madura cattle farmers in Madura island of east Java province of Indonesia. Bangl. J. Anim. Sci. 2014, 43, 68–73. [Google Scholar] [CrossRef]
- Gentry, A. The subfamilies and tribes of the family Bovidae. Mamm. Rev. 1992, 22, 1–32. [Google Scholar] [CrossRef]
- Soubrier, J.; Gower, G.; Chen, K.; Richards, S.M.; Llamas, B.; Mitchell, K.J.; Ho, S.Y.W.; Kosintsev, P.; Lee, M.S.Y.; Baryshnikov, G.; et al. Early cave art and ancient DNA record the origin of European bison. Nat. Commun. 2016, 7, 13158. [Google Scholar] [CrossRef] [PubMed]
- Nijman, I.; Otsen, M.; Verkaar, E.; De Ruijter, C.; Hanekamp, E.; Ochieng, J.; Shamshad, S.; Rege, J.; Hanotte, O.; Barwegen, M.; et al. Hybridization of banteng (Bos javanicus) and zebu (Bos indicus) revealed by mitochondrial DNA, satellite DNA, AFLP and microsatellites. Heredity 2003, 90, 10–16. [Google Scholar] [CrossRef]
- Priyo, T.W., Jr.; Edo, A.; Taura, Y.; Yamato, O.; Ono, T.; Taniguchi, M.; Widodo, O.S.; Islam, M.S.; Maki, S.; Takagi, M. Novel approach for evaluating pregnancy-associated glycoprotein and inflammation markers during the postpartum period in Holstein Friesian cows. Animals 2024, 14, 1459. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).