Simulating Precision Feeding of High-Concentrate Diets with High-Fat Inclusion and Different Plant-Based Saturated, Unsaturated, and Animal Fat Sources in Continuous Culture Fermenters
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatments and Experimental Design
2.2. Continuous Culture Conditions
2.3. Sample Collection and Analysis
2.4. Calculations and Statistical Analysis
3. Results and Discussion
3.1. Diet Composition and Nutrient Inputs
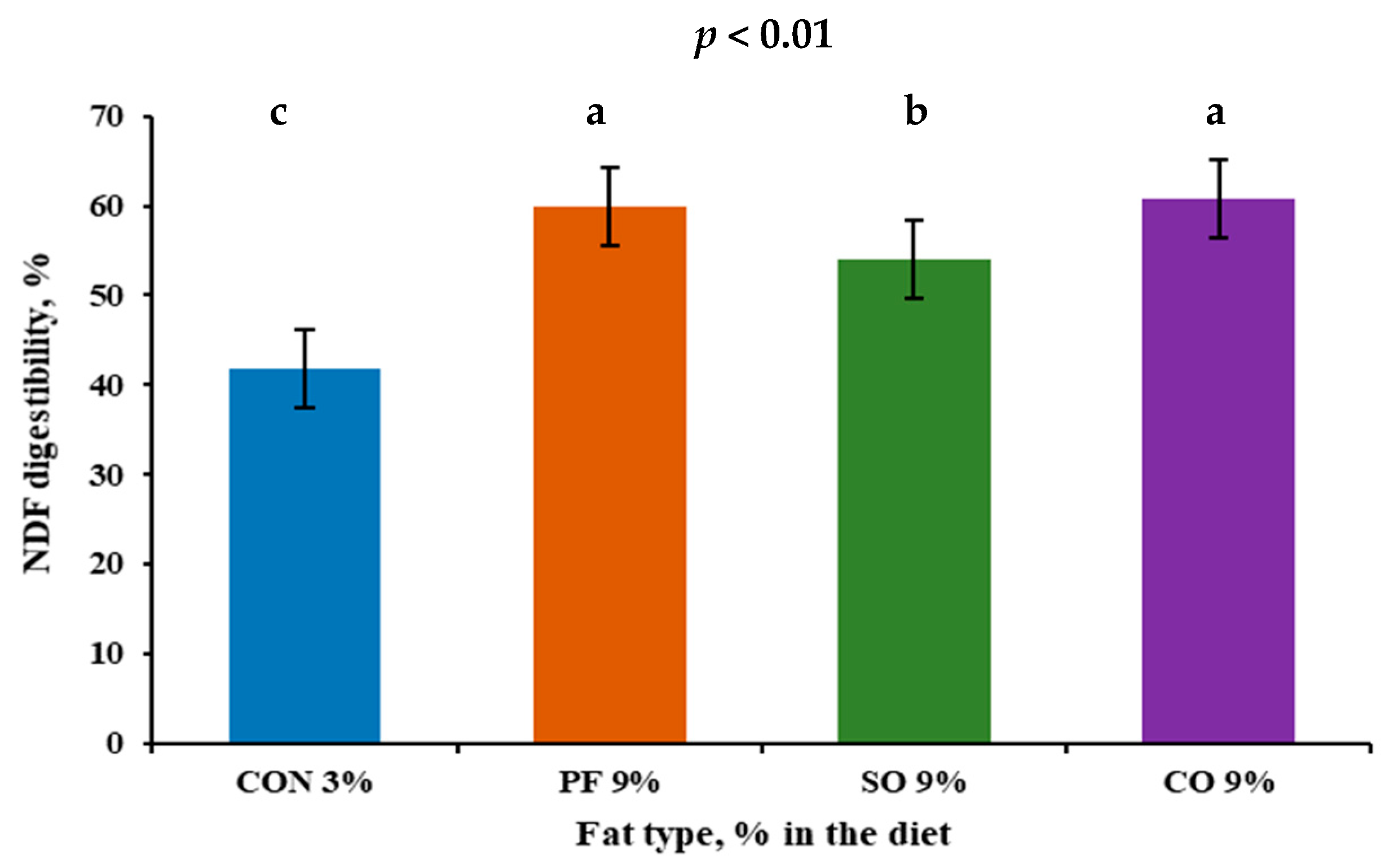
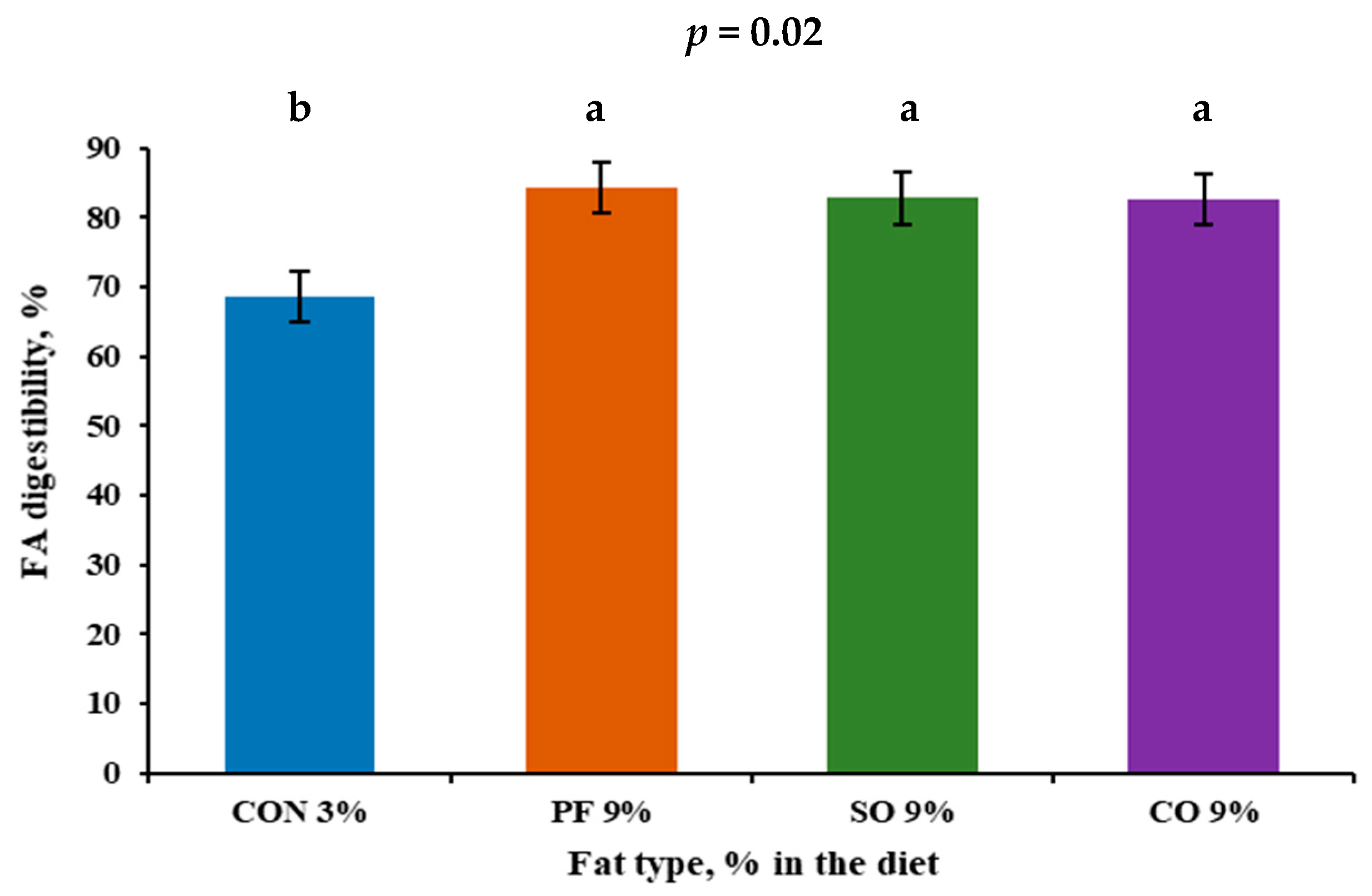
3.2. Digestibility of Nutrients
3.3. Fatty Acid Flows and Biohydrogenation
3.4. Characteristics of Fermentation
| Fat Type, * % in the Diet | ||||||
|---|---|---|---|---|---|---|
| Culture Fermentation | CON 3% | PF 9% | SO 9% | CO 9% | SE | p-Value |
| Total VFA, mM | 111.9 a | 83.4 b | 88.0 b | 66.3 c | 4.39 | <0.01 |
| Individual VFA, mol/100 mol | ||||||
| Acetate | 49.9 a | 47.4 ab | 45.2 bc | 44.3 c | 1.26 | <0.01 |
| Propionate | 31.4 a | 30.2 ab | 31.5 a | 27.1 b | 1.19 | 0.02 |
| Butyrate | 11.8 b | 15.7 ab | 14.3 b | 18.9 a | 1.24 | <0.01 |
| Valerate | 6.05 bc | 5.65 c | 8.22 ab | 8.64 a | 0.81 | <0.01 |
| Isobutyrate | 0.68 b | 0.80 ab | 0.71 ab | 0.98 a | 0.12 | <0.01 |
| Acetate/propionate | 1.62 | 1.58 | 1.46 | 1.66 | 0.08 | 0.22 |
| NH3N, mg/dL | 4.84 d | 5.64 b | 5.09 c | 5.91 a | 0.02 | <0.01 |
| pH | 5.78 d | 6.05 b | 5.94 c | 6.13 a | 0.01 | <0.01 |
| Eh, 1 mV | −296 a | −265 a | −279 a | −360 b | 17.9 | <0.01 |
| rH 2 | 8.35 a | 9.92 a | 9.22 a | 6.90 b | 0.60 | 0.01 |
| Protozoa, 103/mL | 26.0 a | 19.4 c | 16.9 d | 22.1 b | 0.55 | <0.01 |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Fat Type, % in the Diet | ||||
| Nutrient Input, g/d | CON 3% | PF 9% | SO 9% | CO 9% |
| As fed | 53.4 | 47.7 | 47.7 | 47.7 |
| DM | 48.3 | 43.2 | 43.2 | 42.9 |
| OM | 46.1 | 41.1 | 41.0 | 41.0 |
| N | 0.99 | 0.97 | 0.98 | 0.98 |
| EE | 1.70 | 3.70 | 3.76 | 3.57 |
| NDF | 10.0 | 8.57 | 8.74 | 8.74 |
| ADF | 4.76 | 3.98 | 4.15 | 4.15 |
| Starch | 18.9 | 13.8 | 13.7 | 13.6 |
| NFC | 28.2 | 22.8 | 22.3 | 22.5 |
| Ash | 2.13 | 2.09 | 2.24 | 1.96 |
| ME, 1 Mcal/d | 0.12 | 0.12 | 0.12 | 0.12 |
| FA input, mg/d | ||||
| Total | 1401 | 3465 | 3563 | 3360 |
| C8:0 | 0.72 | 2.45 | 0.95 | 1.95 |
| C10:0 | 0.14 | 0.86 | 0.13 | 1.45 |
| C12:0 | 0.72 | 2.18 | 0.71 | 1157 |
| C14:0 | 1.57 | 15.3 | 2.53 | 951 |
| C16:0 | 196 | 811 | 408 | 191 |
| C18:0 | 55 | 158 | 96 | 33.2 |
| C22:0 | 3.30 | 10.5 | 6.84 | 2.24 |
| C24:0 | 6.92 | 43.2 | 34.3 | 12.3 |
| C18:1 | 359 | 1046 | 617 | 255 |
| C18:2 | 728 | 1077 | 1600 | 637 |
| C18:3 | 61.6 | 78.3 | 148 | 52.6 |
| Fractional turnover rate | ||||
| Liquid fraction, %/h | 8.60 | 7.76 | 7.76 | 7.76 |
| Solid fraction, %/h | 3.84 | 3.22 | 3.22 | 3.22 |
| 1 ME (Mcal/d) calculated as ME = (digested OM × 4.409 × 1.01 − 0.45) × 0.82. To represent the increase in energy as fat increased in the diets, ME = (digested OM × 4.409 × 1.01 − 0.45) + (0.0046 × (EE − 3) × 0.82 (modified from [18]). | ||||
References
- Reynolds, C.K.; Tyrrell, H.F.; Reynolds, P.J. Effects of diet forage-to-concentrate ratio and intake on energy metabolism in growing beef heifers: Whole body energy and nitrogen balance and visceral heat production. J. Nutr. 1991, 121, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Zanton, G.I.; Heinrichs, A.J. The effects of controlled feeding of a high-forage or high-concentrate ration on heifer growth and first-lactation milk production. J. Dairy Sci. 2007, 90, 3388–3396. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.K.; Saijpaul, S.; Kaur, K. Effect of supplementation of indigenously prepared rumen protected fat on rumen fermentation in buffaloes. Indian J. Anim. Sci. 2010, 80, 902–905. [Google Scholar]
- Hussein, S.M.; Aguerre, M.J.; Jenkins, T.C.; Bridges, W.C.; Lascano, G.J. Screening Dietary Fat Sources and Concentrations Included in Low- and High-Forage Diets Using an In Vitro Gas Production System. Fermentation 2024, 10, 506. [Google Scholar] [CrossRef]
- Zanton, G.I.; Heinrichs, A.J. Digestion and nitrogen utilization in dairy heifers limit-fed a low or high forage ration at four levels of nitrogen intake. J. Dairy Sci. 2009, 92, 2078–2094. [Google Scholar] [CrossRef] [PubMed]
- Lascano, G.J.; Heinrichs, A.J. Rumen fermentation pattern of dairy heifers fed restricted amounts of low, medium, and high concentrate diets without and with yeast culture. Livest. Sci. 2009, 124, 48–57. [Google Scholar] [CrossRef]
- Lascano, G.J.; Zanton, G.I.; Suarez-Mena, F.X.; Heinrichs, A.J. Effect of limit feeding high- and low-concentrate diets with Saccharomyces cerevisiae on digestibility and on dairy heifer growth and first-lactation performance. J. Dairy Sci. 2009, 92, 5100–5110. [Google Scholar] [CrossRef]
- Palmquist, D.L.; Jenkins, T.C. Fat in lactation rations: Review. J. Dairy Sci. 1980, 63, 1–14. [Google Scholar] [CrossRef]
- Nocek, J.E. Bovine acidosis: Implications on laminitis. J. Dairy Sci. 1997, 80, 1005–1028. [Google Scholar] [CrossRef]
- Azain, M.J. Role of fatty acids in adipocyte growth and development. J. Anim. Sci. 2004, 82, 916–924. [Google Scholar] [CrossRef]
- Jenkins, T.C. Lipid metabolism in the rumen. J. Dairy Sci. 1993, 76, 3851–3863. [Google Scholar] [CrossRef] [PubMed]
- Pantoja, J.; Firkins, J.L.; Eastridge, M.L.; Hull, B.L. Effects of fat saturation and source of fiber on site of nutrient digestion and milk production by lactating dairy cows. J. Dairy Sci. 1994, 77, 2341–2356. [Google Scholar] [CrossRef]
- Machmuller, A.; Solvia, C.R.; Kreuzer, M. Effect of coconut oil and defaunation treatment on methanogenesis in sheep. Repro. Nutr. Dev. 2003, 43, 41–55. [Google Scholar] [CrossRef]
- Pilajun, R.; Wanapat, M. Microbial population in the rumen of swamp buffalo (Bubalus bubalis) as influenced by coconut oil and mangosteen peel supplementation. J. Anim. Physiol. Anim. Nutr. 2013, 97, 439–450. [Google Scholar] [CrossRef]
- Hutchison, S.E.; Kegley, B.; Apple, J.K.; Wistuba, T.J.; Dikeman, M.E.; Rule, D.C. Effects of adding poultry fat in the finishing diet of steers on performance, carcass characteristics, sensory traits, and fatty acid profiles. J. Anim. Sci. 2006, 84, 2426–2435. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Hussein, S.M.; Shaker, A.S. Effect of adding garlic powder and local red sumac to quail diets on productive performance and some blood biochemical characteristics during the growth stage in cages. IOP Conf. Ser. Earth Environ. Sci. 2023, 1262, 072113. [Google Scholar] [CrossRef]
- Swisher, K. Market Report: Down, Down, Down, but an Uptrend Is Coming. Render, 7 April 2015; pp. 10–15. [Google Scholar]
- NRC (National Research Council). The Nutrient Requirements of Dairy Cattle, 7 Rev. ed.; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Anderson, J.L.; Kalscheur, K.F.; Garcia, A.D.; Schingoethe, D.J. Feeding fat from distillers dried grains with solubles to dairy heifers: I. Effects on growth performance and total-tract digestibility of nutrients. J. Dairy Sci. 2015, 98, 5699–5708. [Google Scholar] [CrossRef]
- Anderson, J.L.; Kalscheur, K.F.; Garcia, A.D.; Schingoethe, D.J.; Hippen, A.R. Ensiling characteristics of wet distillers grains mixed with soybean hulls and evaluation of the feeding value for growing Holstein heifers. J. Anim. Sci. 2009, 87, 2113–2123. [Google Scholar] [CrossRef]
- Elliott, J.P.; Drackley, J.K.; Aldrich, C.G.; Merchen, N.R. Effects of saturation and esterification of fat sources on site and extent of digestion in steers: Ruminal fermentation and digestion of organic matter, fiber, and nitrogen. J. Anim. Sci. 1997, 75, 2803–2812. [Google Scholar] [CrossRef]
- Palmquist, D.L.; Conrad, H.R. High fat rations for dairy cows. Effects on feed intake, milk and fat production, and plasma metabolites. J. Dairy Sci. 1978, 61, 890–901. [Google Scholar] [CrossRef]
- Al-Dalawi, R.H.; Al-Hadeedy, I.Y. Influence of different levels of dietary supplementation phytase enzyme upon growth performance, carcass traits and some biochemical parameters of local Japanese quail. Plant Arch. 2019, 19, 2287–2293. [Google Scholar]
- Palmquist, D.L. Influence of source and amount of dietary fat on digestibility in lactating cows. J. Dairy Sci. 1991, 74, 1354–1360. [Google Scholar] [CrossRef]
- Drackley, J.K.; Elliott, J.P. Milk composition, ruminal characteristics, and nutrient utilization in dairy cows fed partially hydrogenated tallow. J. Dairy Sci. 1993, 76, 183–196. [Google Scholar] [CrossRef]
- Oldick, B.S.; Firkins, J.L. Effects of degree of fat saturation on fiber digestion and microbial protein synthesis when diets are fed twelve times daily. J. Anim. Sci. 2000, 78, 2412–2420. [Google Scholar] [CrossRef]
- Lascano, G.J.; Heinrichs, A.J. Effects of feeding different levels of dietary fiber through the addition of corn stover on nutrient utilization of dairy heifers precision-fed high and low concentrate diets. J. Dairy Sci. 2011, 94, 3025–3036. [Google Scholar] [CrossRef]
- Slyter, L.L.; Bryant, M.P.; Wolin, M.J. Effect of pH on population and fermentation in a continuously cultured rumen ecosystem. Appl. Microbiol. 1966, 14, 573–578. [Google Scholar] [CrossRef]
- Teather, R.M.; Sauer, F.D. A naturally compartmented rumen simulation system for the continuous culture of rumen bacteria and protozoa. J. Dairy Sci. 1988, 71, 666–673. [Google Scholar] [CrossRef]
- Lascano, G.J.; Alende, M.; Koch, L.E.; Jenkins, T.C. Changes in fermentation and biohydrogenation intermediates in continuous cultures fed low and high levels of fat with increasing rates of starch degradability. J. Dairy Sci. 2016, 99, 6334–6341. [Google Scholar] [CrossRef] [PubMed]
- Lascano, G.J.; Koch, L.E.; Heinrichs, A.J. Precision feeding dairy heifers a high rumen-degradable protein diet with different proportions of dietary fiber and forage-to concentrate ratios. J. Dairy Sci. 2016, 99, 7175–7190. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.C.; Calsamiglia, S.; Cardozo, P.W.; Vlaeminck, B. Effect of pH and level of concentrate in the diet on the production of biohydrogenation intermediates in a dual-flow continuous culture. J. Dairy Sci. 2009, 92, 4456–4466. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.C.; Bridges, W.C.; Harrison, J.H.; Young, K.M. Addition of potassium carbonate to continuous cultures of mixed ruminal bacteria shifts volatile fatty acids and dairy production of biohydrogenation intermediates. J. Dairy Sci. 2014, 97, 975–984. [Google Scholar] [CrossRef]
- Toledo, M.; Hussein, S.M.; Peña, M.; Aguerre, M.J.; Bridges, W.; Lascano, G.J. Effects of Caffeine Doses on Rumen Fermentation Profile and Nutrient Digestibility Using a Lactating Cow Diet under Continuous Cultures Conditions. Ruminants 2024, 4, 406–417. [Google Scholar] [CrossRef]
- Dai, X.; Paula, E.M.; Lelis, A.L.J.; Silva, L.G.; Brandao, V.L.N.; Monteiro, H.F.; Fan, P.; Ooulson, S.R.; Jeong, K.C.; Faciola, A.P. Effects of lipopolysaccharide dosing on bacterial community composition and fermentation in a dual-flow continuous culture system. J. Dairy Sci. 2019, 102, 334–350. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 15th ed.; AOAC: Arlington, VA, USA, 2000. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Bach Knudson, K.E. Carbohydrate and lignin contents of plant material used in animal feeding. Anim. Feed. Sci. Technol. 1997, 67, 319–338. [Google Scholar] [CrossRef]
- Moody, M.L.; Zanton, G.I.; Daubert, J.M.; Heinrichs, A.J. Nutrient utilization of differing forage-to-concentrate ratios by growing Holstein heifers. J. Dairy Sci. 2007, 90, 5580–5586. [Google Scholar] [CrossRef]
- Chaney, A.L.; Marbach, E.P. Modified reagents for determination of urea and ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef]
- Yang, C.M.J.; Varga, G.A. Effect of three concentrate feeding frequencies on rumen protozoa, rumen digesta kinetics, and milk yield in dairy cows. J. Dairy Sci. 1989, 72, 950–957. [Google Scholar] [CrossRef]
- Ogimoto, K.; Imai, S. Atlas of Rumen Microbiology; Japan Scientific Societies Press: Tokyo, Japan, 1981. [Google Scholar]
- Littell, R.C.; Henry, P.R.; Ammerman, C.B. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 1998, 76, 1216–1231. [Google Scholar] [CrossRef]
- Eng, K.S.; Smith, J.C.; Craig, J.H.; Riewe, M.E. Rate of passage of concentrate and roughage through digestive tract of sheep. J. Anim. Sci. 1964, 23, 1129–1132. [Google Scholar] [CrossRef]
- Colucci, P.E.; MacLeod, G.K.; Grovum, W.L.; McMillan, I.; Barney, D.J. Digesta kinetics in sheep and cattle fed diets with different forage to concentrate ratios at high and low intakes. J. Dairy Sci. 1990, 73, 2143–2156. [Google Scholar] [CrossRef]
- Weld, K.A.; Armentano, L.E. The effects of adding fat to diets of lactating dairy cows on total-tract neutral detergent fiber digestibility: A meta-analysis. J. Dairy Sci. 2017, 100, 1766–1779. [Google Scholar] [CrossRef]
- Pilajun, R.; Wanapat, M. Effect of roughage to concentrate ratio and plant oil supplementation on in vitro fermentation end-products. Pak. J. Nutr. 2014, 13, 492–499. [Google Scholar] [CrossRef]
- Suarez-Mena, F.X.; Lascano, G.J.; Rico, D.E.; Heinrichs, A.J. Effect of forage level and replacing canola meal with dry distillers grains with solubles in precision-fed heifer diets: Digestibility and rumen fermentation. J. Dairy Sci. 2015, 98, 8054–8065. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Rico, D.E.; Ying, Y.; Harvatine, K.J. Effect of a high-palmitic acid fat supplement on milk production and apparent total-tract digestibility in high- and low-milk yield dairy cows. J. Dairy Sci. 2014, 97, 3739–3751. [Google Scholar] [CrossRef] [PubMed]
- Manthey, A.K.; Anderson, J.L. Growth performance, rumen fermentation, nutrient utilization, and metabolic profile of dairy heifers limit-fed distillers dried grains with ad libitum forage. J. Dairy Sci. 2018, 101, 365–375. [Google Scholar] [CrossRef]
- Leaver, J.D.; Campling, R.C.; Holmes, W. The effect of level of feeding on the digestibility of diets for sheep and cattle. Anim. Prod. 1969, 11, 11–18. [Google Scholar] [CrossRef]
- Brown, M.S.; Ponce, C.H.; Pulikanti, R. Adaptation of beef cattle to high-concentrate diets: Performance and ruminal metabolism. J. Anim. Sci. 2006, 84, E25–E33. [Google Scholar] [CrossRef]
- Russell, J.B.; Wilson, D.B. Why are ruminal cellulolytic bacteria unable to digest cellulose at low pH? J. Dairy Sci. 1996, 79, 1503–1509. [Google Scholar] [CrossRef]
- Potu, R.B.; AbuGhazaleh, A.A.; Hastings, D.; Jones, K.; Ibrahim, S.A. The effect of lipid supplements on ruminal bacteria in continuous culture fermenters varies with the fatty acid composition. J. Microbiol. 2011, 49, 216–223. [Google Scholar] [CrossRef]
- Loor, J.J.; Bandara, A.B.P.A.; Herbein, J.H. Characterization of 18:1 and 18:2 isomers produced during microbial biohydrogenation of unsaturated fatty acids from canola and soya bean oil in the rumen of lactating cows. J. Anim. Physiol. Anim. Nutr. 2002, 86, 422–432. [Google Scholar] [CrossRef]
- Varadyova, Z.; Kisidayova, S.; Siroka, P.; Jalc, D. Fatty acid profiles of rumen fluid from sheep fed diets supplemented with various oils and effect on the rumen ciliate population. Czech J. Anim. Sci. 2007, 52, 399–406. [Google Scholar] [CrossRef]
- Kucuk, O.; Hess, B.W.; Rule, D.C. Fatty acid compositions of mixed ruminal microbes isolated from sheep supplemented with soybean oil. Res. Vet. Sci. 2008, 84, 215–224. [Google Scholar] [CrossRef]
- Schmidely, P.; Glasser, F.; Doreau, M.; Sauvant, D. Digestion of fatty acids in ruminants: A meta-analysis of flows and variation factors. 1. Total fatty acids. Animal 2008, 2, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Zened, A.; Enjalbert, F.; Nicot, M.C.; Troegeler-Meynadier, A. Starch plus sunflower oil addition to the diet of dry dairy cows results in a trans-11 to trans-10 shift of biohydrogenation. J. Dairy Sci. 2013, 96, 451–459. [Google Scholar] [CrossRef] [PubMed]
- AbuGhazaleh, A.A.; Jacobson, B.N. Production of trans C18:1 and conjugated linoleic acid in continuous culture fermenters fed diets containing fish oil and sunflower oil with decreasing levels of forage. Animal 2007, 1, 660–665. [Google Scholar] [CrossRef]
- Martin, S.A.; Fonty, G.; Michalet-Doreau, B. Factors affecting the fibrolytic activity of the digestive microbial ecosystems in ruminants. In Gastrointestinal Microbiology in Animals; Martin, S.S., Ed.; Research Signpost: Trivandrum, India, 2002; pp. 1–17. [Google Scholar]
- Jenkins, T.C.; Wallace, R.J.; Moate, P.J.; Mosley, E.E. Recent advances in biohydrogenation of unsaturated fatty acids within the rumen microbial ecosystem. J. Anim. Sci. 2008, 86, 397–412. [Google Scholar] [CrossRef]
- Machmuller, A. Medium-chain fatty acids and their potential to reduce methanogenesis in domestic ruminants. Agric. Ecosyst. Environ. 2006, 112, 107–114. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Cardozo, P.W.; Ferret, A.; Bach, A. Changes in rumen microbial fermentation are due to a combined effect of type of diet and pH. J. Anim. Sci. 2008, 86, 702–711. [Google Scholar] [CrossRef]
- Enjalbert, F.; Garrett, J.E.; Moncoulon, R.; Bayourthe, C.; Chicoteau, P. Effects of yeast culture (Saccharomyces cerevisiae) on ruminal digestion in non-lactating dairy cows. Anim. Feed Sci. Technol. 1999, 76, 195–206. [Google Scholar] [CrossRef]
- Tjardes, K.E.; Faulkner, D.B.; Buskirk, D.D.; Parrett, D.F.; Berger, L.L.; Merchen, N.R.; Ireland, F.A. The influence of processed corn and supplemental fat on digestion of limit-fed diets and performance of beef cows. J. Anim. Sci. 1998, 76, 8–17. [Google Scholar] [CrossRef]
- Eun, J.S.; Fellner, V.; Gumpertz, M.L. Methane production by mixed ruminal cultures incubated in dual-flow fermenters. J. Dairy Sci. 2004, 87, 112–121. [Google Scholar] [CrossRef]
- Russell, J.B.; Onodera, R.; Hino, T. Ruminal protein fermentation: New perspectives on previous contradictions. In Physiological Aspects of Digestion and Metabolism in Ruminants; Tsuda, T., Sasaki, Y., Kawashima, R., Eds.; Academic Press: San Diego, CA, USA, 1991; pp. 681–697. [Google Scholar]
- Manthey, A.K.; Anderson, J.L.; Perry, G.A. Feeding distillers dried grains in replacement of forage in limit-fed dairy heifer rations: Effects on growth performance, rumen fermentation, and total-tract digestibility of nutrients. J. Dairy Sci. 2016, 99, 7206–7215. [Google Scholar] [CrossRef]
- Fahey, G.C.; Berger, L.L. Carbohydrate nutrition of ruminants. In The Ruminant Animal: Digestive Physiology and Nutrition; Church, D.C., Ed.; Prentice Hall Inc.: Upper Saddle River, NJ, USA, 1988; pp. 269–295. [Google Scholar]
- Doreau, M.; Ferlay, A. Effect of dietary lipids on nitrogen metabolism in the rumen: A review. Livest. Prod. Sci. 1995, 43, 97–110. [Google Scholar] [CrossRef]
- Al-Jumaili, W. Effect of oil palm (Elaeis Guineensis JACQ.) leaves methanolic extract on in vitro methanogenesis and gas production in the diets of goats. Kirkuk Univ. J. Agric. Sci. 2022, 13, 177–191. [Google Scholar]
- Pantoja, J.; Firkins, J.L.; Eastridge, M.L. Site of digestion and milk production by cows fed fats differing in saturation, esterification, and chain length. J. Dairy Sci. 1995, 78, 2247–2258. [Google Scholar] [CrossRef]
- Chibisa, G.E.; Gorka, P.; Penner, G.B.; Berthiaume, R.; Mutsvangwa, T. Effects of partial replacement of dietary starch from barley or corn with lactose on ruminal function, short-chain fatty acid absorption, nitrogen utilization, and production performance of dairy cows. J. Dairy Sci. 2015, 98, 2627–2640. [Google Scholar] [CrossRef]
- Suarez-Mena, F.X.; Lascano, G.J.; Heinrichs, A.J. Chewing activities and particle size of rumen digesta and feces of precision-fed dairy heifers fed different forage levels with increasing levels of distillers grains. J. Dairy Sci. 2013, 96, 5184–5193. [Google Scholar] [CrossRef]
- Suarez-Mena, F.X.; Lascano, G.J.; Hussein, S.M.; Heinrichs, A.J. Effects of distillers dried grains with solubles and forage dietary concentration in precision-fed dairy heifer diets: Mineral apparent absorption and retention. Appl. Anim. Sci. 2019, 35, 169–176. [Google Scholar] [CrossRef]
- Julien, C.; Marden, J.P.; Bonnefont, C.; Moncoulon, R.; Monteils, V.; Bayourthe, C. Effects of varying proportions of concentrates on ruminal-reducing power and bacterial community structure in dry dairy cows fed hay-based diets. Animal 2010, 4, 1641–1646. [Google Scholar] [CrossRef]
- Huang, Y.J.; Marden, P.; Julien, C.; Bayourthe, C. Redox potential: An intrinsic parameter of the rumen environment. J. Anim. Physiol. Anim. Nutr. 2018, 102, 393–402. [Google Scholar] [CrossRef]
- Karnati, S.K.R.; Sylvester, J.T.; Ribeiro, C.V.D.M.; Gilligan, L.E.; Firkins, J.L. Investigating unsaturated fat, monensin, or bromoethanesulfonate in continuous cultures retaining ruminal protozoa. I. Fermentation, biohydrogenation, and microbial protein synthesis. J. Dairy Sci. 2009, 92, 3849–3860. [Google Scholar] [CrossRef]
- Mathew, B.; Eastridges, M.L.; Oelker, E.R.; Firkins, J.L.; Karnati, S.K.R. Interactions of monensin with dietary fat and carbohydrate components on ruminal fermentation and production responses by dairy cows. J. Dairy Sci. 2011, 94, 396–409. [Google Scholar] [CrossRef]
- Yang, S.L.; Bu, D.P.; Wang, J.Q.; Hu, Z.Y.; Li, D.; Wei, H.Y.; Zhou, L.Y.; Loor, J.J. Soybean oil and linseed oil supplementation affect profiles of ruminal microorganism in dairy cows. Animal 2009, 3, 1562–1569. [Google Scholar] [CrossRef]
- Ferlay, A.; Chabrot, J.; Elmeddah, Y.; Doreau, M. Ruminal lipid balance and intestinal digestion by dairy cows fed calcium salts of rapeseed oil fatty acids or rapeseed oil. J. Anim. Sci. 1993, 71, 2237–2245. [Google Scholar] [CrossRef]
- Hristov, A.N.; Ivan, M.; McAllister, T.A. In vitro effects of individual fatty acids on protozoal numbers and on fermentation products in ruminal fluid from cattle fed a high-concentrate, barley-based diet. J. Anim. Sci. 2004, 82, 2693–2704. [Google Scholar] [CrossRef]
- Shamoon, S.; Mohamad, S. Effect of Feeding Restricted dry matter intakes on nutrient digestibility, Nitrogen balance and some rumen and blood parameters in karadi lambs. Kirkuk Univ. J. Agric. Sci. 2018, 9, 60–68. [Google Scholar]
- Al-Hadeedy, I.Y.; Ameen, Q.A.; Shaker, A.S.; Mohamed, A.H.; Taha, M.W.; Hussein, S.M. Using the Principal Component Analysis of Body Weight in Three Genetic Groups of Japanese Quail. IOP Conf. Ser. Earth Environ. Sci. 2023, 1252, 012148. [Google Scholar] [CrossRef]
- Newbold, C.J.; Chamberlain, D.G. Lipids as rumen-defaunating agents. Proc. Nutr. Soc. 1988, 47, 154A. [Google Scholar]
- Maia, M.R.G.; Chaudhary, L.C.; Figueres, L.; Wallace, R.J. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie Van Leeuwenhoek 2007, 91, 303–314. [Google Scholar] [CrossRef]
| Fat Type, % in the Diet | ||||
|---|---|---|---|---|
| Ingredient, 1 % | CON 3% | PF 9% | SO 9% | CO 9% |
| Coastal bermudagrass hay | 5.00 | 5.00 | 5.00 | 5.00 |
| Whole corn silage | 30.0 | 30.0 | 30.0 | 30.0 |
| Ground corn | 51.8 | 40.8 | 40.8 | 40.8 |
| Soybean meal (SBM) | 11.2 | 16.4 | 16.4 | 16.4 |
| Mineral mix | 2.00 | 2.00 | 2.00 | 2.00 |
| Fat inclusion | 0.00 | 5.79 | 5.79 | 5.79 |
| Chemical composition | ||||
| DM % | 90.5 | 90.6 | 90.7 | 90.0 |
| OM, % | 95.6 | 95.2 | 94.8 | 95.5 |
| CP, % | 12.8 | 14.0 | 14.2 | 14.2 |
| Soluble CP, % CP | 23.4 | 24.3 | 24.3 | 23.7 |
| NDF, % | 20.8 | 19.8 | 20.2 | 20.4 |
| ADF, % | 9.84 | 9.20 | 9.58 | 9.71 |
| Sugar, % | 3.60 | 4.10 | 4.00 | 3.80 |
| Starch, % | 45.2 | 37.9 | 38.7 | 38.4 |
| Ether extract, % | 3.52 | 8.56 | 8.69 | 8.31 |
| NSC, % | 48.8 | 42.0 | 42.7 | 42.2 |
| NFC, 2 % | 58.5 | 52.8 | 51.7 | 52.6 |
| TDN | 78.9 | 86.0 | 85.4 | 82.4 |
| ME, 3 Mcal/Kg | 2.88 | 3.14 | 3.11 | 3.01 |
| Ash, % | 4.41 | 4.83 | 5.18 | 4.55 |
| Fatty acid, % | ||||
| C8:0 | 0.05 | 0.07 | 0.03 | 0.06 |
| C10:0 | 0.01 | 0.02 | 0.01 | 0.04 |
| C12:0 | 0.05 | 0.06 | 0.02 | 34.5 |
| C14:0 | 0.11 | 0.44 | 0.07 | 28.3 |
| C14:1T | 0.01 | 0.01 | 0.00 | 0.12 |
| C14:1 | 0.08 | 0.08 | 0.04 | 0.01 |
| C16:0 | 14.7 | 23.9 | 11.7 | 6.00 |
| C18:0 | 0.04 | 4.59 | 0.03 | 0.99 |
| C18:1 | 25.7 | 32.2 | 17.3 | 7.59 |
| C18:1–11C | 1.68 | 2.65 | 19.9 | 0.90 |
| C18:2 | 51.9 | 31.3 | 44.9 | 18.9 |
| C18:3 | 4.40 | 2.26 | 4.17 | 1.80 |
| C22:0 | 0.24 | 0.30 | 0.19 | 0.07 |
| C24:0 | 0.49 | 1.25 | 0.96 | 0.37 |
| C22:2 | 0.01 | 0.34 | 0.41 | 0.26 |
| C22:6 | 0.51 | 0.51 | 0.26 | 0.06 |
| Total, mg/g | 28.9 | 80.2 | 82.3 | 78.2 |
| Fat Type, * % in the Diet | ||||||
|---|---|---|---|---|---|---|
| Digestibility, % | CON 3% | PF 9% | SO 9% | CO 9% | SE | p-Value |
| DM | 69.0 c | 80.1 a | 76.3 b | 80.9 a | 0.35 | <0.01 |
| OM | 74.5 c | 84.6 a | 81.4 b | 85.4 a | 0.28 | <0.01 |
| NDF | 41.8 c | 60.0 a | 54.0 b | 60.7 a | 0.65 | <0.01 |
| ADF | 33.9 c | 50.6 a | 46.6 b | 51.1 a | 0.81 | <0.01 |
| FA | 68.6 b | 84.3 a | 82.8 a | 82.6 a | 1.78 | 0.02 |
| Starch | 99.7 c | 99.9 ab | 99.9 b | 99.9 a | 0.01 | <0.01 |
| Fat Type, * % in the Diet | ||||||
|---|---|---|---|---|---|---|
| FA Outflow, mg/d | CON 3% | PF 9% | SO 9% | CO 9% | SE | p-Value |
| Saturated | ||||||
| C8:0 | 16.5 a | 12.8 a | 10.4 ab | 2.15 b | 2.90 | 0.01 |
| C10:0 | 3.52 a | 1.17 b | 1.96 ab | 2.32 ab | 0.64 | 0.13 |
| C12:0 | 1.78 b | 2.09 b | 1.99 b | 641 a | 18.9 | <0.01 |
| C14:0 | 2.41 b | 8.70 b | 3.00 b | 476 a | 25.8 | <0.01 |
| C16:0 | 145 c | 419 a | 248 b | 72.9 c | 27.0 | <0.01 |
| C18:0 | 29.3 c | 501 a | 233 b | 22.3 c | 37.9 | 0.03 |
| C22:0 | 2.20 c | 6.66 a | 4.04 b | 1.62 c | 0.54 | 0.02 |
| C24:0 | 2.70 b | 4.91 ab | 6.18 a | 2.36 b | 0.97 | 0.05 |
| Unsaturated | ||||||
| C18:1 | 237 ab | 230 b | 316 a | 117 c | 29.1 | 0.04 |
| C18:2 | 434 b | 227 c | 587 a | 241 c | 44.9 | 0.04 |
| C18:3 | 24.5 b | 13.5 b | 43.8 a | 16.1 b | 3.81 | 0.05 |
| Total | 1106 b | 2628 a | 2609 a | 2422 a | 102 | 0.01 |
| Biohydrogenation, 1 % | ||||||
| C18:2 | 42.1 c | 78.9 a | 63.3 b | 62.1 b | 3.39 | <0.01 |
| C18:3 | 60.3 b | 82.8 a | 70.6 b | 69.4 b | 3.63 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, S.M.; Jenkins, T.C.; Aguerre, M.J.; Bridges, W.C.; Lascano, G.J. Simulating Precision Feeding of High-Concentrate Diets with High-Fat Inclusion and Different Plant-Based Saturated, Unsaturated, and Animal Fat Sources in Continuous Culture Fermenters. Animals 2025, 15, 2406. https://doi.org/10.3390/ani15162406
Hussein SM, Jenkins TC, Aguerre MJ, Bridges WC, Lascano GJ. Simulating Precision Feeding of High-Concentrate Diets with High-Fat Inclusion and Different Plant-Based Saturated, Unsaturated, and Animal Fat Sources in Continuous Culture Fermenters. Animals. 2025; 15(16):2406. https://doi.org/10.3390/ani15162406
Chicago/Turabian StyleHussein, Saad M., Thomas C. Jenkins, Matias J. Aguerre, William C. Bridges, and Gustavo J. Lascano. 2025. "Simulating Precision Feeding of High-Concentrate Diets with High-Fat Inclusion and Different Plant-Based Saturated, Unsaturated, and Animal Fat Sources in Continuous Culture Fermenters" Animals 15, no. 16: 2406. https://doi.org/10.3390/ani15162406
APA StyleHussein, S. M., Jenkins, T. C., Aguerre, M. J., Bridges, W. C., & Lascano, G. J. (2025). Simulating Precision Feeding of High-Concentrate Diets with High-Fat Inclusion and Different Plant-Based Saturated, Unsaturated, and Animal Fat Sources in Continuous Culture Fermenters. Animals, 15(16), 2406. https://doi.org/10.3390/ani15162406








