Optimizing Rooster Semen Preservation: Effect of Oxygen Exposure, Sample Rotation, and HEPES Buffer Supplementation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Management
2.3. Semen Sample Collection and Extender Preparation
2.4. Semen Evaluation and Dilution
- Sperm motility was assessed using phase-contrast microscopy at 40× magnification on a pre-warmed slide (37 °C). A 5–10 µL semen aliquot was mounted under a coverslip, and progressive motility (PMOT) was recorded. Mean PMOT values were 89.87 ± 1.91% for 0.9% NaCl and 93.14 ± 1.44% for IGGKPh;
- Sperm viability was determined using the eosin–nigrosine staining technique. A 5 µL semen aliquot was mixed with 20 µL of a pre-warmed staining solution (0.6% eosin and 5% nigrosine in distilled water), incubated for 1–3 min, smeared onto a glass slide, air-dried, and examined under oil immersion at 100× magnification. Viable sperm appeared unstained, while non-viable cells exhibited pink-stained heads, as determined by counting a minimum of 300 sperm cells per sample. Mean viability was 92.46 ± 0.45% for 0.9% NaCl and 95.20 ± 0.65% for IGGKPh;
- Sperm concentration was quantified using a hemocytometer. Semen was diluted 1:1000 in a 4% NaCl solution containing eosin to enhance cell visibility. A 10 µL aliquot of the diluted sample was loaded onto the hemocytometer and analyzed at 40× magnification. Mean sperm concentrations were 5.36 ± 0.51 × 109 sperm/mL for 0.9% NaCl and 5.88 ± 0.95 × 109 sperm/mL for IGGKPh;
- The pH of the semen was measured using a portable digital pH meter (HI98100 Checker Plus; Hanna Instruments, Woonsocket, RI, USA). Mean pH values were 7.05 ± 0.080 for 0.9% NaCl and 6.87 ± 0.007 for IGGKPh.
2.5. Lipid Peroxidation
2.6. Experimental Design
2.6.1. Experimental 1: Influence of Oxygen Exposure and Gentle Rotation on Rooster Sperm Quality During Chilled Storage at 5 °C for 24 h
2.6.2. Experimental 2; Effect of HEPES Buffer Supplementation on Rooster Sperm Quality During Simulated AI Handling
2.7. Fertility Assessment
2.8. Statistical Analysis
3. Results
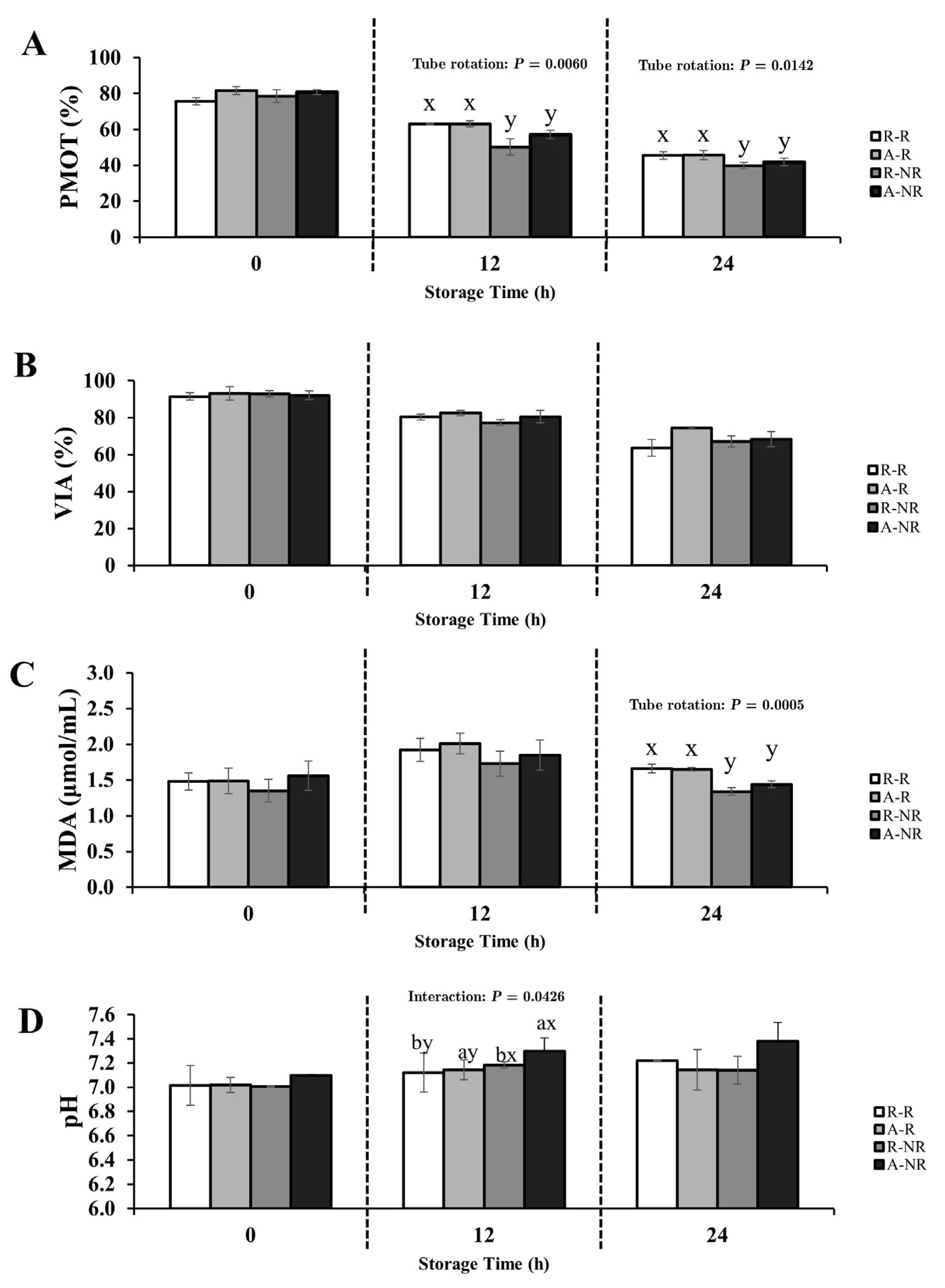
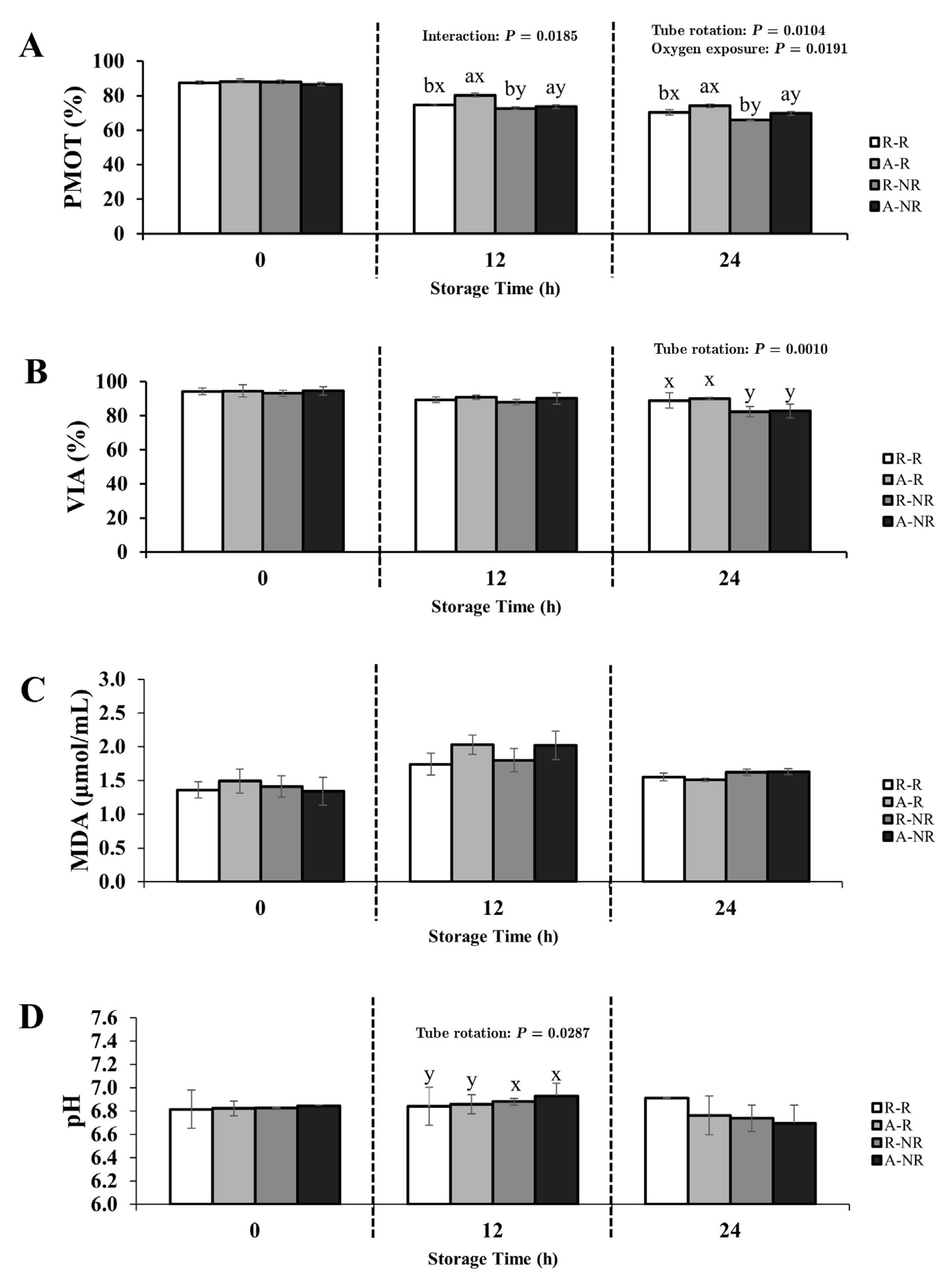
3.1. Experimental 1; Influence of Oxygen Exposure and Gentle Rotation on Rooster Sperm Quality During Chilled Storage at 5 °C for 24 h
3.1.1. Semen Diluted with 0.9% NaCl Extender
3.1.2. Semen Diluted with IGGKPh Extender
3.1.3. Fertility Outcomes
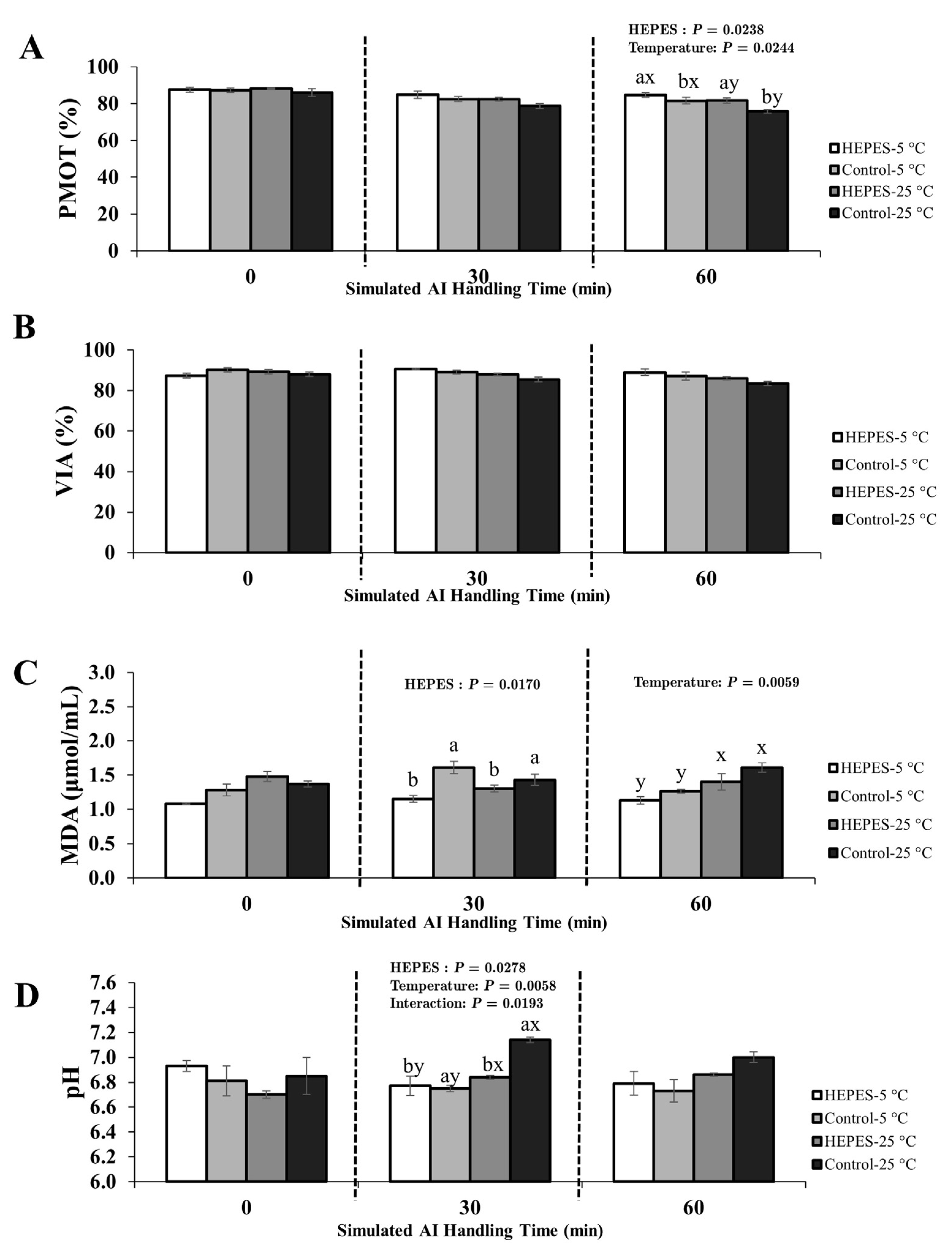
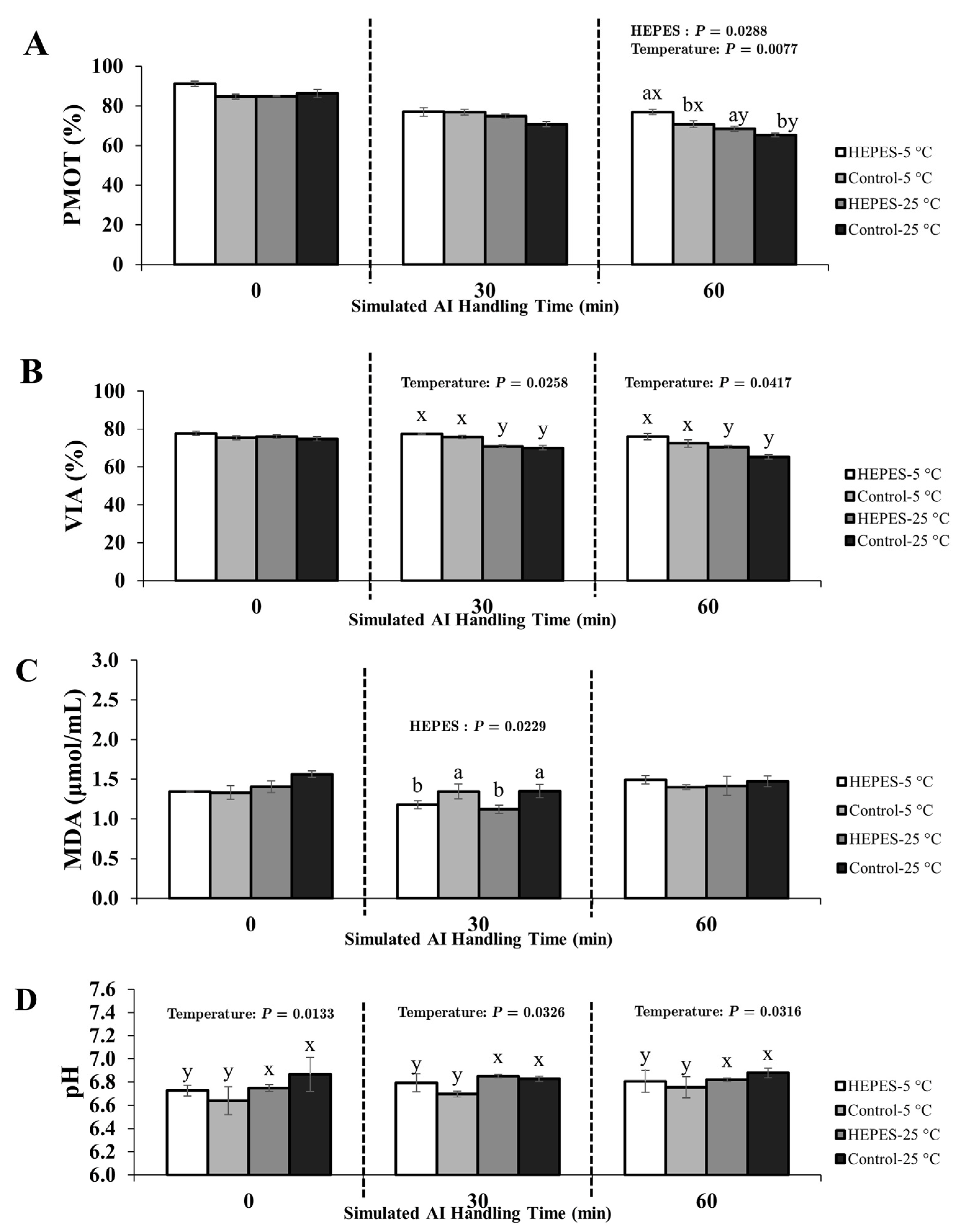
3.2. Experimental 2; Effect of HEPES Buffer Supplementation on Rooster Sperm Quality During Simulated AI Handling
3.2.1. Semen Diluted with 0.9% NaCl Extender
3.2.2. Semen Diluted with IGGKPh Extender
3.2.3. Fertility Outcomes
4. Discussion
4.1. Extender Comparison: 0.9% NaCl vs. IGGKPh
4.2. The Influence of Oxygen Exposure on Rooster Sperm Preservation
4.3. The Impact of Tube Rotation on Sperm Quality
4.4. The Role of HEPES Buffer Supplementation in Enhancing Sperm Preservation During AI Handling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohan, J.; Sharma, S.K.; Kolluri, G.; Dhama, K. History of Artificial Insemination in Poultry, Its Components and Significance. World’s Poult. Sci. J. 2018, 74, 475–488. [Google Scholar] [CrossRef]
- Bustani, G.S.; Baiee, F.H. Semen Extenders: An Evaluative Overview of Preservative Mechanisms of Semen and Semen Extenders. Vet. World 2021, 14, 1220–1233. [Google Scholar] [CrossRef]
- Surai, P.F.; Wishart, G.J. Poultry Artificial Insemination Technology in the Countries of the Former USSR. World’s Poult. Sci. J. 1996, 52, 27–43. [Google Scholar] [CrossRef]
- Łukaszewicz, E. Effects of Semen Filtration and Dilution Rate on Morphology and Fertility of Frozen Gander Spermatozoa. Theriogenology 2001, 55, 1819–1829. [Google Scholar] [CrossRef]
- Sexton, T.J. A New Poultry Semen Extender: 5. Relationship of Diluent Components to Cytotoxic Effects of Dimethylsulfoxide on Turkey Spermatozoa. Poult. Sci. 1980, 59, 1142–1144. [Google Scholar] [CrossRef]
- Chankitisakul, V.; Boonkum, W.; Kaewkanha, T.; Pimprasert, M.; Ratchamak, R.; Authaida, S.; Thananurak, P. Fertilizing Ability and Survivability of Rooster Sperm Diluted with a Novel Semen Extender Supplemented with Serine for Practical Use on Smallholder Farms. Poult. Sci. 2022, 101, 102188. [Google Scholar] [CrossRef] [PubMed]
- Pimpa, J.; Authaida, S.; Boonkum, W.; Rerkyusuke, S.; Janta, C.; Chankitisakul, V. Unveiling the Potential of Aloe Vera Gel Supplementation in a Cooling Extender: A Breakthrough in Enhancing Rooster Sperm Quality and Fertility Ability. Animals 2024, 14, 2290. [Google Scholar] [CrossRef] [PubMed]
- Koedkanmark, T.; Ratchamak, R.; Authaida, S.; Boonkum, W.; Semaming, Y.; Chankitisakul, V. Supplementation of Sperm Cooling Medium with Eurycoma Longifolia Extract Enhances Native Thai Chicken Sperm Quality and Fertility Potential. Front. Vet. Sci. 2024, 11, 1474386. [Google Scholar] [CrossRef]
- Zhandi, M.; Seifi-Ghajalo, E.; Shakeri, M.; Yousefi, A.R.; Sharafi, M.; Seifi-Jamadi, A. Effect of Glutathione Supplementation to Semen Extender on Post-Thawed Rooster Sperm Quality Indices Frozen After Different Equilibration Times. CryoLetters 2020, 41, 92–99. [Google Scholar]
- Masoudi, R.; Sharafi, M.; Pourazadi, L.; Dadashpour Davachi, N.; Asadzadeh, N.; Esmaeilkhanian, S.; Dirandeh, E. Supplementation of Chilling Storage Medium with Glutathione Protects Rooster Sperm Quality. Cryobiology 2020, 92, 260–262. [Google Scholar] [CrossRef]
- Partyka, A.; Niżański, W. Supplementation of Avian Semen Extenders with Antioxidants to Improve Semen Quality—Is It an Effective Strategy? Antioxidants 2021, 10, 1927. [Google Scholar] [CrossRef]
- Price, S.; Aurich, J.; Davies-Morel, M.; Aurich, C. Effects of Oxygen Exposure and Gentamicin on Stallion Semen Stored at 5 and 15 °C. Reprod. Domest. Anim. 2008, 43, 261–266. [Google Scholar] [CrossRef]
- Balogun, K.B.; Stewart, K.R. Effects of Air Exposure and Agitation on Quality of Stored Boar Semen Samples. Reprod. Domest. Anim. 2021, 56, 1200–1208. [Google Scholar] [CrossRef]
- Ben Moula, A.; Hamidallah, N.; Badi, A.; El Fadili, M.; El Amiri, B. Adjusting Ram Semen Preservation: Exploring the Impact of Oxygen Exposure during Liquid Storage. Reprod. Domest. Anim. 2024, 59, e14618. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, B.; Ghosh, S.K.; Lone, S.A.; Prasad, J.K.; Das, G.K.; Katiyar, R.; Mustapha, A.R.; Kumar, A.; Verma, M.R. Effect of Liquid Nitrogen Flushing of Extender on Seminal Antioxidant Profile of Murrah Buffalo during Cryopreservation. Cryoletters 2018, 39, 279–287. [Google Scholar]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative Stress and Sperm Function: A Systematic Review on Evaluation and Management. Arab J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Chankitisakul, V.; Tubtimtong, N.; Boonkum, W.; Vongpralub, T. Effects of Gelatin and Oxytocin Supplementation in a Long-Term Semen Extender on Boar Semen Quality and Fertility Potential. Anim. Biosci. 2024, 37, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.; Rüdiger, K.; Waberski, D. Rotation of Boar Semen Doses During Storage Affects Sperm Quality. Reprod. Domest. Anim. 2015, 50, 684–687. [Google Scholar] [CrossRef]
- Dierberger, H.; Pieper, L.; Jung, M.; Schulze, M. Rotation of Liquid-Preserved Artificial Insemination Doses on Roller Benches Affects Sperm Quality during Storage in Stallions. Reprod. Domest. Anim. 2023, 58, 1413–1419. [Google Scholar] [CrossRef]
- Baicu, S.C.; Taylor, M.J. Acid–Base Buffering in Organ Preservation Solutions as a Function of Temperature: New Parameters for Comparing Buffer Capacity and Efficiency. Cryobiology 2002, 45, 33–48. [Google Scholar] [CrossRef]
- Yániz, J.; Gosálvez, J.; Lopez, C.; Silvestre, M.; Santolaria, P. Effect of Diluent Composition on the Dynamics of Sperm DNA Fragmentation and Other Sperm Quality Parameters in Ram during Incubation at 37 °C. Small Rumin. Res. 2015, 129, 92–96. [Google Scholar] [CrossRef]
- Trentin, J.M.; Centeno, L.A.M.; Balestrin Tde, S.; Minela, T.; Zanatta, G.M.; Pessoa, G.A.; Rubin, M.I.B. Sodium Bicarbonate and HEPES as Buffer Components for Cooling the Semen of Pony Stallions. Semina Ciênc. Agrar. 2018, 39, 631–642. [Google Scholar] [CrossRef]
- Mussa, N.J.; Boonkum, W.; Chankitisakul, V. Semen Quality Traits of Two Thai Native Chickens Producing a High and a Low of Semen Volumes. Vet. Sci. 2023, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Chauychu-noo, N.; Thananurak, P.; Boonkum, W.; Vongpralub, T.; Chankitisakul, V. Effect of Organic Selenium Dietary Supplementation on Quality and Fertility of Cryopreserved Chicken Sperm. Cryobiology 2021, 98, 57–62. [Google Scholar] [CrossRef]
- Tvrdá, E.; Kňažická, Z.; Lukáčová, J.; Schneidgenová, M.; Goc, Z.; Greń, A.; Szabó, C.; Massányi, P.; Lukáč, N. The Impact of Lead and Cadmium on Selected Motility, Prooxidant and Antioxidant Parameters of Bovine Seminal Plasma and Spermatozoa. J. Environ. Sci. Health A 2013, 48, 1292–1300. [Google Scholar] [CrossRef]
- Adebisi, K.A.; Ewuola, E. Comparative Effect of Saline Solutions as Diluents on in Vitro Semen Storage, Egg Fertility and Hatchability in Turkey Hens. Niger. J. Anim. Sci. 2019, 21, 107–116. [Google Scholar]
- Vasicek, J.; Kuzelova, L.; Kulikova, B.; Chrenek, P. Effect of Diluent and Storage Time on Sperm Characteristics of Rooster Insemination Doses. Avian Biol. Res. 2015, 8, 41–46. [Google Scholar] [CrossRef]
- Sexton, T.J. Oxidative and Glycolytic Activity of Chicken and Turkey Spermatozoa. Comp. Biochem. Physiol. B 1974, 48, 59–65. [Google Scholar] [CrossRef]
- Setiawan, R.; Priyadarshana, C.; Tajima, A.; Travis, A.J.; Asano, A. Localisation and Function of Glucose Transporter GLUT1 in Chicken (Gallus gallus domesticus) Spermatozoa: Relationship between ATP Production Pathways and Flagellar Motility. Reprod. Fertil. Dev. 2020, 32, 697–705. [Google Scholar] [CrossRef]
- Amini, M.R.; Kohram, H.; Zare Shahaneh, A.; Zhandi, M.; Sharideh, H.; Nabi, M.M. The Effects of Different Levels of Vitamin E and Vitamin C in Modified Beltsville Extender on Rooster Post-Thawed Sperm Quality. Cell Tissue Bank. 2015, 16, 587–592. [Google Scholar] [CrossRef]
- Mussa, N.J.; Ratchamak, R.; Ratsiri, T.; Vongpralub, T.; Boonkum, W.; Semaming, Y.; Chankitisakul, V. Lipid Profile of Sperm Cells in Thai Native and Commercial Roosters and Its Impact on Cryopreserved Semen Quality. Trop. Anim. Health Prod. 2021, 53, 321. [Google Scholar] [CrossRef]
- Vandemark, N.L.; Salisbury, G.W.; Bratton, R.W. Oxygen Damage to Bull Spermatozoa and Its Prevention by Catalase. J. Dairy Sci. 1949, 32, 353–360. [Google Scholar] [CrossRef]
- Balamurugan, B.; Ghosh, S.K.; Lone, S.A.; Prasad, J.K.; Ramamoorthy, M.; Kumar, A. Correlation between Dissolved Oxygen Level, Antioxidants and Oxidants in Semen Diluted with Partially Deoxygenated Extender at Various Stages of Cryopreservation. CryoLetters 2022, 43, 158–166. [Google Scholar] [CrossRef]
- Williams, A.C.; Ford, W.C. The Role of Glucose in Supporting Motility and Capacitation in Human Spermatozoa. J. Androl. 2001, 22, 680–695. [Google Scholar] [CrossRef]
- Rodriguez-Gil, J. Mammalian Sperm Energy Resources Management and Survival during Conservation in Refrigeration. Reprod. Domest. Anim. 2006, 41, 11–20. [Google Scholar] [CrossRef]
- Wishart, G.J. Maintenance of ATP Concentrations in and of Fertilizing Ability of Fowl and Turkey Spermatozoa in Vitro. J. Reprod. Fertil. 1982, 66, 457–466. [Google Scholar] [CrossRef]
- Nagy, S.Z.; Sinkovics, G.Y.; Kovács, A. Viability and Acrosome Integrity of Rabbit Spermatozoa Processed in a Gelatin-Supplemented Extender. Anim. Reprod. Sci. 2002, 70, 283–286. [Google Scholar] [CrossRef]
- Vyt, P.; Maes, D.; Sys, S.U.; Rijsselaere, T.; Van Soom, A. Air Contact Influences the pH of Extended Porcine Semen. Reprod. Domest. Anim. 2007, 42, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Froman, D.P. Chicken Acrosin: Extraction and Purification. Poult. Sci. 1990, 69, 812–817. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Welch, G.R.; Long, J.A. Mitochondrial Function and Reactive Oxygen Species Action in Relation to Boar Motility. Theriogenology 2008, 70, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Ashizawa, K.; Okauchi, K. Stimulation of Sperm Motility and Oxygen Consumption of Fowl Spermatozoa by a Low Molecular Weight Fraction of Seminal Plasma. J. Reprod. Fertil. 1984, 71, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Ashizawa, K.; Nishiyama, H. Effects of Temperature on the Vigour of Motility, Oxygen Consumption and Duration of Motility of Fowl Spermatozoa under Aerobic Conditions. Jpn. Poult. Sci. 1978, 15, 264–266. [Google Scholar] [CrossRef][Green Version]
- Roy, R.N.; Roy, L.N.; Ashkenazi, S.; Wollen, J.T.; Dunseth, C.D.; Fuge, M.S.; Durden, J.L.; Roy, C.N.; Hughes, H.M.; Morris, B.T.; et al. Buffer Standards for pH Measurement of HEPES for I=0.16 Mol·kg−1 from 5 to 55 °C. J. Solut. Chem. 2009, 38, 449–478. [Google Scholar] [CrossRef][Green Version]
- Ashizawa, K.; Fujiyama, A.; Tsuzuki, Y. Temperature-Dependent Immobilization and Restoration of Fowl Sperm Motility Caused by Intracellular Free Calcium. Jpn. Poult. Sci. 1999, 36, 9–18. [Google Scholar] [CrossRef]
- Getachew, T. A Review Article of Artificial Insemination in Poultry. World’s Vet. J. 2016, 6, 25. [Google Scholar] [CrossRef]
- Lake, P.E.; Ravie, O. Effect on Fertility of Storing Fowl Semen for 24 h at 5° C in Fluids of Different pH. J. Reprod. Fertil. 1979, 57, 149–153. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Extender | Aerobic-Rotated | Aerobic-Non-Rotated | p-Value |
|---|---|---|---|---|
| No. of eggs | 448 | 403 | ||
| Fertility (%) | 0.9%NaCl | N/A | N/A | - |
| IGGKPh | 91.77 ± 3.06 ᵃ | 75.32 ± 6.70 ᵇ | 0.021 | |
| Hatchability (%) | 0.9%NaCl | N/A | N/A | - |
| IGGKPh | 91.58 ± 3.28 | 91.58 ± 4.06 | 0.489 |
| Treatment | No. of Eggs | Parameter | |||
|---|---|---|---|---|---|
| 0.9% NaCl | IGGKPh | ||||
| Fertility (%) | Hatchability (%) | Fertility (%) | Hatchability (%) | ||
| Control-5 °C | 358 | 69.85 ± 2.59 ab | 74.58 ± 4.58 | 69.62 ± 0.38 b | 83.86 ± 3.86 |
| HEPES-5 °C | 400 | 82.80 ± 6.48 a | 78.75 ± 1.25 | 79.59 ± 0.41 a | 93.86 ± 6.14 |
| Control-25 °C | 442 | 57.09 ± 4.45 b | 75.66 ± 1.09 | 66.36 ± 1.65 b | 90.02 ± 2.30 |
| HEPES-25 °C | 437 | 79.50 ± 3.83 a | 73.63 ± 3.04 | 64.92 ± 1.76 b | 83.15 ± 4.58 |
| p-Value | 0.0033 | 0.621 | 0.0037 | 0.3813 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buathalad, K.; Koedkanmark, T.; Boonkum, W.; Chankitisakul, V. Optimizing Rooster Semen Preservation: Effect of Oxygen Exposure, Sample Rotation, and HEPES Buffer Supplementation. Animals 2025, 15, 2391. https://doi.org/10.3390/ani15162391
Buathalad K, Koedkanmark T, Boonkum W, Chankitisakul V. Optimizing Rooster Semen Preservation: Effect of Oxygen Exposure, Sample Rotation, and HEPES Buffer Supplementation. Animals. 2025; 15(16):2391. https://doi.org/10.3390/ani15162391
Chicago/Turabian StyleBuathalad, Khomsan, Thirawat Koedkanmark, Wuttigrai Boonkum, and Vibuntita Chankitisakul. 2025. "Optimizing Rooster Semen Preservation: Effect of Oxygen Exposure, Sample Rotation, and HEPES Buffer Supplementation" Animals 15, no. 16: 2391. https://doi.org/10.3390/ani15162391
APA StyleBuathalad, K., Koedkanmark, T., Boonkum, W., & Chankitisakul, V. (2025). Optimizing Rooster Semen Preservation: Effect of Oxygen Exposure, Sample Rotation, and HEPES Buffer Supplementation. Animals, 15(16), 2391. https://doi.org/10.3390/ani15162391






