Well-Being of the Baltic Herring and Bycatch Fish Species from FAO Major Fishing Areas 27 According to Microplastic Pollution
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
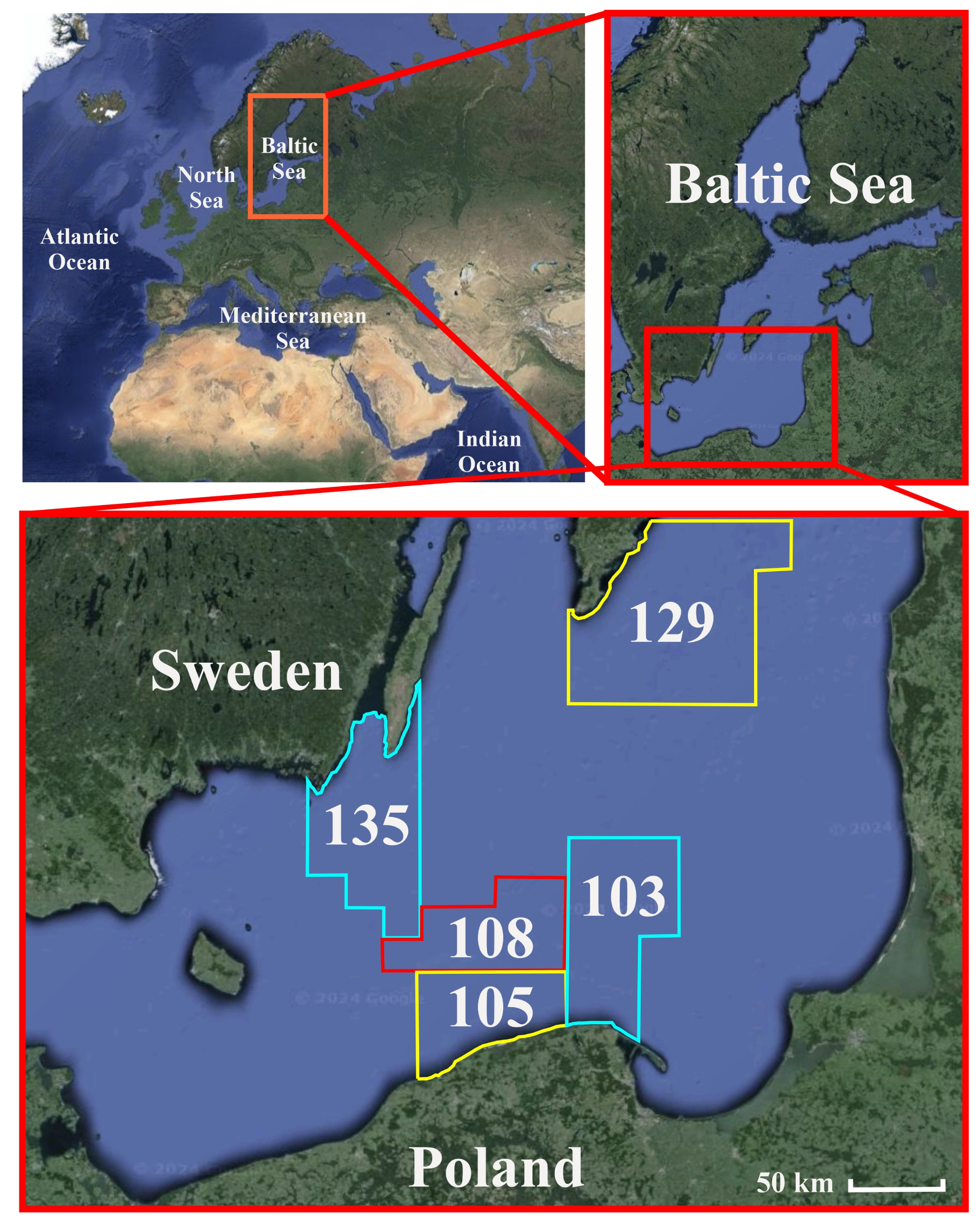
2.1. Study Area
2.2. Baltic Sea Pollution with MPs
2.3. Fish Communities in the Baltic Sea
2.4. Detailed Description of the Studied Species and the Fish Samples’ Collection Protocol
2.5. Sample Preparation
2.6. Contamination Prevention
2.7. Fish Health Condition
2.8. Data Analysis
3. Results
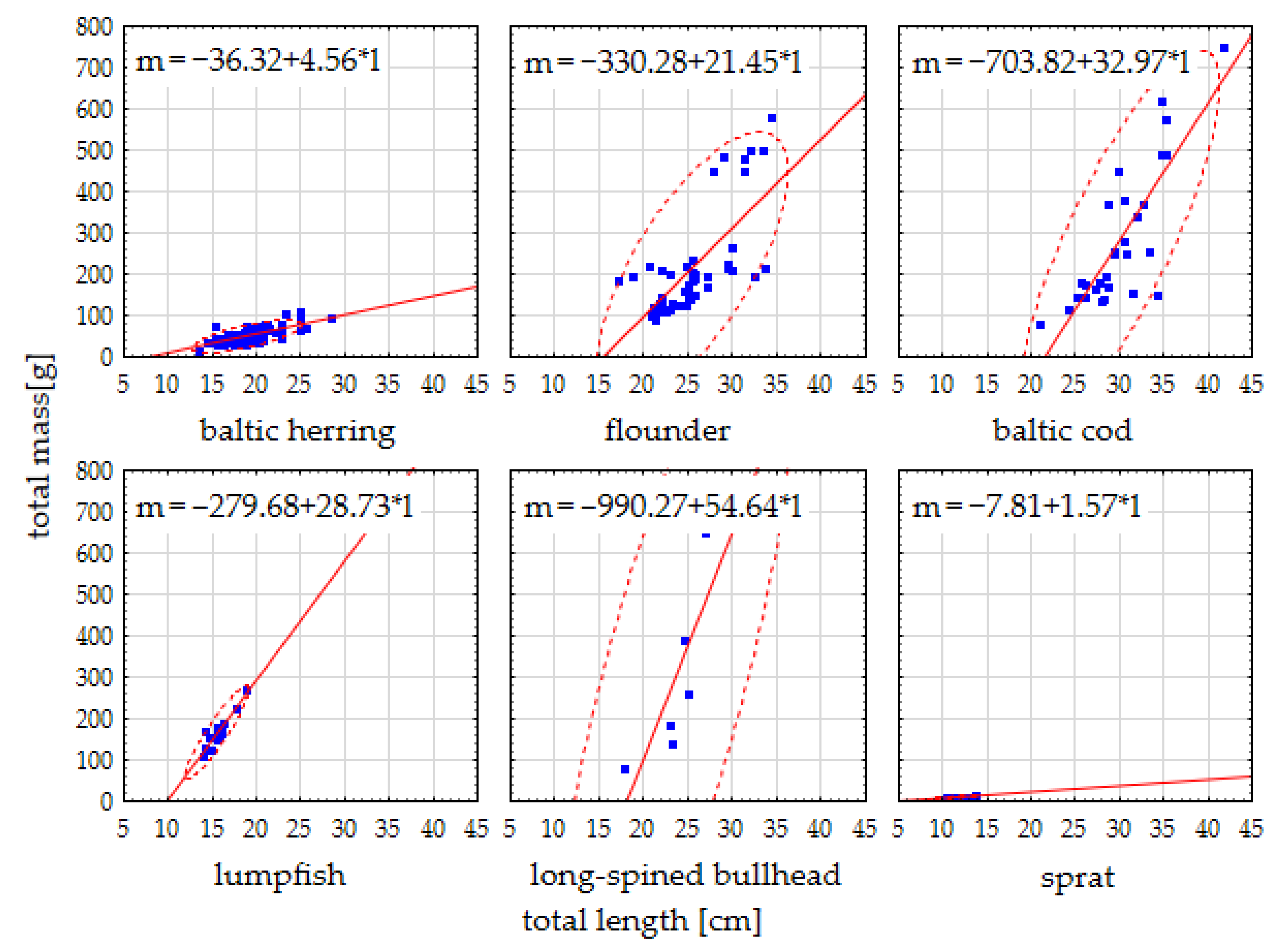
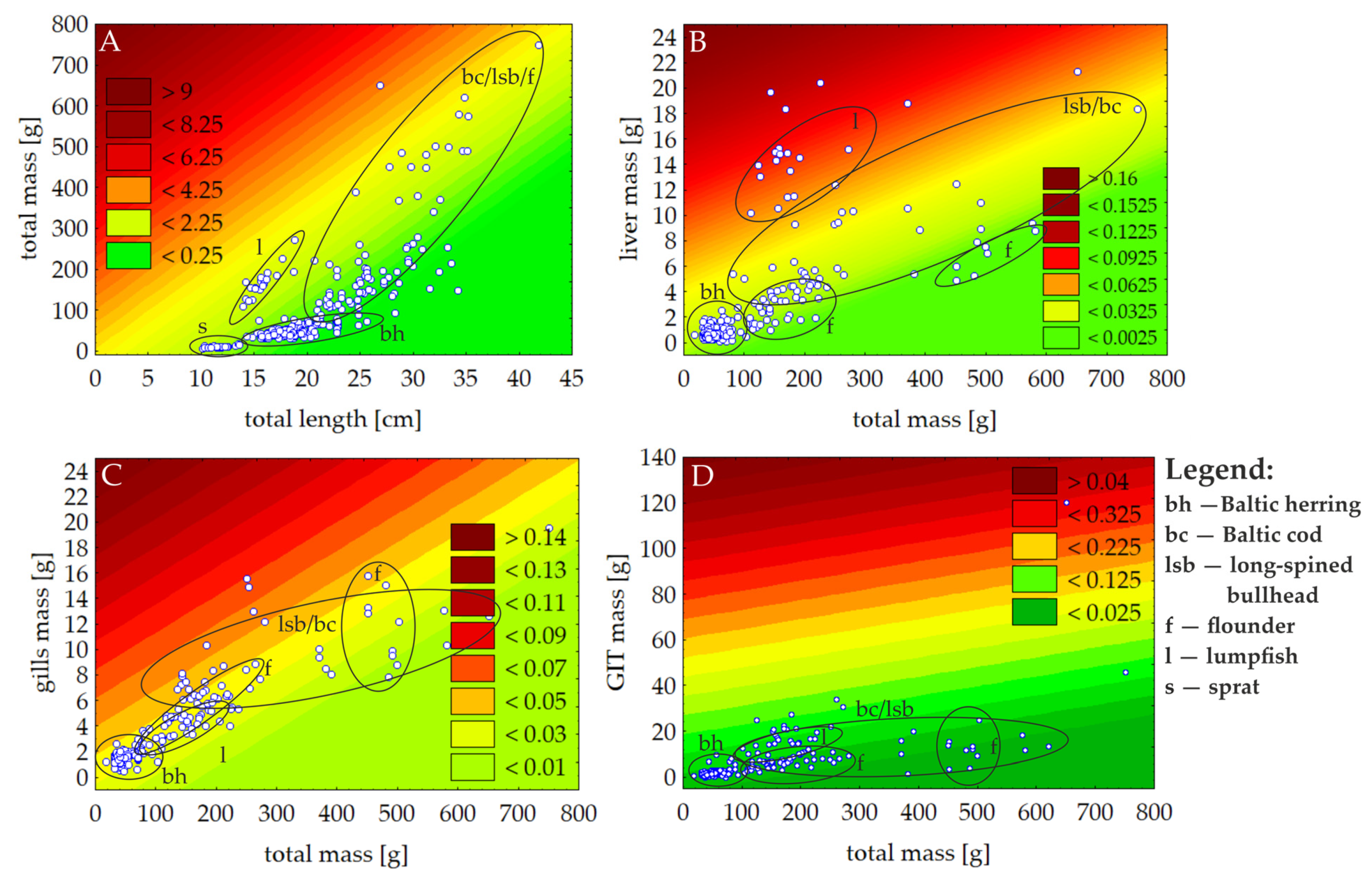
3.1. Fish Biometry
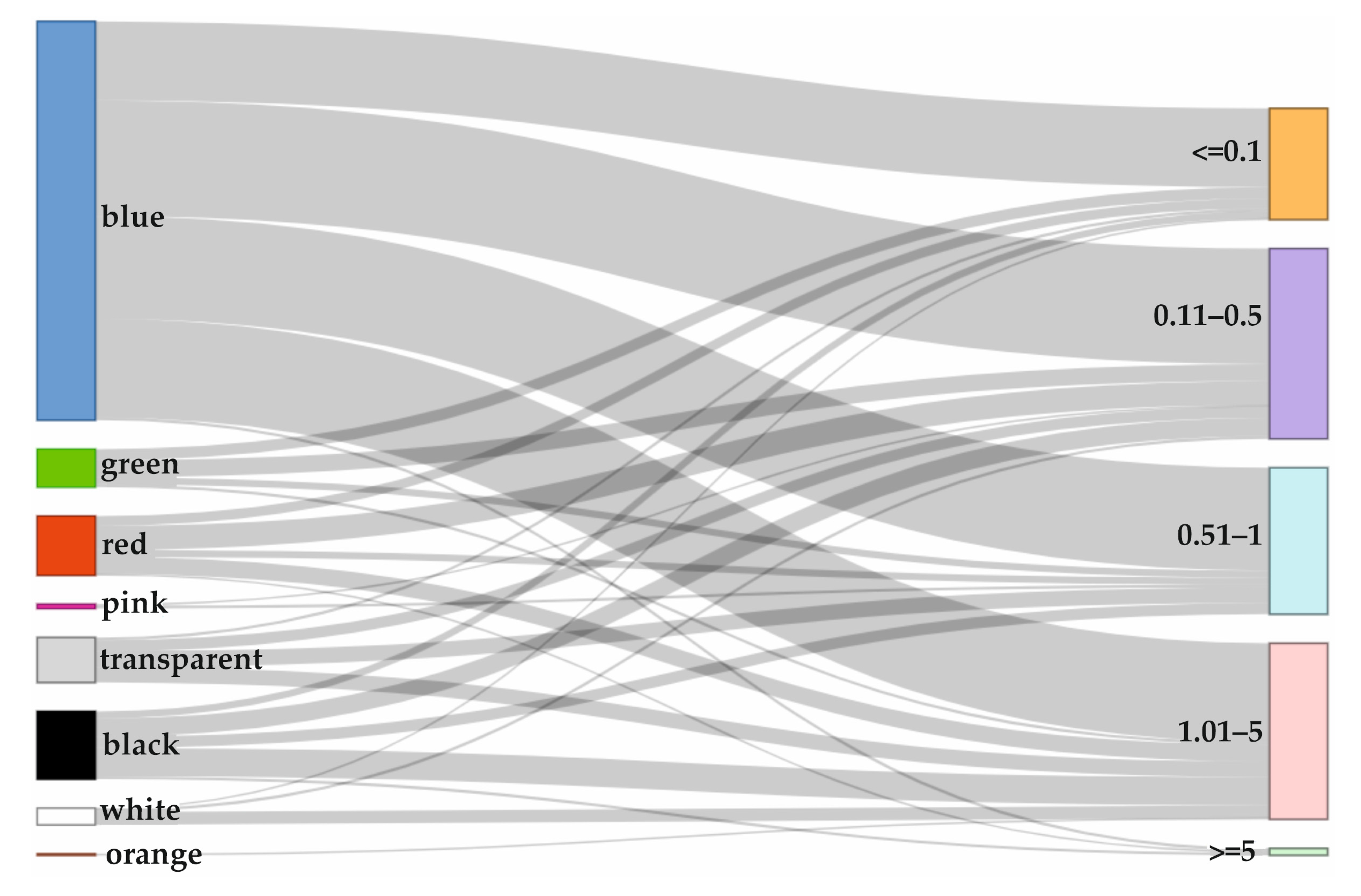
3.2. MPs Contamination According to Species and Organs
3.3. Fish Condition Status
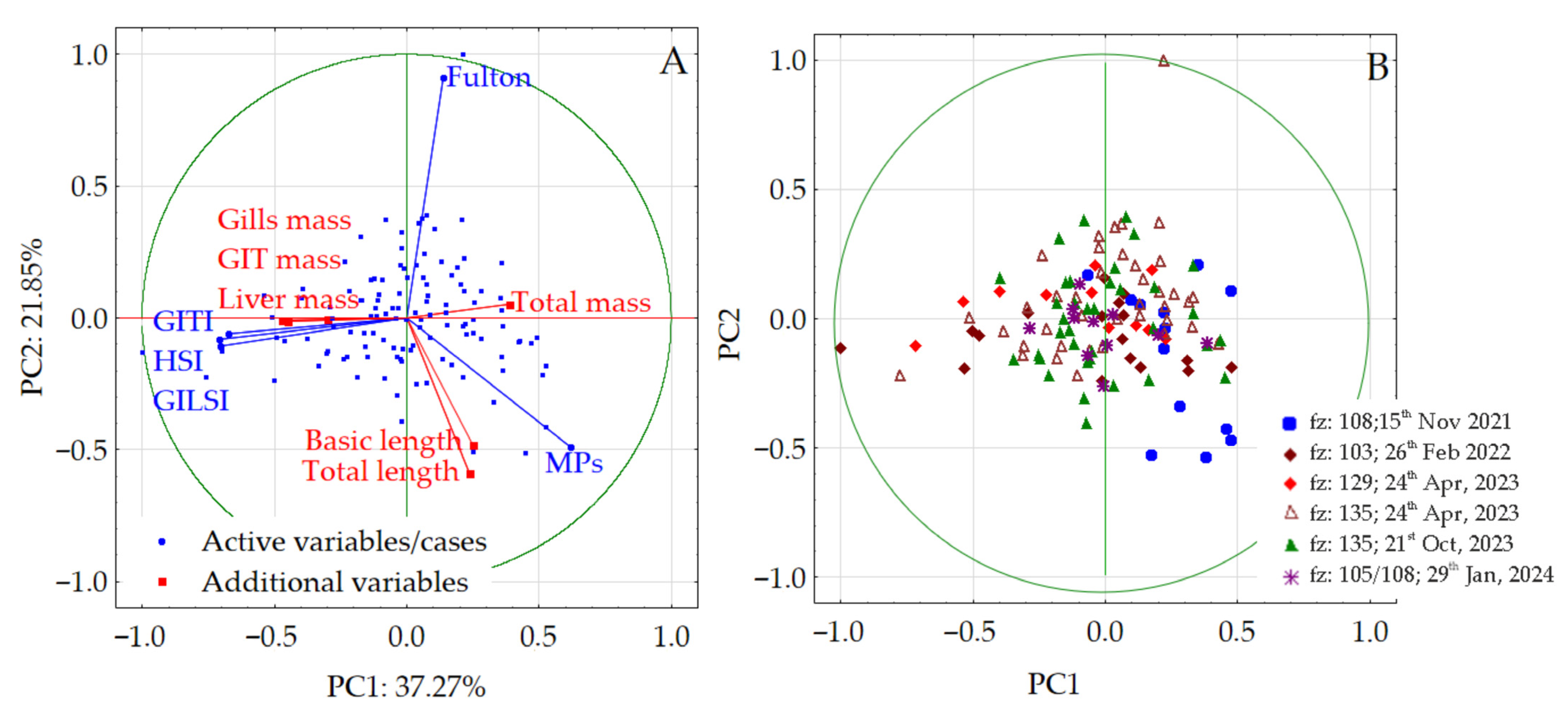
3.4. Multidimensional Analysis
3.5. Physical and Chemical Characterization of MPs
4. Discussion
4.1. Fish Biometry
4.2. MPs Contamination According to Species and Organs
4.2.1. MPs Contamination According to Geographical Region
4.2.2. MPs in Marine Fish Species
4.2.3. Accumulation of MPs in Organs
4.2.4. Physical and Chemical Characterization of MPs
4.3. Fish Condition Status
4.3.1. K Factor
4.3.2. HSI
4.3.3. GILSI
4.3.4. GITI
4.4. Multidimensional Analysis Involving Baltic Herring
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmlund, C.; Hammer, M. Ecological ecosystem services generated by fish populations. Ecol. Econ. 1999, 29, 253–268. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Stoichiometry and homeostasis. In Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002; pp. 1–43. [Google Scholar] [CrossRef]
- Traugott, M.; Thalinger, B.; Wallinger, C.; Sint, D. Fish as predators and prey: DNA-based assessment of their role in food webs. J. Fish. Biol. 2021, 98, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Schiettekatte, N.M.D.; Casey, J.M.; Brandl, S.J.; Mercière, A.; Degregori, S.; Burkepile, D.; Van Wert, J.C.; Ghilardi, M.; Villéger, S.; Parravicini, V. The role of fish feces for nutrient cycling on coral reefs. Oikos 2023, 9, e09914. [Google Scholar] [CrossRef]
- Whitfield, A.K. Fishes and the environmental status of South African estuaries. Fish. Manag. Ecol. 1996, 3, 45–57. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.N.; Ding, H.Y.; Dai, Y.; Ding, S.; Gao, X. Threshold responses in the taxonomic and functional structure of fish assemblages to land use and water quality: A case study from the Taizi River. Water 2019, 11, 661. [Google Scholar] [CrossRef]
- Gebremedhin, S.; Bruneel, S.; Getahun, A.; Anteneh, W.; Goethals, P. Scientific methods to understand fish population dynamics and support sustainable fisheries management. Water 2021, 13, 574. [Google Scholar] [CrossRef]
- Finegold, C. The importance of fisheries and aquaculture to development. In Fisheries, Sustainability and Development; Wramner, P., Cullberg, M., Ackefors, H., Eds.; The Royal Swedish Academy of Agriculture and Forestry: Stockholm, Sweden, 2009; pp. 353–364. [Google Scholar]
- Bosch, A.C.; O’Neill, B.; Sigge, G.O.; Kerwath, S.E.; Hoffman, L.C. Heavy metals in marine fish meat and consumer health: A review. J. Sci. Food Agric. 2016, 96, 32–48. [Google Scholar] [CrossRef]
- Mostofa, K.; Liu, C.Q.; Vione, D.; Gao, K.; Ogawa, H. Sources, factors, mechanisms and possible solutions to pollutants in marine ecosystems. Environ. Pollut. 2013, 182, 461–478. [Google Scholar] [CrossRef]
- Bashir, I.; Lone, F.A.; Bhat, R.A.; Mir, S.A.; Dar, Z.A.; Dar, S.A. Concerns and threats of contamination on aquatic ecosystems. In Bioremediation and Biotechnology; Hakeem, K., Bhat, R., Qadri, H., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Gissi, E.; Manea, E.; Mazaris, A.D.; Fraschetti, S.; Almpanidou, V.; Bevilacqua, S.; Coll, M.; Guarnieri, G.; Lloret-Lloret, E.; Pascual, M.; et al. A review of the combined effects of climate change and other local human stressors on the marine environment. Sci. Total Environ. 2021, 755, 142564. [Google Scholar] [CrossRef]
- Malik, D.S.; Sharma, A.K.; Thakur, S.; Sharma, M. A review on impact of water pollution on freshwater fish species and their aquatic environment. In Advances in Environmental Pollution Management: Wastewater Impacts and Treatment Technologies; AEM: Milwaukee, WI, USA, 2020. [Google Scholar] [CrossRef]
- Dijoux, S.; Boukal, D.S. Community structure and collapses in multichannel food webs: Role of consumer body sizes and mesohabitat productivities. Ecol. Lett. 2021, 24, 1607–1618. [Google Scholar] [CrossRef]
- Laufkötter, C.A.O.; Zscheischler, J.A.O.; Frölicher, T.A.O. High-impact marine heatwaves attributable to human-induced global warming. Science 2020, 369, 1621–1625. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef]
- Galafassi, S.; Sighicelli, M.; Pusceddu, A.; Bettinetti, R.; Cau, A.; Temperini, M.E.; Gillibert, R.; Ortolani, M.; Pietrelli, L.; Zaupa, S.; et al. Microplastic pollution in perch (Perca fluviatilis, Linnaeus 1758) from Italian south-alpine lakes. Environ. Pollut. 2021, 288, 117782. [Google Scholar] [CrossRef]
- Nusair, S.D.; Almasaleekh, M.J.; Rahman, H.R.; Alkhatatbeh, M. Environmental exposure of humans to bromide in the Dead Sea area: Measurement of genotoxicy and apoptosis biomarkers. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 837, 34–41. [Google Scholar] [CrossRef]
- Amelia, T.S.M.; Khalik, W.M.A.W.M.; Ong, M.C.; Shao, Y.; Pan, H.; Bhubalan, K. Marine microplastics as vectors of major ocean pollutants and its hazards to the marine ecosystem and humans. Prog. Earth Planet. Sci. 2021, 8, 12. [Google Scholar] [CrossRef]
- Ding, T.; Wei, L.; Hou, Z.; Li, J.; Zhang, C.; Lin, D. Microplastics altered contaminant behavior and toxicity in natural waters. J. Hazard. Mater. 2022, 425, 127908. [Google Scholar] [CrossRef] [PubMed]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Saad, D. Why Microplastics are exceptional contaminants? In Advances and Challenges in Microplastics; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Hu, K.; Yang, Y.; Zuo, J.; Tian, W.; Wang, Y.; Duan, X.; Wang, S. Emerging microplastics in the environment: Properties, distributions, and impacts. Chemosphere 2022, 297, 134118. [Google Scholar] [CrossRef] [PubMed]
- Vedolin, M.C.; Teophilo, C.Y.S.; Turra, A.; Figueira, R.C.L. Spatial variability in the concentrations of metals in beached microplastics. Mar. Pollut. Bull. 2018, 129, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Dave, P.H.; Kwong, R.W.M.; Wu, M.; Zhong, H. Influence of microplastics on the mobility, bioavailability, and toxicity of heavy metals: A review. Bull. Environ. Contam. Toxicol. 2021, 107, 710–721. [Google Scholar] [CrossRef]
- Munoz, M.; Ortiz, D.; Nieto-Sandoval, J.; de Pedro, Z.M.; Casas, J.A. Adsorption of micropollutants onto realistic microplastics: Role of microplastic nature, size, age, and NOM fouling. Chemosphere 2021, 283, 131085. [Google Scholar] [CrossRef] [PubMed]
- Bucci, K.; Tulio, M.; Rochman, C.M. What is known and unknown about the effects of plastic pollution: A meta-analysis and systematic review. Ecol. Appl. 2020, 30, e02044. [Google Scholar] [CrossRef] [PubMed]
- Issac, M.N.; Kandasubramanian, B. Effect of microplastics in water and aquatic systems. Environ. Sci. Pollut. Res. 2021, 28, 19544–19562. [Google Scholar] [CrossRef] [PubMed]
- Kibria, G.; Haroon, A.K.Y.; Rose, G.; Hossain, M.M.; Nugegoda, D. Pollution Risks, Impacts and Management: Social, Economic, and Environmental Perspectives; Scientific Publishers: Jodhpur, India, 2021. [Google Scholar]
- Su, L.; Deng, H.; Li, B.; Chen, Q.; Pettigrove, V.; Wu, C.; Shi, H. The occurrence of microplastic in specific organs in commercially caught fishes from coast and estuary area of east China. J. Hazard. Mater. 2019, 365, 716–724. [Google Scholar] [CrossRef]
- McIlwraith, H.K.; Kim, J.; Helm, P.; Bhavsar, S.P.; Metzger, J.S.; Rochman, C.M. Evidence of microplastic translocation in wild-caught fish and implications for microplastic accumulation dynamics in food webs. Environ. Sci. Technol. 2021, 55, 12372–12382. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef]
- McCormick, M.I.; Chivers, D.P.; Ferrari, M.; Blandford, M.I.; Nanninga, G.; Richardson, C.; Fakan, E.P.; Vamvounis, G.; Gulizia, A.M.; Allan, B.J. Microplastic exposure interacts with habitat degradation to affect behaviour and survival of juvenile fish in the field. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201947. [Google Scholar] [CrossRef]
- Terra, B.F.; Araújo, F.G.; Calza, C.F.; Lopes, R.; Teixeira, T.P. Heavy metal in tissues of three fish species from different trophic levels in a tropical brazilian river. Water Air Soil Pollut. 2008, 187, 275–284. [Google Scholar] [CrossRef]
- Pereira, S.; Pinto, A.L.; Cortes, R.; Fontaínhas-Fernandes, A.; Coimbra, A.M.; Monteiro, S.M. Gill histopathological and oxidative stress evaluation in native fish captured in Portuguese northwestern rivers. Ecotoxicol. Environ. Saf. 2013, 90, 157–166. [Google Scholar] [CrossRef]
- Velmurugan, B.; Selvanayagam, M.; Cengiz, E.I.; Unlu, E. Histopathological changes in the gill and liver tissues of freshwater fish, Cirrhinus mrigala exposed to dichlorvos. Braz. Arch. Biol. Technol. 2009, 52, 5. [Google Scholar] [CrossRef]
- Thophon, S.; Kruatrachue, M.; Upatham, E.S.; Pokethitiyook, P.; Sahaphong, S.; Jaritkhuan, S. Histopathological alterations of white seabass, Lates calcarifer, in acute and subchronic cadmium exposure. Environ. Pollut. 2003, 121, 307–320. [Google Scholar] [CrossRef]
- Illing, B.; Rummer, J.L. Physiology can contribute to better understanding, management, and conservation of coral reef fishes. Conserv. Physiol. 2017, 5, cox005. [Google Scholar] [CrossRef]
- Madliger, C.L.; Love, O.P.; Hultine, K.R.; Cooke, S.J. The conservation physiology toolbox: Status and opportunities. Conserv. Physiol. 2018, 6, coy029. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.; Migliaccio, K.W.; Li, Y.; Schaffer, B.; Kiker, G.A.; Brown, M.T. Land use disturbance indicators and water quality variability in the Biscayne Bay Watershed, Florida. Ecol. Indic. 2011, 11, 1093–1104. [Google Scholar] [CrossRef]
- Wikelski, M.; Cooke, S.J. Conservation physiology. Trends Ecol. Evol. 2006, 21, 38–46. [Google Scholar] [CrossRef]
- Bolger, T.; Connolly, P.L. The selection of suitable indices for the measurement and analysis of fish condition. J. Fish. Biol. 1989, 34, 171–182. [Google Scholar] [CrossRef]
- Mozsár, A.; Boros, G.; Sály, P.; Antal, L.; Nagy, S.S. Relationship between Fulton’s condition factor and proximate body composition in three freshwater fish species. J. Appl. Ichthyol. 2015, 31, 315–320. [Google Scholar] [CrossRef]
- Mello, L.G.S.; Rose, G.A. Seasonal cycles in weight and condition in Atlantic cod (Gadus morhua L.) in relation to fisheries. ICES J. Mar. Sci. 2005, 62, 1006–1015. [Google Scholar] [CrossRef]
- Ravikumar, T.; Neethiselvan, N.; Jayakumar, N.; Sudhan, C.; Umamaheswari, T.; Padmavathy, P. Length-weight relationships and Fulton’s condition factor (K) for 29 demersal reef fishes caught by longline. Thalassas 2023, 39, 1263–1270. [Google Scholar] [CrossRef]
- Leão-Buchir, J.; Folle, N.M.T.; de Souza, T.L.; Brito, M.P.; de Oliveira, E.C.; de Almeida Roque, A.; Ramsdorf, W.A.; Fávaro, L.F.; Garcia, J.R.E.; Esquivel, L.; et al. Effects of trophic 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) exposure in Oreochromis niloticus: A multiple biomarkers analysis. Environ. Toxicol. Pharmacol. 2021, 87, 103693. [Google Scholar] [CrossRef]
- Lambert, Y.; Dutil, J.D. Condition and energy reserves of Atlantic cod (Gadus morhua) during the collapse of the northern Gulf of St. Lawrence stock. Can. J. Fish. Aquat. Sci. 1997, 54, 2388–2400. [Google Scholar] [CrossRef]
- Bruslé, J.; Anadon, G.G. The structure and function of fish liver. In Fish Morphology, 1st ed.; Taylor & Francis: London, UK, 1996; p. 17. [Google Scholar]
- Dobrzycka-Krahel, A.; Bogalecka, M. The Baltic Sea under anthropopressure—The sea of paradoxes. Water 2022, 14, 3772. [Google Scholar] [CrossRef]
- Szymczycha, B.; Zaborska, A.; Bełdowski, J.; Kuliński, K.; Beszczyńska-Möller, A.; Kędra, M.; Pempkowiak, J. Chapter 4—The Baltic Sea. In World Seas: An Environmental Evaluation, 2nd ed.; Sheppard, C., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 85–111. [Google Scholar] [CrossRef]
- Mutton, A.F.A.; Couper, A.D. Baltic Sea; Encyclopedia Britannica: Chicago, IL, USA, 2024. [Google Scholar]
- Dargahi, B. Environmental impacts of shallow water mining in the Baltic Sea. Front. Mar. Sci. 2023, 10, 1223654. [Google Scholar] [CrossRef]
- Lehmann, A.; Myrberg, K.; Post, P.; Chubarenko, I.; Dailidiene, I.; Hinrichsen, H.H.; Hüssy, K.; Liblik, T.; Meier, H.E.; Lips, U.; et al. Salinity dynamics of the Baltic Sea. Earth Syst. Dyn. 2022, 13, 373–392. [Google Scholar] [CrossRef]
- Schmidt, N.; Garate-Olaizola, M.; Laurila, A. Acclimatizing laboratory-reared hatchling cod (Gadus morhua) to salinity conditions in the Baltic Sea. Aquaculture 2024, 579, 740255. [Google Scholar] [CrossRef]
- Störmer, O. Climate change impacts on coastal waters of the Baltic Sea. In Global Change and Baltic Coastal Zones; Schernewski, G., Hofstede, J., Neumann, T., Eds.; Coastal Research Library 1; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Lampe, R. Küsten und Küstenschutz in Mecklenburg-Vorpommern. Z. Erdkundeunterricht 1996, 9, 364–372. [Google Scholar]
- Gogina, M.; Zettler, M.L. Diversity and distribution of benthic macrofauna in the Baltic Sea: Data inventory and its use for species distribution modelling and prediction. J. Sea Res. 2010, 64, 313–321. [Google Scholar] [CrossRef]
- HELCOM. The Baltic marine environment 1999–2002. In Baltic Sea Environment Proceedings; Helsinki Commission: Washington, DC, USA, 2003; Volume 87. [Google Scholar]
- Storie, J.; Suškevičs, M.; Nevzati, F. Evidence on the impact of Baltic Sea ecosystems on human health and well-being: A systematic map. Environ. Evid. 2010, 10, 30. [Google Scholar] [CrossRef]
- HELCOM. Ecosystem health of the Baltic Sea 2003–2007. In Baltic Sea Environment Proceedings; Helsinki Commission: Washington, DC, USA, 2010; Volume 122. [Google Scholar]
- Asmala, E.; Saikku, L.; Vienonen, S. Import–export balance of nitrogen and phosphorus in food, fodder and fertilizers in the Baltic Sea drainage area. Sci. Total Environ. 2011, 409, 4917–4922. [Google Scholar] [CrossRef]
- HELCOM. Input of nutrients by the seven biggest rivers in the Baltic Sea region. In Baltic Sea Environment Proceedings; Helsinki Commission: Washington, DC, USA, 2018; Volume 161. [Google Scholar]
- Büngener, L.; Postila, H.; Löder, M.G.J.; Laforsch, C.; Ronkanen, A.; Heiderscheidt, E. The fate of microplastics from municipal wastewater in a surface flow treatment wetland. Sci. Total Environ. 2023, 903, 166334. [Google Scholar] [CrossRef] [PubMed]
- Piskuła, P.; Astel, A.M. Microplastics occurrence in two mountainous rivers in the lowland area—A case study of the Central Pomeranian Region, Poland. Microplastics 2022, 1, 167–185. [Google Scholar] [CrossRef]
- Dereszewska, A.; Krasowska, K.; Popek, M. Microplastics in harbor seawaters: A case study in the port of Gdynia. Baltic Sea. Sustainability 2023, 15, 6678. [Google Scholar] [CrossRef]
- Graca, B.; Szewc, K.; Zakrzewska, D.; Dołęga, A.; Szczerbowska-Boruchowska, M. Sources and fate of microplastics in marine and beach sediments of the Southern Baltic Sea—A preliminary study. Environ. Sci. Pollut. Res. 2017, 24, 7650–7661. [Google Scholar] [CrossRef]
- Narloch, I.; Gackowska, A.; Wejnerowska, G. Microplastic in the Baltic Sea: A review of distribution processes, sources, analysis methods and regulatory policies. Environ. Pollut. 2022, 315, 120453. [Google Scholar] [CrossRef]
- Mishra, A.; Buhhalko, N.; Lind, K.; Lips, I.; Liblik, T.; Väli, G.; Lips, U. Spatiotemporal variability of microplastics in the eastern Baltic Sea. Front. Mar. Sci. 2022, 9, 875984. [Google Scholar] [CrossRef]
- Chubarenko, I.; Esiukova, E.; Zobkov, M.; Isachenko, I. Microplastics distribution in bottom sediments of the Baltic Sea Proper. Mar. Pollut. Bull. 2022, 179, 113743. [Google Scholar] [CrossRef]
- Urban-Malinga, B.; Zalewski, M.; Jakubowska, A.; Wodzinowski, T.; Malinga, M.; Pałys, B.; Dąbrowska, A. Microplastics on sandy beaches of the southern Baltic Sea. Mar. Pollut. Bull. 2020, 155, 111170. [Google Scholar] [CrossRef]
- Piskuła, P.; Astel, A.; Pawlik, M. Microplastics in seawater and fish acquired from the corresponding fishing zones of the Baltic Sea. Mar. Pollut. Bull. 2025, 211, 117485. [Google Scholar] [CrossRef]
- Białowąs, M.; Jonko-Sobuś, K.; Pawlak, J.; Polak-Juszczak, L.; Dąbrowska, A.; Urban-Malinga, B. Plastic in digestive tracts and gills of cod and herring from the Baltic Sea. Sci. Total Environ. 2022, 822, 153333. [Google Scholar] [CrossRef]
- Sainio, E.; Lehtiniemi, M.; Set¨al¨a, O. Microplastic ingestion by small coastal fish in the northern Baltic Sea. Finland. Mar. Pollut. Bull. 2021, 172, 112814. [Google Scholar] [CrossRef]
- Beer, S.; Garm, A.; Huwer, B.; Dierking, J.; Nielsen, T.G. No increase in marine microplastic concentration over the last three decades—A case study from the Baltic Sea. Sci. Total Environ. 2018, 621, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Ojaveer, E.; Kalejs, M. Ecology and long-term forecasting of sprat (Sprattus sprattus balticus) stock in the Baltic Sea: A review. Rev. Fish. Biol. Fish. 2010, 20, 203–217. [Google Scholar] [CrossRef]
- Kautsky, L.; Kautsky, N. The Baltic Sea, Including Bothnian Sea and Bothnian Bay. In World 24: Intertidal and Littoral Ecosystems; Mathieson, A.C., Nienhuis, P.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 83–108. [Google Scholar]
- Ojaveer, H.; Jaanus, A.; MacKenzie, B.R.; Martin, G.; Olenin, S.; Radziejewska, T.; Telesh, I.; Zettler, M.T.; Zaiko, A. Status of biodiversity in the Baltic Sea. PLoS ONE 2010, 5, e12467. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, B.R.; Gislason, H.; Möllmann, C.; Köster, F.W. Impact of 21st century climate change on the Baltic Sea fish community and fisheries. Glob. Change Biol. 2007, 13, 1348–1367. [Google Scholar] [CrossRef]
- Eero, M. Reconstructing the population dynamics of sprat (Sprattus sprattus balticus) in the Baltic Sea in the 20th century. ICES J. Mar. Sci. 2012, 69, 1010–1018. [Google Scholar] [CrossRef]
- Aro, E. Fish migration studies in the Baltic Sea: A historical review. ICES Mar. Sci. Symp. 2002, 215, 361–370. [Google Scholar] [CrossRef]
- Pawlikowski, B.; Komar-Szymczak, K. Selected physicochemical and quality indicators of herring from fishery areas in south Baltic. Postęp. Tech. Przetwórstwa Spoż. 2018, 2, 44–48. (In Polish) [Google Scholar]
- Moyano, M.; Illing, B.; Polte, P.; Kotterba, P.; Zablotski, Y.; Gröhsler, T.; Hüdepohl, P.; Cooke, S.J.; Peck, M.A. Linking individual physiological indicators to the productivity of fish populations: A case study of Atlantic herring. Ecol. Indic. 2020, 113, 106146. [Google Scholar] [CrossRef]
- Peck, M.A.; Alheit, J.; Bertrand, A.; Catalán, I.A.; Garrido, S.; Moyano, M.; Rykaczewski, R.R.; Takasuka, A.; van der Lingen, C.D. Small pelagic fish in the new millennium: A bottom-up view of global research effort. Prog. Oceanogr. 2021, 191, 102494. [Google Scholar] [CrossRef]
- Megrey, B.A.; Rose, K.A.; Klumb, R.A.; Hay, D.E.; Werner, F.E.; Eslinger, D.E.; Smith, S.L. A bioenergetics-based population dynamics model of Pacific herring (Clupea harengus pallasi) coupled to a lower trophic level nutrient–phytoplankton–zooplankton model: Description, calibration, and sensitivity analysis. Ecol. Model. 2007, 202, 144–164. [Google Scholar] [CrossRef]
- Cooke, S.J.; Sack, L.; Franklin, C.E.; Farrell, A.P.; Beardall, J.; Wikelski, M.; Chown, S.L. What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv. Physiol. 2013, 1, cot001. [Google Scholar] [CrossRef]
- Mieszkowska, N.; Genner, M.J.; Hawkins, S.J.; Sims, D.W. Atlantic cod: Impacts of climate change and commercial fishing. Adv. Mar. Biol. 2009, 56, 213–273. [Google Scholar] [CrossRef]
- Drinkwater, K.F. The response of Atlantic cod (Gadus morhua) to future climate change. ICES J. Mar. Sci. 2005, 62, 1327–1337. [Google Scholar] [CrossRef]
- Magnussen, E. Food and feeding habits of cod (Gadus morhua) on the Faroe Bank. ICES J. Mar. Sci. 2011, 68, 1909–1917. [Google Scholar] [CrossRef]
- Ojaveer, E.; Kalejs, M. On ecosystem-based regions in the Baltic Sea. J. Mar. Syst. 2008, 74, 672–685. [Google Scholar] [CrossRef]
- Orio, A.; Bergström, U.; Casini, M.; Erlandsson, M.; Eschbaum, R.; Hüssy, K.; Lehmann, A.; Ložys, L.; Ustups, D.; Florin, A.B. Characterizing and predicting the distribution of Baltic Sea flounder (Platichthys flesus) during the spawning season. J. Sea Res. 2017, 126, 46–55. [Google Scholar] [CrossRef]
- Nissling, A.; Dahlman, G. Fecundity of flounder, Pleuronectes flesus, in the Baltic Sea—Reproductive strategies in two sympatric populations. J. Sea Res. 2010, 64, 190–198. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase, version 02/2011; World Wide Web Electronic Publication: Geneva, Switzerland, 2004; Available online: https://www.fishbase.org/ (accessed on 18 November 2024).
- Gibson, S.E.; Alexander, D.; Biesecker, D.; Fisher, R.R.; Guhathakurta, M.; Hudson, H.; Thompson, B.J. Modeling CMEs in three dimensions using an analytic MHD model. In Solar Wind Nine; Habbal, S.R., Esser, R., Hollweg, J.V., Iseberg, P.A., Eds.; American Institute of Physics: Woodbury, NY, USA, 1999; p. 645. [Google Scholar]
- Pampoulie, C.; Skirnisdottir, S.; Olafsdottir, G.; Helyar, S.; Thorsteinsson, V.; Jónsson, S.; Fréchet, A.; Durif, C.M.F.; Sherman, S.; Lampart-Kałużniacka, M.; et al. Genetic structure of the lumpfish Cyclopterus lumpus across the North Atlantic. ICES J. Mar. Sci. 2014, 71, 2390–2397. [Google Scholar] [CrossRef]
- Piskuła, P.; Astel, A.M. Occurrence of microplastics in commercial fishes from aquatic ecosystems of northern Poland. Ecohydrol. Hydrobiol. 2024, 24, 492–505. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. 10—Microplastic identification techniques. In Microplastic Pollutants; Crawford, C.B., Quinn, B., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2017; pp. 219–267. [Google Scholar] [CrossRef]
- Zobkov, M.B.; Esiukova, E.E. Microplastics in a marine environment: Review of methods for sampling, processing, and analyzing microplastics in water, bottom sediments, and coastal deposits. Oceanology 2018, 58, 137–143. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, X.; Hou, C.; Wu, Y.; Teng, J.; Zhang, C.; Tan, H.; Shan, E.; Zhang, W.; Zhao, J. Microplastic uptake in commercial fishes from the Bohai Sea, China. Chemosphere 2021, 263, 127962. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Monitoring Guidance for Marine Litter in European Seas; Draft Report; Marine Strategy Framework Directive GES Technical Subgroup on Marine Litter (TSG-ML): Brussels, Belgium, 2013. [Google Scholar]
- Piskuła, P.; Astel, A.M. Microplastics in commercial fishes and by-catch from selected FAO Major Fishing Areas of the southern Baltic Sea. Animals 2023, 13, 458. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, B.G.; Brown, M.L.; Willis, D.W. Relative weight (Wr) status and current use in fisheries assessment and management. Rev. Fish. Sci. 2000, 8, 1–44. [Google Scholar] [CrossRef]
- Lusher, A.L.; McHugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Ogonowski, M.; Wenman, V.; Danielsson, S.; Gorokhova, E. Ingested Microplastic Is Not Correlated to HOC Concentrations in Baltic Sea Herring; CEST: Rhodes, Greece, 2017. [Google Scholar]
- Lenz, R.; Enders, K.; Beer, S.; Sørensen, T.K.; Stedmon, C.A. Analysis of microplastic in the stomachs of herring and cod from the North Sea and Baltic Sea. DTU Aqua Natl. Inst. Aquat. Resour. 2016, 10, 30. [Google Scholar] [CrossRef]
- Kennedy, A.B.; Sankey, W.; Riall, H. The Thermal Efficiency of Steam Engines. Minutes Proc. Inst. Civ. Eng. 1898, 134, 278–312. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Davidson, D.; Marshall, C.T. Are morphometric indices accurate indicators of stored energy in herring Clupea harengus? J. Fish Biol. 2010, 76, 913–929. [Google Scholar] [CrossRef]
- Óskarsson, G.J.; Kjesbu, O.S.; Slotte, A. Predictions of realised fecundity and spawning time in Norwegian spring-spawning herring (Clupea harengus). J. Sea Res. 2002, 48, 59–79. [Google Scholar] [CrossRef]
- McPherson, L.R.; Slotte, A.; Kvamme, C.; Meier, S.; Marshall, C.T. Inconsistencies in measurement of fish condition: A comparison of four indices of fat reserves for Atlantic herring (Clupea harengus). ICES J. Mar. Sci. 2011, 68, 52–60. [Google Scholar] [CrossRef]
- Pascoe, E.S. Quantifying Interannual Variability in the Condition of Young-of-Year Pacific Herring (Clupea pallasi) in the Strait of Georgia, BC. Ph.D. Thesis, School of Earth and Ocean Sciences, Southampton, UK, 2018. Available online: https://dspace.library.uvic.ca/items/88c72a96-140f-4814-b84a-2551f8573dd6/full (accessed on 18 November 2024).
- Arula, T.; Ojaveer, H.; Shpilev, H. Individual fecundity of the autumn spawning Baltic herring Clupea harengus membras L. Estonian J. Ecol. 2012, 61, 119–134. [Google Scholar] [CrossRef]
- Lind, Y.; Huovila, T.; Käkelä, R. A retrospective study of fatty acid composition in Baltic herring (Clupea harengus membras) caught at three locations in the Baltic Sea (1973–2009). ICES J. Mar. Sci. 2018, 75, 330–339. [Google Scholar] [CrossRef]
- Ojaveer, H.; Tomkiewicz, J.; Arula, T.; Klais, R. Female ovarian abnormalities and reproductive failure of autumn-spawning herring (Clupea harengus membras) in the Baltic Sea. ICES J. Mar. Sci. 2015, 72, 2332–2340. [Google Scholar] [CrossRef]
- Vainikka, A.; Mollet, F.; Casini, M.; Gårdmark, A. Spatial variation in growth, condition and maturation reaction norms of the Baltic herring Clupea harengus membras. Mar. Ecol. Prog. Ser. 2009, 383, 285–294. [Google Scholar] [CrossRef]
- Persson, M. Changes in Condition of Herring (Clupea harengus) in Swedish Coastal Waters; Södertörn University College, School of Life Sciences: Stockholm County, Sweden, 2010. [Google Scholar]
- Bucholtz, R.H.; Tomkiewicz, J.; Nyengaard, J.R.; Andersen, J.B. Oogenesis, fecundity and condition of Baltic herring (Clupea harengus L.): A stereological study. Fish. Res. 2013, 145, 100–113. [Google Scholar] [CrossRef]
- Podolska, M.; Horbowy, J. Infection of Baltic herring (Clupea harengus membras) with Anisakis simplex larvae, 1992–1999: A statistical analysis using generalized linear models. ICES J. Mar. Sci. 2003, 60, 85–93. [Google Scholar] [CrossRef]
- Strzelczak, A.; Balejko, J.; Szymczak, M.; Witczak, A. Effect of protein denaturation temperature on rheological properties of Baltic Herring (Clupea harengus membras) muscle tissue. Foods 2021, 10, 829. [Google Scholar] [CrossRef]
- Tserkova, F. Growth parameters of the Black Sea sprat (Sprattus sprattus L.) during the period November 2010–March 2012 along the Bulgarian Black Sea coast. Bulg. J. Agric. Sci. 2013, 19 (Suppl. S1), 109–113. [Google Scholar]
- Panayotova, M. Growth Parameters of the Black Sea Sprat (Sprattus sprattus L.) During the Period 1998–2000 Along the Bulgarian Black Sea Coast. Proc. Inst. Oceanol. 2001, 3, 163–169, Bulgarian Academy of Sciences. Available online: https://www.io-bas.bg/downloads/oc3-16.pdf (accessed on 18 November 2024).
- Vitale, F.; Mittermayer, F.; Krischansson, B.; Johansson, M.; Casini, M. Growth and maturity of sprat (Sprattus sprattus) in the Kattegat and Skagerrak, eastern North Sea. Aquat. Living Resour. 2015, 28, 127–137. [Google Scholar] [CrossRef]
- Brendeland, Ø. Spatial Variability in Life History Traits of Sprat (Sprattus sprattus) in Norwegian Fjords Suggests Low Mixing of Adults Between the Fjords. Master’s Thesis, University of Bergen, Bergen, Norway, 2022. [Google Scholar]
- Nadolna-Ałtyn, K.; Szostakowska, B.; Podolska, M. Sprat (Sprattus sprattus) as a possible source of invasion of marine predators with Contracaecum osculatum in the southern Baltic Sea. Russ. J. Mar. Biol. 2018, 44, 471–476. [Google Scholar] [CrossRef]
- Keinänen, M.; Käkelä, R.; Ritvanen, T.; Myllylä, T.; Pönni, J.; Vuorinen, P.J. Fatty acid composition of sprat (Sprattus sprattus) and herring (Clupea harengus) in the Baltic Sea as potential prey for salmon (Salmo salar). Helgol. Mar. Res. 2017, 71, 4. [Google Scholar] [CrossRef]
- ICES CM. ACFM:01. Report of the Working Group on the Assessment of Northern Shelf Demersal Stocks; ICES: Copenhagen, Denmark, 2001; p. 421. [Google Scholar]
- De Vries, A.N.; Govoni, D.; Árnason, S.H.; Carlsson, P. Microplastic ingestion by fish: Body size, condition factor and gut fullness are not related to the amount of plastics consumed. Mar. Pollut. Bull. 2020, 151, 110827. [Google Scholar] [CrossRef] [PubMed]
- Chouinard, G.A.; Swain, D.P. Depth-dependent variation in condition and length-at-age of Atlantic cod (Gadus morhua) in the southern Gulf of St. Lawrence. Can. J. Fish. Aquat. Sci. 2002, 59, 1451–1459. [Google Scholar] [CrossRef]
- Lilly, G.R.; Shelton, A.; Brattey, J.; Cadigan, N.; Murphy, E.F.; Stansbury, D.E.; Davis, M.B.; Morgan, M.J. An Assessment of the cod Stock in NAFO Divisions 2J + 3KL. In NAFO SCR Doc. 99-28; N4084. 1999. Available online: https://www.nafo.int/Portals/0/PDFs/sc/1999/scr99-028.pdf (accessed on 18 November 2024).
- ICES CM. ACFM:01. Report of the Working Group on the Assessment of Demersal Stocks in the North Sea and Skagerrak; ICES: Copenhagen, Denmark, 2002; p. 548. [Google Scholar]
- Kjesbu, O.S. The spawning activity of cod, Gadus morhua L. J. Fish Biol. 1989, 34, 195–206. [Google Scholar] [CrossRef]
- Lloret, J.; Rätz, H.J. Condition of cod (Gadus morhua) off Greenland during 1982–1998. Fish. Res. 2000, 48, 79–86. [Google Scholar] [CrossRef]
- Rätz, H.J.; Stein, M.; Lloret, J. Variation in growth and recruitment of Atlantic cod (Gadus morhua) off Greenland during the second half of the twentieth century. J. Northw. Atl. Fish. Sci. 1999, 25, 161–170. [Google Scholar] [CrossRef]
- Stansbury, D.E. An Assessment of the Cod Stock in NAFO Divisions 3NO. In NAFO SCR Doc. 01/72; N4450; 2001; p. 64. Available online: https://www.nafo.int/Portals/0/PDFs/sc/2001/scr01-072.pdf (accessed on 18 November 2024).
- Mion, M.; Thorsen, A.; Vitale, F.; Dierking, J.; Herrmann, J.P.; Huwer, B.; von Dewitz, B.; Casini, M. Effect of fish length and nutritional condition on the fecundity of distressed Atlantic cod Gadus morhua from the Baltic Sea. J. Fish Biol. 2018, 92, 1016–1034. [Google Scholar] [CrossRef]
- Kraus, G.; Pelletier, D.; Dubreuil, J.; Möllmann, C.; Hinrichsen, H.H.; Bastardie, F.; Vermard, Y.; Mahévas, S. A model-based evaluation of Marine Protected Areas: The example of eastern Baltic cod (Gadus morhua callarias L.). ICES J. Mar. Sci. 2009, 66, 109–121. [Google Scholar] [CrossRef]
- Rummel, C.D.; Löder, M.G.J.; Fricke, N.; Lang, T.; Griebeler, E.M.; Janke, M.; Gerdts, G. Plastic ingestion by pelagic and demersal fish from the North Sea and Baltic Sea. Mar. Pollut. Bull. 2016, 102, 134–141. [Google Scholar] [CrossRef]
- Kerambrun, E.; Henry, F.; Cornille, V.; Courcot, L.; Amara, R. A combined measurement of metal bioaccumulation and condition indices in juvenile European flounder, Platichthys flesus, from European estuaries. Chemosphere 2013, 91, 498–505. [Google Scholar] [CrossRef]
- Amara, R.; Selleslagh, J.; Billon, G.; Minier, C. Growth and condition of 0-group European flounder, Platichthys flesus as indicator of estuarine habitat quality. Hydrobiologia 2009, 627, 87–98. [Google Scholar] [CrossRef]
- Henry, F.; Filipuci, I.; Billon, G.; Courcot, L.; Kerambrun, E.; Amara, R. Metal concentrations, growth and condition indices in European juvenile flounder (Platichthys flesus) relative to sediment contamination levels in four Eastern English Channel estuaries. J. Environ. Monit. 2012, 14, 3211–3219. [Google Scholar] [CrossRef]
- Mendes, C.V.R. Factors Affecting Early Life Patterns of the European Flounder Platichthys flesus in a Nursery Habitat. Ph.D. Thesis, University of Porto, Porto, Portugal, 2019. Available online: https://repositorio-aberto.up.pt/bitstream/10216/119268/2/319524.pdf (accessed on 18 November 2024).
- Freitas, V.; Santos, D.; Silva, D.M.; Cunha, J.; Rodrigues, S.M.; Neves, V.; Rocha, E.; Martinho, F.; Ramos, S. Spatial and temporal patterns of gonadal maturation and spawning in European flounder Platichthys flesus at its southern continental edge. Fish. Res. 2024, 269, 106864. [Google Scholar] [CrossRef]
- Kleinkauf, A.; Connor, L.; Swarbreck, D.; Levene, C.; Walker, P.; Johnson, P.J.; Leah, R.T. General condition biomarkers in relation to contaminant burden in European flounder (Platichthys flesus). Ecotoxicol. Environ. Saf. 2004, 58, 335–355. [Google Scholar] [CrossRef]
- Kopecka, J.; Pempkowiak, J. Temporal and spatial variations of selected biomarker activities in flounder (Platichthys flesus) collected in the Baltic proper. Ecotoxicol. Environ. Saf. 2008, 70, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, H.; Wennhage, H.; Lappalainen, A.; Ådjers, K.; Rask, M.; Norkko, A. Decline of flounder (Platichthys flesus (L.)) at the margin of the species’ distribution range. J. Sea Res. 2015, 105, 1–9. [Google Scholar] [CrossRef]
- Nissling, A.; Larsson, R. Population specific sperm production in European flounder Platichthys flesus: Adaptation to salinity at spawning. J. Fish Biol. 2018, 93, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Umaru, J.A.; Annune, P.A.; Cheikyula, J.O.; Okomoda, V.T. Some biometric parameters of four selected fish species in Doma Dam, Nasarawa State, Nigeria. Int. J. Aquac. 2015, 5, 31. [Google Scholar] [CrossRef][Green Version]
- Roots, O.; Schramm, K.W.; Simmm, M.; Henkelmann, B.; Lankov, A. Polychlorinated dibenzo-p-dioxins and dibenzofurans in Baltic herring and sprat in the north-eastern part of the Baltic Sea. Proc. Estonian. Acad. Sci. Biol. 2006, 55, 51–60. [Google Scholar][Green Version]
- Dos Santos Schmidt, T.C.; Hay, D.E.; Sundby, S.; Devine, J.A.; Óskarsson, G.J.; Slotte, A.; Wuenschel, M.J.; Lajus, D.; Johannessen, A.; van Damme, C.J.G.; et al. Adult body growth and reproductive investment vary markedly within and across Atlantic and Pacific herring: A meta-analysis and review of 26 stocks. Rev. Fish Biol. Fish. 2021, 31, 685–708. [Google Scholar] [CrossRef]
- Wheeler, J.P.; Purchase, C.F.; Macdonald, P.D.M.; Fill, R.; Jacks, L.; Wang, H.; Ye, C. Temporal changes in maturation, mean length-at-age, and condition of spring-spawning Atlantic herring (Clupea harengus) in Newfoundland waters. ICES J. Mar. Sci. 2009, 66, 1800–1807. [Google Scholar] [CrossRef]
- Frantzen, S.; Måge, A.; Iversen, S.A.; Julshamn, K. Seasonal variation in the levels of organohalogen compounds in herring (Clupea harengus) from the Norwegian Sea. Chemosphere 2011, 85, 179–187. [Google Scholar] [CrossRef]
- Cardinale, M.; Casini, M.; Arrhenius, F. The influence of biotic and abiotic factors on the growth of sprat (Sprattus sprattus) in the Baltic Sea. Aquat. Living Resour. 2002, 15, 273–281. [Google Scholar] [CrossRef]
- Simm, M.; Roots, O.; Kotta, J.; Lankov, A.; Henkelmann, B.; Shen, H.; Schramm, K.W. PCDD/Fs in sprat (Sprattus sprattus balticus) from the Gulf of Finland, the Baltic Sea. Chemosphere 2006, 65, 1570–1575. [Google Scholar] [CrossRef]
- Moore, C.; Lynch, D.; Clarke, M.; Officer, R.; Mills, J.; Louis-Defour, J.; Brophy, D. Age verification of north Atlantic sprat. Fish. Res. 2019, 213, 144–150. [Google Scholar] [CrossRef]
- Casarsa, L.; Diez, M.J.; Madirolas, A.; Cabreira, A.G.; Buratti, C.C. Morphometric description of schools from two different stocks of the southernmost sprat Sprattus fuegensis. Fish. Res. 2019, 212, 29–34. [Google Scholar] [CrossRef]
- Van Beveren, E.; Bonhommeau, S.; Fromentin, J.M.; Bigot, J.L.; Bourdeix, J.H.; Brosset, P.; Roos, D.; Saraux, C. Rapid changes in growth, condition, size and age of small pelagic fish in the Mediterranean. Mar. Biol. 2014, 161, 1809–1822. [Google Scholar] [CrossRef]
- Sinovcic, G.; Franicevic, M.; Zorica, B.; Ciles-Kec, V. Length-weight and length-length relationships for 10 pelagic fish species from the Adriatic Sea (Croatia). J. Appl. Ichthyol. 2004, 20, 156–158. [Google Scholar] [CrossRef]
- Kalaycı, F.; Samsun, N.; Bilgin, S.; Samsun, O. Length-weight relationship of 10 fish species caught by bottom trawl and midwater trawl from the Middle Black Sea, Turkey. Turk. J. Fish. Aquat. Sci. 2007, 7, 1. [Google Scholar]
- Kasapoğlu, N. Age, growth, and mortality of exploited stocks: Anchovy, Sprat, Mediterranean Horse Mackerel, Whiting, and Red Mullet in the Southeastern Black Sea. Aquat. Sci. Eng. 2018, 33, 39–49. [Google Scholar] [CrossRef]
- Stancheva, M.; Merdzhanova, A.; Petrova, E.; Petrova, D. Heavy metals and proximate composition of Black Sea sprat (Sprattus sprattus) and goby (Neogobius melanostomus). Bulg. J. Agric. Sci. 2013, 19 (Suppl. S1), 35–41. [Google Scholar]
- Zuyev, G.V.; Bondarev, V.A.; Samotoi, I.V. Local overfishing of the Black Sea sprat (Sprattus sprattus: Clupeidae, Pisces) and intraspecies differentiation. Mar. Biol. J. 2018, 3, 35–45. [Google Scholar] [CrossRef]
- Solberg, I.; Røstad, A.; Kaartvedt, S. Ecology of overwintering sprat (Sprattus sprattus). Prog. Oceanogr. 2015, 138 Pt A, 116–135. [Google Scholar] [CrossRef][Green Version]
- Svedäng, H.; Hornborg, S. Historic changes in length distributions of three Baltic cod (Gadus morhua) stocks: Evidence of growth retardation. Ecol. Evol. 2017, 7, 6089–6102. [Google Scholar] [CrossRef]
- Cardinale, M.; Modin, J. Changes in size-at-maturity of Baltic cod (Gadus morhua) during a period of large variations in stock size and environmental conditions. Fish. Res. 1999, 41, 285–295. [Google Scholar] [CrossRef]
- Kraus, G.; Müller, A.; Trella, K.; Köuster, F.W. Fecundity of Baltic cod: Temporal and spatial variation. J. Fish Biol. 2000, 56, 1327–1341. [Google Scholar] [CrossRef]
- Pachur, M.E.; Horbowy, J. Food composition and prey selection of cod, Gadus morhua (Actinopterygii: Gadiformes: Gadidae), in the southern Baltic Sea. Acta Ichthyol. Piscat. 2013, 43, 109–118. [Google Scholar] [CrossRef]
- Kraus, G.; Tomkiewicz, J.; Diekmann, R.; Köster, F.W. Seasonal prevalence and intensity of follicular atresia in Baltic cod Gadus morhua callarias L. J. Fish Biol. 2008, 72, 831–847. [Google Scholar] [CrossRef]
- Svedäng, H.; Hornborg, S. Selective fishing induces density-dependent growth. Nat. Commun. 2014, 5, 4152. [Google Scholar] [CrossRef]
- Florin, A.B.; Höglund, J. Population structure of flounder (Platichthys flesus) in the Baltic Sea: Differences among demersal and pelagic spawners. Heredity 2008, 101, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Napierska, D.; Baršienė, J.; Mulkiewicz, E.; Podolska, M.; Rybakovas, A. Biomarker responses in flounder Platichthys flesus from the Polish coastal area of the Baltic Sea and applications in biomonitoring. Ecotoxicology 2009, 18, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Polak-Juszczak, L. Trace metals in flounder, Platichthys flesus (Linnaeus, 1758), and sediments from the Baltic Sea and the Portuguese Atlantic coast. Environ. Sci. Pollut. Res. 2013, 20, 7424–7432. [Google Scholar] [CrossRef] [PubMed]
- Borg, J.P.G.; Westerbom, M.; Lehtonen, H. Sex-specific distribution and diet of Platichthys flesus at the end of spawning in the northern Baltic Sea. J. Fish Biol. 2014, 84, 937–951. [Google Scholar] [CrossRef]
- Sahin, T.; Gunes, E.; Aydin, I.; Polat, H. Reproductive characteristics and egg development in flounder (Pleuronectes flesus luscus) in the Southern Black Sea. IJA 2008, 60, 20–26. [Google Scholar] [CrossRef]
- Lampart-Kaluzniacka, M.; Heese, T. Morphological characteristics of South Baltic lumpfish, Cyclopterus lumpus l., 1758. Acta Ichthyol. Piscat. 2000, 30, 99–110. [Google Scholar] [CrossRef]
- Engebretsen, S.; Aldrin, M.; Lunde, L.; Austad, M.; Rafoss, T.; Danielsen, O.R.; Lindhom, A.; Boissonnot, L.; Jansen, P.A. Condition factor tailored to lumpfish (Cyclopterus lumpus) used as cleaner fish in salmonid farms. Aquac. Rep. 2024, 35, 101996. [Google Scholar] [CrossRef]
- Engebretsen, S.; Aldrin, M.; Qviller, L.; Stige, L.C.; Rafoss, T.; Danielsen, O.R.; Lindhom, A.; Jansen, P.A. Salmon lice (Lepeophtheirus salmonis) in the stomach contents of lumpfish (Cyclopterus lumpus) sampled from Norwegian fish farms: Relationship between lice grazing and operational conditions. Aquaculture 2023, 563, 738967. [Google Scholar] [CrossRef]
- Whittaker, B.A.; Consuegra, S.; Garcia de Leaniz, C. Genetic and phenotypic differentiation of lumpfish (Cyclopterus lumpus) across the North Atlantic: Implications for conservation and aquaculture. PeerJ 2015, 6, e5974. [Google Scholar] [CrossRef]
- Lovell, J.M.; Findlay, M.M.; Moate, R.M.; Pilgrim, D.A. The polarization of inner ear ciliary bundles from a scorpaeniform fish. J. Fish Biol. 2005, 66, 836–846. [Google Scholar] [CrossRef]
- Cardinale, M.; Arrhenius, F. Decreasing weight-at-age of Atlantic herring (Clupea harengus) from the Baltic Sea between 1986 and 1996: A statistical analysis. ICES J. Mar. Sci. 2000, 57, 882–893. [Google Scholar] [CrossRef][Green Version]
- Casini, M.; Rouyer, T.; Bartolino, V.; Larson, N.; Grygiel, W. Density-dependence in space and time: Opposite synchronous variations in population distribution and body condition in the Baltic Sea sprat (Sprattus sprattus) over three decades. PLoS ONE 2014, 9, e92278. [Google Scholar] [CrossRef] [PubMed]
- Shelton, P.A.; Sinclair, A.F.; Chouinard, G.A.; Mohn, R.; Duplisea, D.E. Fishing under low productivity conditions is further delaying recovery of Northwest Atlantic cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 2006, 63, 235–238. [Google Scholar] [CrossRef]
- Casini, M.; Cardinale, M.; Arrhenius, F. Feeding preferences of herring (Clupea harengus) and sprat (Sprattus sprattus) in the southern Baltic Sea. ICES J. Mar. Sci. 2004, 61, 1267–1277. [Google Scholar] [CrossRef]
- Heikinheimo, O. Interactions between cod, herring and sprat in the changing environment of the Baltic Sea: A dynamic model analysis. Ecol. Modell. 2011, 222, 1731–1742. [Google Scholar] [CrossRef]
- Casini, M.; Cardinale, M.; Hjelm, J. Inter-annual variation in herring, Clupea harengus, and sprat, Sprattus sprattus, condition in the central Baltic Sea: What gives the tune? Oikos 2006, 112, 638–650. [Google Scholar] [CrossRef]
- Aps, R.; Lassen, H. Recovery of depleted Baltic Sea fish stocks: A review. ICES J. Mar. Sci. 2010, 67, 1856–1860. [Google Scholar] [CrossRef]
- Olsson, J.; Bergström, L.; Gårdmark, A. Abiotic drivers of coastal fish community change during four decades in the Baltic Sea. ICES J. Mar. Sci. 2012, 69, 961–970. [Google Scholar] [CrossRef]
- Yao, C.L.; Somero, G.N. The impact of acute temperature stress on hemocytes of invasive and native mussels (Mytilus galloprovincialis and Mytilus californianus): DNA damage, membrane integrity, apoptosis and signaling pathways. J. Exp. Biol. 2012, 215, 4267–4277. [Google Scholar] [CrossRef]
- Willig, M.R.; Kaufam, D.M.; Stevens, R.D. Latitudinal gradients of biodiversity: Pattern, process, scale, and synthesis. Annu. Rev. Ecol. System. 2003, 34, 273–309. [Google Scholar] [CrossRef]
- Fonds, M.; Cronie, R.; Vethaak, A.D.; Van Der Puyl, P. Metabolism, food consumption and growth of plaice (Pleuronectes platessa) and flounder (Platichthys flesus) in relation to fish size and temperature. Neth. J. Sea Res. 1992, 29, 127–143. [Google Scholar] [CrossRef]
- Neill, W.H.; Miller, J.M.; Van Der Veer, H.W.; Winemiller, K.O. Ecophysiology of marine fish recruitment: A conceptual framework for understanding interannual variability. Neth. J. Sea Res. 1994, 32, 135–152. [Google Scholar] [CrossRef]
- Fincham, J.I.; Rijnsdorp, A.D.; Engelhard, G.H. Shifts in the timing of spawning in sole linked to warming sea temperatures. J. Sea Res. 2013, 75, 69–76. [Google Scholar] [CrossRef]
- Neuheimer, A.; Thresher, R.; Lyle, J.; Semmens, J.M. Tolerance limit for fish growth exceeded by warming waters. Nature Clim. Change 2011, 1, 110–113. [Google Scholar] [CrossRef]
- Castellani, C.; Edwards, M. Marine Plankton: A Practical Guide to Ecology, Methodology, and Taxonomy; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Steffensen, J.F. Respiratory systems and metabolic rates. Fish Physiol. 2005, 22, 203–238. [Google Scholar] [CrossRef]
- Aydin, İ.; Firidin, Ş.; Öztürk, R.; Alemdağ, M.; Terzi̇, Y.; Eroğlu, O. Genetically Distinct European Flounder (Platichthys flesus L.) Matriline in the Black Sea. Thalassas 2024, 40, 115–123. [Google Scholar] [CrossRef]
- Soliño, L.; Vidal-Liñán, L.; Pérez, P.; García-Barcelona, S.; Baldó, F.; Gago, J. Microplastic occurrence in deep-sea fish species Alepocephalus bairdii and Coryphaenoides rupestris from the Porcupine Bank (North Atlantic). Sci. Total Environ. 2022, 834, 155150. [Google Scholar] [CrossRef]
- Maaghloud, H.; Houssa, R.; Ouansafi, S.; Bellali, F.; El Bouqdaoui, K.; Charouki, N.; Fahde, A. Ingestion of microplastics by pelagic fish from the Moroccan Central Atlantic coast. Environ. Pollut. 2020, 261, 114194. [Google Scholar] [CrossRef]
- Jonathan, M.P.; Sujitha, S.B.; Rodriguez-Gonzalez, F.; Villegas, L.E.; Hernández-Camacho, C.J.; Sarkar, S.K. Evidences of microplastics in diverse fish species off the Western Coast of Pacific Ocean, Mexico. Ocean Coast. Manag. 2021, 204, 105544. [Google Scholar] [CrossRef]
- Digka, N.; Tsangaris, C.; Torre, M.; Anastasopoulou, A.; Zeri, C. Microplastics in mussels and fish from the Northern Ionian Sea. Mar. Pollut. Bull. 2018, 135, 30–40. [Google Scholar] [CrossRef]
- Güven, O.; Gökdağ, K.; Jovanović, B.; Kıdeyş, A.E. Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environ. Pollut. 2017, 223, 286–294. [Google Scholar] [CrossRef]
- Abadi, Z.T.R.; Abtahi, B.; Grossart, H.P.; Khodabandeh, S. Microplastic content of Kutum fish, Rutilus frisii kutum in the southern Caspian Sea. Sci. Total Environ. 2021, 752, 141542. [Google Scholar] [CrossRef]
- Ding, J.; Ju, P.; Ran, Q.; Li, J.; Jiang, F.; Cao, W.; Zhang, J.; Sun, C. Elder fish means more microplastics? Alaska pollock microplastic story in the Bering Sea. Sci. Adv. 2023, 9, eadf5897. [Google Scholar] [CrossRef]
- Gao, S.; Yan, K.; Liang, B.; Shu, R.; Wang, N.; Zhang, S. The different ways microplastics from the water column and sediment accumulate in fish in Haizhou Bay. Sci. Total Environ. 2023, 854, 158575. [Google Scholar] [CrossRef]
- Abbasi, A.; Sadeghi, P.; Abadi, Z.T.R. Characterization of microplastics in digestive tract of commercial fish species from the Oman Sea. Mar. Pollut. Bull. 2023, 197, 115769. [Google Scholar] [CrossRef]
- Schernewski, G.; Radtke, H.; Hauk, R.; Baresel, C.; Olshammar, M.; Osinski, R.; Oberbeckmann, S. Transport and Behavior of Microplastics Emissions from Urban Sources in the Baltic Sea. Front. Environ. Sci. 2020, 8, 579361. [Google Scholar] [CrossRef]
- Cardinale, M.; Casini, M.; Arrhenius, F.; Håkansson, N. Diel spatial distribution and feeding activity of herring (Clupea harengus) and sprat (Sprattus sprattus) in the Baltic Sea. Aquat. Living Resour. 2003, 16, 283–292. [Google Scholar] [CrossRef]
- Kulatska, N.; Woods, P.J.; Elvarsson, B.; Bartolino, V. Size-selective competition between cod and pelagic fisheries for prey. ICES J. Mar. Sci. 2021, 78, 1872–1886. [Google Scholar] [CrossRef]
- Bošković, N.; Joksimović, D.; Perošević-Bajčeta, A.; Peković, M.; Bajt, O. Distribution and characterization of microplastics in marine sediments from the Montenegrin coast. J. Soils Sediments 2022, 22, 2958–2967. [Google Scholar] [CrossRef]
- Rodríguez-Rey, M.; Whittaker, B. The global ecological niche of lumpfish (Cyclopterus lumpus) and predicted range shifts under climate change. Hydrobiologia 2023, 850, 2089–2100. [Google Scholar] [CrossRef]
- McGoran, A.R.; Clark, P.F.; Morritt, D. Presence of microplastic in the digestive tracts of European flounder, Platichthys flesus, and European smelt, Osmerus eperlanus, from the River Thames. Environ. Pollut. 2017, 220, 744–751. [Google Scholar] [CrossRef]
- Jabeen, K.; Su, L.; Li, J.; Yang, D.; Tong, C.; Mu, J.; Shi, H. Microplastics and mesoplastics in fish from coastal and fresh waters of China. Environ. Pollut. 2017, 221, 141–149. [Google Scholar] [CrossRef]
- Koongolla, J.B.; Lin, L.; Pan, Y.F.; Yang, C.P.; Sun, D.R.; Liu, S.; Xu, X.R.; Maharana, D.; Huang, J.S.; Li, H.X. Occurrence of microplastics in gastrointestinal tracts and gills of fish from Beibu Gulf, South China Sea. Environ. Pollut. 2020, 258, 113734. [Google Scholar] [CrossRef]
- Keerthika, K.; Padmavathy, P.; Rani, V.; Jeyashakila, R.; Aanand, S.; Kutty, R. Contamination of microplastics, surface morphology and risk assessment in beaches along the Thoothukudi coast, Gulf of Mannar region. Environ. Sci. Pollut. Res. 2022, 29, 75525–75538. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, T.; Li, Y.; He, X.; Wang, R.; Xu, J.; Gao, G. The accumulation of microplastics in fish from an important fish farm and mariculture area, Haizhou Bay, China. Sci. Total Environ. 2019, 696, 133948. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, C.; Wang, S.; Sun, D.; Zhou, A.; Xie, S.; Xu, G.; Zou, J. Occurrence of microplastics in the gastrointestinal tract and gills of fish from Guangdong, South China. J. Mar. Sci. Eng. 2021, 9, 981. [Google Scholar] [CrossRef]
- Jaafar, N.; Azfaralariff, A.; Musa, S.M.; Mohamed, M.; Yusoff, A.H.; Lazim, A.M. Occurrence, distribution and characteristics of microplastics in gastrointestinal tract and gills of commercial marine fish from Malaysia. Sci. Total Environ. 2021, 799, 149457. [Google Scholar] [CrossRef]
- Zheng, S.; Tang, S.; Wang, W.X. Microplastics and nanoplastics induced differential respiratory damages in tilapia fish Oreochromis niloticus. J. Hazard. Mater. 2024, 465, 133181. [Google Scholar] [CrossRef]
- Trani, A.; Mezzapesa, G.; Piscitelli, L.; Mondelli, D.; Nardelli, L.; Belmonte, G.; Toso, A.; Piraino, S.; Panti, C.; Baini, M.; et al. Microplastics in water surface and in the gastrointestinal tract of target marine organisms in Salento coastal seas (Italy, Southern Puglia). Environ. Pollut. 2023, 316, 120702. [Google Scholar] [CrossRef]
- Seetapan, K.; Prommi, T.O. Microplastics in commercial fish digestive tracts from freshwater habitats in Northern Thailand. Ecol. Montenegrina 2023, 68, 48–65. [Google Scholar] [CrossRef]
- Atamanalp, M.; Köktürk, M.; Uçar, A.; Duyar, H.A.; Özdemir, S.; Parlak, V.; Esenbuğa, N.; Alak, G. Microplastics in tissues (brain, gill, muscle and gastrointestinal) of Mullus barbatus and Alosa immaculata. Arch. Environ. Contam. Toxicol. 2021, 81, 460–469. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef]
- Su, Y.; Lin, H.C. Analyses of microplastics in the digestive tract of bottom-trawled fishes in Southwest Taiwan. Reg. Stud. Mar. Sci. 2023, 57, 102756. [Google Scholar] [CrossRef]
- De Sales-Ribeiro, C.; Brito-Casillas, Y.; Fernandez, A.; Caballero, M. An end to the controversy over the microscopic detection and effects of pristine microplastics in fish organs. Sci. Rep. 2020, 10, 12434. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef]
- Jovanović, B.; Gökdağ, K.; Güven, O.; Emre, Y.; Whitley, E.M.; Kideys, A.E. Virgin microplastics are not causing imminent harm to fish after dietary exposure. Mar. Pollut. Bull. 2018, 130, 123–131. [Google Scholar] [CrossRef]
- Fuglem, B.; Jirillo, E.; Bjerkås, I.; Kiyono, H.; Nochi, T.; Yuki, Y.; Raida, M.; Fischer, U.; Koppang, E.O. Antigen-sampling cells in the salmonid intestinal epithelium. Dev. Comp. Immunol. 2010, 34, 768–774. [Google Scholar] [CrossRef]
- Løvmo, S.D.; Speth, M.T.; Repnik, U.; Koppang, E.O.; Griffiths, G.W.; Hildahl, J.P. Translocation of nanoparticles and Mycobacterium marinum across the intestinal epithelium in zebrafish and the role of the mucosal immune system. Dev. Comp. Immunol. 2017, 67, 508–518. [Google Scholar] [CrossRef]
- Handy, R.D.; Henry, T.B.; Scown, T.M.; Johnston, B.D.; Tyler, C.R. Manufactured nanoparticles: Their uptake and effects on fish—A mechanistic analysis. Ecotoxicology 2008, 17, 396–409. [Google Scholar] [CrossRef]
- Guerrera, M.C.; Aragona, M.; Porcino, C.; Fazio, F.; Laurà, R.; Levanti, M.; Montalbano, G.; Germanà, G.; Abbate, F.; Germanà, A. Micro and nano plastics distribution in fish as model organisms: Histopathology, blood response and bioaccumulation in different organs. Appl. Sci. 2021, 11, 5768. [Google Scholar] [CrossRef]
- Tepe, Y.; Aydın, H.; Ustaoğlu, F.; Kodat, M. Occurrence of microplastics in the gastrointestinal tracts of four most consumed fish species in Giresun, the Southeastern Black Sea. Environ. Sci. Pollut. Res. Int. 2024, 43, 55336–55345. [Google Scholar] [CrossRef]
- Suaria, G.; Avio, C.G.; Mineo, A.; Lattin, G.L.; Magaldi, M.G.; Belmonte, G.; Moore, C.J.; Regoli, F.; Aliani, S. The Mediterranean Plastic Soup: Synthetic polymers in Mediterranean surface waters. Sci. Rep. 2016, 6, 37551. [Google Scholar] [CrossRef]
- Mondal, P.; Hoque, M.S.; Rahman, M.A.; Hasan, M.M.; Chakma, S.; Islam, M.S.; Shahjahan, M. Occurrence, characteristics and distribution of microplastics in commercial marine fishes of the Bay of Bengal. Mar. Pollut. Bull. 2024, 208, 117020. [Google Scholar] [CrossRef]
- Cordova, M.R.; Riani, E.; Shiomoto, A. Microplastics ingestion by blue panchax fish (Aplocheilus sp.) from Ciliwung Estuary, Jakarta, Indonesia. Mar. Pollut. Bull. 2020; 161, Pt B, 111763. [Google Scholar] [CrossRef]
- Yin, J.; Ju, Y.; Qian, H.; Wang, J.; Miao, X.; Zhu, Y.; Zhou, L.; Ye, L. Nanoplastics and microplastics may be damaging our livers. Toxics 2022, 10, 586. [Google Scholar] [CrossRef]
- Sun, X.; Li, Q.; Zhu, M.; Liang, J.; Zheng, S.; Zhao, Y. Ingestion of microplastics by natural zooplankton groups in the northern South China Sea. Mar. Pollut. Bull. 2017, 115, 217–224. [Google Scholar] [CrossRef]
- Botterell, Z.L.R.; Beaumont, N.; Dorrington, T.; Steinke, M.; Thompson, R.C.; Lindeque, P.K. Bioavailability and effects of microplastics on marine zooplankton: A review. Environ. Pollut. 2019, 245, 98–110. [Google Scholar] [CrossRef]
- Clere, I.K.; Ahmmed, F.; Remoto, P.; Fraser-Miller, S.J.; Gordon, K.C.; Komyakova, V.; Allan, B.J.M. Quantification and characterization of microplastics in commercial fish from southern New Zealand. Mar. Pollut. Bull. 2022, 184, 114121. [Google Scholar] [CrossRef]
- Kasamesiri, P.; Thaimuangphol, W. Microplastics ingestion by freshwater fish in the Chi river, Thailand. Int. J. Geomate 2020, 18, 114–119. [Google Scholar] [CrossRef]
- Hamed, M.; Martyniuk, C.J.; Lee, J.-S.; Shi, H.; Sayed, A.L.-D. Distribution, abundance, and composition of microplastics in market fishes from the Red and Mediterranean seas in Egypt. J. Sea Res. 2023, 194, 102407. [Google Scholar] [CrossRef]
- Santonicola, S.; Volgare, M.; Di Pace, E.; Mercogliano, R.; Cocca, M.; Raimo, G.; Colavita, G. Research and characterization of fibrous microplastics and natural microfibers in pelagic and benthic fish species of commercial interest. Ital. J. Food Saf. 2023, 12, 11032. [Google Scholar] [CrossRef]
- Scacco, U.; Mancini, E.; Marcucci, F.; Tiralongo, F. Microplastics in the deep: Comparing dietary and plastic ingestion data between two Mediterranean bathyal opportunistic feeder species, Galeus melastomus and Coelorinchus caelorhincus, through stomach content analysis. J. Mar. Sci. Eng. 2022, 10, 624. [Google Scholar] [CrossRef]
- Carrillo-Barragán, P.; Fitzsimmons, C.; Lloyd-Hartley, H.; Tinlin-Mackenzie, A.; Scott, C.; Sugden, H. Fifty-year study of microplastics ingested by brachyuran and fish larvae in the central English North Sea. Environ. Pollut. 2024, 342, 123060. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics—The Facts 2019. An Analysis of European Plastic Production, Demand and Waste Data. Plastics Europe. 2019. Available online: https://plasticseurope.org/wp-content/uploads/2021/10/2019-Plastics-the-facts.pdf (accessed on 2 April 2025).
- McKeen, L.W. Environmentally friendly polymers. Plast. Des. Libr. 2017, 13, 305–323. [Google Scholar] [CrossRef]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Úbeda, B.; Hernández-León, S.; Palma, Á.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef]
- Galloway, T.S. Micro- and nano-plastics and human health. Mar. Anthropog. Litter 2015, 343–366. [Google Scholar] [CrossRef]
- Herbinger, C.M.; Friars, G.W. Correlation between condition factor and total lipid content in Atlantic salmon, Salmo salar L. Aquac. Res. 1991, 22, 527–529. [Google Scholar] [CrossRef]
- Chellappa, S.; Huntingford, F.A.; Strang, R.H.C.; Thomson, R.Y. Condition factor and hepatosomatic index as estimates of energy status in male three-spined stickleback. J. Fish Biol. 1995, 47, 775–787. [Google Scholar] [CrossRef]
- Barrett, C.J.; Johnson, M.L.; Hall, N.J.; Hull, S.L. The first use of Fulton’s K for assessing and comparing the conditions of inter-tidal fish populations. Mar. Ecol. 2016, 37, 42–45. [Google Scholar] [CrossRef]
- Imsland, A.K.; Reynolds, P.; Hangstad, T.A.; Jónsdóttir, Ó.; Noble, T.; Wilson, M.; Mackie, J.A.; Elvegård, T.A.; Urskog, T.C.; Mikalsen, B. Feeding behaviour and growth of lumpfish (Cyclopterus lumpus L.) fed with feed blocks. Aquac. Res. 2018, 49, 2006–2012. [Google Scholar] [CrossRef]
- Imsland, A.D.K.; Frogg, N.; Stefansson, S.O.; Reynolds, P. Improving sea lice grazing of lumpfish (Cyclopterus lumpus L.) by feeding live feeds prior to transfer to Atlantic salmon (Salmo salar L.) net-pens. Aquaculture 2019, 511, 734224. [Google Scholar] [CrossRef]
- Imsland, A.K.D.; Berg, M.S.; Haugland, G.T.; Eliasen, K. Comparing body density of lumpfish (Cyclopterus lumpus) to different operational welfare indicators. Fishes 2022, 7, 284. [Google Scholar] [CrossRef]
- Imsland, A.K.D.; Reynolds, P.; Lorentzen, M.; Eilertsen, R.A.; Micallef, G.; Tvenning, R. Improving survival and health of lumpfish (Cyclopterus lumpus L.) by the use of feed blocks and operational welfare indicators (OWIs) in commercial Atlantic salmon cages. Aquaculture 2020, 527, 735476. [Google Scholar] [CrossRef]
- Rajasilta, M.; Laine, P.; Paranko, J. Current growth, fat reserves and somatic condition of juvenile Baltic herring (Clupea harengus membras) reared in different salinities. Helgol. Mar. Res. 2011, 65, 59–66. [Google Scholar] [CrossRef]
- Neuenfeldt, S.; Bartolino, V.; Orio, A.; Andersen, K.H.; Andersen, N.G.; Niiranen, S.; Bergström, U.; Ustups, D.; Kulatska, N.; Casini, M. Feeding and grow of Atlantic cods (Gadus morhua L.) in the east Baltic Sea under environmental change. ICES J. Mar. Sci. 2020, 77, 624–632. [Google Scholar] [CrossRef]
- Eriksson, H.; Albert, J.; Albert, S.; Warren, R.; Pakoa, K.; Andrew, N. The role of fish and fisheries in recovering from natural hazards: Lessons learned from Vanuatu. Environ. Sci. Policy 2017, 76, 50–58. [Google Scholar] [CrossRef]
- Bryhn, A.C.; Bergek, S.; Bergström, U.; Casini, M.; Dahlgren, E.; Ek, C.; Hjelm, J.; Königson, S.; Ljungberg, P.; Lundström, K.; et al. Which factors can affect the productivity and dynamics of cod stocks in the Baltic Sea, Kattegat and Skagerrak? Ocean Coast. Manag. 2022, 223, 106154. [Google Scholar] [CrossRef]
- Ighwela, K.A.; Ahmad, A.B.; Abol-Munafi, A.B. The selection of viscerosomatic and hepatosomatic indices for the measurement and analysis of Oreochromis niloticus condition fed with varying dietary maltose levels. Int. J. Fauna Biol. 2014, 1, 18–20. [Google Scholar]
- Sadekarpawar, S.; Parikh, P. Gonadosomatic and hepatosomatic indices of freshwater fish Oreochromis mossambicus in response to a plant nutrient. World J. Zool. 2013, 8, 110–118. [Google Scholar] [CrossRef]
- Dambo, A.; Solomon, S.G.; Ayuba, V.O.; Okayi, R.G. Study on condition factor and hepatosomatic index of Bagrus bayad (Forsskal, 1775) and Synodontis nigrita (Valenciennes, 1840) from Kangimi Reservoir, Kaduna State, Nigeria. BAJOPAS 2021, 14, 192–197. [Google Scholar] [CrossRef]
- Popović, N.T.; Čižmek, L.; Babić, S.; Strunjak-Perović, I.; Čož-Rakovac, R. Fish liver damage related to the wastewater treatment plant effluents. Environ. Sci. Pollut. Res. 2023, 30, 48739–48768. [Google Scholar] [CrossRef]
- Austin, B. The effects of pollution on fish health. J. Appl. Microbiol. 1998, 85, 234S–242S. [Google Scholar] [CrossRef]
- Vethaak, A.D.; Jol, J.G. Diseases of flounder Platichthys flesus in Dutch coastal and estuarine waters, with particular reference to environmental stress factors. I. Epizootiology of gross lesions. DAO 1996, 26, 81–97. [Google Scholar] [CrossRef]
- Lang, T.; Wosniok, W.; Baršienė, J.; Broeg, K.; Kopecka, J.; Parkkonen, J. Liver histopathology in Baltic flounder (Platichthys flesus) as indicator of biological effects of contaminants. Mar. Pollut. Bull. 2006, 53, 488–496. [Google Scholar] [CrossRef]
- Schubert, S.; Keddig, N.; Gerwinski, W.; Neukirchen, J.; Kammann, U.; Haarich, M.; Hanel, R.; Theobald, N. Persistent organic pollutants in Baltic herring (Clupea harengus)—An aspect of gender. Environ. Monit. Assess. 2016, 188, 368. [Google Scholar] [CrossRef]
- Sweidan, A.H.; El-Bendary, N.; Hegazy, O.M.; Hassanien, A.E.; Snasel, V. Water pollution detection system based on fish gills as a biomarker. Procedia Comput. Sci. 2015, 65, 601–611. [Google Scholar] [CrossRef]
- Pandey, S.; Parvez, S.; Ansari, R.A.; Ali, M.; Kaur, M.; Hayat, F.; Ahmad, F.; Raisuddin, S. Effects of exposure to multiple trace metals on biochemical, histological and ultrastructural features of gills of a freshwater fish, Channa punctata Bloch. Chem. Biol. Interact. 2008, 174, 183–192. [Google Scholar] [CrossRef]
- Aslam, S.; Yousafzai, A.M. Chromium toxicity in fish: A review article. J. Entomol. Zool. Stud. 2017, 5, 1483–1488. [Google Scholar]
- Rohani, M.F. Pesticides toxicity in fish: Histopathological and hemato-biochemical aspects—A review. Emerg. Contam. 2023, 9, 100234. [Google Scholar] [CrossRef]
- Da Cruz, A.L.; Prado, T.M.; da Silva Maciel, L.; Couto, R.D. Environmental effects on the gills and blood of Oreochromis niloticus exposed to rivers of Bahia, Brazil. Ecotoxicol. Environ. Saf. 2015, 111, 23–31. [Google Scholar] [CrossRef]
- Steinberg, C.E.W. Diets and digestive tracts—‘your food determines your intestine’. In Aquatic Animal Nutrition; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Kleinow, K.M.; James, M.O. Response of the teleost gastrointestinal system to xenobiotics. In Target Organ Toxicity in Marine and Freshwater Teleosts, 1st ed.; Schlenk, D., Benson, W.H., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 322–386. [Google Scholar]
- Bandowe, B.A.M.; Bigalke, M.; Boamah, L.; Nyarko, E.; Saalia, F.K.; Wilcke, W. Polycyclic aromatic compounds (PAHs and oxygenated PAHs) and trace metals in fish species from Ghana (West Africa): Bioaccumulation and health risk assessment. Environ. Int. 2014, 65, 135–146. [Google Scholar] [CrossRef]
- Ajima, M.N.O.; Nnodi, P.C.; Ogo, O.A.; Adaka, G.S.; Osuigwe, D.I.; Njoku, D.C. Bioaccumulation of heavy metals in Mbaa River and the impact on aquatic ecosystem. Environ. Monit. Assess. 2015, 187, 768. [Google Scholar] [CrossRef]
- Filgueiras, A.V.; Preciado, I.; Cartón, A.; Gago, J. Microplastic ingestion by pelagic and benthic fish and diet composition: A case study in the NW Iberian shelf. Mar. Pollut. Bull. 2020, 160, 111623. [Google Scholar] [CrossRef]
- Lopes, C.; Ambrosino, A.C.; Figueiredo, C.; Caetano, M.; Santos, M.M.; Garrido, S.; Raimundo, J. Microplastic distribution in different tissues of small pelagic fish of the Northeast Atlantic Ocean. Sci. Total Environ. 2023, 901, 166050. [Google Scholar] [CrossRef]
- Menéndez, D.; Blanco-Fernandez, C.; Machado-Schiaffino, G.; Ardura, A.; Garcia-Vazquez, E. High microplastics concentration in liver is negatively associated with condition factor in the Benguela hake Merluccius polli. Ecotoxicol. Environ. Saf. 2023, 262, 115135. [Google Scholar] [CrossRef]
- Sbrana, A.; Valente, T.; Scacco, U.; Bianchi, J.; Silvestri, C.; Palazzo, L.; de Lucia, G.A.; Valerani, C.; Ardizzone, G.; Matiddi, M. Spatial variability and influence of biological parameters on microplastic ingestion by Boops boops (L.) along the Italian coasts (Western Mediterranean Sea). Environ. Pollut. 2020, 263, 114429. [Google Scholar] [CrossRef]
- Welden, N.A.C.; Cowie, P.R. Long-term microplastic retention causes reduced body condition in the langoustine, Nephrops norvegicus. Environ. Pollut. 2016, 218, 895–900. [Google Scholar] [CrossRef]
- Critchell, K.; Hoogenboom, M.O. Effects of microplastic exposure on the body condition and behaviour of planktivorous reef fish (Acanthochromis polyacanthus). PLoS ONE 2018, 13, e0193308. [Google Scholar] [CrossRef]
- Wright, S.L.; Rowe, D.; Thompson, R.C.; Galloway, T.S. Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol. 2013, 23, R1031–R1033. [Google Scholar] [CrossRef]
- Watts, A.J.R.; Urbina, M.A.; Corr, S.; Lewis, C.; Galloway, T.S. Ingestion of plastic microfibers by the crab Carcinus maenas and its effect on food consumption and energy balance. Environ. Sci. Technol. 2015, 49, 14597–14604. [Google Scholar] [CrossRef]
- Lohmann, R. Microplastics are not important for the cycling and bioaccumulation of organic pollutants in the oceans—But should microplastics be considered POPs themselves? Integr. Environ. Assess. Manag. 2017, 13, 460–465. [Google Scholar] [CrossRef]
- Compa, M.; Ventero, A.; Iglesias, M.; Deudero, S. Ingestion of microplastics and natural fibres in Sardina pilchardus (Walbaum, 1792) and Engraulis encrasicolus (Linnaeus, 1758) along the Spanish Mediterranean coast. Mar. Pollut. Bull. 2018, 128, 89–96. [Google Scholar] [CrossRef]

| Species (Latin Name, Feeding Features, Habitat Zone) | Variable [Unit] | N | Mean | Median | Minimum | Maximum | S.D. |
|---|---|---|---|---|---|---|---|
| Baltic herring (Clupea harengus, planktivore, pelagic) | Total mass [g] | 128 | 50.08 | 45.87 | 16.87 | 108.04 | 15.71 |
| Liver mass [g] | 125 | 0.75 | 0.61 | 0.12 | 2.51 | 0.45 | |
| GIT mass [g] | 126 | 1.86 | 1.70 | 0.04 | 9.73 | 1.18 | |
| Gills mass [g] | 126 | 1.61 | 1.67 | 0.51 | 2.98 | 0.48 | |
| Total length [cm] | 128 | 18.93 | 18.70 | 13.50 | 28.30 | 2.48 | |
| Basic length [cm] | 117 | 16.16 | 15.70 | 11.40 | 23.50 | 2.39 | |
| K factor | 128 | 0.75 | 0.71 | 0.39 | 2.04 | 0.22 | |
| HSI | 125 | 0.02 | 0.01 | 0.00 | 0.06 | 0.01 | |
| GILSI | 126 | 0.03 | 0.03 | 0.01 | 0.08 | 0.01 | |
| GITI | 126 | 0.04 | 0.04 | 0.00 | 0.17 | 0.02 | |
| flounder (Platichthys flesus, carnivore, demersal) | Total mass [g] | 46 | 217.13 | 189.12 | 88.30 | 580.74 | 126.02 |
| Liver mass [g] | 46 | 3.76 | 3.53 | 1.02 | 8.77 | 1.95 | |
| GIT mass [g] | 46 | 9.19 | 7.97 | 2.41 | 25.11 | 5.45 | |
| Gills mass [g] | 46 | 6.23 | 5.35 | 3.05 | 15.80 | 2.97 | |
| Total length [cm] | 46 | 25.52 | 24.95 | 17.10 | 34.30 | 4.14 | |
| Basic length [cm] | 46 | 20.95 | 20.20 | 15.20 | 30.80 | 3.61 | |
| K factor | 46 | 1.29 | 1.11 | 0.57 | 3.71 | 0.59 | |
| HSI | 46 | 0.02 | 0.02 | 0.01 | 0.05 | 0.01 | |
| GILSI | 46 | 0.03 | 0.03 | 0.02 | 0.04 | 0.01 | |
| GITI | 46 | 0.05 | 0.04 | 0.01 | 0.20 | 0.03 | |
| Baltic cod (Gadus morhua, omnivore, demersal/pelagic) | Total mass [g] | 30 | 284.64 | 248.47 | 78.14 | 750.12 | 170.69 |
| Liver mass [g] | 27 | 8.22 | 6.39 | 1.74 | 18.83 | 5.02 | |
| GIT mass [g] | 27 | 10.86 | 7.88 | 1.47 | 45.96 | 8.36 | |
| Gills mass [g] | 27 | 9.21 | 8.14 | 3.64 | 19.53 | 3.79 | |
| Total length [cm] | 30 | 29.98 | 29.40 | 20.80 | 41.80 | 4.22 | |
| Basic length [cm] | 30 | 24.74 | 24.80 | 16.80 | 32.10 | 3.69 | |
| K factor | 30 | 0.98 | 1.00 | 0.37 | 1.70 | 0.31 | |
| HSI | 27 | 0.03 | 0.03 | 0.01 | 0.11 | 0.02 | |
| GILSI | 27 | 0.04 | 0.04 | 0.02 | 0.06 | 0.01 | |
| GITI | 27 | 0.04 | 0.04 | 0.00 | 0.09 | 0.02 | |
| lumpfish (Cyclopterus lumpus, carnivore, demersal) | Total mass [g] | 19 | 165.54 | 158.87 | 110.06 | 271.70 | 39.04 |
| Liver mass [g] | 15 | 13.93 | 14.32 | 10.23 | 20.46 | 2.49 | |
| GIT mass [g] | 15 | 18.55 | 19.67 | 10.04 | 30.58 | 5.16 | |
| Gills mass [g] | 15 | 4.60 | 4.58 | 2.86 | 7.73 | 1.30 | |
| Total length [cm] | 19 | 15.49 | 15.50 | 13.90 | 18.80 | 1.25 | |
| Basic length [cm] | 19 | 12.94 | 12.80 | 11.80 | 15.10 | 0.89 | |
| K factor | 19 | 4.41 | 4.22 | 3.90 | 5.94 | 0.49 | |
| HSI | 15 | 0.09 | 0.09 | 0.06 | 0.11 | 0.02 | |
| GILSI | 15 | 0.03 | 0.03 | 0.02 | 0.04 | 0.00 | |
| GITI | 15 | 0.11 | 0.11 | 0.08 | 0.14 | 0.02 | |
| long-spined bullhead (Taurulus bubalis, carnivore, demersal) | Total mass [g] | 6 | 284.61 | 221.92 | 80.73 | 650.35 | 208.58 |
| Liver mass [g] | 6 | 12.49 | 9.80 | 5.40 | 21.32 | 6.46 | |
| GIT mass [g] | 6 | 36.02 | 23.79 | 5.84 | 120.42 | 42.74 | |
| Gills mass [g] | 6 | 9.23 | 9.20 | 3.45 | 13.03 | 3.56 | |
| Total length [cm] | 6 | 23.33 | 23.85 | 17.80 | 26.80 | 3.06 | |
| Basic length [cm] | 6 | 18.93 | 19.60 | 15.20 | 20.80 | 2.03 | |
| K factor | 6 | 1.97 | 1.62 | 1.14 | 3.38 | 0.86 | |
| HSI | 6 | 0.06 | 0.05 | 0.02 | 0.14 | 0.04 | |
| GILSI | 6 | 0.04 | 0.05 | 0.02 | 0.06 | 0.02 | |
| GITI | 6 | 0.11 | 0.12 | 0.04 | 0.19 | 0.06 | |
| sprat (Sprattus sprattus, planktivore, pelagic) | Total mass [g] | 28 | 10.03 | 9.81 | 6.09 | 15.34 | 1.95 |
| Total length [cm] | 28 | 11.36 | 11.40 | 10.10 | 13.60 | 0.92 | |
| Basic length [cm] | 28 | 9.58 | 9.50 | 8.30 | 11.50 | 0.76 | |
| K factor | 28 | 0.69 | 0.66 | 0.48 | 1.13 | 0.12 |
| Species | NMPs/Nall | Share of Fish with MPs | MPs Found in Organs (Items), (%) | Total | ||
|---|---|---|---|---|---|---|
| Liver | Gills | Gastrointestinal Tract | ||||
| Baltic herring | 80/128 | 63% | 33(15%) | 99 (45%) | 89 (40%) | 221 |
| Baltic cod | 17/30 | 57% | 9 (18%) | 25 (49%) | 17 (33%) | 51 |
| flounder | 28/46 | 61% | 19 (16%) | 47 (39%) | 54 (45%) | 120 |
| lumpfish | 15/19 | 79% | 10 (16%) | 39 (63%) | 13 (21%) | 62 |
| long-spined bullhead | 4/6 | 67% | 1 (12%) | 4 (50%) | 3 (38%) | 8 |
| sprat | 6/28 | 21% | n.c. | n.c. | n.c. | 9 |
| total | 150/257 | 59% | 72 (15%) | 214 (45%) | 176 (37%) | 471 |
| Species | Location | Length | Mass | References | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baltic herring | 10–16 | 17–22 | 23–28 | 29–34 | >35 | 10–50 | 50–100 | 101–150 | 151–200 | 201–250 | 251–300 | 301–350 | 351–400 | 401–450 | >450 | |||
| BS | southern BS | current study | ||||||||||||||||
| southern BS | [119] | |||||||||||||||||
| central BS | [118] | |||||||||||||||||
| northern BS | [113] | |||||||||||||||||
| Estonian coast | [149] | |||||||||||||||||
| Northeast Pacific | [150] | |||||||||||||||||
| Northwest Atlantic | ||||||||||||||||||
| Northeast Atlantic | ||||||||||||||||||
| North Atlantic | [151] | |||||||||||||||||
| Norwegian Sea | [150] | |||||||||||||||||
| [152] | ||||||||||||||||||
| North Sea | [109] | |||||||||||||||||
| [111] | ||||||||||||||||||
| [138] | ||||||||||||||||||
| sprat | 5–7 | 7.1–9 | 9.1–11 | 11.1–13 | >13 | 1–2 | 2.1–4 | 4.1–6 | 6.1–8 | 8.1–10 | 10.1–12 | 12.1–14 | 14.1–16 | 16.1–18 | >18 | |||
| BS | southern BS | current study | ||||||||||||||||
| Baltic Proper | [153] | |||||||||||||||||
| [154] | ||||||||||||||||||
| Atlantic | [155] | |||||||||||||||||
| [156] | ||||||||||||||||||
| Mediterranean Sea | [157] | |||||||||||||||||
| Adriatic Sea | [158] | |||||||||||||||||
| Black Sea | [159] | |||||||||||||||||
| [160] | ||||||||||||||||||
| [161] | ||||||||||||||||||
| [162] | ||||||||||||||||||
| North Sea | [163] | |||||||||||||||||
| Baltic cod | 20–30 | 31–40 | 41–50 | 51–60 | >60 | 200–300 | 301–400 | 401–500 | 501–600 | 601–700 | 701–800 | 801–900 | 901–1000 | 1000–1200 | >1200 | |||
| BS | southern BS | current study | ||||||||||||||||
| Bornholm coast | [164] | |||||||||||||||||
| Baltic Proper | [165] | |||||||||||||||||
| Bornholm Basin/Gdansk Deep | [166] | |||||||||||||||||
| southern BS | [167] | |||||||||||||||||
| Bornholm Basin | [168] | |||||||||||||||||
| southern BS | [73] | |||||||||||||||||
| eastern BS | [169] | |||||||||||||||||
| flounder | 15–20 | 21–25 | 26–30 | 31–35 | >36 | 200–300 | 301–400 | 401–500 | 501–600 | 601–700 | 701–800 | 801–900 | 901–1000 | 1001–1200 | >1200 | |||
| BS | southern BS | current study | ||||||||||||||||
| Hanö Bight, Gotland coast | [147] | |||||||||||||||||
| all BS | [170] | |||||||||||||||||
| Polish coast | [171] | |||||||||||||||||
| Polish coast | [172] | |||||||||||||||||
| northern BS | [173] | |||||||||||||||||
| Atlantic Ocean | [172] | |||||||||||||||||
| [140] | ||||||||||||||||||
| [143] | ||||||||||||||||||
| North Sea | [147] | |||||||||||||||||
| Black Sea | [174] | |||||||||||||||||
| lumpfish | 15–20 | 21–30 | 31–40 | 41–45 | >45 | 100–200 | 201–300 | 301–400 | 401–500 | 501–600 | 601–700 | 701–800 | 801–900 | 901–1000 | >1000 | |||
| BS | southern BS | current study | ||||||||||||||||
| central, southern BS | [175] | |||||||||||||||||
| Norwegian fish farms | [176,177] | |||||||||||||||||
| Western Atlantic | [178] | |||||||||||||||||
| Eastern Atlantic | ||||||||||||||||||
| English Channel | ||||||||||||||||||
| long-spined bullhead | 5–10 | 11–15 | 16–20 | 21–25 | >25 | 50–100 | 101–200 | 201–300 | 301–400 | 401–500 | 501–600 | 601–700 | 701–800 | 801–900 | >900 | |||
| BS | current study | |||||||||||||||||
| Southern England | [179] | |||||||||||||||||
| period | mass | length | ||||||||||||||||
| 1960–1979 | ||||||||||||||||||
| 1980–1999 | ||||||||||||||||||
| 2000–2009 | ||||||||||||||||||
| 2010–2019 | ||||||||||||||||||
| 2020–2024 | ||||||||||||||||||
| Species | Location | K Factor | References | ||
|---|---|---|---|---|---|
| Baltic herring | <0.9 | 0.91–1.1 | >1.1 | ||
| central BS | current study; [114,116,119] | ||||
| northern and eastern BS | [114,116] | ||||
| [115] | |||||
| southwest BS | [120] | ||||
| Atlantic Ocean | [109] | ||||
| [110] | |||||
| [111] | |||||
| North Sea | [112] | ||||
| sprat | BS | current study; [125,126] | |||
| Black Sea | [121,122] | ||||
| North Sea | [123] | ||||
| [124] | |||||
| Baltic cod | BS | current study | |||
| [136] | |||||
| [137] | |||||
| [138] | |||||
| Atlantic | [127,128,129,130] | ||||
| [133,134,135] | |||||
| North Sea | [131] | ||||
| [132] | |||||
| Irish Sea | [127] | ||||
| flounders | BS | current study | |||
| [145] | |||||
| [146] | |||||
| [138] | |||||
| [147] | |||||
| Atlantic | [139] | ||||
| [140] | |||||
| [141,142] | |||||
| [143] | |||||
| Irish Sea | [144] | ||||
| long-spined bullhead | BS | current study | |||
| Atlantic | [250] | ||||
| lumpfish | 3–3.5 | 3.51–4.5 | 4.51–5.5 | ||
| BS | current study | ||||
| North Sea (salmon farms) | [251] | ||||
| [252,253] | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piskuła, P.; Astel, A.M. Well-Being of the Baltic Herring and Bycatch Fish Species from FAO Major Fishing Areas 27 According to Microplastic Pollution. Animals 2025, 15, 2381. https://doi.org/10.3390/ani15162381
Piskuła P, Astel AM. Well-Being of the Baltic Herring and Bycatch Fish Species from FAO Major Fishing Areas 27 According to Microplastic Pollution. Animals. 2025; 15(16):2381. https://doi.org/10.3390/ani15162381
Chicago/Turabian StylePiskuła, Paulina, and Aleksander Maria Astel. 2025. "Well-Being of the Baltic Herring and Bycatch Fish Species from FAO Major Fishing Areas 27 According to Microplastic Pollution" Animals 15, no. 16: 2381. https://doi.org/10.3390/ani15162381
APA StylePiskuła, P., & Astel, A. M. (2025). Well-Being of the Baltic Herring and Bycatch Fish Species from FAO Major Fishing Areas 27 According to Microplastic Pollution. Animals, 15(16), 2381. https://doi.org/10.3390/ani15162381






