Evaluation of an Ipsilateral Uterine Horn Resection and Ovariectomy Surgical Model in Gilts for Embryo Collection
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Gilt Selection and Pre-Surgery Cycling
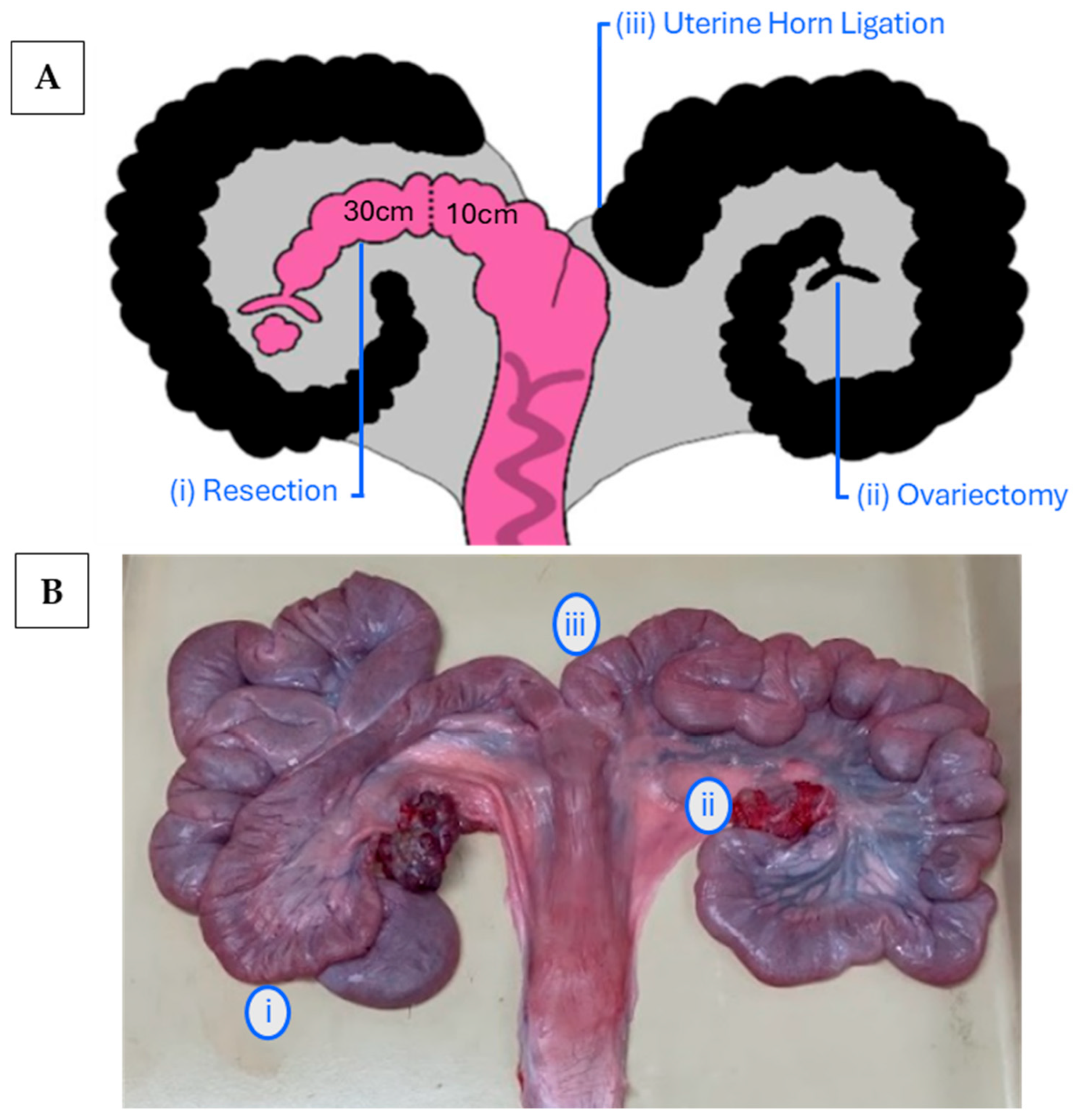
2.3. Surgery
2.4. Ovary Dissection—Contralateral Ovariectomy
2.5. Animal Husbandry and Post-Surgical Management
2.6. Collection and Assessment of Reproductive Tissues
2.7. Statistical Analysis
3. Results
3.1. Estrous Cyclicity
3.2. Reproductive Tract Measurements
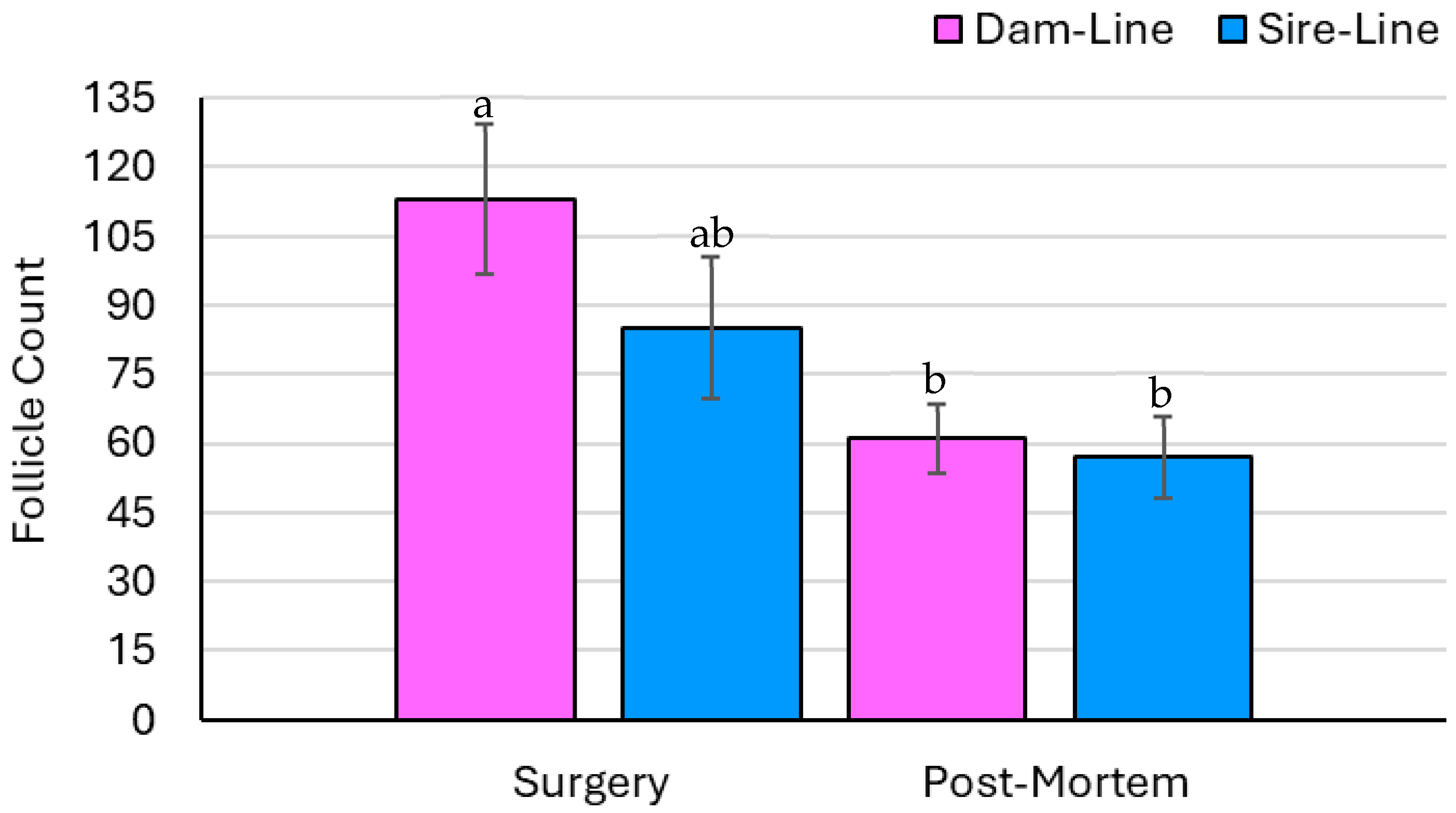
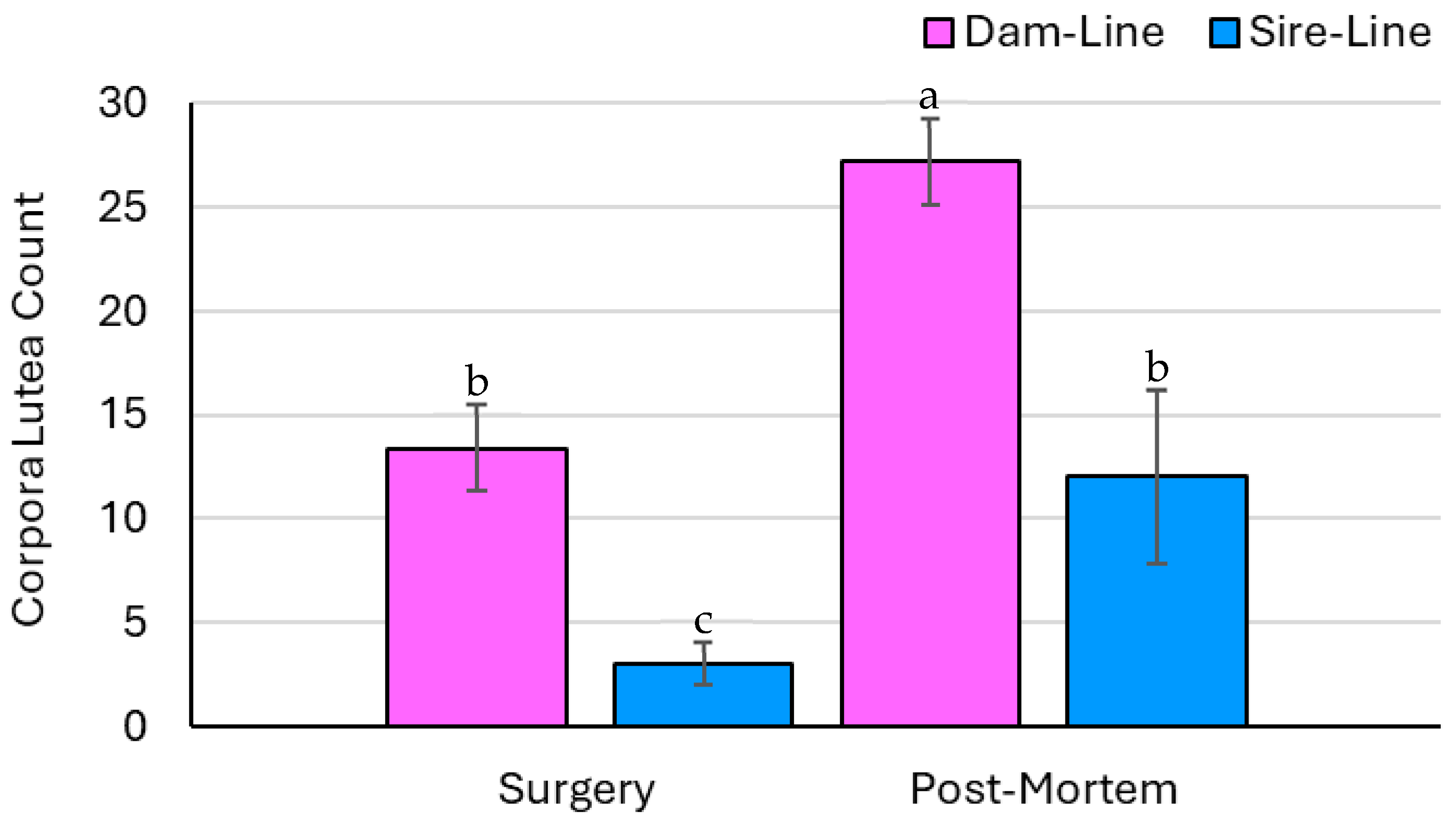
3.3. Ovary Parameters—Count Data
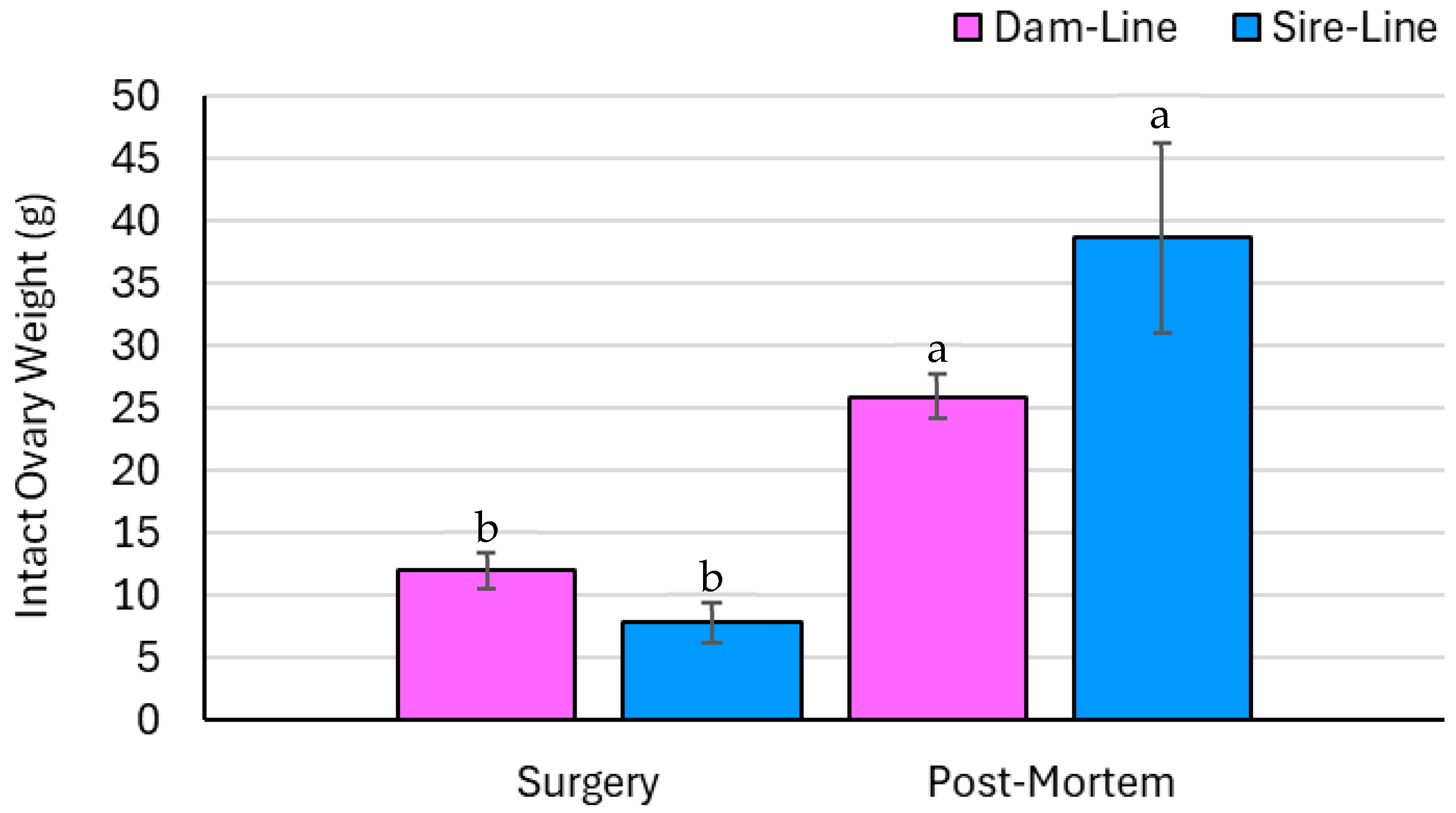
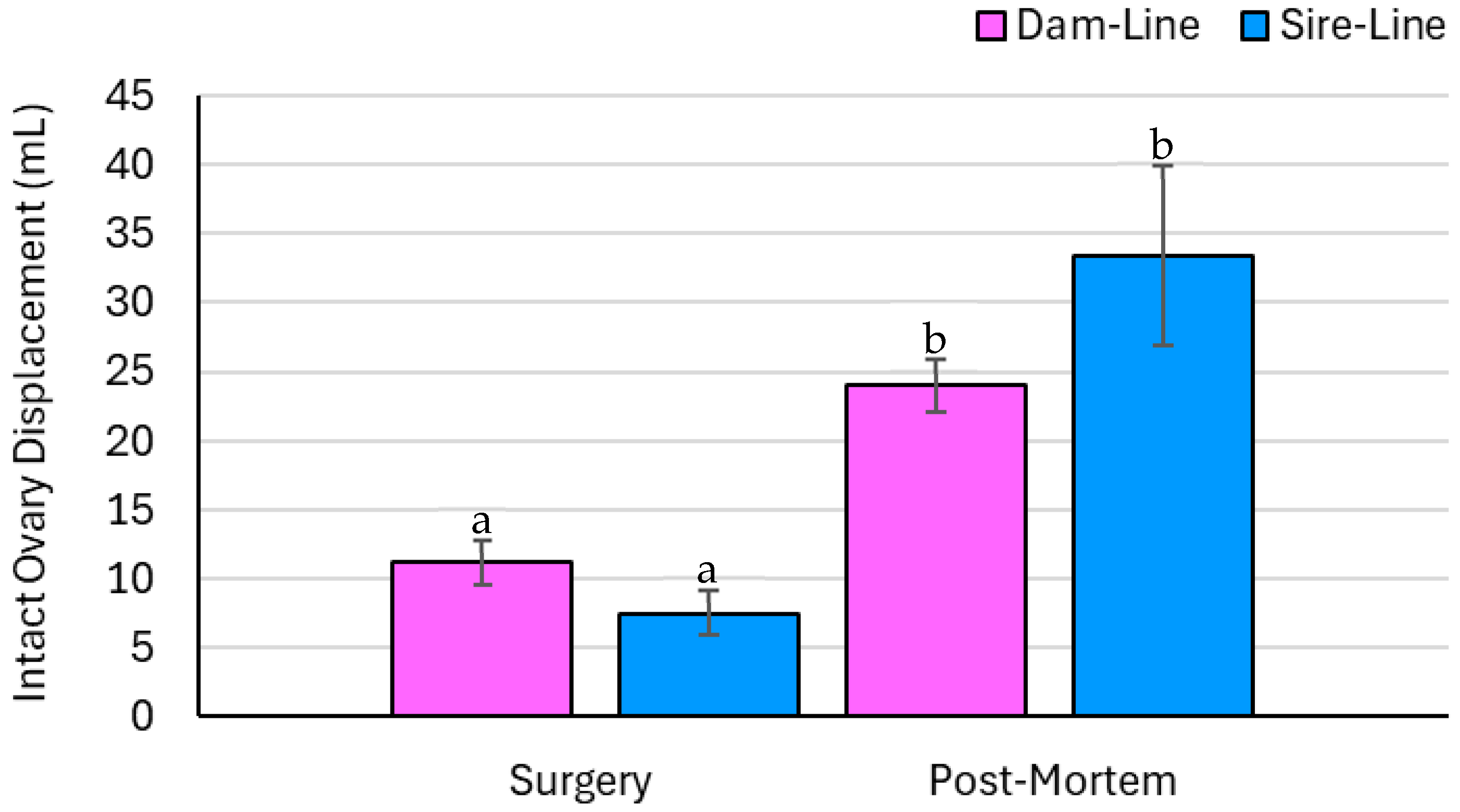
3.4. Ovary Parameters—Measured Data
3.5. Post-Slaughter Embryo Recovery
4. Discussion
4.1. Ovulatory Compensation from Surgery to Slaughter
4.2. Physiological Outcomes of Ipsilateral Uterine Horn Resection Surgery
4.3. Post-Slaughter Embryo Collection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CL | Corpora lutea |
| d | Day |
| h | Hour |
| IM | Intramuscular |
| IV | Intravenous |
| LSM | Least squares mean |
| P0 | Parity zero |
| PGF2α | Prostaglandin F2alpha |
| SD | Standard deviation |
| UH | Uterine horn |
| UTJ | Utero-tubal junction |
References
- OECD; FAO. Agricultural Outlook 2021–2030; OECD Publishing: Paris, France, 2021; pp. 163–177. [Google Scholar]
- Kim, S.W.; Gormley, A.; Jang, K.B.; Duarte, M.E. Current status of global pig production: An overview and research trends. Anim. Biosci. 2024, 37, 719–729. [Google Scholar] [CrossRef]
- Vonderohe, C.E.; Brizgys, L.A.; Richert, J.A.; Radcliffe, J.S. Swine production: How sustainable is sustainability? Anim. Front. 2022, 12, 7–17. [Google Scholar] [CrossRef]
- Koketsu, Y.; Tani, S.; Iida, R. Factors for improving reproductive performance of sows and herd productivity in commercial breeding herds. Porc. Health Manag. 2017, 3, 1. [Google Scholar] [CrossRef]
- Peltoniemi, O.; Björkman, S.; Oropeza-Moe, M.; Oliviero, C. Developments of reproductive management and biotechnology in the pig. Anim. Reprod. 2019, 16, 524–538. [Google Scholar] [CrossRef]
- Martinez, E.A.; Cuello, C.; Parrilla, I.; Rodriguez-Martinez, H.; Roca, J.; Vazquez, J.L.; Vazquez, J.M.; Gil, M.A. Design, development, and application of a non-surgical deep uterine embryo transfer technique in pigs. Anim. Front. 2013, 3, 40–47. [Google Scholar] [CrossRef]
- Viana, J.H.M. 2022 Statistics of Embryo Production and Transfer in Domestic Farm Animals; Embryo Technology Newsletter: Champaign, IL, USA, 2023; pp. 1–25. [Google Scholar]
- Martinez, C.A.; Nohalez, A.; Parrilla, I.; Vazquez, J.L.; Roca, J.; Cuello, C.; Rodriguez-Martinez, H.; Martinez, E.A.; Gil, M.A. Surgical embryo collection but not nonsurgical embryo transfer compromises postintervention prolificacy in sows. Theriogenology 2017, 87, 316–320. [Google Scholar] [CrossRef]
- Hazeleger, W.; Kemp, B. Recent developments in pig embryo transfer. Theriogenology 2001, 56, 1321–1331. [Google Scholar] [CrossRef]
- Cameron, R.; Durack, M.; Fogarty, R.; Putra, D.; McVeigh, J. Practical experience with commercial embryo transfer in pigs. Aust. Vet. J. 1989, 66, 314–318. [Google Scholar] [CrossRef]
- Wollenberg, C.; Wentz, I.; Blum, B.; Holtz, W. Survival of pig embryos flushed from the reproductive tract immediately or two hours after slaughter of donors. J. Anim. Sci. 1990, 68, 2023–2026. [Google Scholar] [CrossRef] [PubMed]
- Singh, E.L.; Thomas, F.C. Embryo transfer as a means of controlling the transmission of viral infections. IX. The in vitro exposure of zona pellucida-intact porcine embryos to swine vesicular disease virus. Theriogenology 1987, 27, 443–449. [Google Scholar] [CrossRef]
- Shisong, C.; Wrathall, A.E. The importance of the zona pellucida for disease control in livestock by embryo transfer. Br. Vet. J. 1989, 145, 129–140. [Google Scholar] [CrossRef]
- Eaglesome, M.D.; Hare, W.C.; Singh, E.L. Embryo transfer: A discussion on its potential for infectious disease control based on a review of studies on infection of gametes and early embryos by various agents. Can. Vet. J. 1980, 21, 106–112. [Google Scholar] [PubMed]
- Hazeleger, W.; Bouwman, E.G.; Noordhuizen, J.P.T.M.; Kemp, B. Effect of superovulation induction on embryonic development on day 5 and subsequent development and survival after nonsurgical embryo transfer in pigs. Theriogenology 2000, 53, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.K.; Quan, H.X.; Vu, H.T.T.; Nguyen, H.L.T.; Nguyen, H.T.; Hoang, A.T.; Le, D.V.; Phan, H.T.; Nguyen, N.T.T.; Pham, Y.K.T.; et al. Influence of superovulation on the production of native Vietnamese pig embryos in vivo and embryonic survival after vitrified-thawed. Livest. Sci. 2023, 276, 105322. [Google Scholar] [CrossRef]
- Martinez, E.A.; Angel, M.A.; Cuello, C.; Sanchez-Osorio, J.; Gomis, J.; Parrilla, I.; Vila, J.; Colina, I.; Diaz, M.; Reixach, J.; et al. Successful non-surgical deep uterine transfer of porcine morulae after 24 hour culture in a chemically defined medium. PLoS ONE 2014, 9, e104696. [Google Scholar] [CrossRef]
- Dalin, A.M. Ovarian follicular activity during the luteal phase in gilts. J. Vet. Med. A 1987, 34, 592–601. [Google Scholar] [CrossRef]
- Sell-Kubiak, E. Selection for litter size and litter birthweight in Large White pigs: Maximum, mean and variability of reproduction traits. Animal 2021, 15, 100352. [Google Scholar] [CrossRef]
- Elbert, K.; Matthews, N.; Wassmuth, R.; Tetens, J. Effects of sire line, birth weight and sex on growth performance and carcass traits of crossbred pigs under standardized environmental conditions. Arch. Anim. Breed. 2020, 63, 367–376. [Google Scholar] [CrossRef]
- Senger, P.L. Pathways to Pregnancy and Parturition, 3rd ed.; Current Conceptions Inc.: Pullman, WA, USA, 2012. [Google Scholar]
- Guthrie, H.D.; Cooper, B.S.; Welch, G.R.; Zakaria, A.D.; Johnson, L.A. Atresia in follicles grown after ovulation in the pig: Measurement of increased apoptosis in granulosa cells and reduced follicular fluid estradiol-17β. Biol. Reprod. 1995, 52, 920–927. [Google Scholar] [CrossRef]
- Brinkley, H.J.; Wickersham, E.W.; First, N.L.; Casida, L.E. Effect of unilateral ovariectomy on the structure and function of the corpora lutea of the pig. Endocrinology 1964, 74, 462–467. [Google Scholar] [CrossRef]
- Małopolska, M.M.; Tuz, R.; Schwarz, T.; Ekanayake, D.L.; D’Ambrosio, J.; Ahmadi, B.; Nowicki, J.; Tomaszewska, E.; Grzesiak, M.; Bartlewski, P.M. Correlates of reproductive tract anatomy and uterine histomorphometrics with fertility in swine. Theriogenology 2021, 165, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Sharif-Islam, M.; Van Der Werf, J.H.J.; Wood, B.J.; Hermesch, S. The predicted benefits of genomic selection on pig breeding objectives. J. Anim. Breed. Genet. 2024, 141, 685–701. [Google Scholar] [CrossRef]
- Reproto, R.O. Genetic selection and advances in swine breeding: A review of its impact on sow’s reproductive traits. Int. J. Res. Rev. 2020, 7, 41–52. [Google Scholar]
- Christenson, R.K.; Leymaster, K.A.; Young, L.D. Justification of unilateral hysterectomy-ovariectomy as a model to evaluate uterine capacity in swine. J. Anim. Sci. 1987, 65, 738–744. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hayashi, S.; Ohtubo, Y.; Honda, A.; Mizuno, J.; Hirano, S. Nonsurgical embryo collection from sows with surgically shortened uteri. Theriogenology 1989, 32, 123–129. [Google Scholar] [CrossRef]
- Gretser, S.; Weber, K.J.; Braun, Y.; Harter, P.N.; Rolle, U.; McNally, J.; Gradhand, E. Tissue shrinkage of resected specimens in hirschsprung’s disease: Why pediatric surgeons think the bowel specimen was longer than indicated in the pathology report. Pediatr. Dev. Pathol. 2023, 26, 287–291. [Google Scholar] [CrossRef]
- Goldstein, N.S.; Soman, A.; Sacksner, J. Disparate surgical margin lengths of colorectal resection specimens between in vivo and in vitro measurements: The effects of surgical resection and formalin fixation on organ shrinkage. Am. J. Clin. Pathol. 1999, 111, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.C.; Dziuk, P.J. Procedures for measuring length of the pig uterus. J. Anim. Sci. 1988, 66, 1712–1720. [Google Scholar] [CrossRef]
- Soede, N.M.; Langendijk, P.; Kemp, B. Reproductive cycles in pigs. Anim. Reprod. Sci. 2011, 124, 251–258. [Google Scholar] [CrossRef]
- Castagna, C.D.; Peixoto, C.H.; Bortolozzo, F.P.; Wentz, I.; Neto, G.B.; Ruschel, F.C. Ovarian cysts and their consequences on the reproductive performance of swine herds. Anim. Reprod. Sci. 2004, 81, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Tummaruk, P.; Kesdangsakonwut, S. Factors affecting the incidence of cystic ovaries in replacement gilts. Comp. Clin. Path 2012, 21, 1–7. [Google Scholar] [CrossRef]
- Hazeleger, W.; Kemp, B.; Soede, N.M.; Noordhuizen, J.P.T.M.; Lende, T.V.D. Transcervical embryo collection and reproductive performance of sows with resectioned uteri. Reprod. Domest. Anim. 1994, 29, 38–45. [Google Scholar] [CrossRef]
- De Rensis, F.; Saleri, R.; Tummaruk, P.; Techakumphu, M.; Kirkwood, R.N. Prostaglandin F2α and control of reproduction in female swine: A review. Theriogenology 2012, 77, 1–11. [Google Scholar] [CrossRef]
- Hazeleger, W.; Van Der Meulen, J.; Van Der Lende, T. A method for transcervical embryo collection in the pig. Theriogenology 1989, 32, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Gadsby, J.E.; Balapure, A.K.; Britt, J.H.; Fitz, T.A. Prostaglandin F2α receptors on enzyme-dissociated pig luteal cells throughout the estrous cycle. Endocrinology 1990, 126, 787–795. [Google Scholar] [CrossRef]
- Brüssow, K.-P.; Rátky, J.; Antosik, P.; Kempisty, B.; Jaśkowski, J.M. Embryo transfer in swine—An indispensable key for the application of reproductive techniques. Electron. J. Pol. Agric. Univ. 2018, 21. [Google Scholar] [CrossRef]
- Ducro-Steverink, D.W.B.; Peters, C.G.W.; Maters, C.C.; Hazeleger, W.; Merks, J.W.M. Reproduction results and offspring performance after non-surgical embryo transfer in pigs. Theriogenology 2004, 62, 522–531. [Google Scholar] [CrossRef]
- Scofield, A.M.; Clegg, F.G.; Lamming, G.E. Embryonic mortality and uterine infection in the pig. Reproduction 1974, 36, 353–361. [Google Scholar] [CrossRef]
- Chan, H.C.; Chen, H.; Ruan, Y.; Sun, T. Physiology and pathophysiology of the epithelial barrier of the female reproductive tract. In Biology and Regulation of Blood-Tissue Barriers, 1st ed.; Cheng, C.Y., Ed.; Springer: New York, NY, USA, 2013; pp. 193–217. [Google Scholar]
- López-Albors, O.; Llamas-López, P.J.; Ortuño, J.Á.; Latorre, R.; García-Vázquez, F.A. In vivo measurement of pH and CO2 levels in the uterus of sows through the estrous cycle and after insemination. Sci. Rep. 2021, 11, 3194. [Google Scholar] [CrossRef]
- Nichol, R.; Hunter, R.H.F.; Cooke, G.M. Oviduct fluid pH in intact and unilaterally ovariectomized pigs. Can. J. Physiol. Pharmacol. 1997, 75, 1069–1074. [Google Scholar] [CrossRef]
- Grahofer, A.; Björkman, S.; Peltoniemi, O. Diagnosis of endometritis and cystitis in sows: Use of biomarkers. J. Anim. Sci. 2020, 98, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Soo, S.; Alberto, R.; Timothy, J. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology 2004, 101, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Margraf, A.; Ludwig, N.; Zarbock, A.; Rossaint, J. Systemic inflammatory response syndrome after surgery: Mechanisms and protection. Anesth. Analg. 2020, 131, 1693–1707. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, S.; Zhou, X. pH sensing at the intersection of tissue homeostasis and inflammation. Trends Immunol. 2023, 44, 807–825. [Google Scholar] [CrossRef]
| Unfertilized Oocyte | Fertilized Oocyte | 2-Cell | 4-Cell | 8-Cell | Morula |
|---|---|---|---|---|---|
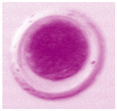 |  | 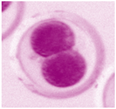 | 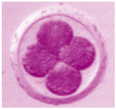 | 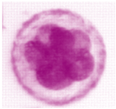 |  |
| Measured (cm) | Mean (SD) | Minimum | Maximum |
|---|---|---|---|
| Intact Uterine Horn | 97.00 (15.98) | 69.00 | 121.00 |
| Resection | |||
| Utero-tubal Junction—Anastomosis | 23.79 (4.14) | 18.00 | 29.50 |
| Anastomosis—Uterine Horn | 12.64 (2.39) | 8.50 | 14.50 |
| Total Length | 36.63 (3.49) | 32.00 | 41.00 |
| Uterine Body | 6.56 (2.09) | 3.50 | 10.00 |
| Cervix | 18.31 (5.04) | 8.50 | 25.00 |
| Vagina | |||
| Cranial | 19.63 (5.01) | 13.00 | 28.00 |
| Caudal | 12.44 (0.94) | 11.00 | 14.00 |
| Total Length | 32.06 (5.05) | 25.50 | 40.00 |
| Oviduct | |||
| Ipsilateral | 28.50 (0.82) | 25.50 | 32.00 |
| Contralateral | 27.88 (1.69) | 21.00 | 35.50 |
| Unfertilized Oocyte | Fertilized Oocyte | 2–4 Cell | 4–8 Cell | Morula | Total |
|---|---|---|---|---|---|
| 18 | 4 | 1 | 37 | 30 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ewasiuk, M.E.; Uwiera, R.R.E.; Zak, L.J.; Grindflek, E.; Dyck, M.K. Evaluation of an Ipsilateral Uterine Horn Resection and Ovariectomy Surgical Model in Gilts for Embryo Collection. Animals 2025, 15, 2366. https://doi.org/10.3390/ani15162366
Ewasiuk ME, Uwiera RRE, Zak LJ, Grindflek E, Dyck MK. Evaluation of an Ipsilateral Uterine Horn Resection and Ovariectomy Surgical Model in Gilts for Embryo Collection. Animals. 2025; 15(16):2366. https://doi.org/10.3390/ani15162366
Chicago/Turabian StyleEwasiuk, Mikayla E., Richard R. E. Uwiera, Louisa J. Zak, Eli Grindflek, and Michael K. Dyck. 2025. "Evaluation of an Ipsilateral Uterine Horn Resection and Ovariectomy Surgical Model in Gilts for Embryo Collection" Animals 15, no. 16: 2366. https://doi.org/10.3390/ani15162366
APA StyleEwasiuk, M. E., Uwiera, R. R. E., Zak, L. J., Grindflek, E., & Dyck, M. K. (2025). Evaluation of an Ipsilateral Uterine Horn Resection and Ovariectomy Surgical Model in Gilts for Embryo Collection. Animals, 15(16), 2366. https://doi.org/10.3390/ani15162366





