Prevalence and Seroprevalence of Infectious Bronchitis Virus and Infectious Laryngotracheitis Virus in Backyard Poultry in Central Chile
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
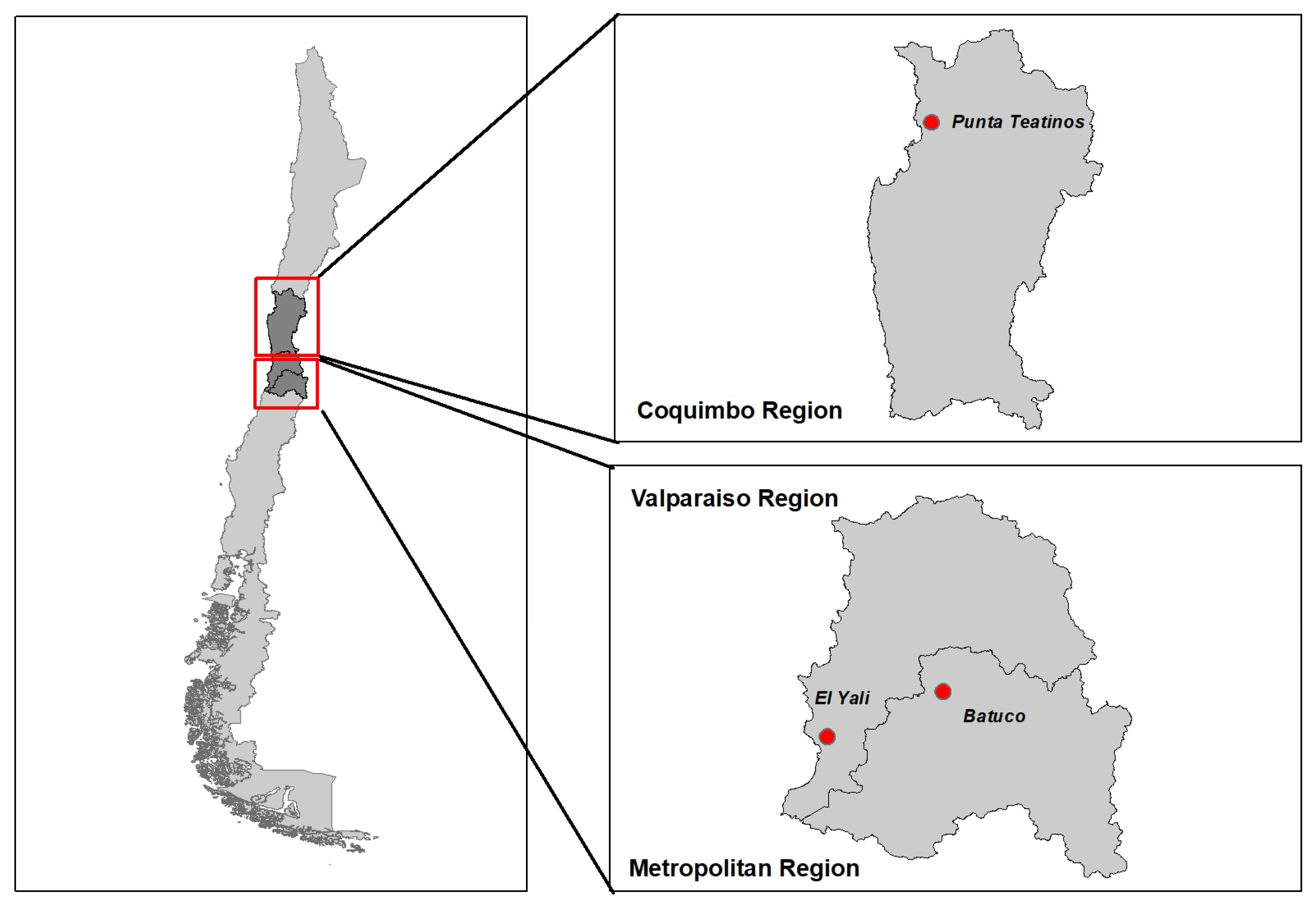
2.1. Study Area and Study Design
2.2. Laboratory Analysis
2.3. Data Analysis
3. Results
3.1. Serological Surveillance
3.2. Molecular Surveillance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPS | backyard production system |
| IBV | infectious bronchitis virus |
| ILTV | infectious laryngotracheitis virus |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| qPCR | Quantitative Polymerase Chain Reaction |
| CI | confidence interval |
| COVID-19 | Coronavirus Disease 2019 |
References
- Mottet, A.; Tempio, G. Global poultry production: Current state and future outlook and challenges. World’s Poult. Sci. J. 2017, 73, 245–256. [Google Scholar] [CrossRef]
- OECD/FAO. OECD-FAO Agricultural Outlook 2021–2030; OECD Publishing: Paris, France, 2021. [Google Scholar]
- Sonaiya, E.; Swan, S. Small-Scale Poultry Production, Technical Guide Manual; FAO Animal Production and Health: Rome, Italy, 2004; Volume 1. [Google Scholar]
- Alders, R.G.; Campbell, A.; Costa, R.; Guèye, E.F.; Ahasanul Hoque, M.; Perezgrovas-Garza, R.; Rota, A.; Wingett, K. Livestock across the world: Diverse animal species with complex roles in human societies and ecosystem services. Anim. Front. 2021, 11, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Reardon, T.; Kennedy, A.; DeFries, R.S.; Koo, J.; Minten, B.; Takeshima, H.; Thornton, P. The future of small farms: Innovations for inclusive transformation. In Science and Innovations for Food Systems Transformation; Springer International Publishing: Cham, Swizerland, 2023; pp. 191–205. [Google Scholar]
- von Braun, J.; Afsana, K.; Fresco, L.O.; Hassan, M.H.A. Science and Innovations for Food Systems Transformation; Springer Nature: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Di Pillo, F.; Anríquez, G.; Alarcón, P.; Jimenez-Bluhm, P.; Galdames, P.; Nieto, V.; Schultz-Cherry, S.; Hamilton-West, C. Backyard poultry production in Chile: Animal health management and contribution to food access in an upper middle-income country. Prev. Vet. Med. 2019, 164, 41–48. [Google Scholar] [CrossRef]
- Hamilton-West, C.; Rojas, H.; Pinto, J.; Orozco, J.; Hervé-Claude, L.; Urcelay, S. Characterization of backyard poultry production systems and disease risk in the central zone of Chile. Res. Vet. Sci. 2012, 93, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Baumberger, C.; Di Pillo, F.; Galdames, P.; Oyarzun, C.; Marambio, V.; Jimenez-Bluhm, P.; Hamilton-West, C. Swine Backyard Production Systems in Central Chile: Characterizing Farm Structure, Animal Management, and Production Value Chain. Animals 2023, 13, 2000. [Google Scholar] [CrossRef]
- Jimenez-Bluhm, P.; Di Pillo, F.; Bahl, J.; Osorio, J.; Schultz-Cherry, S.; Hamilton-West, C. Circulation of influenza in backyard productive systems in central Chile and evidence of spillover from wild birds. Prev. Vet. Med. 2018, 153, 1–6. [Google Scholar] [CrossRef]
- Bravo-Vasquez, N.; Baumberger, C.; Jimenez-Bluhm, P.; Di Pillo, F.; Lazo, A.; Sanhueza, J.; Schultz-Cherry, S.; Hamilton-West, C. Risk factors and spatial relative risk assessment for influenza A virus in poultry and swine in backyard production systems of central Chile. Vet. Med. Sci. 2020, 6, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Vasquez, N.; Di Pillo, F.; Lazo, A.; Jiménez-Bluhm, P.; Schultz-Cherry, S.; Hamilton-West, C. Presence of influenza viruses in backyard poultry and swine in El Yali wetland, Chile. Prev. Vet. Med. 2016, 134, 211–215. [Google Scholar] [CrossRef]
- Ayala, A.J.; Yabsley, M.J.; Hernandez, S.M. A review of pathogen transmission at the backyard chicken–wild bird interface. Front. Vet. Sci. 2020, 7, 539925. [Google Scholar] [CrossRef]
- Jones, P.; Niemi, J.; Christensen, J.-P.; Tranter, R.; Bennett, R. A review of the financial impact of production diseases in poultry production systems. Anim. Prod. Sci. 2018, 59, 1585–1597. [Google Scholar] [CrossRef]
- Di Pillo, F.; Jimenez-Bluhm, P.; Baumberger, C.; Marambio, V.; Galdames, P.; Monti, G.; Schultz-Cherry, S.; Hamilton-West, C. Movement Restriction and Increased Surveillance as Efficient Measures to Control the Spread of Highly Pathogenic Avian Influenza in Backyard Productive Systems in Central Chile. Front. Vet. Sci. 2020, 7, 424. [Google Scholar] [CrossRef] [PubMed]
- Chaves Hernández, A. Poultry and avian diseases. In Encyclopedia of Agriculture and Food Systems; Elsevier: Amsterdam, The Netherlands, 2014; pp. 504–520. [Google Scholar]
- Laamiri, N.; Fällgren, P.; Zohari, S.; Ben Ali, J.; Ghram, A.; Leijon, M.; Hmila, I. Accurate detection of avian respiratory viruses by use of multiplex PCR-based luminex suspension microarray assay. J. Clin. Microbiol. 2016, 54, 2716–2725. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, H. Infectious laryngotracheitis: A review. Braz. J. Poult. Sci. 2003, 5, 157–168. [Google Scholar] [CrossRef]
- Ou, S.-C.; Giambrone, J.J. Infectious laryngotracheitis virus in chickens. World J. Virol. 2012, 1, 142. [Google Scholar] [CrossRef]
- Agunos, A.; Pierson, F.W.; Lungu, B.; Dunn, P.A.; Tablante, N. Review of nonfoodborne zoonotic and potentially zoonotic poultry diseases. Avian Dis. 2016, 60, 553–575. [Google Scholar] [CrossRef]
- Goraya, M.U.; Ali, L.; Younis, I. Innate immune responses against avian respiratory viruses. Hosts Viruses 2017, 4, 78. [Google Scholar] [CrossRef]
- Brown Jordan, A.; Gongora, V.; Hartley, D.; Oura, C. A review of eight high-priority, economically important viral pathogens of poultry within the Caribbean region. Vet. Sci. 2018, 5, 14. [Google Scholar] [CrossRef]
- Seifi, S.; Asasi, K.; Mohammadi, A. Natural co-infection caused by avian influenza H9 subtype and infectious bronchitis viruses in broiler chicken farms. Vet. Arh. 2010, 80, 269–281. [Google Scholar]
- Abdo, W.; Magouz, A.; El-Khayat, F.; Kamal, T. Acute Outbreak of Co-Infection of Fowl Pox and Infectious Laryngotracheitis Viruses in Chicken in Egypt. Pak. Vet. J. 2017, 37, 321–325. [Google Scholar]
- ChileCarne. La Industria en Cifras. Resumen de Cifras. 2021. Available online: https://www.chilecarne.cl/la-industria-en-cifras/ (accessed on 22 August 2023).
- INE. Censo Agropecuario y Forestal; Instituto Nacional de Estadística: Santiago, Chile, 2021. Available online: https://www.ine.gob.cl/censoagropecuario (accessed on 11 August 2025).
- Cubillos, A.; Ulloa, J.; Cubillos, V.; Cook, J.K. Characterisation of strains of infectious bronchitis virus isolated in Chile. Avian Pathol. 1991, 20, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.C.; McFarlane, R.; Ulloa, J. Detection and characterization of infectious bronchitis virus in Chile by RT-PCR and sequence analysis. Arch. Med. Vet. 2006, 38, 175–178. [Google Scholar]
- Guzmán, M.; Sáenz, L.; Hidalgo, H. Molecular and Antigenic Characterization of GI-13 and GI-16 Avian Infectious Bronchitis Virus Isolated in Chile from 2009 to 2017 Regarding 4/91 Vaccine Introduction. Animals 2019, 9, 656. [Google Scholar] [CrossRef] [PubMed]
- Verdugo, C.; Pinto, A.; Ariyama, N.; Moroni, M.; Hernandez, C. Molecular identification of avian viruses in neotropic cormorants (Phalacrocorax brasilianus) in Chile. J. Wildl. Dis. 2019, 55, 105–112. [Google Scholar] [CrossRef]
- Gatica, T.; Salgado, S.; Reyes, H.; Loncoman, C. Development of a qPCR Tool for Detection, Quantification, and Molecular Characterization of Infectious Laryngotracheitis Virus Variants in Chile from 2019 to 2023. Animals 2025, 15, 1623. [Google Scholar] [CrossRef]
- Di Castri, F.; Hajek, E.R. Bioclimatología de Chile; Universidad Católica de Chile: Santiago, Chile, 1976. [Google Scholar]
- Aguayo, M.; Pauchard, A.; Azócar, G.; Parra, O. Cambio del uso del suelo en el centro sur de Chile a fines del siglo XX: Entendiendo la dinámica espacial y temporal del paisaje. Rev. Chil. Hist. Nat. 2009, 82, 361–374. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.; Gonzalez, L.A. InfoStat. [Programa de Cómputo] 2011; Versión 24-03-2011. Available online: http://www.infostat.com.ar/ (accessed on 6 August 2025).
- Baumberger, C.; Anríquez, G.; Galdames, P.; Palma, T.; Gonzalez, M.A.; Orozco, K.; Oyarzun, C.; Rojas, C.; Marambio, V.; Ruiz, S. Exposure Practices to Animal-Origin Influenza A Virus at the Animal–Human Interface in Poultry and Swine Backyard Farms. Zoonoses Public Health 2025, 72, 42–54. [Google Scholar] [CrossRef]
- Conan, A.; Goutard, F.L.; Sorn, S.; Vong, S. Biosecurity measures for backyard poultry in developing countries: A systematic review. BMC Vet. Res. 2012, 8, 240. [Google Scholar] [CrossRef]
- Iqbal, M. Controlling avian influenza infections: The challenge of the backyard poultry. J. Mol. Genet. Med. Int. J. Biomed. Res. 2009, 3, 119. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.A.; Amin, Z.; Rodrigues, K.F.; Saallah, S.; Shaarani, S.M.; Sarker, S.; Siddiquee, S. Infectious bronchitis virus (gammacoronavirus) in poultry farming: Vaccination, immune response and measures for mitigation. Vet. Sci. 2021, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Ruiz, E.; Ramirez-Cruz, G.; Camara Gamboa, E.; Alexander, D.; Gough, R. A serological survey for avian infectious bronchitis virus and Newcastle disease virus antibodies in backyard (free-range) village chickens in Mexico. Trop. Anim. Health Prod. 2000, 32, 381–390. [Google Scholar] [CrossRef]
- Pohjola, L.; Tammiranta, N.; Ek-Kommonen, C.; Soveri, T.; Hänninen, M.-L.; Fredriksson Ahomaa, M.; Huovilainen, A. A survey for selected avian viral pathogens in backyard chicken farms in Finland. Avian Pathol. 2017, 46, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q.; Zhuang, Q.-Y.; Wang, K.-C.; Liu, S.; Shao, J.-Z.; Jiang, W.-M.; Hou, G.-Y.; Li, J.-P.; Yu, J.-M.; Li, Y.-P. Identification and survey of a novel avian coronavirus in ducks. PLoS ONE 2013, 8, e72918. [Google Scholar] [CrossRef]
- Hughes, L.A.; Savage, C.; Naylor, C.; Bennett, M.; Chantrey, J.; Jones, R. Genetically diverse coronaviruses in wild bird populations of northern England. Emerg. Infect. Dis. 2009, 15, 1091. [Google Scholar] [CrossRef]
- Woo, P.; Lau, S.; Lam, C.; Lau, C.; Tsang, A.; Lau, J.; Bai, R.; Teng, J.; Tsang, C.; Wang, M. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar] [CrossRef] [PubMed]
- Miłek, J.; Blicharz-Domańska, K. Coronaviruses in avian species–review with focus on epidemiology and diagnosis in wild birds. J. Vet. Res. 2018, 62, 249. [Google Scholar] [CrossRef] [PubMed]
| Study Area | Pathogen | Seroprevalence (n (%)) | Total BPSs (n) | Seroprevalence 95% CI (%) |
|---|---|---|---|---|
| Batuco | IBV | 27 (93.1) | 29 | 83.9–100.0 |
| ILTV | 26 (89.7) | 29 | 78.6–100.0 | |
| El Yali | IBV | 30 (100.0) | 30 | 88.4–100.0 |
| ILTV | 25 (83.3) | 30 | 70.0–96.7 | |
| Punta Teatinos | IBV | 27 (93.1) | 29 | 83.9–100.0 |
| ILTV | 24 (82.8) | 29 | 69.0–96.5 |
| Pathogen | Sampling Season | Prevalence (n (%)) | Total BPSs (n) | Prevalence 95% CI (%) |
|---|---|---|---|---|
| IBV | 2021 | 3 (27.3) | 11 | 1.0–53.6 |
| ILTV | 2021 | 8 (72.7) | 11 | 46.4–99.9 |
| IBV | 2024 | 1 (5.0) | 20 | 0.0–14.6 |
| ILTV | 2024 | 5 (25.0) | 20 | 6.0–44.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumberger, C.; Di Pillo, F.; Tapia, D.; Coloma, C.; Orozco, K.; Galdames, P.; Oyarzun, C.; Gárate, D.; Torreblanca, C.; Ruiz, S.; et al. Prevalence and Seroprevalence of Infectious Bronchitis Virus and Infectious Laryngotracheitis Virus in Backyard Poultry in Central Chile. Animals 2025, 15, 2364. https://doi.org/10.3390/ani15162364
Baumberger C, Di Pillo F, Tapia D, Coloma C, Orozco K, Galdames P, Oyarzun C, Gárate D, Torreblanca C, Ruiz S, et al. Prevalence and Seroprevalence of Infectious Bronchitis Virus and Infectious Laryngotracheitis Virus in Backyard Poultry in Central Chile. Animals. 2025; 15(16):2364. https://doi.org/10.3390/ani15162364
Chicago/Turabian StyleBaumberger, Cecilia, Francisca Di Pillo, David Tapia, Claudio Coloma, Katherinne Orozco, Pablo Galdames, Cristobal Oyarzun, Diego Gárate, Camila Torreblanca, Soledad Ruiz, and et al. 2025. "Prevalence and Seroprevalence of Infectious Bronchitis Virus and Infectious Laryngotracheitis Virus in Backyard Poultry in Central Chile" Animals 15, no. 16: 2364. https://doi.org/10.3390/ani15162364
APA StyleBaumberger, C., Di Pillo, F., Tapia, D., Coloma, C., Orozco, K., Galdames, P., Oyarzun, C., Gárate, D., Torreblanca, C., Ruiz, S., Jimenez-Bluhm, P., & Hamilton-West, C. (2025). Prevalence and Seroprevalence of Infectious Bronchitis Virus and Infectious Laryngotracheitis Virus in Backyard Poultry in Central Chile. Animals, 15(16), 2364. https://doi.org/10.3390/ani15162364






