Analgesic and Gastrointestinal Effects of Methadone in Horses Undergoing Orchiectomy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
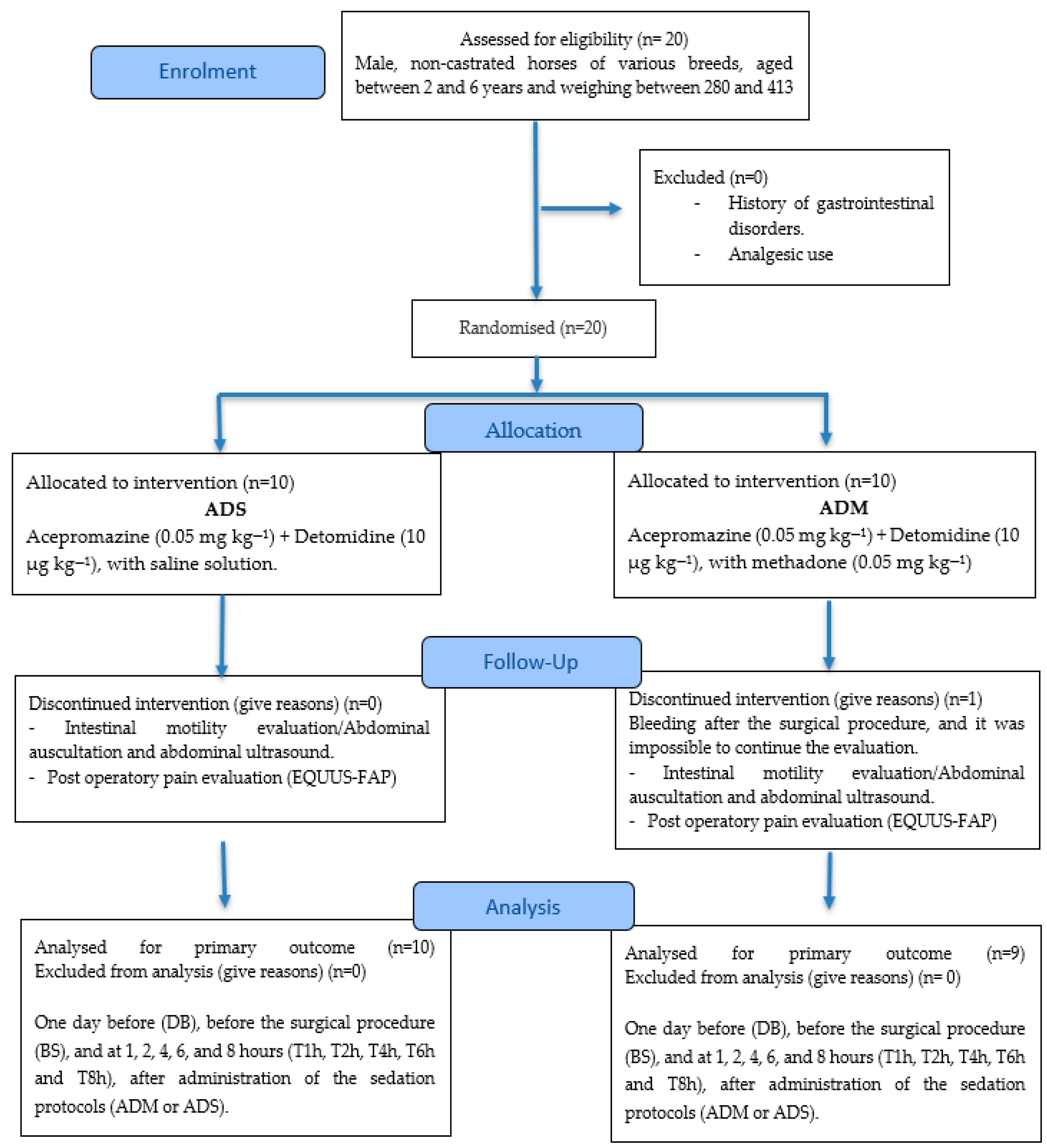
2.1. Animals and Experimental Design
2.2. Surgical Procedure and Sedation Protocols
2.3. Physiological Parameters
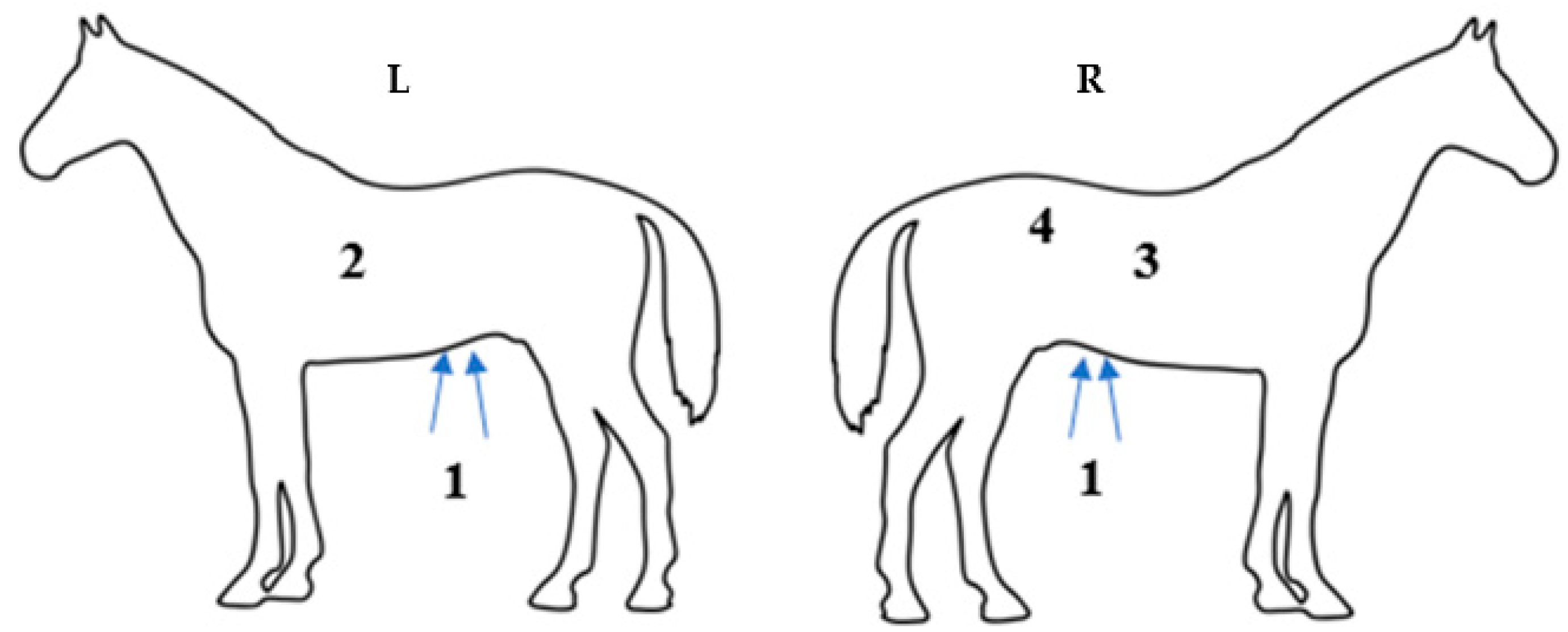
2.4. Intestinal Motility Evaluation/Abdominal Auscultation
2.5. Intestinal Motility Evaluation/Abdominal Ultrasound Evaluation
2.6. Postoperatory Pain Evaluation
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lorena, S.E.R.S.; Luna, S.P.L.; Lascelles, B.D.X.; Corrente, J.E. Attitude of Brazilian veterinarians in the recognition and treatment of pain in horses and cattle. Vet. Anaesth. Analg. 2013, 40, 410–418. [Google Scholar] [CrossRef]
- Cardona, D.; Hernández, D. Evaluación y tratamiento polimodal del dolor musculo esquelético y abdominal en equinos. Rev. Sist. Prod. Agroecol. 2020, 11, 67–100. [Google Scholar] [CrossRef]
- Nannarone, S.; Giannettoni, G.; Laurenza, G.; Giontella, A.; Moretti, G. Methadone or Butorphanol as Pre-Anaesthetic Agents Combined with Romifidine in Horses Undergoing Elective Surgery: Qualitative Assessment of Sedation and Induction. Animals 2021, 11, 2572. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.; Luna, S.; Rosa, A.; Quarterone, C.; Crosignani, N.; Taylor, P.M.; Pantoja, J.C.; Puoli, J.N. Antinociceptive effects of methadone combined with detomidine or acepromazine in horses. Equine Vet. J. 2016, 48, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Clutton, R.E. Opioid Analgesia in Horses. Vet. Clin. N. Am. Equine Pract. 2010, 26, 493–514. [Google Scholar] [CrossRef]
- Gozalo-Marcilla, M.; Luna, S.P.; Crosignani, N.; Filho, J.N.P.; Possebon, F.S.; Pelligand, L.; Taylor, P.M. Sedative and antinociceptive effects of different combinations of detomidine and methadone in standing horses. Vet. Anaesth. Analg. 2017, 44, 1116–1127. [Google Scholar] [CrossRef]
- Tessier, C.; Pitaud, J.P.; Thorin, C.; Touzot-Jourde, G. Systemic morphine administration causes gastric distention and hyperphagia in healthy horses. Equine Vet. J. 2019, 51, 653–657. [Google Scholar] [CrossRef]
- de Oliveira, F.; Pignaton, W.; Teixeira, F.; Queiroz-Neto, A.; Puoli-Filho, J.N.P.; Scognamillo, M.V.R.; Viveiros, B.M.; Luna, S.P.L. Antinociceptive and Behavioral Effects of Methadone Alone or in Combination with Detomidine in Conscious Horses. J. Equine Vet. Sci. 2014, 34, 380–386. [Google Scholar] [CrossRef]
- Freeman, S.L.; England, G.C.W. Effect of romifidine on gastrointestinal motility, assessed by transrectal ultrasonography. Equine Vet. J. 2001, 33, 570–576. [Google Scholar] [CrossRef]
- Boscan, P.; Van-Hoogmoed, L.M.; Farver, T.B.; Snyder, J.R. Evaluation of the effects of the opioid agonist morphine on gastrointestinal tract function in horses. Am. J. Vet. Res. 2006, 67, 992–997. [Google Scholar] [CrossRef]
- Valverde, A. Alpha-2 agonists as pain therapy in horses. Vet. Clin. N. Am. Equine Pract. 2010, 26, 515–532. [Google Scholar] [CrossRef]
- Gozalo-Marcilla, M.; Luna, S.P.L.; Moreira da Silva, R.; Crosignani, N.; Lopes, N.P.; Taylor, P.M.; Pelligand, L. Characterisation of the in vivo interactions between detomidine and methadone in horses: Pharmacokinetic and pharmacodynamic modelling. Equine Vet. J. 2019, 51, 517–529. [Google Scholar] [CrossRef]
- Fernandes, L.; De Souza, A.; Dominghette, A.; Pelegrini, G.; Carvalho, T.H.; Moreira, V.J. Orquiectomia em Equinos: Técnicas Cirúrgicas e suas Complicações. Braz. J. Dev. 2021, 7, 110097–110106. [Google Scholar] [CrossRef]
- Sasaki, N.; Murata, A.; Lee, I.; Yamada, H. Evaluation of equine cecal motility by auscultation, ultrasonography and electrointestinography after jejunocecostomy. Res. Vet. Sci. 2008, 84, 305–310. [Google Scholar] [CrossRef]
- Goma, N.; Uhlig, A.; Schusser, G.F. Effect of Buscopan® compositum on the motility of the duodenum, cecum and left ventral colon in healthy conscious horses. Berl. Munch. Tierarztl. Wochenschr. 2011, 124, 168–174. [Google Scholar]
- Vanderbroek, A.R.; Reef, V.B.; Aitken, M.R.; Stefanovski, D.; Southwood, L.L. Assessing gastrointestinal motility in healthy horses comparing auscultation, ultrasonography and an acoustic gastrointestinal surveillance biosensor: A randomised, blinded, controlled crossover proof of principle study. Equine Vet. J. 2019, 51, 246–251. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M.; El-ashker, M.R. Reference Values and Repeatability of Transabdominal Ultrasonographic Gastrointestinal Tract Thickness and Motility in Healthy Donkeys (Equus asinus). J. Equine Vet. Sci. 2020, 2, 103–153. [Google Scholar] [CrossRef]
- Van Loon, P.A.M.; Van Dierendonck, M.C. Monitoring acute equine visceral pain with the Equine Utrecht University Scale for Composite Pain Assessment (EQUUS COMPASS) and the Equine Utrecht University Scale for Facial Assessment of Pain (EQUUS FAP): A scale-construction study. Vet. J. 2015, 206, 356–364. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Bagatini, A.; Gomes, C.R.; Zanettini, M.; Rezer, G. Dexmedetomidina: Farmacologia e uso clínico. Braz. J. Anesthesiol. 2002, 52, 606–617. [Google Scholar] [CrossRef]
- Fantoni, D.T.; Futema, F.; Cortopassi, S.; Lopes, L.C.; Mirandola, R.; Ferreira, M.A. Avaliação comparativa entre a acepromazina, detomidina e romifidina em eqüinos. Cienc. Rural 1999, 29, 45–50. [Google Scholar] [CrossRef]
- Yamashita, K.; Tsubakishita, S.; Futaok, S.; Ueda, I.; Hamaguchi, H.; Seno, T.; Katoh, S.; Izumisawa, Y.; Kotani, T.; Muir, W.W. Cardiovascular effects of medetomidine, detomidine, and xylazine in horses. J. Vet. Med. Sci. 2000, 62, 1025–1032. [Google Scholar] [CrossRef]
- Castro, M.L.; Silva, R.V.; Silva, A.K.; Wenceslau, R.R.; Beier, S.L.; Fagundes, N.; Pimenta, E.L.M.; Lima, J.T.B.; Winter, I.C.; Palhares, M.S. Sedative and cardiorespiratory effects of detomidine combined or not with diazepam in horses subjected to dental examination. Res. Soc. Dev. 2023, 12, e12912338283. [Google Scholar] [CrossRef]
- Love, E.J.; Taylor, P.M.; Clark, C. Analgesic effect of butorphanol in ponies following castration. Equine Vet. J. 2009, 41, 552–556. [Google Scholar] [CrossRef]
- Dalla-Costa, E.; Minero, M.; Lebelt, D.; Stucke, D.; Canali, E.; Leach, M.E. Development of the horse grimace scale (HGS) as a pain assessment tool in horses undergoing routine castration. PLoS ONE 2014, 9, e92281. [Google Scholar] [CrossRef]
- Van Dierendonck, M.C.; Van Loon, J.P.A.M. Monitoring acute equine visceral pain with the Equine Utrecht University Scale for Composite Pain Assessment (EQUUS COMPASS) and the Equine Utrecht University Scale for Facial Assessment of Pain (EQUUS FAP): A validation study. Vet. J. 2016, 216, 175–177. [Google Scholar] [CrossRef]
- Van Loon, J.P.A.M.; Van Dierendonck, M.C. Monitoring equine head-related pain with the Equine Utrecht University scale for facial assessment of pain (EQUUS-FAP). Vet. J. 2017, 220, 88–90. [Google Scholar] [CrossRef]
- Haunhorst, F.R.; Hopster, K.; Schmicke, M.; Bienert-Zeit, A.; Kästner, S. Clinical effect of buprenorphine or butorphanol, in combination with detomidine and diazepam, on sedation and postoperative pain after cheek tooth extraction in horses. Can. Vet. J. 2022, 63, 39–46. [Google Scholar]
- Sanz, M.G.; Sellon, D.C.; Cary, J.A.; Hines, M.T.; Farnsworth, K.D. Analgesic effects of butorphanol tartrate and phenylbutazone administered alone and in combination in young horses undergoing routine castration. J. Am. Vet. Med. Assoc. 2009, 235, 1194–1203. [Google Scholar] [CrossRef]
- Gozalo-Marcilla, M.; Rodrigues de Oliveira, A.; Werneck-Fonseca, M.; Possebon, F.S.; Pelligand, L.; Taylor, P.M.; Luna, S.P.L. Sedative and antinociceptive effects of different detomidine constant rate infusions, with or without methadone in standing horses. Equine Vet. J. 2018, 51, 530–536. [Google Scholar] [CrossRef]
- Emanuel, D.; Kästner, S.B.R.; Delarocque, J.; Grob, A.J.; Bienert-Zeit, A. Influence of Butorphanol, Buprenorphine and Levomethadone on Sedation Quality and Postoperative Analgesia in Horses Undergoing Cheek Tooth Extraction. Vet. Sci. 2022, 9, 174. [Google Scholar] [CrossRef]
- Cohen, N.D.; Lester, G.D.; Sanchez, L.C.; Merritt, A.M.; Roussel, A.J., Jr. Evaluation of risk factors associated with development of postoperative ileus in horses. J. Am. Vet. Med. Assoc. 2004, 225, 1070–1078. [Google Scholar] [CrossRef]
- Mitchell, C.F.; Malone, E.D.; Sage, A.M.; Niksich, K. Evaluation of gastrointestinal activity patterns in healthy horses using B mode and Doppler ultrasonography. Can. Vet. J. 2005, 46, 134–140. [Google Scholar]
- Martin-Flores, M.; Campoy, L.; Kinsley, M.A.; Mohammed, H.O.; Gleed, R.D.; Cheetham, J. Analgesic and gastrointestinal effects of epidural morphine in horses after laparoscopic cryptorchidectomy under general anesthesia. Vet. Anaesth. Analg. 2014, 41, 430–437. [Google Scholar] [CrossRef]
- Merritt, A.M.; Burrow, J.A.; Hartless, C.S. Effect of xylazine, detomidine, and a combination of xylazine and butorphanol on equine duodenal motility. Am. J. Vet. Res. 1998, 59, 619–623. [Google Scholar] [CrossRef]
- Haralambus, R.; Juri, M.; Mokry, A.; Jenner, F. The impact of opioid administration on the incidence of post-anaesthetic colic in horses. Front. Pain Res. 2024, 5, 1347548. [Google Scholar] [CrossRef]
- Gosnell, B.A.; Lipton, J.M. Opioid peptide effects on feeding in rabbits. Peptides 1986, 7, 745–747. [Google Scholar] [CrossRef]
- Katsuura, Y.; Heckmann, J.A.; Taha, S.A. Mu-Opioid receptor stimulation in the nucleus accumbens elevates fatty tastant intake by increasing palatability and suppressing satiety signals. Am. J. Physiol. Regul. Integr. Compr. Physiol. 2011, 301, 244–254. [Google Scholar] [CrossRef]
- Epstein, K.L.; Hall, M.D. Effect of Nasogastric Tube Placement, Manipulation, and Fluid Administration on Transcutaneous Ultrasound Visualization and Assessment of Stomach Position in Healthy Unfed and Fed Horses. Animals 2022, 12, 3433. [Google Scholar] [CrossRef]
- Le Jeune, S.; Whitcomb, M.B. Ultrasound of the equine acute abdomen. Vet. Clin. N. Am. Equine Pract. 2014, 30, 353–381. [Google Scholar] [CrossRef]
| Parameter | Group | N | Time (T) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DB | BS | T1h | T2h | T4h | T6h | T8h | |||
| HR | ADM | 10 | 39 (6.9) Aa | 31 (4.0) Ac | 32 (5.5) Ac | 31 (3.8) Ac | 33 (5.2) Abc | 35 (3.6) Aab | 37 (4.5) Aa |
| ADS | 9 | 42 (15.0) Aa | 40 (12.1) Aabc | 34 (6.4) Ac | 34 (8.0) Ac | 36 (7.4) Abc | 42 (8.5) Abc | 42 (8.9) Aa | |
| fR | ADM | 10 | 24 (9.0) Aa | 18 (5.0) Ab | 14 (4.2) Ac | 13 (2.8) Ac | 13 (3.3) Ac | 12 (2.7) Ac | 13 (4.2) Ac |
| ADS | 9 | 21 (4.4) Aa | 17 (10.4) Ab | 9 (3.2) Ac | 8 (2.2) Ac | 12 (6.3) Abc | 15 (8.6) Abc | 15 (7.7) Abc | |
| RT | ADM | 10 | 37.8 (0.3) Ab | 37.2 (0.5) Ac | 36.4 (0.3) Ad | 36.4 (0.4) Ad | 37.4 (0.7) Ac | 38.0 (0.7) Aab | 38.2 (0.7) Aa |
| ADS | 9 | 37.7 (0.2) Ab | 37.4 (0.4) Ac | 36.7 (0.8) Ad | 36.7 (0.5) Ad | 37.2 (0.4) Ac | 37.9 (0.6) Aab | 38.3 (0.4) Aa | |
| CRT | ADM | 10 | 2 (0.0) Aa | 2 (0.0) Aa | 2 (0.0) Aa | 2 (0.3) Aa | 2 (0.4) Aa | 2 (0.0) Aa | 2 (0.0) Aa |
| ADS | 9 | 2 (0.0) Aa | 2 (0.0) Aa | 2 (0.0) Aa | 2 (0.0) Aa | 2 (0.0) Aa | 2 (0.3) Aa | 2 (0.0) Aa | |
| Parameter | Group | N | Time (T) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DB | BS | T1h | T2h | T4h | T6h | T8h | |||
| LDQA | ADM | 10 | 2 (2–2) Aab | 2 (2–2) Aabc | 1.5 (1–2) Ac | 2 (1–2) Abc | 2 (2–2.75) Aa | 2 (2–2) Aabc | 2 (2–2) Aabc |
| ADS | 9 | 2 (2–2) Aa | 2 (2–2) Aa | 1 (0–1) Bc | 1 (1–2) Ab | 2 (2–2) Ba | 2 (1–2) Aab | 2 (2–2) Aab | |
| LVQA | ADM | 10 | 2 (2–2) Ab | 2 (2–2) Ab | 1 (1–1.75) Ac | 2 (2–2) Ab | 3 (2–3) Aa | 2 (2–2) Ab | 2 (2–2) Ab |
| ADS | 9 | 2 (2–2) Aa | 2 (2–2) Aa | 1 (1–1) Ac | 1 (1–2) Bbc | 2 (2–2) Bab | 2 (2–2) Aa | 2 (2–2) Aa | |
| RDQA | ADM | 10 | 2 (2–2) Aa | 2 (2–2) Aa | 1 (1–1) Ab | 2 (2–2) Aa | 2 (2–2) Aa | 2 (2–2) Aa | 2 (2–2) Aa |
| ADS | 9 | 2 (2–2) Aa | 2 (2–2) Aa | 1 (0–1) Bb | 1 (1–2) Aa | 1 (1–2) Ba | 2 (2–2) Aa | 2 (2–2) Aa | |
| RVQA | ADM | 10 | 2 (2–2) Aa | 2 (2–2) Aa | 1 (1–2) Ab | 2 (2–2) Aab | 2 (2–2) Aa | 2 (2–2) Aa | 2 (2–2) Aa |
| ADS | 9 | 2 (2–2) Aa | 2 (2–2) Aa | 1 (1–1) Bb | 1 (1–2) Aab | 2 (1–2) Aa | 2 (1–2) Aa | 2 (2–2) Aa | |
| Parameter | Group | N | Time (T) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DB | BS | T1h | T2h | T4h | T6h | T8h | |||
| USRVC | ADM | 10 | 2 (2–2) Aa | 2 (2–2) Aa | 2 (2–2) Aa | 2 (1–2) Aa | 2 (2–2) Aa | 2 (2–2) Aa | 2 (2–2) Aa |
| ADS | 9 | 2 (2–3) Aa | 2 (1–2) Aa | 2 (2–2) Aa | 2 (2–3) Aa | 2 (2–2) Aa | 1 (1–2) Ba | 2 (2–2) Aa | |
| USLVC | ADM | 10 | 2 (2–2) Aa | 2 (2–2) Aa | 2 (1–2) Aa | 2 (1.25–2) Aa | 2 (2–2) Aa | 2 (2–2) Aa | 2 (2–2) Aa |
| ADS | 9 | 2 (2–3) Aa | 2 (1–2) Aab | 1 (1–2) Ab | 2 (1–2) Aab | 2 (1–2) Aab | 2 (2–2) Aab | 2 (2–2) Aab | |
| USCEC | ADM | 10 | 2.5 (2–3) Aa | 2 (2–2) Aab | 1 (1–1.75) Ab | 2 (2–2.75) Aab | 2.5 (2–3) Aab | 2 (2–3) Aa | 2 (2–2) Aab |
| ADS | 9 | 2 (1–3) Aa | 2 (1–2) Aa | 1 (1–2) Aa | 2 (1–2) Aa | 2 (1–2) Aa | 1 (1–2) Ba | 2 (2–3) Aa | |
| USDOU | ADM | 10 | 2.5 (2–3) Aa | 3 (2–3) Aa | 2 (2–2) Aa | 2 (2–2) Aa | 2.5 (2–3) Aa | 2 (2–3.5) Aa | 3 (2.25–3) Aa |
| ADS | 9 | 3 (3–3) Aa | 3 (2–3) Aa | 2 (2–3) Aa | 2 (2–3) Aa | 2 (2–3) Aa | 3 (2–3) Aa | 2 (2–3) Aa | |
| Time (T) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Group | N | DB | BS | T1h | T2h | T4h | T6h | T8h |
| IC ST | ADM | 10 | 12.5 (12–14) Ab | 10 (9.25–11) Ac | 10 (10–10.75) Ac | 10 (10–10) Ac | 13 (11.25–13.75) Ab | 13 (12–14) Ab | 15 (13–15) Aa |
| IC ST | ADS | 9 | 10 (10–11) Bab | 9 (8–10) Ac | 9 (8–11) Abc | 9 (8–10) Ac | 10 (9–11) Babc | 11 (10–13) Ba | 11 (10–13) Ba |
| Parameter | Group | N | Time | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DB | BS | T1h | T2h | T4h | T6h | T8H | |||
| EQUUS-FAP | ADM | 10 | 0 (0–0) Abc | 0 (0–0) Ac | 2 (2–3) Aa | 1 (0–2) Aa | 0 (0–0) Ac | 0 (0–0) Ac | 0 (0–0) Ac |
| ADS | 9 | 0 (0–0) Ad | 0 (0–1) Acd | 3 (1–4) Aa | 3 (3–4) Ba | 1 (1–2) Bab | 1 (0–2) Bbc | 1 (0–2) Abcd | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado Moreno, N.; Alves Moreira, J.; Araujo De Oliveira, L.; Sanches Gontijo, A.; Castilho Baldi, M.L.; Rocha Wenceslau, R.; Beier, S.L. Analgesic and Gastrointestinal Effects of Methadone in Horses Undergoing Orchiectomy. Animals 2025, 15, 2358. https://doi.org/10.3390/ani15162358
Maldonado Moreno N, Alves Moreira J, Araujo De Oliveira L, Sanches Gontijo A, Castilho Baldi ML, Rocha Wenceslau R, Beier SL. Analgesic and Gastrointestinal Effects of Methadone in Horses Undergoing Orchiectomy. Animals. 2025; 15(16):2358. https://doi.org/10.3390/ani15162358
Chicago/Turabian StyleMaldonado Moreno, Natalya, Júlia Alves Moreira, Luiza Araujo De Oliveira, Amaranta Sanches Gontijo, Maria Luiza Castilho Baldi, Raphael Rocha Wenceslau, and Suzane Lilian Beier. 2025. "Analgesic and Gastrointestinal Effects of Methadone in Horses Undergoing Orchiectomy" Animals 15, no. 16: 2358. https://doi.org/10.3390/ani15162358
APA StyleMaldonado Moreno, N., Alves Moreira, J., Araujo De Oliveira, L., Sanches Gontijo, A., Castilho Baldi, M. L., Rocha Wenceslau, R., & Beier, S. L. (2025). Analgesic and Gastrointestinal Effects of Methadone in Horses Undergoing Orchiectomy. Animals, 15(16), 2358. https://doi.org/10.3390/ani15162358







