Light Color Influences Incubation Characteristics, Postnatal Growth, and Stress Physiology with a Lack of Expression Changes of Myf5 and Myf6 Genes in Gerze Native Chicken
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Material
2.2. Incubation Conditions
2.3. Post-Hatch Procedures
2.4. Chick Start and Grow-Out Period
2.5. Slaughter and Muscle Sampling
2.6. Plasma Serotonin Evaluation
2.7. Plasma Corticosterone Evaluation
2.8. Total RNA Isolation
2.9. Complementary DNA (cDNA) Synthesis
2.10. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.11. Statistical Analysis
3. Results
3.1. Pre-Incubation Characteristics
3.2. Hatch Window and Incubation Efficiency
3.3. Chick Quality and Hatchability Parameters
3.4. Post-Hatch Growth Performance
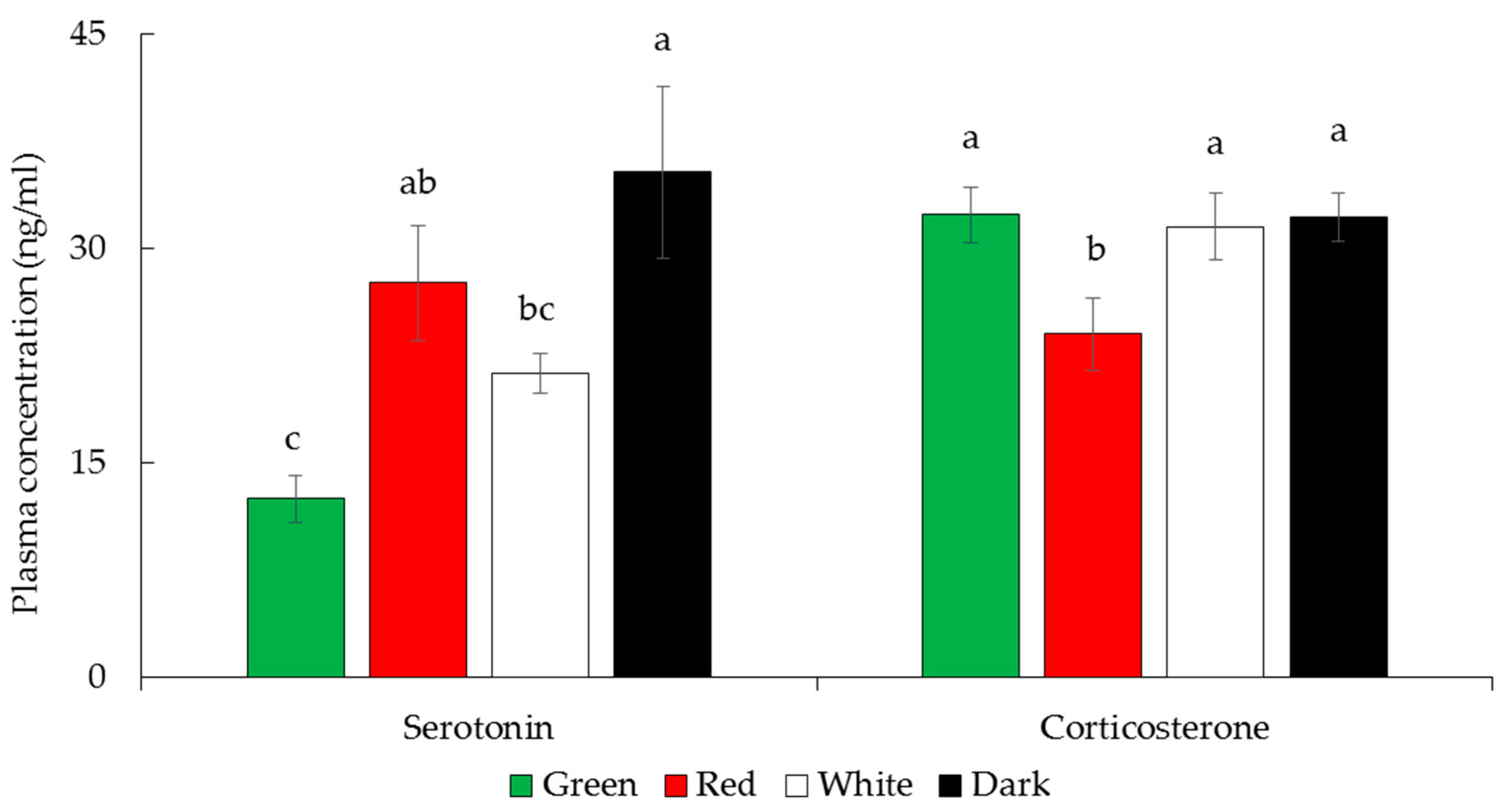
3.5. Plasma Corticosterone and Serotonin Hormone Concentrations
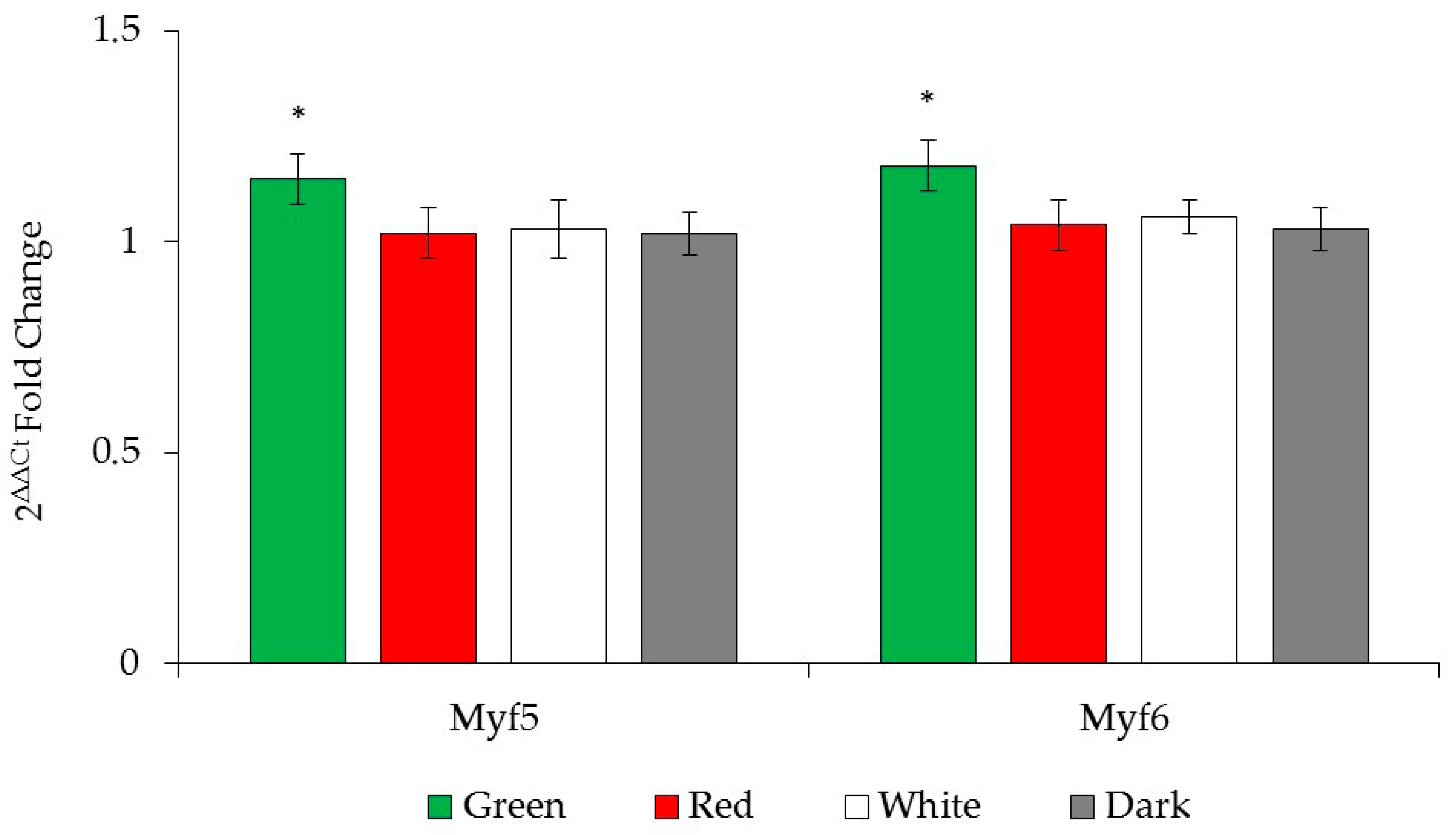
3.6. Myogenic Gene Expression
4. Discussion
4.1. Spectral Modulation of Embryonic Development
4.2. Impacts on Hatchability and Early Chick Vitality
4.3. Long-Term Growth Trajectories and Behavioral Implications
4.4. Molecular Regulation of Muscle Development
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noiva, R.M.; Menezes, A.C.; Peleteiro, M.C. Influence of temperature and humidity manipulation on chicken embryonic development. BMC Vet. Res. 2014, 10, 234. [Google Scholar] [CrossRef]
- Sampaio, S.A.; Oliveira, R.F.d.; Borges, K.F.; Gouveia, A.B.V.S.; Silva, J.M.S.d.; Santos, A.J.; Carrijo, M.S.; Santos, F.R.d.; Araújo Neto, F.R.d.; Gomide, A.P.C.; et al. Influence of monochromatic light during incubation on the production and metabolism of low-temperature broiler chicks. Animals 2024, 14, 1620. [Google Scholar] [CrossRef]
- Archer, G.S.; Mench, J.A. Natural incubation patterns and the effects of exposing eggs to light at various times during incubation on post-hatch fear and stress responses in broiler (meat) chickens. Appl. Anim. Behav. Sci. 2014, 152, 44–51. [Google Scholar] [CrossRef]
- Archer, G.S.; Mench, J.A. The effects of the duration and onset of light stimulation during incubation on the behavior, plasma melatonin levels, and productivity of broiler chickens. J. Anim. Sci. 2014, 92, 1753–1758. [Google Scholar] [CrossRef] [PubMed]
- Archer, G.S. Exposing broiler eggs to green, red, and white light during incubation. Animal 2017, 11, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Archer, G.S.; Mench, J.A. The effects of light stimulation during incubation on indicators of stress susceptibility in broilers. Poult. Sci. 2013, 92, 3103–3108. [Google Scholar] [CrossRef] [PubMed]
- Archer, G.S. Spectrum of white light during incubation: Warm vs. cool white LED lighting. Int. J. Poult. Sci. 2016, 15, 34–348. [Google Scholar]
- Dimond, S.J. Effects of photic stimulation before hatching on the development of fear in chicks. J. Comp. Physiol. Psychol. 1968, 65, 320–324. [Google Scholar] [CrossRef]
- Archer, G.S.; Mench, J.A. Exposing avian embryos to light affect posthatch anti-predator fear responses. Appl. Anim. Behav. Sci. 2016, 186, 80–84. [Google Scholar] [CrossRef]
- Cooper, J.B. Effect of light during incubation on hatchability of turkey eggs. Poult. Sci. 1972, 51, 1105–1108. [Google Scholar] [CrossRef]
- Shafey, T.M.; Al-Mohsen, T.H. Embryonic growth, hatching time and hatchability performance of meat breeder eggs incubated under continuous green light. Asian-Australas. J. Anim. Sci. 2002, 15, 1702–1707. [Google Scholar] [CrossRef]
- Shafey, T.M. Effect of lighted incubation on embryonic growth and hatchability performance of two strains of layer breeder eggs. Br. Poult. Sci. 2004, 45, 223–229. [Google Scholar] [CrossRef]
- Archer, G.S.; Jeffrey, D.; Tucker, Z. Effect of the combination of white and red LED lighting during incubation on layer, broiler, and Pekin duck hatchability. Poult. Sci. 2017, 96, 2670–2675. [Google Scholar] [CrossRef]
- Iwuchukwu, G.A.; Gökçek, D.; Özdemir, Z. Myogenic regulator genes responsible for muscle development in farm animals. BSJ Agric. 2024, 7, 418–428. [Google Scholar] [CrossRef]
- Hluchý, S.; Toman, R.; Cabaj, M.; Adamkovičová, M. The effect of white and monochromatic lights on chicken hatching. Sci. Pap. Anim. Sci. 2012, 45, 408–410. [Google Scholar]
- Tong, Q.; McGonnell, I.; Demmers, T.; Roulston, N.; Bergoug, H.; Romanini, C.; Verhelst, R.; Guinebretiere, M.; Eterradossi, N.; Berckmans, D. Effect of a photoperiodic green light programme during incubation on embryo development and hatch process. Animal 2018, 12, 765–773. [Google Scholar] [CrossRef]
- Huth, J.C.; Archer, G.S. Effects of LED lighting during incubation on layer and broiler hatchability, chick quality, stress susceptibility, and post-hatch growth. Poult. Sci. 2015, 94, 3052–3058. [Google Scholar] [CrossRef]
- Rozenboim, I.; Piestun, Y.; Mobarkey, N.; Barak, M.; Hoyzman, A.; Halevy, O. Monochromatic light stimuli during embryogenesis enhance embryo development and posthatch growth. Poult. Sci. 2004, 83, 1413–1419. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, X.D.; Wang, X.F.; Li, J.L.; Gao, F.; Zhou, G.H. Green light-emitting diodes light stimuli during incubation enhances posthatch growth without disrupting normal eye development of broiler embryos and hatchlings. Asian-Australas. J. Anim. Sci. 2016, 29, 1562–1568. [Google Scholar] [CrossRef]
- Rozenboim, I.; Huisinga, R.; Halevy, O.; El Halawani, M.E. Effect of embryonic photostimulation on the posthatch growth of turkey poults. Poult. Sci. 2003, 82, 1181–1187. [Google Scholar] [CrossRef]
- Halevy, O.; Piestun, Y.; Rozenboim, I.; Yablonka-Reuveni, Z. In ovo exposure to monochromatic green light promotes skeletal muscle cell proliferation and affects myofiber growth in posthatch chicks. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, 1062–1070. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.J.; Qiao, X.; Yue, H.Y.; Wu, S.G.; Yao, J.H.; Qi, G.H. Effect of monochromatic light stimuli during embryogenesis on muscular growth, chemical composition, and meat quality of breast muscle in male broilers. Poult. Sci. 2011, 91, 1026–1031. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.J.; Wang, J.; Wu, S.G.; Qiao, X.; Yue, H.Y.; Yao, J.H.; Qi, G.H. Stimulation with monochromatic green light during incubation alters satellite cell mitotic activity and gene expression in relation to embryonic and posthatch muscle growth of broiler chickens. Animal 2014, 8, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Şekeroğlu, A.; Özen, N. Gerze ve Denizli tavuk ırklarının bazı verim özellikleri bakımından karşılaştırılması. Akdeniz Üniv. Ziraat Fak. Derg. 1997, 10, 41–57. [Google Scholar]
- Aksoy, F.T.; Atasoy, F.; Onbaşılar, E.E.; Apaydın, S. The possibilities of sexing day-old Denizli fowl by means of feathering characteristics. Turk. J. Vet. Anim. Sci. 2002, 26, 567–575. [Google Scholar]
- Özdoğan, N.; Gürcan, İ.S.; Bilgen, A. Egg weight and egg weight repeatability of Denizli and Gerze local hen breeds. Livest. Stud. 2007, 47, 21–28. [Google Scholar]
- Boerjan, M. Chick vitality and uniformity. Int. Hatch. Pract. 2006, 20, 7–8. [Google Scholar]
- Nagy, B.; Bognár, Z.; Csabai, T.J.; Fekete, N.; Buzás, E.I.; Kovács, Á.F.; Szekeres-Barthó, J.; Pállinger, É. Effects of light exposure during IVF: Transcriptomic analysis of murine embryos and embryo-derived EVs. Front. Immunol. 2025, 16, 1429252. [Google Scholar] [CrossRef]
- Abdulateef, S.; Saed, Z.J.; Al-Bayar, M.A.; Mustafa, R.D.; Mohammed, N.A.; Shawkat, S.S.; Mohammed, T.T. Impact of the light colors on embryonic development, hatchability, chick quality in broiler chicken. Tikrit J. Agric. Sci. 2024, 24, 37–49. [Google Scholar] [CrossRef]
- Riedel, C.; Tzschentke, B. The effect of light during incubation on embryonic development and post-hatch behaviour, health and performance of domestic chicken. Lohmann Inf. 2023, 1–21. Available online: https://lohmann-breeders.com/lohmanninfo/the-effect-of-light-during-incubation-on-embryonic-development-and-post-hatch-behaviour-health-and-performance-of-domestic-chicken/ (accessed on 2 July 2025).
- Wang, P.; Sun, Y.; Fan, J.; Zong, Y.; Li, Y.; Shi, L.; Isa, A.M.; Wang, Y.; Ni, A.; Ge, P.; et al. Effects of monochromatic green light stimulation during embryogenesis on hatching and posthatch performance of four strains of layer breeder. Poult. Sci. 2020, 99, 5501–5508. [Google Scholar] [CrossRef]
- El-Sabrout, K.; Khalil, M. Effect of LED lighting on hatchability and chick performance of chicken eggs. Pak. J. Zool. 2017, 49, 2323–2325. [Google Scholar] [CrossRef]
- Rozenboim, I.; El Halawani, M.E.; Kashash, Y.; Piestun, Y.; Halevy, O. The effect of monochromatic photostimulation on growth and development of broiler birds. Gen. Comp. Endocrinol. 2013, 190, 214–219. [Google Scholar] [CrossRef]
- Drozdová, A.; Kaňková, Z.; Bilčík, B.; Zeman, M. Prenatal effects of red and blue light on physiological and behavioral parameters of broiler chickens. Czech J. Anim. Sci. 2021, 66, 412–419. [Google Scholar] [CrossRef]
- Ahmad, F.; Sharif, M.; Ashraf, M.; Riaz, M.; Shoaib, M.; Siddique, T.; Ahmed, A.; Sheir, A.H.; Yaseen, M.A.; Hamid, M.M.A. Effect of different light intensities and colors on growth performance and reproductive characteristics in Japanese quails. Turk. J. Vet. Anim. Sci. 2023, 47, 185–193. [Google Scholar] [CrossRef]
- Senaratna, D.; Samarakone, T.; Gunawardena, W. Red color light at different intensities affects the performance, behavioral activities and welfare of broilers. Anim. Biosci. 2016, 29, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.M.E.; Siddiqui, S.A.; Shehata, A.M.; Biswas, A.; Abougabal, M.S.; Kamal, A.M.; Mesalam, N.M.; Elsayed, M.A.; Yang, B.; Ebeid, T.A.; et al. Impact of light wavelength on growth and welfare of broiler chickens—Overview and future perspective. Ann.Anim. Sci. 2024, 24, 731–748. [Google Scholar] [CrossRef]
- Newberry, R.C.; Hunt, J.R.; Gardiner, E.E. Influence of light intensity on behavior and performance of broiler chickens. Poult. Sci. 1988, 67, 1020–1025. [Google Scholar] [CrossRef]
- Rault, J.L.; Clark, K.; Groves, P.J.; Cronin, G.M. Light intensity of 5 or 20 lux on broiler behavior, welfare, and productivity. Poult. Sci. 2017, 96, 779–787. [Google Scholar] [CrossRef]
- Abdulateef, S.M.; Al-Bayar, M.A.; Majid, A.A.; Shawkat, S.S.; Tatar, A.; Al-Ani, M.Q. Effect of exposure to different light colors on embryonic development and neurophysiological traits in the chick embryo. Vet. World 2021, 14, 1284–1289. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, H.; Zhou, S.; Han, Q.; Peng, R.; Jiang, X. Changes in embryonic development, juvenile growth and physiological adaptation of the cuttlefish Sepia pharaonis in response to photoperiod manipulation. J. Oceanol. Limnol. 2022, 40, 2012–2027. [Google Scholar] [CrossRef]
- Gnocchi, D.; Leoni, S.; Incerpi, S.; Bruscalupi, G. 3,5,3′-Triiodothyronine (T 3) stimulates cell proliferation through the activation of the PI3K/Akt pathway and reactive oxygen species (ROS) production in chick embryo hepatocytes. Steroids 2012, 77, 589–595. [Google Scholar] [CrossRef]
- Dishon, L.; Avital-Cohen, N.; Malamud, D.; Heiblum, R.; Druyan, S.; Porter, T.E.; Gumułka, M.; Rozenboim, I. In-ovo monochromatic green light photostimulation enhances embryonic somatotropic axis activity. Poult. Sci. 2017, 96, 1884–1890. [Google Scholar] [CrossRef]
- Buckingham, M.; Rigby, P.W.J. Gene regulatory networks and transcriptional mechanisms that control Myogenesis. Dev. Cell 2014, 28, 225–238. [Google Scholar] [CrossRef]
- Braun, T.; Gautel, M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat. Rev. Mol. Cell Biol. 2011, 12, 349–361. [Google Scholar] [CrossRef]


| Category | Criteria for Scoring |
|---|---|
| Reflex | Chicks take more than 2 s to return to normal position when flipped onto their back |
| Navel | Abnormalities such as small white or black button-shaped belly, yellow residue, or open navel |
| Legs | Red hocks, swollen legs, or leg deformities |
| Beak | Red spots, egg white contamination on the nostrils, or deformities |
| Abdomen | Complete exhaustion or remaining hardness from egg yolk |
| Genes | Primer Sequences (5′-3′) | Product Size (bp) | Gen Bank |
|---|---|---|---|
| Mfy5-F | CGGAAGGCAGCCACTATGAG | 150 | NM_001030363 |
| Mfy5-R | GAGGCTTTCGATGTACCTGATG | ||
| Mfy6-F | GCCTGCAAAACCTGCAAGAG | 150 | NM_001030746 |
| Mfy6-R | AGTCCGCCTTTTCAGAGCCT | ||
| GAPDH-F | CTGCCCAGAACATCATCCCA | 139 | K01458 |
| GAPDH-R | CGGCAGGTCAGGTCAACAAC |
| Parameters | Group | p | |||
|---|---|---|---|---|---|
| Green | Red | White | Dark | ||
| EN | 114 | 114 | 114 | 108 | 1000 |
| EIW (g) | 59.068 ± 0.302 | 59.394 ± 0.347 | 58.691 ± 0.323 | 58.241 ± 0.332 | 0.540 |
| ETW (g) | 52.901 ± 0.301 | 53.505 ± 0.318 | 52.390 ± 0.314 | 52.010 ± 0.343 | 0.928 |
| ESL (g) | 6.167 ± 0.078 | 5.889 ± 0.122 | 6.301 ± 0.119 | 6.231 ± 0.082 | 0.125 |
| Parameters | Group | p | |||
|---|---|---|---|---|---|
| Green | Red | White | Dark | ||
| HE (%) | 87.72 a | 81.58 a | 78.07 b | 79.63 a | 0.042 |
| H (%) | 90.91 | 86.92 | 79.46 | 84.31 | 0.739 |
| F (%) | 110.00 | 107 | 98.25 | 94.44 | 0.999 |
| UD (%) | 0.00 | 0.00 | 2.68 | 0.00 | 0.666 |
| EED (%) | 0.00 | 1.87 | 0.00 | 0.00 | 0.548 |
| MED (%) | 0.91 | 0.00 | 0.00 | 1.96 | 0.681 |
| LED (%) | 8.18 | 11.21 | 17.86 | 13.73 | 0.129 |
| TED (%) | 9.09 | 13.08 | 17.86 | 15.69 | 0.096 |
| CW (g) | 40.86 ± 0.29 a | 41.98 ± 0.34 a | 40.28 ± 0.32 b | 40.17 ± 0.36 b | <0.001 |
| ECR (%) | 69.17 a | 70.62 a | 68.63 b | 68.97 b | <0.001 |
| PS | 9.78 ± 0.53 | 9.70 ± 0.05 | 9.57 ± 0.07 | 9.56 ± 0.08 | 0.516 |
| Parameter (g) | Group | p | |||
|---|---|---|---|---|---|
| Green | Red | White | Dark | ||
| n = 81 | n = 62 | n = 69 | n = 70 | ||
| 4.W | 304.52 ± 4.97 a | 283.03 ± 5.27 b | 301.61 ± 5.73 a | 309.93 ± 7.10 a | 0.009 |
| 6.W | 436.17 ± 6.78 | 440.65 ± 8.33 | 424.77 ± 7.76 | 448.69 ± 8.86 | 0.653 |
| 8.W | 601.99 ± 9.82 b | 611.90 ± 12.00 b | 605.10 ± 10.20 b | 648.10 ± 12.20 a | 0.031 |
| 10.W | 796.50 ± 13.30 | 825.60 ± 15.90 | 823.20 ± 14.80 | 840.40 ± 15.80 | 0.660 |
| 12.W | 956.10 ± 15.50 | 1014.80 ± 20.00 | 1019.30 ± 18.70 | 1004.50 ± 18.40 | 0.545 |
| 14.W | 1060.30 ± 18.10 b | 1196.90 ± 24.00 a | 1182.60 ± 22.90 a | 1126.20 ± 22.50 a,b | <0.001 |
| 4–6W | 131.65 ± 3.67 b | 157.61 ± 4.30 a | 123.14 ± 4.60 b | 138.76 ± 10.40 a | 0.002 |
| 4–8W LWG | 297.47 ± 6.13 b | 328.89 ± 8.23 a | 303.82 ± 6.37 b | 338.20 ± 13.20 a | 0.002 |
| 4–10W LWG | 492.00 ± 10.10 b | 542.60 ± 12.40 a | 522.70 ± 10.90 a,b | 530.50 ± 16.50 a,b | 0.029 |
| 4–12W LWG | 651.60 ± 12.7 b | 731.80 ± 17.00 a | 718.90 ± 15.10 a | 694.50 ± 18.90 a,b | 0.002 |
| 4–14 W LWG | 755.80 ± 16.00 c | 913.80 ± 21.70 a | 880.70 ± 19.30 a,b | 816.30 ± 22.90 b,c | <0.001 |
| Green | Red | White | Darkness | p | |
|---|---|---|---|---|---|
| DLWG | |||||
| Myf5 | 0.329 | 0.129 | 0.146 | 0.207 | 0.214 |
| Myf6 | 0.418 | 0.158 | 0.163 | 0.143 | 0.117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwuchukwu, G.A.; Şen, U.; Önder, H.; Cilavdaroğlu, E.; Yamak, U.S. Light Color Influences Incubation Characteristics, Postnatal Growth, and Stress Physiology with a Lack of Expression Changes of Myf5 and Myf6 Genes in Gerze Native Chicken. Animals 2025, 15, 2347. https://doi.org/10.3390/ani15162347
Iwuchukwu GA, Şen U, Önder H, Cilavdaroğlu E, Yamak US. Light Color Influences Incubation Characteristics, Postnatal Growth, and Stress Physiology with a Lack of Expression Changes of Myf5 and Myf6 Genes in Gerze Native Chicken. Animals. 2025; 15(16):2347. https://doi.org/10.3390/ani15162347
Chicago/Turabian StyleIwuchukwu, Godswill Arinzechukwu, Uğur Şen, Hasan Önder, Elif Cilavdaroğlu, and Umut Sami Yamak. 2025. "Light Color Influences Incubation Characteristics, Postnatal Growth, and Stress Physiology with a Lack of Expression Changes of Myf5 and Myf6 Genes in Gerze Native Chicken" Animals 15, no. 16: 2347. https://doi.org/10.3390/ani15162347
APA StyleIwuchukwu, G. A., Şen, U., Önder, H., Cilavdaroğlu, E., & Yamak, U. S. (2025). Light Color Influences Incubation Characteristics, Postnatal Growth, and Stress Physiology with a Lack of Expression Changes of Myf5 and Myf6 Genes in Gerze Native Chicken. Animals, 15(16), 2347. https://doi.org/10.3390/ani15162347





