Impact of Amirthalingamia macracantha Larvae on Nile Tilapia (Oreochromis niloticus): A Morpho-Histopathological Perspective
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Fish Collection
2.2. Parasitological Examination
2.3. Histopathological Examination
2.3.1. Paraffin Processing Technique
2.3.2. Quantitative Morphometrical Analysis
2.4. Statistical Analysis
3. Results
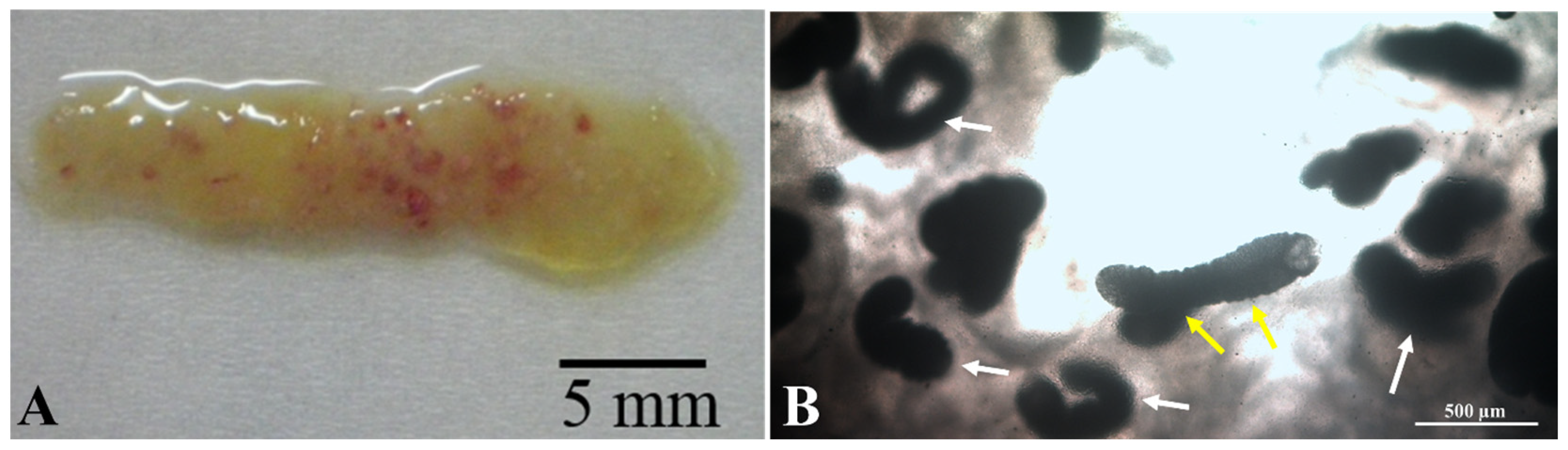
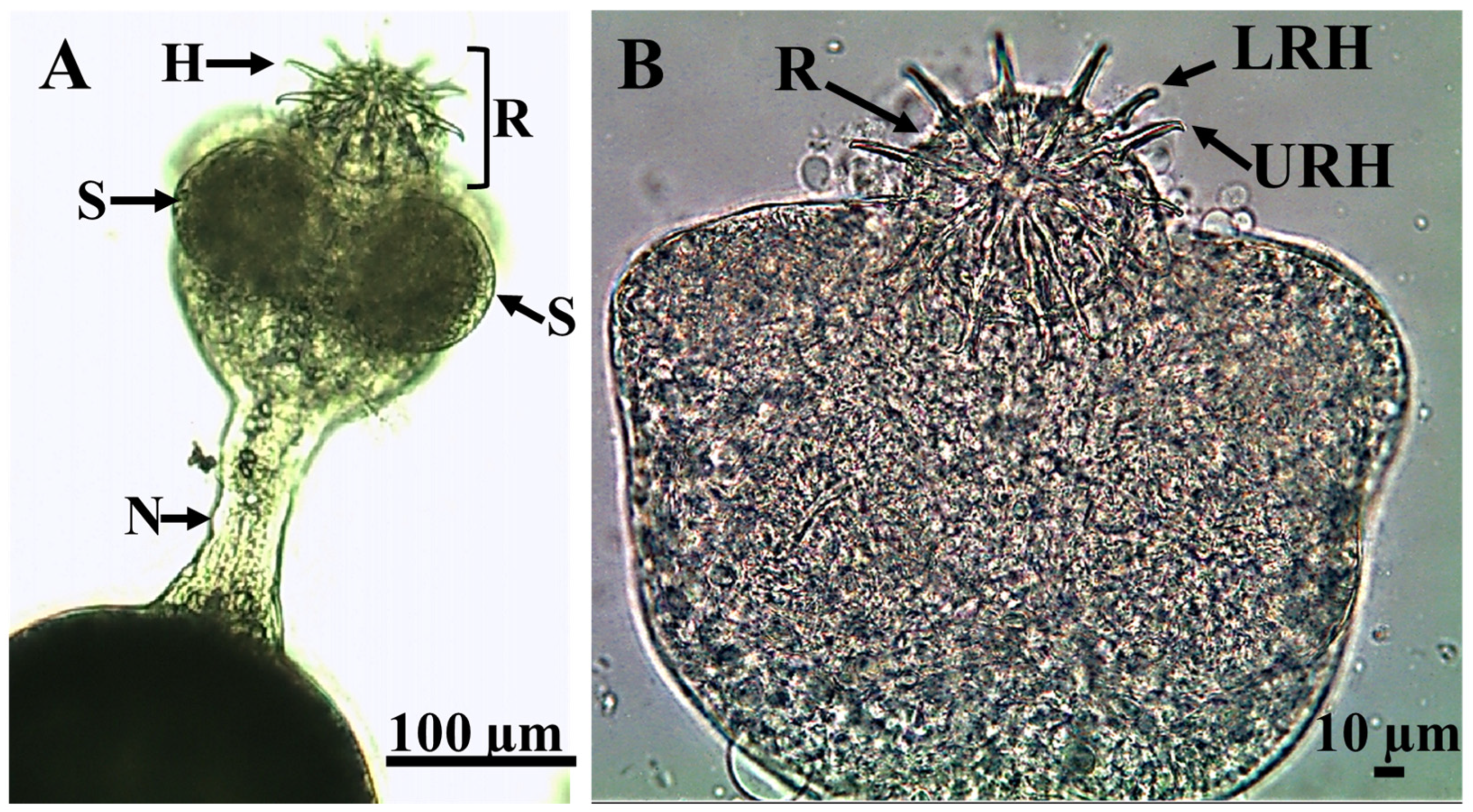
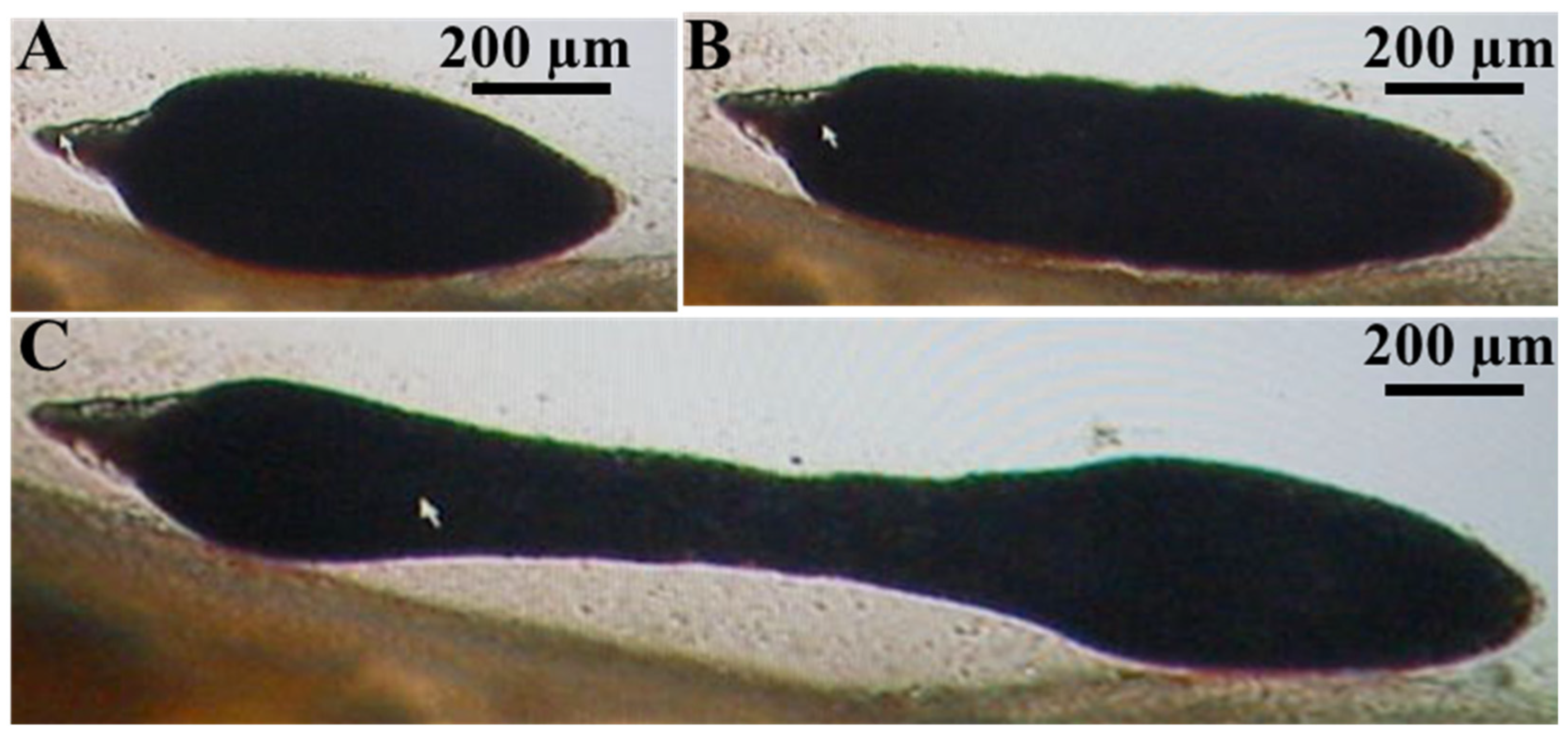
3.1. Parasitological Examination
Parasite Movement Description
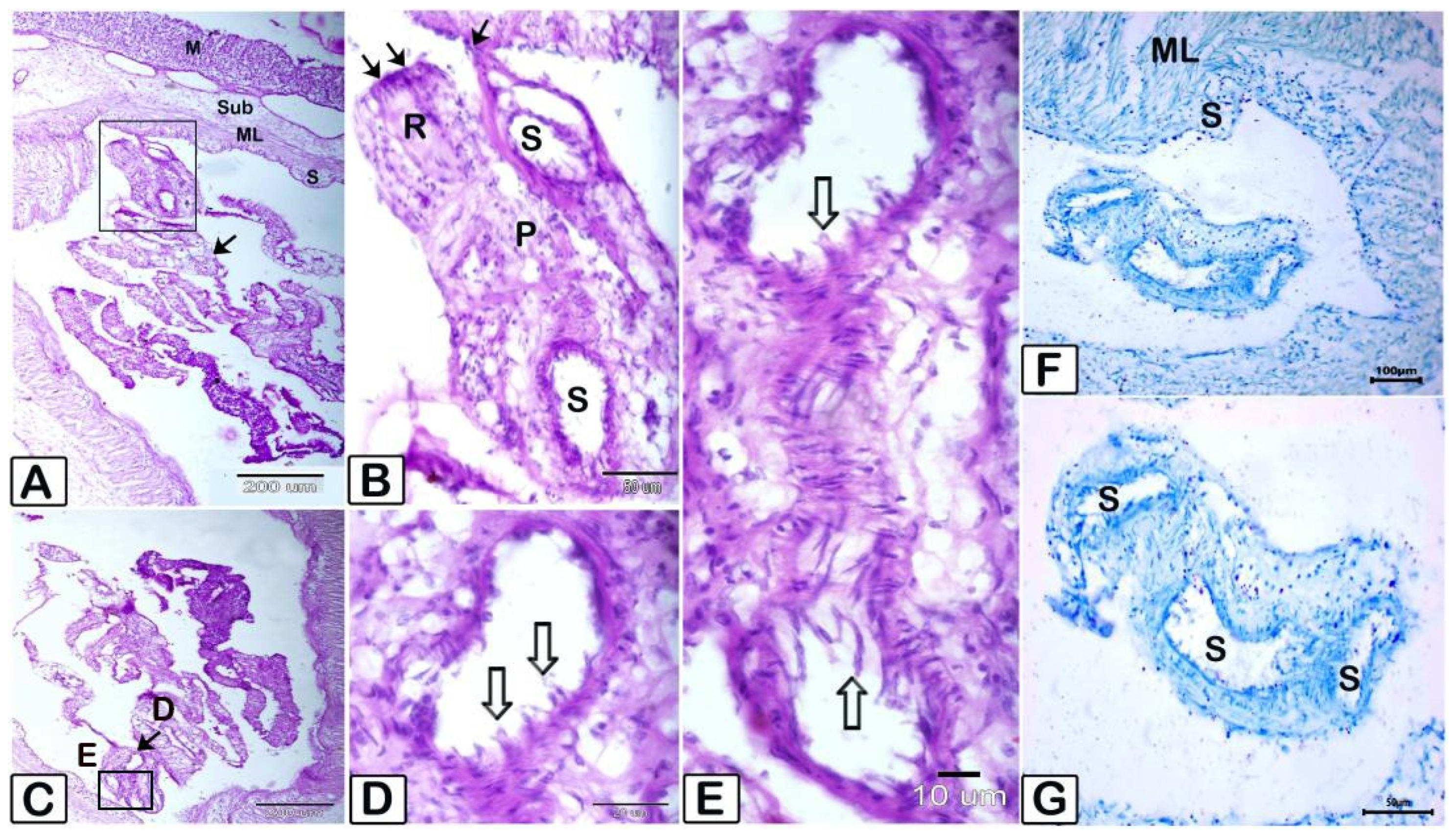
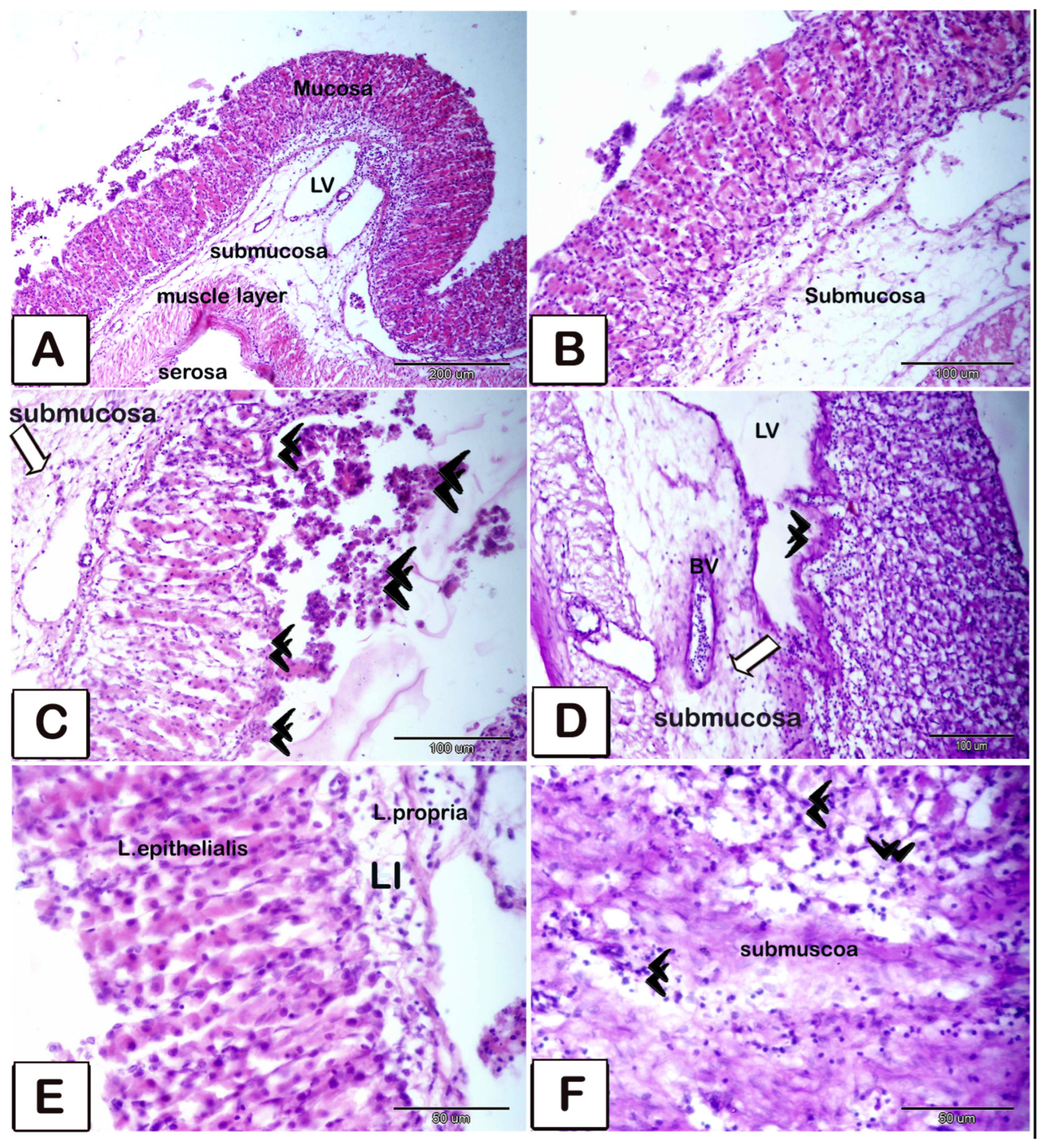
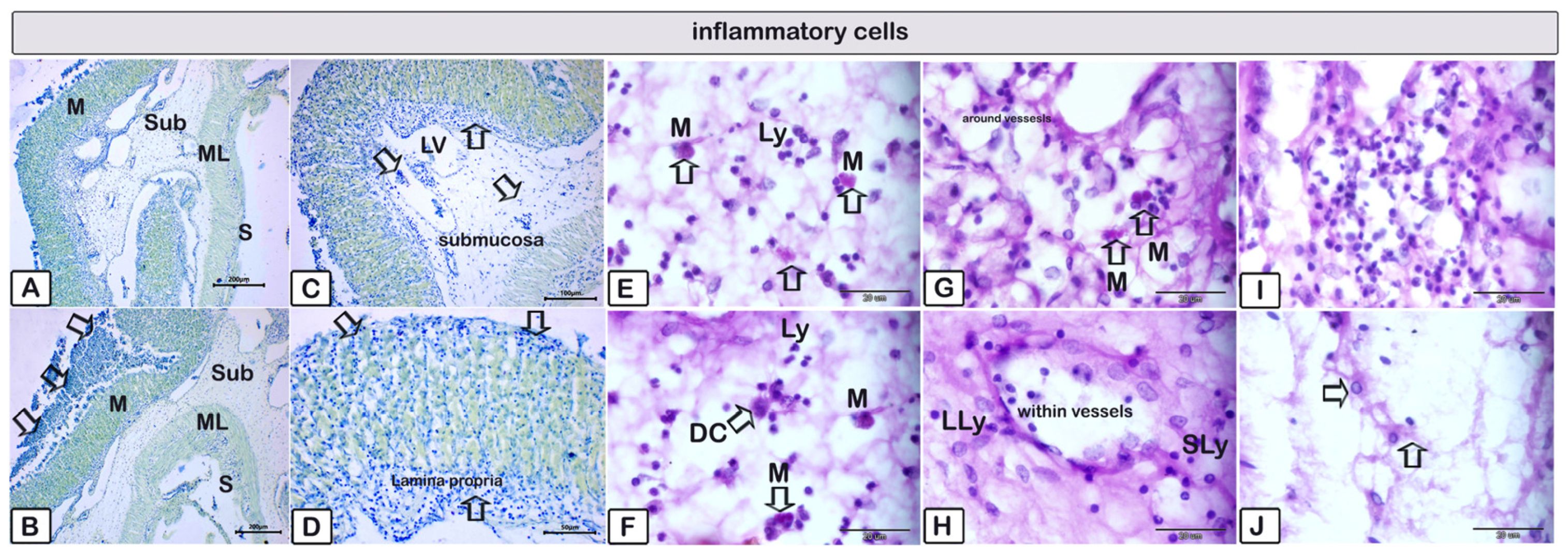
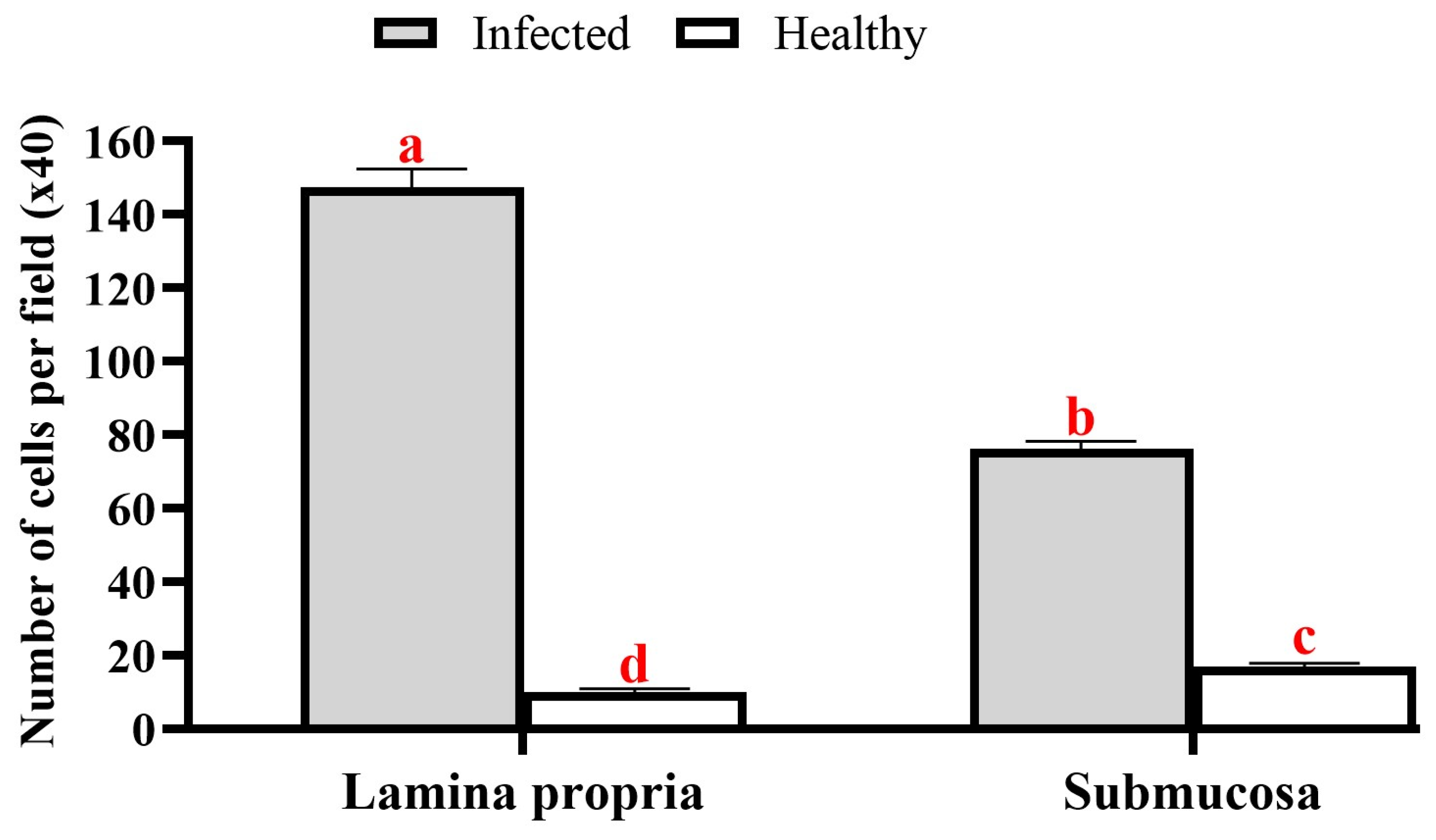
3.2. Histopathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture (2020). Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- El-Sayed, A.F.M.; Fitzsimmons, K. From Africa to the world—The journey of Nile tilapia. Rev. Aquac. 2023, 15, 6–21. [Google Scholar] [CrossRef]
- Bhujel, R.C. A review of strategies for the management of Nile tilapia (Oreochromis niloticus) broodfish in seed production systems, especially hapa-based systems. Aquaculture 2000, 181, 37–59. [Google Scholar] [CrossRef]
- Eknath, A.E.; Hulata, G. Use and exchange of genetic resources of Nile tilapia (Oreochromis niloticus). Rev. Aquac. 2009, 1, 197–213. [Google Scholar] [CrossRef]
- Ojwala, R.A.; Otachi, E.O.; Kitaka, N.K. Effect of water quality on the parasite assemblages infecting Nile tilapia in selected fish farms in Nakuru County, Kenya. Parasitol. Res. 2018, 117, 3459–3471. [Google Scholar] [CrossRef]
- Konstantinidis, I.; Anastasiadi, D.; Sætrom, P.; Nedoluzhko, A.V.; Mjelle, R.; Podgorniak, T.; Piferrer, F.; Fernandes, J.M. Epigenetic mapping of the somatotropic axis in Nile tilapia reveals differential DNA hydroxymethylation marks associated with growth. Genomics 2021, 113, 2953–2964. [Google Scholar] [CrossRef]
- Morrison, C.; Wright, J., Jr. A study of the histology of the digestive tract of the Nile tilapia. J. Fish Biol. 1999, 54, 597–606. [Google Scholar] [CrossRef]
- Shinn, A.P.; Avenant-Oldewage, A.; Bondad-Reantaso, M.G.; Cruz-Laufer, A.J.; García-Vásquez, A.; Hernández-Orts, J.S.; Kuchta, R.; Longshaw, M.; Metselaar, M.; Pariselle, A.; et al. A global review of problematic and pathogenic parasites of farmed tilapia. Rev. Aquac. 2023, 15, 92–153. [Google Scholar] [CrossRef]
- Caira, J.N.; JensEn, K.; Georgiev, B.B.; Kuchta, R.; Littlewood, T.; Mariaux, J.; Scholz, T.; Tkach, V.V.; Waeschenbach, A. An overview of the tapeworms of vertebrate bowels of the earth. In Planetary Biodiversity Inventory (2008–2017): Tapeworms from the Vertebrate Bowels of the Earth; University of Kansas, Natural History Museum: Lawrence, KS, USA, 2018. [Google Scholar]
- Uhrová, L. Diverzita Larválních Stádií Tasemnic Čeledi Gryporhynchidae (Cestoda: Cyclophyllidea) z Cichlidníchryb (Perciformes: Cichlidae) Jižní Afriky; University of South Bohemia in České Budějovice the Faculty of Natural Science: České Budějovice, Czech Republic, 2016. [Google Scholar]
- Scholz, T.; Bray, R.A.; Kuchta, R.; Řepová, R. Larvae of gryporhynchid cestodes (Cyclophyllidea) from fish: A review. Folia Parasitol. 2004, 51, 131–152. [Google Scholar] [CrossRef]
- Scholz, T.; Boane, C.; Saraiva, A. New metacestodes of gryporhynchid tapeworms (Cestoda: Cyclophyllidea) from carp (Cyprinus carpio Linnaeus, 1758) from Mozambique, Africa. Comp. Parasitol. 2008, 75, 315–320. [Google Scholar] [CrossRef]
- Akoll, P.; Konecny, R.; Mwanja, W.W.; Schiemer, F. Infection patterns of Nile tilapia (Oreochromis niloticus L.) by two helminth species with contrasting life styles. Parasitol. Res. 2012, 110, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Aloo, P. A comparative study of helminth parasites from the fish Tilapia zillii and Oreochromis leucostictus in Lake Naivasha and Oloidien Bay, Kenya. J. Helminthol. 2002, 76, 95–102. [Google Scholar] [CrossRef]
- Bray, R.A. A new genus of dilepidid cestode in Tilapia nilotica (L., 1757) and Phalacrocorax carbo (L., 1758) in Sudan. J. Nat. Hist. 1974, 8, 589–596. [Google Scholar] [CrossRef]
- Florio, D.; Gustinelli, A.; Caffara, M.; Turci, F.; Quaglio, F.; Konečný, R.; Nikowitz, T.; Wathuta, E.M.; Magana, A.E.M.; Otachi, E.O.; et al. Veterinary and public health aspects in tilapia (Oreochromis niloticus niloticus) aquaculture in Kenya, Uganda and Ethiopia. Ittiopatologia 2009, 6, 51–93. [Google Scholar]
- Gulelat, Y.; Yimer, E.; Asmare, K.; Bekele, J. Study on parasitic helminths infecting three fish species from Koka reservoir, Ethiopia. SINET Ethiop. J. Sci. 2013, 36, 73–80. [Google Scholar]
- Shvydka, S.; Sarabeev, V.; Estruch, V.D.; Cadarso-Suárez, C. Optimum Sample Size to Estimate Mean Parasite Abundance in Fish Parasite Surveys. Helminthologia 2018, 55, 52–59. [Google Scholar] [CrossRef]
- Eissa, A.E. Clinical and Laboratory Manual of Fish Diseases; LAP LAMBERT Academic Publishing: Saarbrücken, Germany, 2016. [Google Scholar]
- Noga, E.J. Fish Disease: Diagnosis and Treatment, 2nd ed.; Iowa State University Press: Ames, IA, USA, 2010. [Google Scholar]
- Kildea, M.A.; Allan, G.L.; Kearney, R.E. Accumulation and clearance of the anaesthetics clove oil and AQUI-S™ from the edible tissue of silver perch (Bidyanus bidyanus). Aquaculture 2004, 232, 265–277. [Google Scholar] [CrossRef]
- Abdallah, E.S.H.; Hamouda, A.H. Livoneca redmanii Leach, 1818 (Cymothoidae) a parasitic isopod infesting the gills of the European seabass, Dicentrarchus labrax (Linnaeus, 1758): Morphological and molecular characterization study. BMC Vet. Res. 2022, 18, 330. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, E.S.H.; Hamouda, A.H. Morphological and molecular characterization of Lernanthropus kroyeri, a copepod infesting the gills of European seabass Dicentrarchus labrax. Egypt. J. Aquat. Res. 2023, 49, 49–55. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; Hassan, E.S.; Mohie, H.; Nour El Deen, E.A.; Kuraa, H.M.M.; Hanna, H.N.S. Parasitic infections of the gills of wild African Sharptooth Catfish (Clarias gariepinus). Assiut Vet. Med. J. 2018, 64, 31–39. [Google Scholar] [CrossRef]
- Thabit, H.; Abdallah, E.S.H. Morphological and molecular identification of third-stage Contracaecum larvae (Nematoda: Anisakidae) parasitizing Nile perch Lates niloticus in Egypt. Aquac. Res. 2022, 53, 4869–4881. [Google Scholar] [CrossRef]
- Hassan, E.S.; Mahmoud, M.M.; Metwally, A.M.; Moktar, D.M. Lamproglena monodi (Copepoda: Lernaeidae), infesting gills of Oreochromis niloticus and Tilapia zillii. Glob. J. Fish. Aquac. Res. 2013, 6, 1–16. [Google Scholar]
- Abdallah, E.S.H.; Al Tayip, A.M.; Nasr, S.K.A.E.; Sayed, G.M.; Elkamel, A.A.E. Acanthogyrus tilapiae Infections in Wild and Cultured Nile tilapia Oreochromis niloticus. Assiut Vet. Med. J. 2017, 63, 44–50. [Google Scholar]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Reiczigel, J.; Marozzi, M.; Fábián, I.; Rózsa, L. Biostatistics for parasitologists—A primer to quantitative parasitology. Trends Parasitol. 2019, 35, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D. Some Simple Methods for the Assessment of Tropical Fish Stocks; FAO fisheries technical paper No 234; FAO: Rome, Italy, 1983; Volume 52. [Google Scholar]
- Moustafa, M.; Sayed, R.; Zayed, A.; El-Hafeez, A.H. Morphological and morphometric study of the development of seminiferous epithelium of donkey (Equus asinus) from birth to maturity. J. Cytol. Histol. 2015, 6, 1. [Google Scholar]
- Hassan, M.S.; Abd-Allah, E.A.; El-Amir, Y.O.; Fayed, H.; Abdelhafeez, H.H. Histopathological Investigation and Plasma Energy Metabolic Profile as A diagnostic Tools of Postpartum Metritis and its Implication on Reproductive Performance of Dairy Cows. Egypt. J. Vet. Sci. 2025, 1–16. [Google Scholar] [CrossRef]
- Gaafar, A.Y.; Hassan Abdullah, E.S.; Mahmoud, M.M.; Younes, A.M.; Nakai, T. Pathological and immunohistochemical studies following the experimental infection of ayu (Plecoglossus altivelis) by Edwardsiella ictaluri. Microsc. Res. Tech. 2021, 84, 460–470. [Google Scholar] [CrossRef]
- D’Iglio, C.; Albano, M.; Famulari, S.; Spanò, N.; Rinelli, P.; Savoca, S.; Capillo, G. Basic Intersexuality (Abnormal Hermaphroditism) in the Blackmouth Catshark, Galeus melastomus,(Rafinesque, 1810), from the Southern Tyrrhenian Sea (Central Mediterranean Sea). Fishes 2022, 7, 120. [Google Scholar] [CrossRef]
- Fournie, J.W.; Krol, R.M.; Hawkins, W.E. Fixation of fish tissues. In The Laboratory Fish; Elsevier: Amsterdam, The Netherlands, 2000; pp. 569–578. [Google Scholar]
- Marino, F.; Licata, L.; Albano, M.; Ieni, A.; Di Caro, G.; Macrì, B. Angioleiomyoma in a conger (Conger conger). Dis. Aquat. Org. 2016, 119, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hakeem, S.S.; Fadladdin, Y.A.J.; Khormi, M.A.; Abd-El-Hafeez, H.H. Modulation of the intestinal mucosal and cell-mediated response against natural helminth infection in the African catfish Clarias gariepinus. BMC Vet. Res. 2024, 20, 335. [Google Scholar] [CrossRef] [PubMed]
- Joyeux, C.; Baer, J. Mission Saharienne Augieras-Draper, 1927–1928. Cestodes. Bull. Mus. Nat. Hist. Nat. 1930, 2, 217–223. [Google Scholar]
- Fuhrmann, O. Die Cestoden der Vögel des weissen Nils. In Results of the Swedish Zoological Expedition to Egypt and the White Nile, 1901; Jägerskiöld, L.A., Ed.; BioStor: Miami, FL, USA, 1909; Part 3; pp. 1–55. [Google Scholar]
- Joyeux, C.; Baer, J. Notices helminthologiques. Bull. Soc. zool. Fr. 1935, 60, 482–501. [Google Scholar]
- Adamba, S.W.K.; Otachi, E.O.; Ong’ondo, G.O. Parasite communities of Oreochromis niloticus baringoensis (Trewavas, 1983) in relation to selected water quality parameters in the springs of Lorwai Swamp and Lake Baringo, Kenya. Acta Parasitol. 2020, 65, 441–451. [Google Scholar] [CrossRef]
- Otachi, E.O. Parasites of Fish and Trace Elements in Lakes Naivasha and Turkana, Kenya. PhD Thesis, Universität Wien, Wien, Austria, 2013. [Google Scholar]
- Scholz, T.; Vanhove, M.; Smit, N.; Jayasundera, Z.; Gelnar, M. A Guide to the Parasites of African Freshwater Fishes; Royal Belgian Institute of Natural Sciences Brussels: Bruxelles, Belgium, 2018; Volume 18. [Google Scholar]
- Ortega-Olivares, M.; García-Varela, M. Phylogenetic relationships of the family Gryporhynchidae (Cestoda: Cyclophyllidea) inferred through SSU and LSU rDNA sequences. J. Helminthol. 2019, 93, 763–771. [Google Scholar] [CrossRef]
- Scholz, T.; Tavakol, S.; Uhrová, L.; Brabec, J.; Přikrylová, I.; Mašová, Š.; Šimková, A.; Halajian, A.; Luus-Powell, W.J. An annotated list and molecular data on larvae of gryporhynchid tapeworms (Cestoda: Cyclophyllidea) from freshwater fishes in Africa. Syst. Parasitol. 2018, 95, 567–590. [Google Scholar] [CrossRef]
- Rufai, M.A.; Adetona, A.J. Parasites fauna of farmed African catfish (Clarias gariepinus) in Osogbo, Osun State, Southwestern Nigeria. Ann. West Univ. Timisoara. Ser. Biol. 2017, 20, 123–130. [Google Scholar]
- Oso, J.; Ayodele, I.; Fagbuaro, O. Food and feeding habits of Oreochromis niloticus (L.) and Sarotherodon galilaeus (L.) in a tropical reservoir. World J. Zool. 2006, 1, 118–121. [Google Scholar]
- Dempster, P.; Beveridge, M.; Baird, D. Herbivory in the tilapia Oreochromis niloticus: A comparison of feeding rates on phytoplankton and periphyton. J. Fish Biol. 1993, 43, 385–392. [Google Scholar] [CrossRef]
- Getachew, T.; Fernando, C. The food habits of an herbivorous fish (Oreochromis niloticus Linn.) in Lake Awasa, Ethiopia. Hydrobiologia 1989, 174, 195–200. [Google Scholar] [CrossRef]
- Akoll, P.; Konecny, R.; Mwanja, W.W.; Nattabi, J.K.; Agoe, C.; Schiemer, F. Parasite fauna of farmed Nile tilapia (Oreochromis niloticus) and African catfish (Clarias gariepinus) in Uganda. Parasitol. Res. 2012, 110, 315–323. [Google Scholar] [CrossRef]
- Tadesse, B. Prevalence and adundance of fish parasites in Bomosa Cage Systems and lakes Babogaya and Awassa, Ethiopia. Master’s Thesis, Delft Unesco-IHEna, Delft, the Netherlands, 2009. [Google Scholar]
- Khan, R. Host-parasite interactions in some fish species. J. Parasitol. Res. 2012. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; Freeman, R.S. The role of plerocercoids in the biology of Proteocephalus ambloplitis (Cestoda) maturing in smallmouth bass. Can. J. Zool. 1973, 51, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, A. Limnological studies of and primary production in temple pond ecosystems. Hydrobiologia 1976, 48, 117–123. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Devi, P. Water quality guidelines for the management of pond fish culture. Int. J. Environ. Sci. 2013, 3, 1980–2009. [Google Scholar]
- Sures, B. Environmental parasitology: Relevancy of parasites in monitoring environmental pollution. Trends Parasitol. 2004, 20, 170–177. [Google Scholar] [CrossRef]
- Dirican, S.; Çilek, S. Condition factors of seven Cyprinid fish species from Çamligöze dam lake on central Anatolia, Turkey. Afr. J. Agric. Res. 2012, 7, 4460–4464. [Google Scholar] [CrossRef]
- Soni, N.; Ujjania, N. Length-weight relationship and condition factor of indian major carps of Vallabhsagar Reservoir, Gujarat, India. Indian J. Fish. 2017, 64, 186–189. [Google Scholar] [CrossRef]
- Olurin, K.; Aderibigbe, O. Length-weight relationship and condition factor of pond reared juvenile Oreochromis niloticus. World J. Zool. 2006, 1, 82–85. [Google Scholar]
- Benesh, D.P.; Chubb, J.C.; Parker, G.A. Adaptive division of growth and development between hosts in helminths with two-host life cycles. Evolution 2022, 76, 1971–1985. [Google Scholar] [CrossRef] [PubMed]
- McSorley, H.J.; Maizels, R.M. Helminth infections and host immune regulation. Clin. Microbiol. Rev. 2012, 25, 585–608. [Google Scholar] [CrossRef]
- Lu, F.; Huang, S. The roles of mast cells in parasitic protozoan infections. Front. Immunol. 2017, 8, 363. [Google Scholar] [CrossRef]
- Jiménez, M.; Cervantes-García, D.; Córdova-Dávalos, L.E.; Pérez-Rodríguez, M.J.; Gonzalez-Espinosa, C.; Salinas, E. Responses of mast cells to pathogens: Beneficial and detrimental roles. Front. Immunol. 2021, 12, 685865. [Google Scholar] [CrossRef]
- Reite, O.B. Mast cells/eosinophilic granule cells of salmonids: Staining properties and responses to noxious agents. Fish Shellfish Immunol. 1997, 7, 567–584. [Google Scholar] [CrossRef]
- Brown, L.F.; Detmar, M.; Tognazzi, K.; Abu-Jawdeh, G.; Iruela-Arispe, L. Uterine smooth muscle cells express functional receptors (flt-1 and KDR) for vascular permeability factor/vascular endothelial growth factor. Lab. Investig. 2019, 76, 245–255. [Google Scholar]
- Abraham, S.N.; St. John, A.L. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 2010, 10, 440–452. [Google Scholar] [CrossRef]
- Dawicki, W.; Marshall, J.S. New and emerging roles for mast cells in host defence. Curr. Opin. Immunol. 2007, 19, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Young, H.A.; Spitzer, J.H.; Visintin, A.; Segal, D.M. Histamine regulates cytokine production in maturing dendritic cells, resulting in altered T cell polarization. J. Clin. Investig. 2001, 108, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Mera-Loor, G.B.; Santana-Piñeros, A.M.; Reyes-Mero, B.M.; Cruz-Quintana, Y. Parvitaenia cochlearii (Cestoda: Gryporhynchidae) en cultivo de chame Dormitator latifrons en Ecuador. Rev. MVZ Córdoba 2023, 28, e2954. [Google Scholar] [CrossRef]







| Character | ||||
|---|---|---|---|---|
| Reference | Current study | Bray [15] | Scholz, Bray, Kuchta and Řepová [11] | Uhrová [10] |
| Host | Oreochromis niloticus | Tilapia nilotica | Haplochromis acidens Pseudocrenilabrus philander Tilapia sparrmanii | |
| Country | Egypt | Sudan | Zimbabwe | |
| Infected organ | stomach & intestine | Liver | Liver | |
| Metacestode length | 1228.5 ± 34.7 µm | 2600–4200 µm | 3000–5000 µm | |
| Metacestode width | 312.6 ± 36 µm | 1300–1800 µm | ||
| Scolex diameter | 314.94 ± 4.8 µm | 850–1120 µm | ||
| Suckers’ diameter | 73.93 ± 0.95 µm | 290–360 µm | ||
| Lower row Total length | 26.21 ± 0.13 µm Handle (H) 13.25 ± 0.4 µm Blade (B) 13.66 ± 0.03 µm B/H ratio 1.03: 1 | 260–280 µm | 240–290 µm Handle 144–160 µm Blade 157–184 µm B/H ratio 0.98–1.11 | 258–270 µm Handle 130–145 µm Blade 150–172 µm B/H ratio 1.15–1.2 |
| Upper row Total length | 4 hooks (49.3 ± 1 µm) Handle 26.65 ± 0.36 µm Blade 24.43 ± 0.42 µm B/H ratio 1:1.1 | 4 hooks (450–455 µm) | 4 hooks (448–480 µm) Handle 240–296 Blade 272–296 B/H ratio 1.00–1.16 | 4 large hooks (465–475 µm) Handle 230–280 Blade 170–280 B/H ratio 1.00–1.18 |
| 6 hooks (42.8 ± 0.6 µm) Handle 24.48 ± 0.19 µm Blade 21.52 ± 0.43 µm B/H ratio 1:1.1 | 6 hooks (400–430 µm)
| 6 hooks (390–450 µm) Handle 224–256 µm Blade 140–280 µm B/H ratio 1.00–1.18 | 6 small hooks (420–430 µm) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, E.S.H.; Mahmoud, M.M.; Abdel-Hafeez, H.H.; Albano, M.; Capillo, G.; Metwally, A.M.; Areshi, S.M.; Alardan, D.; El Sherry, Y.M.I. Impact of Amirthalingamia macracantha Larvae on Nile Tilapia (Oreochromis niloticus): A Morpho-Histopathological Perspective. Animals 2025, 15, 2334. https://doi.org/10.3390/ani15162334
Abdallah ESH, Mahmoud MM, Abdel-Hafeez HH, Albano M, Capillo G, Metwally AM, Areshi SM, Alardan D, El Sherry YMI. Impact of Amirthalingamia macracantha Larvae on Nile Tilapia (Oreochromis niloticus): A Morpho-Histopathological Perspective. Animals. 2025; 15(16):2334. https://doi.org/10.3390/ani15162334
Chicago/Turabian StyleAbdallah, Ebtsam Sayed Hassan, Mahmoud Mostafa Mahmoud, Hanan Hassan Abdel-Hafeez, Marco Albano, Gioele Capillo, Asmaa Mohamed Metwally, Sultan Mohammed Areshi, Dalal Alardan, and Yosra M. I. El Sherry. 2025. "Impact of Amirthalingamia macracantha Larvae on Nile Tilapia (Oreochromis niloticus): A Morpho-Histopathological Perspective" Animals 15, no. 16: 2334. https://doi.org/10.3390/ani15162334
APA StyleAbdallah, E. S. H., Mahmoud, M. M., Abdel-Hafeez, H. H., Albano, M., Capillo, G., Metwally, A. M., Areshi, S. M., Alardan, D., & El Sherry, Y. M. I. (2025). Impact of Amirthalingamia macracantha Larvae on Nile Tilapia (Oreochromis niloticus): A Morpho-Histopathological Perspective. Animals, 15(16), 2334. https://doi.org/10.3390/ani15162334









