Effects of Dietary Baicalin on Aflatoxin B1-Induced Growth Performance and Liver Health in Ducklings
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animal Experiment Design
2.3. Serum Biochemical Assay
2.4. Histopathological Observation
2.5. Statistical Analysis
3. Results
3.1. Growth Performance of Ducklings Exposed to Different Doses of AFB1
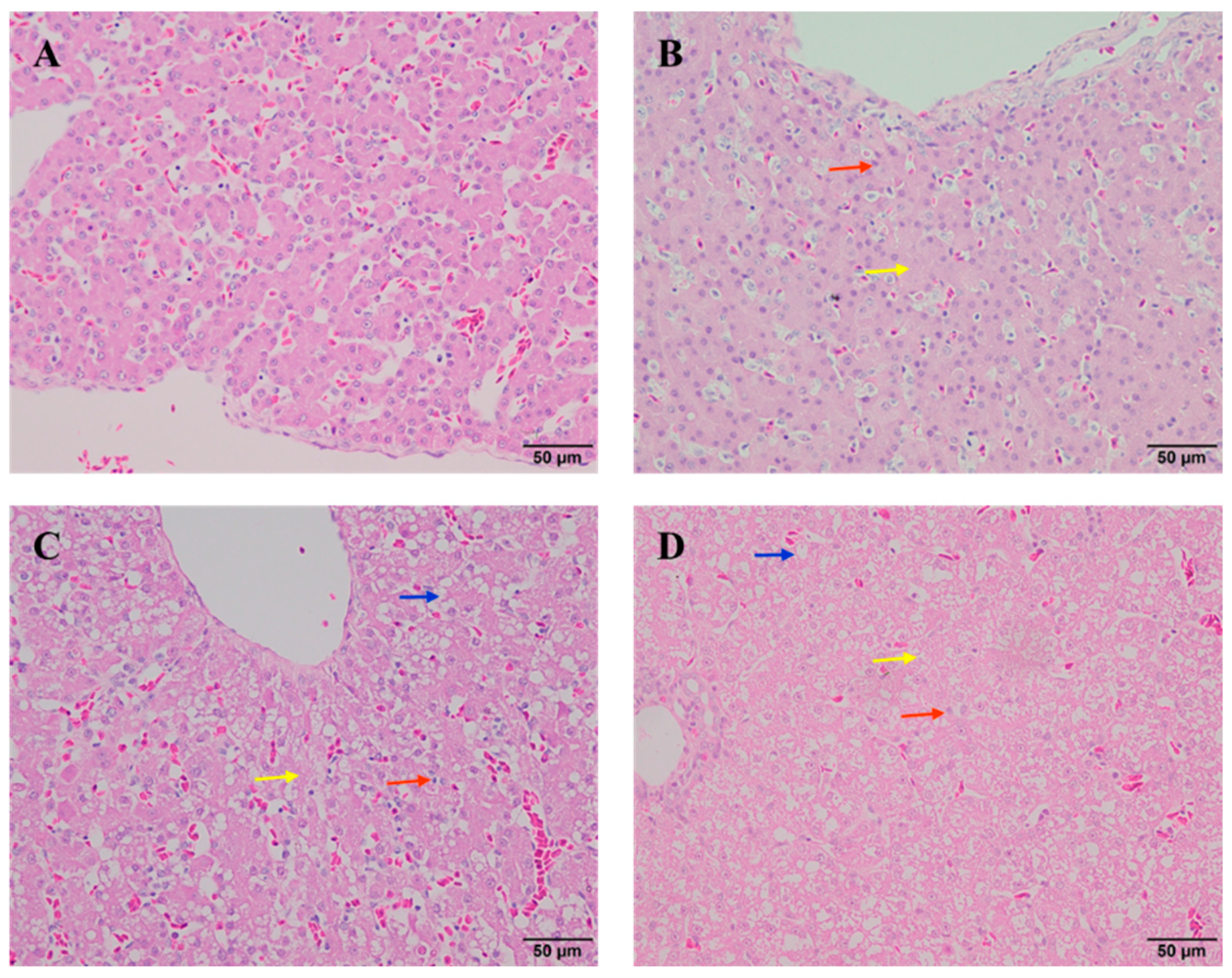
3.2. The Effect of Different Doses of AFB1 on Liver Histopathology of Ducklings
3.3. The Effect of Baicalin on Growth Performance of AFB1-Exposed Ducklings
3.4. The Effect of Baicalin on Serum Biochemical Parameters of AFB1-Exposed Ducklings
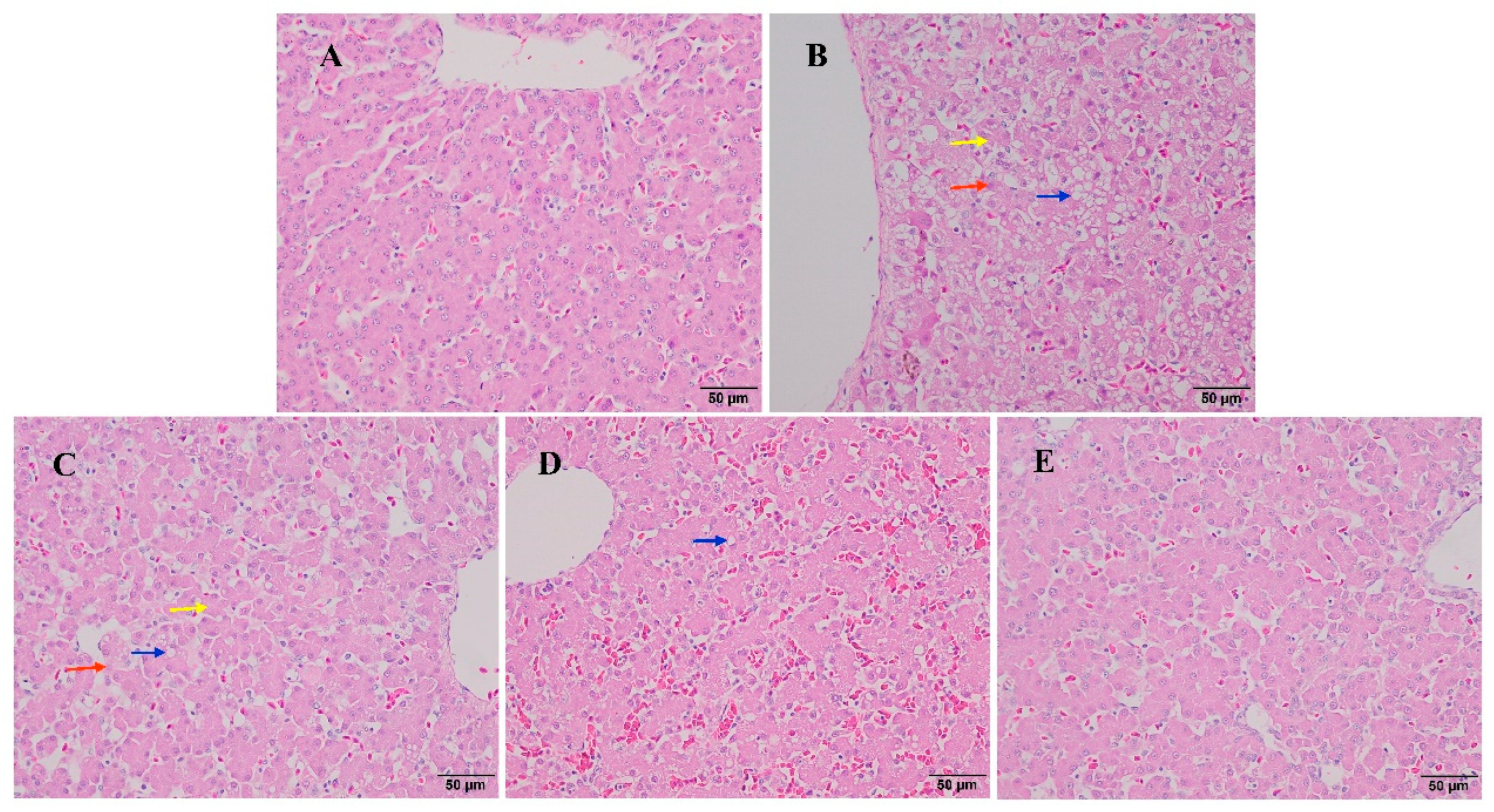
3.5. The Effect of Baicalin on Liver Histopathological Changes in AFB1-Exposed Ducklings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADFI | Average daily feed intake |
| ADG | Average daily gain |
| AFB1 | Aflatoxin B1 |
| ALB | Albumin |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| FCR | Feed-to-gain ratio |
| GGT | Gamma-glutamyltransferase |
| H&E | Hematoxylin and eosin |
References
- Wu, S.; Feng, K.; Niu, J.; Xu, J.; Mo, H.; She, X.; Yu, S.B.; Li, Z.T.; Yan, S. Acyclic cucurbit[n]uril-enabled detection of aflatoxin b1 via host-guest chemistry and bioluminescent immunoassay. Toxins 2025, 17, 104. [Google Scholar] [CrossRef]
- Lu, Q.; Hu, Y.; Nabi, F.; Li, Z.; Janyaro, H.; Zhu, W.; Liu, J. Effect of penthorum chinense pursh compound on afb1-induced immune imbalance via jak/stat signaling pathway in spleen of broiler chicken. Vet. Sci. 2023, 10, 521. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin b1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Yang, H.; Wang, Y.; Pang, Q.; Jiao, Y.; Shan, A.; Feng, X. Dietary curcumin alleviated aflatoxin b1-induced acute liver damage in ducks by regulating nlrp3-caspase-1 signaling pathways. Foods 2021, 10, 3086. [Google Scholar] [CrossRef]
- Limaye, A.; Yu, R.C.; Chou, C.C.; Liu, J.R.; Cheng, K.C. Protective and detoxifying effects conferred by dietary selenium and curcumin against afb1-mediated toxicity in livestock: A review. Toxins 2018, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; He, Y.; Gao, X.; Liu, H.; Rao, G.; Su, Q.; Ruan, Z.; Tang, Z.; Hu, L. Curcumin attenuates afb1-induced duck liver injury by inhibiting oxidative stress and lysosomal damage. Food Chem. Toxicol. 2023, 172, 113593. [Google Scholar] [CrossRef]
- Yu, A.; Wang, H.; Cheng, Q.; Rajput, S.A.; Qi, D. The effects of aflatoxin b(1) on liver cholestasis and its nutritional regulation in ducks. Toxins 2024, 16, 239. [Google Scholar] [CrossRef] [PubMed]
- Tarasconi, L.; Dazuk, V.; Molosse, V.L.; Cécere, B.G.O.; Deolindo, G.L.; Mendes, R.E.; Gloria, E.M.; Ternus, E.M.; Galli, G.M.; Paiano, D.; et al. Nursery pigs fed with feed contaminated by aflatoxin b1 (aspergillus flavus) and anti-mycotoxin blend: Pathogenesis and negative impact on animal health and weight gain. Microb. Pathog. 2024, 186, 106474. [Google Scholar] [CrossRef]
- Ye, X.; Yang, Y.; Yao, Q.; Huang, M.; Balasubramanian, B.; Jha, R.; Liu, W. The ameliorative role of phlorotannin on aflatoxin b(1)-induced liver oxidative stress and mitochondrial injury is related to the activation of nrf2 and nrf1 signaling pathways in broilers. J. Anim. Sci. Biotechnol. 2025, 16, 75. [Google Scholar] [CrossRef]
- Zhang, M.; Jiao, P.; Wang, X.; Sun, Y.; Liang, G.; Xie, X.; Zhang, Y. Evaluation of growth performance, nitrogen balance and blood metabolites of mutton sheep fed an ammonia-treated aflatoxin b1-contaminated diet. Toxins 2022, 14, 361. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.K.; ul Haq, I.; Ijaz, S.; Javed, N.; Akbar, N. Molecular and analytical approaches based characterization of aflatoxins producing aspergillus species affecting groundnut. Pak. J. Agr. Sci. 2024, 61, 931–940. [Google Scholar]
- Saleemi, M.K.; Raza, A.; Khatoon, A.; Zubair, M.; Xu, Y.P.; Murtaza, B.; Li, X.Y.; Jamil, M.; Imran, M.; Muhammad, F.; et al. Toxic effects of aflatoxin b1 on hematobiochemical and histopathological parameters of juvenile white leghorn male birds and their amelioration with vitamin e and moringa oleifera. Pak. Vet. J. 2023, 43, 405–411. [Google Scholar]
- Tarhane, S.; Dursun, I.; Mor, B.; Tarhane, A.K.; Kiziltepe, S.; Filazi, I.; Güven, A. Determination of the mycotoxin activity of filamentous fungi isolated from the intestinal region of adult honey bees by the pcr and uhplc-orbitrap-hrms methods. Kafkas Univ. Vet. Fak. 2023, 29, 473–481. [Google Scholar] [CrossRef]
- Sadimantara, M.S.; Argo, B.D.; Sucipto, S.; Hendrawan, Y. Prediction of aflatoxin contamination in cocoa beans using uv-fluorescence imaging and artificial neural networks for enhanced detection. J. Glob. Innov. Agric. Sci. 2024, 12, 315–325. [Google Scholar] [CrossRef]
- Yang, J.Y.; Li, M.; Zhang, C.L.; Liu, D. Pharmacological properties of baicalin on liver diseases: A narrative review. Pharmacol. Rep. 2021, 73, 1230–1239. [Google Scholar] [CrossRef]
- Jiang, M.; Li, Z.; Qin, X.; Chen, L.; Zhu, G. Regulatory role of flavonoid baicalin from scutellaria baicalensis on ampk: A review. Am. J. Chin. Med. 2025, 53, 771–801. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, Y.; Zhao, C.; Zhao, B.; Wang, J. The pharmacological efficacy of baicalin in inflammatory diseases. Int. J. Mol. Sci. 2023, 24, 9317. [Google Scholar] [CrossRef]
- Su, L.; Wang, R.; Qiu, T.; Wang, J.; Meng, J.; Zhu, J.; Wang, D.; Wu, Y.; Liu, J. The protective effect of baicalin on duck hepatitis a virus type 1-induced duck hepatic mitochondria dysfunction by activating nuclear erythroid 2-related factor 2/antioxidant responsive element signaling pathway. Poult. Sci. 2021, 100, 101032. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, S.; Jiang, L.; Gao, X.; Liu, W.; Zhu, X.; Huang, W.; Zhao, H.; Wei, Z.; Wang, K.; et al. Baicalin protects against zearalenone-induced chicks liver and kidney injury by inhibiting expression of oxidative stress, inflammatory cytokines and caspase signaling pathway. Int. Immunopharmacol. 2021, 100, 108097. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Zhang, J.; Zhou, H.; Qiu, Y.; Guo, P.; Lu, Q.; Xiong, J. Baicalin attenuates aflatoxin b(1)-induced hepatotoxicity via suppressing c-jun-n-terminal kinase-mediated cell apoptosis. Mycotoxin Res. 2024, 40, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Luo, J.Z.; Lan, C.L.; Teng, X.; Ge, B.; Liu, J.Q.; Xie, H.X.; Yang, K.J.; Qin, C.J.; Zhou, X.; et al. Baicalin protects against hepatocyte injury caused by aflatoxin b(1) via the tp53-related ferroptosis pathway. Ecotoxicol. Environ. Saf. 2024, 281, 116661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.Y.; Qi, M.; Gao, X.; Zhao, L.; Liu, J.; Gu, C.Q.; Song, W.J.; Krumm, C.S.; Sun, L.H.; Qi, D.S. Response of the hepatic transcriptome to aflatoxin b1 in ducklings. Toxicon 2016, 111, 69–76. [Google Scholar] [CrossRef]
- Ma, M.; Wang, Q.; Liu, Y.; Li, G.; Liu, L.; Wang, G.; Guo, Y.; Huang, S.; Ma, Q.; Ji, C.; et al. Bacillus cota laccase improved the intestinal health, amino acid metabolism and hepatic metabolic capacity of pekin ducks fed naturally contaminated afb(1) diet. J. Anim. Sci. Biotechnol. 2024, 15, 138. [Google Scholar] [CrossRef]
- GB 13078-2017; National Standard of the People’s Republic of China GB 13078-2017 Feed Hygiene Standards. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2025; Volume NY/T 2071.
- Park, S.W.; Lee, C.H.; Kim, Y.S.; Kang, S.S.; Jeon, S.J.; Son, K.H.; Lee, S.M. Protective effect of baicalin against carbon tetrachloride-induced acute hepatic injury in mice. J. Pharmacol. Sci. 2008, 106, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, Y.; Yang, X.; Liang, B.; Gao, H.; Yang, T. A potential role of baicalin to inhibit apoptosis and protect against acute liver and kidney injury in rat preeclampsia model. Biomed. Pharmacother. 2018, 108, 1546–1552. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Bai, R.; Wang, M.; Du, S. Baicalin attenuates alcoholic liver injury through modulation of hepatic oxidative stress, inflammation and sonic hedgehog pathway in rats. Cell Physiol. Biochem. 2016, 39, 1129–1140. [Google Scholar] [CrossRef]
- Ganguly, R.; Kumar, R.; Pandey, A.K. Baicalin provides protection against fluoxetine-induced hepatotoxicity by modulation of oxidative stress and inflammation. World J. Hepatol. 2022, 14, 729–743. [Google Scholar] [CrossRef]
- Chu, Y.; Wang, H.; Xu, X.; Ji, Y.; Zhao, Y.; Yu, Q.; Rajput, S.A.; Xue, Y.; Qi, D. Protective effect of lipoic acid on oxidative stress and tissue damage induced by aflatoxin b(1) in young laying hens. Toxins 2025, 17, 184. [Google Scholar] [CrossRef]
- Pożarska, A.; Karpiesiuk, K.; Kozera, W.; Czarnik, U.; Dąbrowski, M.; Zielonka, Ł. Afb1 toxicity in human food and animal feed consumption: A review of experimental treatments and preventive measures. Int. J. Mol. Sci. 2024, 25, 5305. [Google Scholar] [CrossRef]
- Karimi Torshizi, M.A.; Sedaghat, A. A consortium of detoxifying bacteria mitigates the aflatoxin b1 toxicosis on performance, health, and blood constituents of laying hens. Poult. Sci. 2023, 102, 102601. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Wang, L.; Yang, P.; Liu, Z.; Rajput, S.A.; Hassan, M.; Qi, D. Epigallocatechin gallate and glutathione attenuate aflatoxin b(1)-induced acute liver injury in ducklings via mitochondria-mediated apoptosis and the nrf2 signalling pathway. Toxins 2022, 14, 876. [Google Scholar] [CrossRef]
- Taranu, I.; Hermenean, A.; Bulgaru, C.; Pistol, G.C.; Ciceu, A.; Grosu, I.A.; Marin, D.E. Diet containing grape seed meal by-product counteracts afb1 toxicity in liver of pig after weaning. Ecotoxicol. Environ. Saf. 2020, 203, 110899. [Google Scholar] [CrossRef]
- Ofori-Attah, E.; Hashimoto, M.; Oki, M.; Kadowaki, D. Therapeutic effect of natural products and dietary supplements on aflatoxin-induced nephropathy. Int. J. Mol. Sci. 2024, 25, 2849. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, S.; Cao, H.; Wu, H.; Liang, Y.; Teng, C.B.; Yu, H.F. Detoxification of aflatoxin b(1) by phytochemicals in agriculture and food science. J. Agric. Food Chem. 2024, 72, 14481–14497. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Tian, E.; Hao, Z.; Tang, S.; Wang, Z.; Sharma, G.; Jiang, H.; Shen, J. Aflatoxin b1 toxicity and protective effects of curcumin: Molecular mechanisms and clinical implications. Antioxidants 2022, 11, 2031. [Google Scholar] [CrossRef]
- Lumsangkul, C.; Kaewtui, P.; Huanhong, K.; Tso, K.H. Antioxidative and antimycotoxigenic efficacies of thunbergia laurifolia lindl. For addressing aflatoxicosis in cherry valley ducks. Toxins 2024, 16, 334. [Google Scholar] [CrossRef]
- Feng, G.D.; He, J.; Ao, X.; Chen, D.W. Effects of maize naturally contaminated with aflatoxin b1 on growth performance, intestinal morphology, and digestive physiology in ducks. Poult. Sci. 2017, 96, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.Q.; Wang, Y.Z.; Zhao, C.X.; Zhao, B.Y.; Wang, J.G. Baicalin: An active natural product with potential medicinal values. J. Asian Nat. Prod. Res. 2025, 27, 1087–1111. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, S.; Ge, H.; Wang, H. The protective role of baicalin regulation of autophagy in cancers. Cytotechnology 2025, 77, 33. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, K.; Liu, K.; Pei, H.; Shi, K.; He, Z.; Zong, Y.; Du, R. Protective effect of baicalin on chlorpyrifos-induced liver injury and its mechanism. Molecules 2023, 28, 7771. [Google Scholar] [CrossRef]
- Bao, M.; Ma, Y.; Liang, M.; Sun, X.; Ju, X.; Yong, Y.; Liu, X. Research progress on pharmacological effects and new dosage forms of baicalin. Vet. Med. Sci. 2022, 8, 2773–2784. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Feng, T.; Yan, Z.; Zhu, D.; Lin, H.; Iqbal, M.; Deng, D.; Kulyar, M.F.; Shen, Y. Hedyotis diffusa alleviate aflatoxin b1-induced liver injury in ducks by mediating nrf2 signaling pathway. Ecotoxicol. Environ. Saf. 2023, 249, 114339. [Google Scholar] [CrossRef] [PubMed]
- Saghir, S.A.M.; Al Hroob, A.M.; Al-Tarawni, A.H.; Abdulghani, M.A.M.; Tabana, Y.; Aldhalmi, A.K.; Mothana, R.A.; Al-Yousef, H.M. Effect of lactiplantibacillus plantarum on the growth, hemato-biochemical, inflammation, apoptosis, oxidative stress markers, involved gens and histopathological alterations in growing rabbits challenged with aflatoxin b1. Poult. Sci. 2024, 103, 104002. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Y.; Wang, F.; Yang, X.; Yao, F.; Ming, K.; Yuan, W.; Zeng, L.; Liu, J. Antiviral effect of baicalin phospholipid complex against duck hepatitis a virus type 1. Poult. Sci. 2018, 97, 2722–2732. [Google Scholar] [CrossRef]
- Liang, S.; Deng, X.; Lei, L.; Zheng, Y.; Ai, J.; Chen, L.; Xiong, H.; Mei, Z.; Cheng, Y.C.; Ren, Y. The comparative study of the therapeutic effects and mechanism of baicalin, baicalein, and their combination on ulcerative colitis rat. Front. Pharmacol. 2019, 10, 1466. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, Q. Aflatoxin b(1) in poultry liver: Toxic mechanism. Toxicon 2023, 233, 107262. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, Q.; Wu, J.; Wu, W.; Jiang, J.; Yan, H.; Huang, J.; Sun, Y.; Deng, Y. The metabolism and biotransformation of afb(1): Key enzymes and pathways. Biochem. Pharmacol. 2022, 199, 115005. [Google Scholar] [CrossRef]
- Chen, Y.; Li, R.; Chang, Q.; Dong, Z.; Yang, H.; Xu, C. Lactobacillus bulgaricus or lactobacillus rhamnosus suppresses nf-κb signaling pathway and protects against afb1-induced hepatitis: A novel potential preventive strategy for aflatoxicosis? Toxins 2019, 11, 17. [Google Scholar] [CrossRef]
- Li, X.; Lv, Z.; Chen, J.; Nepovimova, E.; Long, M.; Wu, W.; Kuca, K. Bacillus amyloliquefaciens b10 can alleviate liver apoptosis and oxidative stress induced by aflatoxin b1. Food Chem. Toxicol. 2021, 151, 112124. [Google Scholar] [CrossRef]
- Su, Q.; Pan, H.; Hong, P.; You, Y.; Wu, Y.; Zou, J.; Sun, J.; Rao, G.; Liao, J.; Tang, Z.; et al. Protective effect of curcumin against endoplasmic reticulum stress and lipid metabolism disorders in afb1-intoxicated duck liver. Mycotoxin Res. 2025, 41, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yang, K.; He, Z.; Su, L.; Tan, X.; Li, Z.; Song, W.; Cao, L.; Ma, Y. Tianjihuang compound alleviates aflatoxin b(1)-induced hepatic steatosis and fibrosis by targeting pparα-tgf-β pathway in ducklings. Poult. Sci. 2025, 104, 105006. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Li, S.; Wang, P.; Zhu, D.; Xu, Z.; Wang, L.; Li, A.; Kulyar, M.F.; Shen, Y. Hepatoprotective effects of radix bupleuri extract on aflatoxin b1-induced liver injury in ducks. Ecotoxicol. Environ. Saf. 2024, 283, 116781. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, Q.; Zhang, X.; Yang, X.; Shi, Y.; Li, Y.; Song, M. Curcumin alleviates aflatoxin b(1)-induced liver pyroptosis and fibrosis by regulating the jak2/nlrp3 signaling pathway in ducks. Foods 2023, 12, 1006. [Google Scholar] [CrossRef]
| Indicators | Control | AFB1 (μg/kg Body Weight/Day) | SEM | p Value | ||
|---|---|---|---|---|---|---|
| 6 | 12 | 24 | ||||
| ADG (g/day) | 28.64 a | 27.57 a | 24.29 b | 21.74 b | 0.779 | 0.001 |
| ADFI (g/day) | 58.66 | 56.06 | 52.87 | 50.77 | / | / |
| FCR | 1.98 | 2.03 | 2.17 | 2.31 | / | / |
| Indicators | Control (A) | AFB1 (B) | 25BA (C) | 50BA (D) | 100BA (E) | SEM | p Value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| A vs. B | C vs. B | D vs. B | E vs. B | |||||||
| ADG (g/day) | 26.48 | 23.10 | 24.16 | 26.68 | 27.84 | 0.531 | 0.005 | 0.942 | 0.162 | 0.015 |
| ADFI (g/day) | 51.60 | 49.83 | 44.99 | 48.14 | 48.80 | 1.118 | 0.572 | 0.474 | 0.969 | 0.995 |
| FCR | 1.96 | 2.13 | 1.86 | 1.84 | 1.75 | 0.050 | 0.074 | 0.173 | 0.141 | 0.035 |
| Indicators | Control (A) | AFB1 (B) | 25BA (C) | 50BA (D) | 100BA (E) | SEM | p Value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| A vs. B | C vs. B | D vs. B | E vs. B | |||||||
| ALB (g/L) | 11.76 | 10.43 | 11.77 | 11.83 | 12.24 | 0.214 | 0.040 | 0.027 | 0.030 | 0.005 |
| AST (U/L) | 22.00 | 35.13 | 22.60 | 20.50 | 21.00 | 2.078 | 0.013 | 0.010 | 0.002 | 0.005 |
| ALT (U/L) | 49.25 | 63.00 | 44.30 | 49.86 | 44.70 | 2.257 | 0.015 | 0.002 | 0.026 | 0.003 |
| GGT (U/L) | 4.40 | 7.10 | 5.00 | 5.33 | 4.70 | 0.193 | 0.020 | 0.063 | 0.160 | 0.027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Zhang, X.; Zhang, J.; Wang, X.; Wen, D.; Guo, P.; Xiong, J.; Qiu, Y. Effects of Dietary Baicalin on Aflatoxin B1-Induced Growth Performance and Liver Health in Ducklings. Animals 2025, 15, 2325. https://doi.org/10.3390/ani15162325
Lu Q, Zhang X, Zhang J, Wang X, Wen D, Guo P, Xiong J, Qiu Y. Effects of Dietary Baicalin on Aflatoxin B1-Induced Growth Performance and Liver Health in Ducklings. Animals. 2025; 15(16):2325. https://doi.org/10.3390/ani15162325
Chicago/Turabian StyleLu, Qirong, Xue Zhang, Jie Zhang, Xinyue Wang, Defeng Wen, Pu Guo, Jianglin Xiong, and Yinsheng Qiu. 2025. "Effects of Dietary Baicalin on Aflatoxin B1-Induced Growth Performance and Liver Health in Ducklings" Animals 15, no. 16: 2325. https://doi.org/10.3390/ani15162325
APA StyleLu, Q., Zhang, X., Zhang, J., Wang, X., Wen, D., Guo, P., Xiong, J., & Qiu, Y. (2025). Effects of Dietary Baicalin on Aflatoxin B1-Induced Growth Performance and Liver Health in Ducklings. Animals, 15(16), 2325. https://doi.org/10.3390/ani15162325




