Reproductive Challenges in Ruminants Under Heat Stress: A Review of Follicular, Oocyte, and Embryonic Responses
Simple Summary
Abstract
1. Introduction
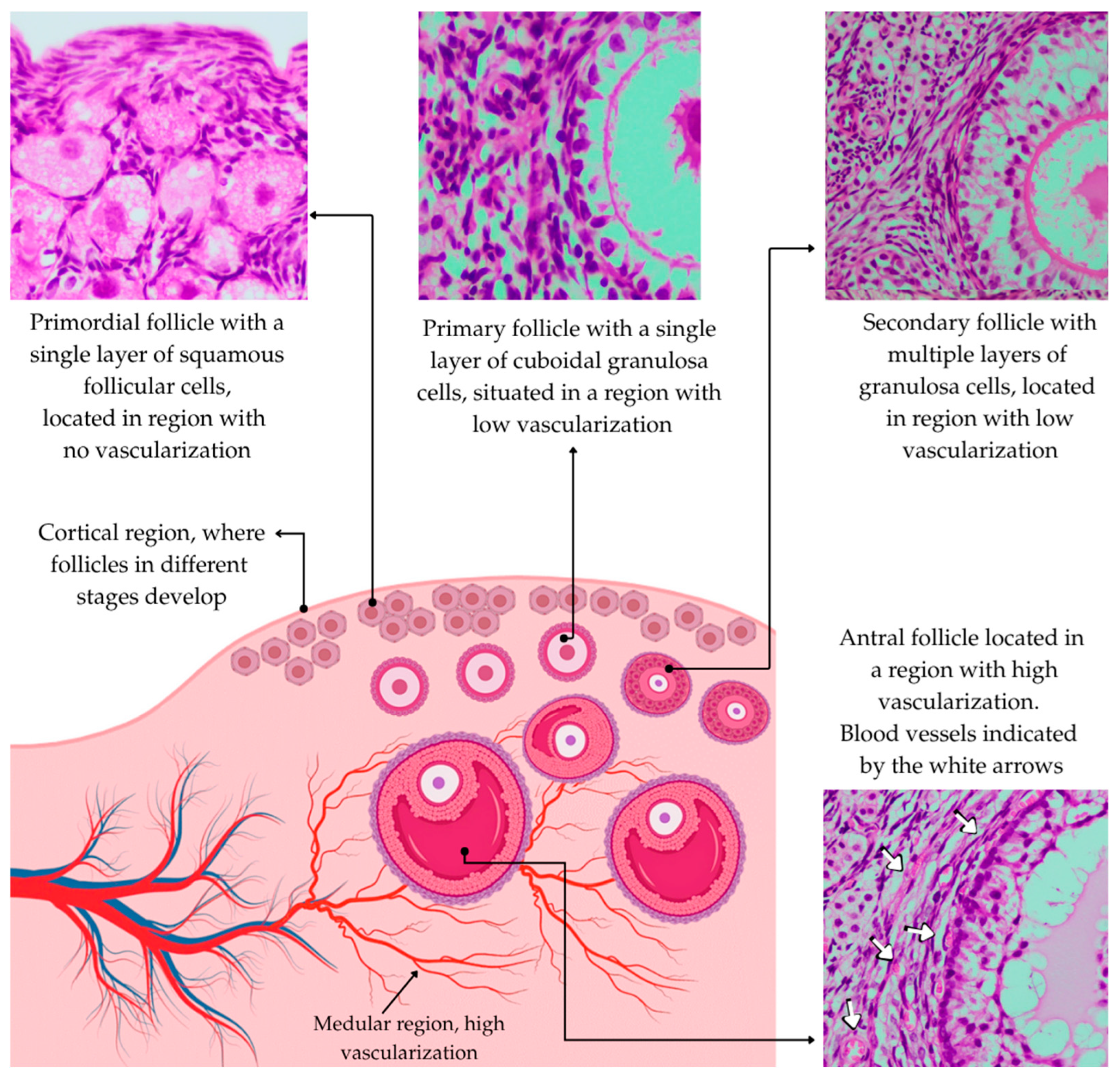
2. Effects of Heat Stress on Early Follicles
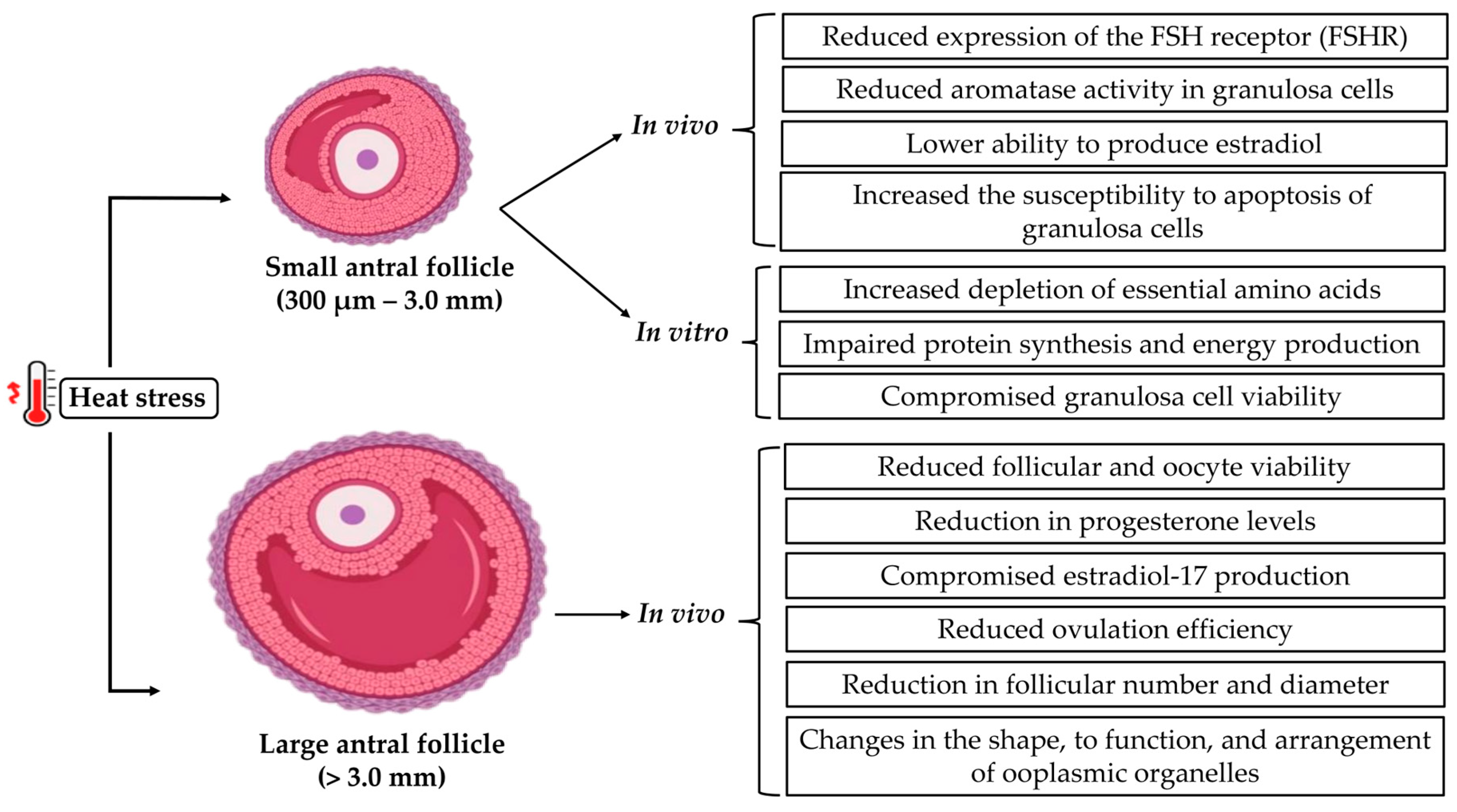
3. Effects of Heat Stress on Antral Follicles
4. Effects of Heat Stress on Steroidogenic Cells and Pathways
5. Heat Stress Affects Oocyte Maturation
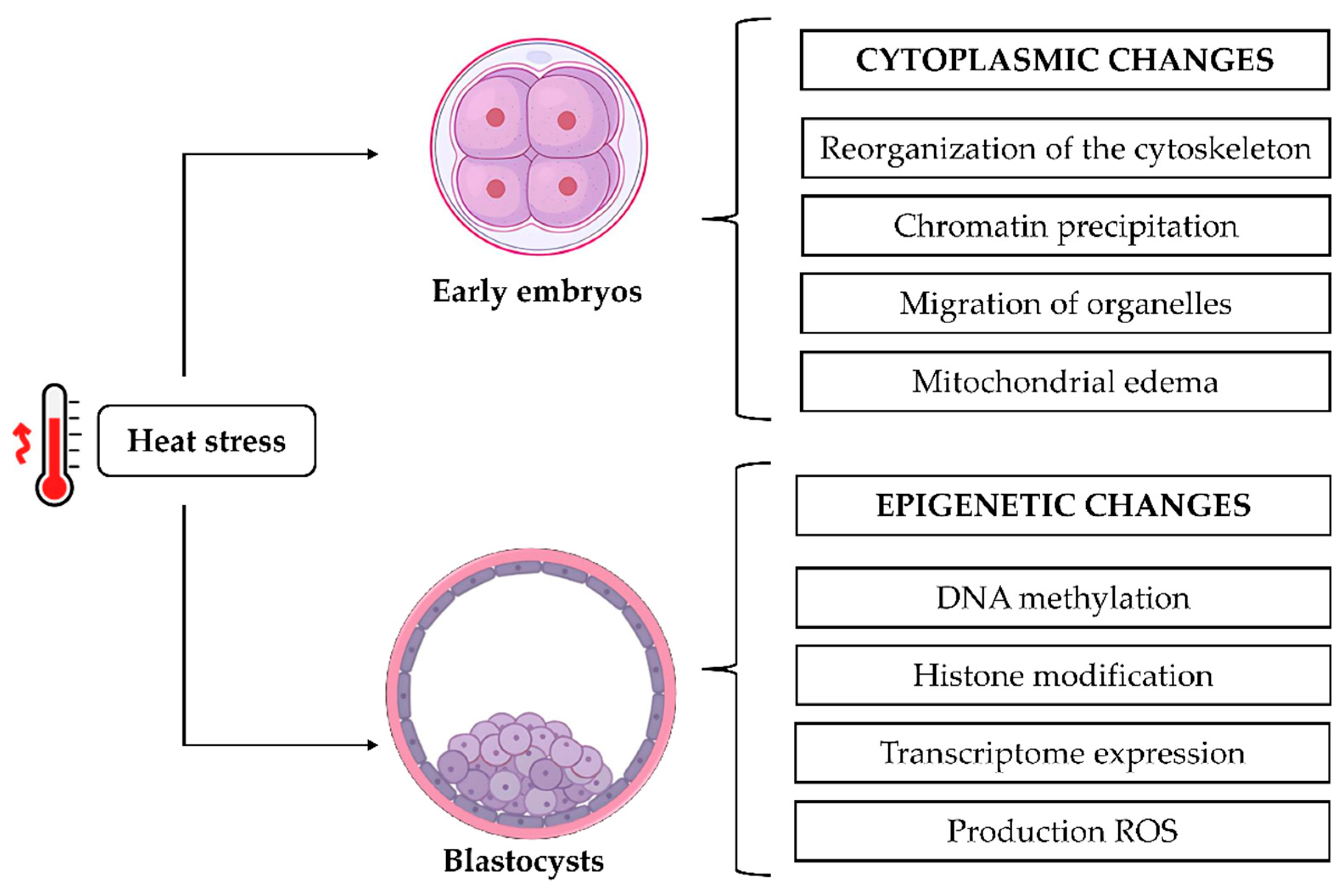
6. Heat Stress and Embryonic Development
7. Final Considerations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pulido-Rodríguez, L.F.; Titto, C.G.; de Andrade Bruni, G.; Froge, G.A.; Fuloni, M.F.; Payan-Carrera, R.; Henrique, F.L.; de Mira Geraldo, A.C.A.P.; Pereira, A.M.F. Effect of Solar Radiation on Thermoregulatory Responses of Santa Inês Sheep and their Crosses with Wool and Hair Dorper Sheep. Small Rumin. Res. 2021, 202, 106470. [Google Scholar] [CrossRef]
- Yan, G.; Li, H.; Shi, Z. Evaluation of Thermal Indices as the Indicators of Heat Stress in Dairy Cows in a Temperate Climate. Animals 2021, 11, 2459. [Google Scholar] [CrossRef]
- Cartwright, S.L.; McKechnie, M.; Schmied, J.; Livernois, A.M.; Mallard, B.A. Effect of In-Vitro Heat Stress Challenge on the Function of Blood Mononuclear Cells from Dairy Cattle Ranked as High, Average and Low Immune Responders. BMC Vet. Res. 2021, 17, 233. [Google Scholar] [CrossRef]
- Thornton, P.; Nelson, G.; Mayberry, D.; Herrero, M. Impacts of Heat Stress on Global Cattle Production during the 21st Century: A Modelling Study. Lancet Planet. Health 2022, 6, e192–e201. [Google Scholar] [CrossRef]
- Gujar, G.; Tiwari, M.; Yadav, N.; Monika, D. Heat Stress Adaptation in Cows –Physiological Responses and Underlying Molecular Mechanisms. J. Therm. Biol. 2023, 118, 103740. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.F.; Chiu, C.L.; Liu, Y.H.; Chang, C.H.; Shao, J.C.; Guo, S.S.; Liao, Y.L.; Chen, C.H.; Tseng, C.D.; Chao, P.J.; et al. Establishment and Risk Factor Assessment of the Abnormal Body Temperature Probability Prediction Model (abtp) for Dairy Cattle. Sci. Rep. 2024, 14, 14557. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, S.L.; Schmied, J.; Livernois, A.M.; Mallard, B.A. Effect of In-Vivo Heat Challenge on Physiological Parameters and Function of Peripheral Blood Mononuclear Cells in Immune Phenotyped Dairy Cattle. Vet. Immunol. Immunopathol. 2022, 246, 110405. [Google Scholar] [CrossRef]
- Herbut, P.; Angrecka, S.; Godyń, D.; Hoffmann, G. The Physiological and Productivity Effects of Heat Stress in Cattle—A Review. Ann. Anim. Sci. 2019, 19, 579–593. [Google Scholar] [CrossRef]
- de Aguiar, L.H.; Hyde, K.A.; Pedroza, G.H.; Denicol, A.C. Heat Stress Impairs In Vitro Development of Preantral Follicles of Cattle. Anim. Reprod. Sci. 2020, 213, 106277. [Google Scholar] [CrossRef]
- Ali, A.I.M.; Sandi, S.; Fariani, A.; Darussalam, A. Physiological Changes and Behavioral Responses in Heat-Stressed Goats under Humid Tropical Environment. Int. J. Biometeorol. 2023, 67, 1757–1764. [Google Scholar] [CrossRef]
- Feng, X.; Li, C.; Zhang, H.; Zhang, P.; Shahzad, M.; Du, W.; Zhao, X. Heat-Stress Impacts on Developing Bovine Oocytes: Unraveling Epigenetic Changes, Oxidative Stress, and Developmental Resilience. Int. J. Mol. Sci. 2024, 25, 4808. [Google Scholar] [CrossRef]
- Carvajal, M.A.; Alaniz, A.J.; Gutiérrez-Gómez, C.; Vergara, P.M.; Sejian, V.; Bozinovic, F. Increasing Importance of Heat Stress for Cattle Farming under Future Global Climate Scenarios. Sci. Total Environ. 2021, 801, 149661. [Google Scholar] [CrossRef]
- Romo-Barron, C.B.; Diaz, D.; Portillo-Loera, J.J.; Romo-Rubio, J.A.; Jimenez-Trejo, F.; Montero-Pardo, A. Impact of Heat Stress on the Reproductive Performance and Physiology of Ewes: A Systematic Review and Meta-Analyses. Int. J. Biometeorol. 2019, 63, 949–962. [Google Scholar] [CrossRef]
- Danso, F.; Iddrisu, L.; Lungu, S.E.; Zhou, G.; Ju, X. Effects of Heat Stress on Goat Production and Mitigating Strategies: A Review. Animals 2024, 14, 1793. [Google Scholar] [CrossRef]
- Dovolou, E.; Giannoulis, T.; Nanas, I.; Amiridis, G.S. Heat Stress: A Serious Disruptor of the Reproductive Physiology of Dairy Cows. Animals 2023, 13, 1846. [Google Scholar] [CrossRef] [PubMed]
- Hooper, H.B.; Silva, P.D.S.; de Oliveira, S.A.; Meringhe, G.K.F.; Lacasse, P.; Negrão, J.A. Effect of Heat Stress in Late Gestation on Subsequent Lactation Performance and Mammary Cell Gene Expression of Saanen Goats. J. Dairy Sci. 2020, 103, 1982–1992. [Google Scholar] [CrossRef]
- Adjassin, J.S.; Assani, A.S.; Bani, A.A.; Sanni Worogo, H.S.; Adégbeïga Alabi, C.D.; Comlan Assogba, B.G.; Virgile Azando, E.B.; Alkoiret, I.T. Impact of Heat Stress on Reproductive Performances in Dairy Goats under Tropical Sub-Humid Environment. Heliyon 2022, 8, e08971. [Google Scholar] [CrossRef]
- Roth, Z. Heat Stress Reduces Maturation and Developmental Capacity in Bovine Oocytes. Reprod. Fertil. Dev. 2021, 33, 66–75. [Google Scholar] [CrossRef]
- Murugan, R.; Saraswat, P.; Sinha, S. Heat Stress in Dairy Cows: A Comprehensive Examination of Wellbeing, Milk Yield, Sexual Health. Rev. Electron. Vet. 2023, 24, 522–532. [Google Scholar]
- Ozawa, M.; Tabayashi, D.; Latief, T.A.; Shimizu, T.; Oshima, I.; Kanai, Y. Alterations in Follicular Dynamics and Steroidogenic Abilities Induced by Heat Stress During Follicular Recruitment in Goats. Reproduction 2005, 129, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Diaz, F.A.; Gutierrez-Castillo, E.J.; Foster, B.A.; Hardin, P.T.; Bondioli, K.R.; Jiang, Z. Evaluation of Seasonal Heat Stress on Transcriptomic Profiles and Global DNA Methylation of Bovine Oocytes. Front. Genet. 2021, 12, 699920. [Google Scholar] [CrossRef]
- Maddahi, A.; Saberivand, A.; Hamali, H.; Jafarpour, F.; Saberivand, M. Exploring the Impact of Heat Stress on Oocyte Maturation and Embryo Development in Dairy Cattle using a Culture Medium Supplemented with Vitamins E, C, and Coenzyme Q10. J. Therm. Biol. 2024, 119, 103759. [Google Scholar] [CrossRef]
- Amaral, C.S.; Koch, J.; Correa Júnior, E.E.; Bertolin, K.; Mujica, L.K.S.; Fiorenza, M.F.; Rosa, S.G.; Nogueira, C.W.; Comim, F.V.; Portela, V.V.M.; et al. Heat Stress on Oocyte or Zygote Compromises Embryo Development, Impairs Interferon Tau Production and Increases Reactive Oxygen Species and Oxidative Stress in Bovine Embryos Produced In Vitro. Mol. Reprod. Dev. 2020, 87, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Stefanska, B.; Pruszynska-Oszmalek, E.; Fievez, V.; Purwin, C.; Nowak, W. Impact of Heat Stress during Close-up Dry Period on Performance, Fertility and Immunometabolic Blood indices of Dairy Cows: Prospective Cohort Study. Sci. Rep. 2024, 14, 21211. [Google Scholar] [CrossRef]
- Roth, Z. Influence of Heat Stress on Reproduction in Dairy Cows—Physiological and Practical Aspects. J. Anim. Sci. 2020, 98, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Chawicha, T.G.; Mummed, Y.Y. An Overview of how Heat Stress Impacts Dairy Cattle Fertility. Multidiscip. Rev. 2022, 5, e2022014. [Google Scholar] [CrossRef]
- Longo, M.; Liuzzi, F.; De Carlini, S.; La Marca, A. The Role of LH in Follicle Development: From Physiology to new Clinical Implications. Reprod. Biol. Endocrinol. 2025, 23, 22. [Google Scholar] [CrossRef]
- Torres-Júnior, J.D.S.; de FA Pires, M.; de Sa, W.F.; Ferreira, A.D.M.; Viana, J.H.; Camargo, L.S.; Ramos, A.A.; Folhadella, I.M.; Polisseni, J.; de Freitas, C.; et al. Effect of Maternal Heat-Stress on Follicular Growth and Oocyte Competence in Bos indicus cattle. Theriogenology 2008, 69, 155–166. [Google Scholar] [CrossRef]
- Hernandez-Fonseca, H.J.; Bosch, P.; Miller, D.M.; Wininger, J.D.; Massey, J.B.; Brackett, B.G. Time course of follicular development after bovine ovarian tissue transplantation in male non-obese diabetic severe combined immunodeficient mice. Fertil. Steril. 2005, 83 (Suppl. 1), 1180–1187. [Google Scholar] [CrossRef]
- Denicol, A.C.; Siqueira, L.G.B. Maternal Contributions to Pregnancy Success: From Gamete Quality to Uterine Environment. Anim. Reprod. 2023, 8, e20230085. [Google Scholar] [CrossRef]
- Hansen, P.J. Antecedents of Mammalian Fertility: Lessons from the Heat-Stressed Cow Regarding the Importance of Oocyte Competence for Fertilization and Embryonic Development. Anim. Front. 2013, 3, 34–39. [Google Scholar] [CrossRef][Green Version]
- Cardone, D.A.; Cáceres, A.R.R.; Sanhueza, M.A.; Bruna, F.A.; Laconi, M.R. Effects of Short-Term In Vitro Heat Stress on Bovine Preantral Follicles. Livest. Sci. 2022, 264, 105076. [Google Scholar] [CrossRef]
- Paes, V.M.; Vieira, L.A.; Correia, H.H.V.; Sa, N.A.R.; Moura, A.A.A.; Sales, A.D.; Rodrigues, A.P.R.; Magalhães-Padilha, D.M.; Santos, F.W.; Apgar, G.A.; et al. Effect of Heat Stress on the Survival and Development of In Vitro Cultured Bovine Preantral Follicles and on In Vitro Maturation of Cumulus–Oocyte Complex. Theriogenology 2016, 86, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, A.L.R.; Luna, H.S. Morfometria e Número de Células da Granulosa de Folículos Pré-antrais Bovinos Submetidos ao Estresse Calórico In Vitro. Acta Vet. Bras. 2008, 2, 85–88. [Google Scholar] [CrossRef]
- Nabenishi, H.; Ohta, H.; Nishimoto, T.; Morita, T.; Ashizawa, K.; Tsuzuki, Y. The Effects of Cysteine Addition During In Vitro Maturation on the Developmental Competence, ROS, GSH and Apoptosis Level of Bovine Oocytes Exposed to Heat Stress. Zygote 2012, 20, 249–259. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Ahmed, S.M.; El-Gammal, Z.; Shouman, S.; Ahmed, A.; Mansour, R.; El-Badri, N. Oocyte Aging: The Role of Cellular and Environmental Factors and Impact on Female Fertility. Adv. Exp. Med. Biol. 2020, 1247, 109–123. [Google Scholar]
- Johnson, H.D. Bioclimates and Livestock. In Bioclimatology and the Adaptation of Livestock, Chapter 1; Johnson, H.D., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1987; pp. 3–16. [Google Scholar]
- Gwazdauskas, F.C.; Thatcher, W.W.; Wilcox, C.J. Physiological, Environmental, and Hormonal Factors at Insemination Which May Affect Conception. J. Dairy Sci. 1973, 56, 873–877. [Google Scholar] [CrossRef]
- Oliveira, M.E.F.; Ferreira, R.M.; Mingoti, G.Z. Local and Systemic Control of Bovine Follicular Growth and Selection. Braz. J. Anim. Reprod. 2011, 35, 418–432. [Google Scholar]
- Baumgarten, S.C.; Stocco, C. Granulosa Cells. Encycl. Reprod. 2018, 2, 8–13. [Google Scholar] [CrossRef]
- Kawano, K.; Sakaguchi, K.; Ninpetch, N.; Yanagawa, Y.; Katagiri, S. Physiological High Temperatures Alter the Amino Acid Metabolism of Bovine Early Antral Follicles. J. Reprod. Dev. 2024, 70, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, E.C.; Nascimento, D.R.; Barrozo, L.G.; Paulino, L.R.F.M.; de Assis, E.I.T.; Silva, J.R.V. Acquisition of Gonadotropin Dependence by Early Antral Follicles and the Challenges to Promote Their Growth In Vitro. Ciênc. Anim. Bras. 2024, 25, e75908E. [Google Scholar] [CrossRef]
- Varnosfaderani, S.; Hajian, M.; Jafarpour, F.; Ghazvini Zadegan, F.; Nasr-Esfahani, M.H. Granulosa Secreted Factors Improve the Developmental Competence of Cumulus Oocyte Complexes from Small Antral Follicles in Sheep. PLoS ONE 2020, 15, e0229043. [Google Scholar] [CrossRef]
- Kawano, K.; Sakaguchi, K.; Madalitso, C.; Ninpetch, N.; Kobayashi, S.; Furukawa, E.; Yanagawa, Y.; Katagiri, S. Effect of Heat Exposure on the Growth and Developmental Competence of Bovine Oocytes Derived from Early Antral Follicles. Sci. Rep. 2022, 12, 8857. [Google Scholar] [CrossRef]
- Cortvrindt, R.; Smitz, J. In Vitro Follicle Growth: Achievements in Mammalian Species. Reprod. Domest. Anim. 2001, 36, 3–9. [Google Scholar] [CrossRef]
- Sammad, A.; Umer, S.; Shi, R.; Zhu, H.; Zhao, X.; Wang, Y. Dairy Cow Reproduction under the Influence of Heat Stress. J. Anim. Physiol. Nutr. 2020, 104, 978–986. [Google Scholar] [CrossRef]
- Gómez-Guzmán, J.A.; Parra-Bracamonte, G.M.; Velazquez, M.A. Impact of Heat Stress on Oocyte Developmental Competence and Pre-Implantation Embryo Viability in Cattle. Animals 2024, 14, 2280. [Google Scholar] [CrossRef]
- Cavalcanti, G.S.; Carvalho, K.C.; da Silva Ferreira, C.; Alvarez, P.A.C.; Monteleone, P.A.A.; Baracat, E.C.; Soares Júnior, J.M. Granulosa Cells and Follicular Development: A Brief Review. Rev. Assoc. Med. Bras. 2023, 69, e20230175. [Google Scholar] [CrossRef]
- Batalha, I.M.; Maylem, E.R.S.; Spicer, L.J.; Bello, C.A.P.; Archilia, E.C.; Schütz, L.F. Effects of Asprosin on Estradiol and Progesterone Secretion and Proliferation of Bovine Granulosa Cells. Mol. Cell. Endocrinol. 2023, 565, 111890. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. Steroidogenic Acute Regulatory Protein (Star), A Novel Mitochondrial Cholesterol Transporter. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2007, 1771, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Galano, M.; Venugopal, S.; Papadopoulos, V. Role of STAR and SCP2/SCPx in the Transport of Cholesterol and Other Lipids. Int. J. Mol. Sci. 2022, 23, 12115. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.; Rosal, K.; Chung, B. Function of CYP11A1 in the mitochondria. Mol. Cell. Endocrinol. 2017, 441, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Redouane, S.; Harmak, H.; Elkarhat, Z.; Charoute, H.; Malki, A.; Barakat, A.; Rouba, H. Exploring the impact of CYP11A1’s missense SNPs on the interaction between CYP11A1 and cholesterol: A comprehensive structural analysis and MD simulation study. Comput. Biol. Chem. 2023, 106, 107937. [Google Scholar] [CrossRef] [PubMed]
- Haire, A.; Bai, J.; Zhao, X.; Song, Y.; Zhao, G.; Dilixiati, A.; Li, J.; Sun, W.Q.; Wan, P.; Fu, X.; et al. Identifying the Heat Resistant Genes by Multi-Tissue Transcriptome Sequencing Analysis in Turpan Black sheep. Theriogenology 2022, 179, 78–86. [Google Scholar] [CrossRef]
- Cheewasopit, W.; Laird, M.; Glister, C.; Knight, P.G. Myostatin is Expressed in Bovine Ovarian Follicles and Modulates Granulosal and Thecal Steroidogenesis. Reproduction 2018, 156, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.S. Hormonal Control of Gene Expression in the Ovary. Endocr. Rev. 1994, 15, 725–751. [Google Scholar] [CrossRef]
- Alemu, T.W.; Pandey, H.O.; Salilew Wondim, D.; Gebremedhn, S.; Neuhof, C.; Tholen, E.; Holker, M.; Schellander, K.; Tesfaye, D. Oxidative and Endoplasmic Reticulum Stress Defense Mechanisms of Bovine Granulosa Cells Exposed to Heat Stress. Theriogenology 2018, 110, 130–141. [Google Scholar] [CrossRef]
- Khan, A.; Dou, J.; Wang, Y.; Jiang, X.; Khan, M.Z.; Luo, H.; Usman, T.; Zhu, H. Evaluation of Heat Stress Effects on Cellular and Transcriptional Adaptation of Bovine Granulosa Cells. J. Anim. Sci. Biotechnol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Khan, A.; Khan, M.Z.; Dou, J.; Umer, S.; Xu, H.; Sammad, A.; Zhu, H.-B.; Wang, Y. RNAi-Mediated Silencing of Catalase Gene Promotes Apoptosis and Impairs Proliferation of Bovine Granulosa Cells under Heat Stress. Animals 2020, 10, 1060. [Google Scholar] [CrossRef]
- Szalai, S.; Bodnár, Á.; Fébel, H.; Bakony, M.; Jurkovich, V. Effects of Heat Stress on Estrus Expression and Pregnancy in Dairy Cows. Animals 2025, 15, 1688. [Google Scholar] [CrossRef]
- Fialho, A.L.L.; Souza-Cáceres, M.B.; Silva, W.A.L.; Arruda, E.D.S.; Kischel, H.; Ribeiro-Ferreira, M.G.C.; Medeiros, C.F.; Silva, J.R.; Oliveira, M.V.M.; Ferraz, A.L.J.; et al. Efeito do Estresse Térmico Calórico Agudo e Crônico Sobre a Qualidade Oocitária de Bovinos de Raças Adaptadas. Arq. Bras. Med. Vet. Zootec. 2018, 70, 64–72. [Google Scholar] [CrossRef]
- Faheem, M.S.; Chhillar, S.; Saraf, K.K.; Singh, I.; Sharma, R.K.; Singh, A.K. Adaptive and Biological Responses of Buffalo Granulosa Cells Exposed to Heat Stress under In Vitro Condition. Reprod. Domest. Anim. 2021, 11, 794. [Google Scholar] [CrossRef]
- Sammad, A.; Hu, L.; Luo, H.; Abbas, N.; Zhang, S.; Yan, P.; Zhao, S. Transcriptome Reveals Granulosa Cells Coping through Redox, Inflammatory and Metabolic Mechanisms under Acute Heat Stress. Cells 2022, 11, 1443. [Google Scholar] [CrossRef]
- Li, L.; Wu, J.; Luo, M.; Sun, Y.; Wang, G. The Effect of Heat Stress on Gene Expression, Synthesis of Steroids, and Apoptosis in Bovine Granulosa Cells. Cell Stress Chaperones 2016, 21, 467–475. [Google Scholar] [CrossRef]
- Sammad, A.; Hu, L.; Luo, H.; Abbas, N.; Zhang, S.; Yan, P.; Zhao, S. Joint Transcriptome and Metabolome Analysis Prevails the Biological Mechanisms Underlying the Pro-Survival Fight In Vitro Heat-Stressed Granulosa Cells. Biology 2022, 11, 839. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Zhang, Y.; Yang, Q.; Wang, Y.; Zhang, S. Acute Heat Stress Regulates Estradiol Synthesis in Ovine Ovarian Granulosa Cells through the SREBPs/MVK–LHR Pathway. Anim. Reprod. Sci. 2025, 272, 107649. [Google Scholar] [CrossRef]
- Menon, K.M.J.; Menon, B. Regulation of Luteinizing Hormone Receptor Expression by An RNA Binding Protein: Role of Erk Signaling. Indian J. Med. Res. 2014, 140 (Suppl. 1), S112–S119. [Google Scholar] [PubMed]
- Wang, Y.R.; Tian, H.B.; Li, Y.K.; Wang, Y.; Jiao, P.; Zhang, Y.; Zhao, J.L. Heme Oxygenase 1 Regulates Apoptosis Induced by Heat Stress in Bovine Ovarian Granulosa Cells via the ERK1/2 Pathway. J. Cell. Physiol. 2019, 234, 3961–3972. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, X.; Li, Y.; Zhang, S.; Yang, Q.; Wang, Y. Advances in the Effects of Heat Stress on Ovarian Granulosa Cells: Unveiling Novel Ferroptosis Pathways. Vet. Sci. 2024, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, C.; Elsheikh, N.A.H.; Li, C.; Yang, F.; Wang, G.; Li, L. HO-1 Reduces Heat Stress-Induced Apoptosis in Bovine Granulosa Cells by Suppressing Oxidative Stress. Aging 2019, 11, 5535–5547. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Lee, A.S. Role of the Unfolded Protein Response, GRP78 and GRP94 in Organ Homeostasis. J. Cell. Physiol. 2015, 230, 1413–1420. [Google Scholar] [CrossRef]
- de Souza, V.D.G.P.; de Souza, G.T.; de Lemos, D.R.; de Oliveira Guimarães, J.M.; Quintão, C.C.R.; Munk, M.; Saraiva, N.Z.; Luiz de Almeida Camargo, S. Heat Shock during In Vitro Maturation of Bovine Oocytes Disturbs bta-miR-19b and DROSHA Transcripts Abundance after In Vitro Fertilization. Reprod. Domest. Anim. 2021, 56, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Feltes, G.L.; Negri, R.; Raidan, F.S.S.; Viana, A.F.P.; Feres, L.F.R.; Ribeiro, V.M.P.; Cobuci, J.A. Impact of Heat Stress on Genetic Evaluation of Oocyte and Embryo Production in Gir Dairy Cattle. Trop. Anim. Health Prod. 2024, 56, 7. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, M.Z.; Umer, S.; Khan, I.M.; Xu, H.; Zhu, H.; Wang, Y. Cellular and Molecular Adaptation of Bovine Granulosa Cells and Oocytes under Heat Stress. Animals 2020, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, D.-S.; Choi, I.; Kim, D.; Son, J.; Jeon, E.; Jung, D.; Han, M.; Ha, S.; Hwang, S. Effects of Heat Stress on Conception in Holstein and Jersey Cattle and Oocyte Maturation In Vitro. J. Anim. Sci. Technol. 2023, 65, 324–335. [Google Scholar] [CrossRef]
- Wrzecińska, M.; Kowalczyk, A.; Kordan, W.; Cwynar, P.; Czerniawska-Piątkowska, E. Disorder of Biological Quality and Autophagy Process in Bovine Oocytes Exposed to Heat Stress and the Effectiveness of In Vitro Fertilization. Int. J. Mol. Sci. 2023, 24, 11164. [Google Scholar] [CrossRef]
- Mzedawee, H.R.H.; Kowsar, R.; Moradi-Hajidavaloo, R.; Shiasi-Sardoabi, R.; Sadeghi, K.; Nasr-Esfahani, M.H.; Hajian, M. Heat Shock Interferes with the Amino Acid Metabolism of Bovine Cumulus-Oocyte Complexes In Vitro: A Multistep Analysis. Amino Acids 2024, 56, 2. [Google Scholar] [CrossRef]
- Klabnik, J.L.; Beever, J.E.; Payton, R.R.; Lamour, K.H.; Schrick, F.N.; Edwards, J.L. A Step Toward Understanding Direct Impacts of a Higher Estrus-Associated Temperature (HEAT): Transcript Level Changes in Cumulus–Oocyte Complexes Directly Exposed to Acute Elevated Temperature. Animals 2025, 15, 517. [Google Scholar] [CrossRef]
- Serra, E.; Gadau, S.D.; Leoni, G.G.; Naitana, S.; Succu, S. Seasonal Effect on Developmental Competence, Oxidative Status and Tubulin Assessment of Prepubertal Ovine Oocyte. Animals 2021, 11, 1886. [Google Scholar] [CrossRef]
- Özmen, Ö.; Karaman, K. Transcriptome Analysis and Potential Mechanisms of Bovine Oocytes under Seasonal Heat Stress. Anim. Biotechnol. 2023, 34, 1179–1195. [Google Scholar] [CrossRef]
- Barakat, I.A.; Khalil, W.K.; Al-Himaidi, A.R. Curcacyclin A and B Modulate Heat Stress-Induced Apoptosis in Sheep Oocytes during In Vitro Maturation. Small Rumin. Res. 2016, 136, 187–196. [Google Scholar] [CrossRef]
- Ahmadi, E.; Nazari, H.; Hossini-Fahraji, H. Low Developmental Competence and High Tolerance to Thermal Stress of Ovine Oocytes in the Warm Compared with the Cold Season. Trop. Anim. Health Prod. 2019, 51, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Pöhland, R.; Souza-Cáceres, M.B.; Datta, T.K.; Vanselow, J.; da Silva, W.A.L.; Cardoso, C.J.T.; de Sterza, F.A.M. Influence of Thermal Stress During In Vitro Maturation on the Developmental Competence of Oocytes and Embryos and the Expression of Sirtuins in Cumulus Oocyte Complexes in Cattle. Semina 2025, 46, 149–168. [Google Scholar] [CrossRef]
- Gharibzadeh, Z.; Riasi, A.; Ostadhosseini, S.; Hosseini, S.M.; Hajian, M.; Nasr-Esfahani, M.H. Effects of Heat Shock during the Early Stage of Oocyte Maturation on the Meiotic Progression, Subsequent Embryonic Development and Gene Expression in Ovine. Zygote 2015, 23, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Rowinski, J.R.; Rispoli, L.A.; Payton, R.R.; Schneider, L.G.; Schrick, F.N.; McLean, K.J.; Edwards, J.L. Impact of an Acute Heat Shock during In Vitro Maturation on Interleukin 6 and its Associated Receptor Component Transcripts in Bovine Cumulus-Oocyte Complexes. Anim. Reprod. 2020, 17, e20200221. [Google Scholar] [CrossRef]
- Santos Junior, E.R.; Chaves, R.M.; Lima, P.F.; Oliveira, M.A.L. Avaliação de Oócitos Caprinos submetidos a Estresse Calórico Induzido durante a Maturação In Vitro. Acta Sci. Vet. 2013, 41, 1159. [Google Scholar]
- Diaz, F.A.; Gutierrez, E.J.; Foster, B.A.; Hardin, P.T.; Bondioli, K.R. Effect of In Vivo and In Vitro Heat Stress on DNA Methylation and DNA Hydroxymethylation of Bovine Oocytes and Early Embryos. Theriogenology 2025, 240, 117400. [Google Scholar] [CrossRef]
- Deluao, J.C.; Winstanley, Y.; Robker, R.L.; Pacella-Ince, L.; Gonzalez, M.B.; McPherson, N.O. Reactive Oxygen Species in the Mammalian Pre-Implantation Embryo. Reproduction 2022, 164, F95–F108. [Google Scholar] [CrossRef]
- Carvalheira, L.D.R.; Leite Albeny, A.C.; Silva, E.B.M.; Borges, Á.M. Heat Shock on Bovine Embryos from Day 2.5–3 Selects the Most Competent for Progression to the Blastocyst Stage. Theriogenology 2024, 230, 21–27. [Google Scholar] [CrossRef]
- Morales-Cruz, J.L.; Calderon-Leyva, G.; Angel-García, O.; Guillen-Muñoz, J.M.; Santos-Jimenez, Z.; Mellado, M.; Pessoa, L.G.; Guerrero-Gallego, H.Z. The Effect of Month of Harvesting and Temperature–Humidity Index on the Number and Quality of Oocytes and In Vitro Embryo Production in Holstein Cows and Heifers. Biology 2023, 12, 1174. [Google Scholar] [CrossRef]
- Rosales-Martínez, F.; Becerril-Pérez, C.M.; Rosendo-Ponce, A.; Riaño-Gaya, A.; Cortez-Romero, C.; Gallegos-Sánchez, J.; Romo-García, S. In Vitro Embryos of Romosinuano and Tropical Milking Cattle During Three Seasons in Veracruz, Mexico. Animals 2024, 14, 1922. [Google Scholar] [CrossRef]
- Martín-Maestro, A.; Sánchez-Ajofrin, I.; Iniesta-Cuerda, M.; Medina-Chávez, D.; Maside, C.; Fernández-Santos, M.R.; Garde, J.; Soler, A.J. Heat Stress Affects the Functionality of the Ovine Cumulus-Oocyte Complex and Subsequent In Vitro Embryo Production. Sci. Rep. 2025, 15, 17163. [Google Scholar] [CrossRef]
- Kim, W.-S.; Ghassemi Nejad, J.; Roh, S.-G.; Lee, H.-G. Heat-Shock Proteins Gene Expression in Peripheral Blood Mononuclear Cells as an Indicator of Heat Stress in Beef Calves. Animals 2020, 10, 895. [Google Scholar] [CrossRef]
- Hariyono, D.N.H.; Prihandini, P.W. Association of Selected Gene Polymorphisms with Thermotolerance Traits in Cattle—A Review. Anim. Biosci. 2022, 35, 1635. [Google Scholar] [CrossRef]
- Gupta, M.; Vaidya, M.; Kumar, S.; Singh, G.; Osei-Amponsah, R.; Chauhan, S.S. Heat Stress: A Major Threat to Ruminant Reproduction and Mitigating Strategies. Int. J. Biometeorol. 2024, 69, 209–224. [Google Scholar] [CrossRef]
- Rakib, M.R.H.; Messina, V.; Gargiulo, J.I.; Lyons, N.A.; Garcia, S.C. Potential use of HSP70 as an Indicator of Heat Stress in Dairy Cows—A Review. J. Dairy Sci. 2024, 107, 11597–11610. [Google Scholar] [CrossRef] [PubMed]
- Jianfang, W.; Raza, S.H.A.; Pant, S.D.; Juan, Z.; Prakash, A.; Abdelnour, S.A.; Aloufi, B.H.; Mahasneh, Z.M.H.; Amin, A.A.; Shokrollahi, B.; et al. Exploring Epigenetic and Genetic Modulation in Animal Responses to Thermal Stress. Mol. Biotechnol. 2024, 67, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.U.; Nawaz, A.; Rehman, M.S.; Ali, M.A.; Dilshad, S.M.R.; Yang, C. Prospects of HSP70 as a Genetic Marker for Thermo-Tolerance and Immuno-Modulation in Animals under Climate Change Scenario. Anim. Nutr. 2019, 5, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Gómez, J.S.; Uribe-García, H.F.; Herrera-Sánchez, M.P.; Lozano-Villegas, K.J.; Rodríguez-Hernández, R.; Rondón-Barragán, I.S. Heat Stress on Cattle Embryo: Gene Regulation and Adaptation. Heliyon 2021, 7, e06570. [Google Scholar] [CrossRef]
- Amitha, J.P.; Krishnan, G.; Bagath, M.; Sejian, V.; Bhatta, R. Heat Stress Impact on the Expression Patterns of Different Reproduction Related Genes in Malabari Goats. Theriogenology 2019, 131, 169–176. [Google Scholar] [CrossRef]
- Camargo, L.S.A.; Aguirre-Lavin, T.; Adenot, P.; Araujo, T.D.; Mendes, V.R.A.; Louro, I.D.; Beaujean, N.; Souza, E.D. Heat Shock During In Vitro Maturation Induces Chromatin Modifications in the Bovine Embryo. Reproduction 2019, 158, 313–322. [Google Scholar] [CrossRef]
- Stamperna, K.; Giannoulis, T.; Dovolou, E.; Kalemkeridou, M.; Nanas, I.; Dadouli, K.; Moutou, K.; Mamuris, Z.; Amiridis, G.S. The Effects of Heat Shock Protein 70 Addition in the Culture Medium on the Development and Quality of In Vitro Produced Heat Shocked Bovine Embryos. Animals 2021, 11, 3347. [Google Scholar] [CrossRef] [PubMed]
- Faheem, M.S.; Ghanem, N.; Samy, R.; Barkawi, A.H. Molecular, Enzymatic Responses and In Vitro Embryonic Developmental Competency of Heat-Shocked Buffalo Embryos Co-Cultured with Granulosa Cells Monolayer. Theriogenology 2023, 211, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.I.; Ezra, E.; Gershoni, M. Broad Phenotypic Impact of the Effects of Transgenerational Heat Stress in Dairy Cattle: A Study of Four Consecutive Generations. Genet. Sel. Evol. 2021, 53, 69. [Google Scholar] [CrossRef] [PubMed]
| Experimental Condition | Effects on Steroidogenesis |
|---|---|
| Bovine granulosa cells cultured at 40 °C and 41 °C | Heat stress caused a 45% reduction in progesterone production at both temperatures, and estradiol levels decreased by 60%, with a more pronounced reduction at 40–41 °C. This was associated with the downregulation of CYP11A1 and STAR, increased reactive oxygen species (ROS), and mitochondrial dysfunction [57]. |
| Cows exposed to heat for 30 and 60 days before ovum pick-up (OPU) | Progesterone production decreased due to LH alteration. Estradiol production and follicular development declined, with altered gonadotropins (increased FSH, decreased LH), reduced oocyte viability, and decreased Bone Morphogenetic Protein (BMP15) and Growth Differentiation Factor 9 (GDF9) [60]. |
| Pantaneira and Girolando cows exposed to heat stress for 60 days | Chronic heat stress reduced oocyte viability in Pantaneira cows due to decreased estradiol production. Girolando cows maintained oocyte competence through efficient thermoregulation under tropical conditions [61]. |
| Bovine granulosa cells cultured in vitro at 43 °C | Progesterone and estradiol production had significant reductions. There were 256 differentially expressed genes (DEGs) and 51 altered metabolites, impacting Transforming Growth Factor Beta (TGF-β) and Vascular Endothelial Growth Factor (VEGF) pathways and modifying amino acid metabolism [65]. |
| Bovine granulosa cells cultured in vitro at 43 °C | Progesterone and estradiol concentrations decreased. There were 330 DEGs (75 upregulated, 255 downregulated), increased ROS and apoptosis, and alterations in the TP53 and Regulatory Associated Protein of mTOR Complex 1 (RPTOR) pathways, indicating metabolic reprogramming [63] |
| Experimental Condition | Damage Caused by Heat Stress |
|---|---|
| Oocytes cultured in vitro at 41 °C for 12 h and then at 38.5 °C in bovine species | DNA methylation and DNA hydroxymethylation Reduced ATP levels Reduced mitochondrial distribution and mitochondrial DNA copies Reduced oocyte transzonal projections Reduced in mRNA expression of GDF9, BMP15, and Mitogen-activated protein kinase 1- MAPK1 [11] |
| Oocytes cultured in vitro under moderate high (40 °C) and low (37 °C) stress in bovine species | Impaired embryonic development rates [83] |
| Bovine oocytes cultured in vitro at 41 °C | Reduced transzonal projections [78] |
| Oocytes cultured in vitro from 38.5 °C to 40.5 °C (for 6 h) and reduced again to 38.5 °C in bovine species | Decreased expression of Interferon Tau and increased reactive oxygen species [23] |
| Bovine oocytes cultured in vitro (41 °C for 12 h) | Disrupted the abundance of transcripts from bta-miR-19b and DROSHA and impaired embryonic development rates [72] |
| Bovine oocytes cultured in vivo (THI over 75) | Negative effects on the quality and number of health oocytes (Grade I); differentially expressed genes in oocyte at GV and MII stages [75] |
| Bovine oocytes cultured in vitro at 40.5 °C | Depletion/appearance of amino acids [77] |
| Ovine oocytes cultured in vitro at 41 °C | Abnormal chromatin configurations and reduced cleavage rates [84] |
| Ovine oocytes cultured in vitro at 42 °C | Increased expression of BAX, C-myc, Caspase 3, and P53 and decreased expression of BCL-2 [81] |
| Ovine oocytes cultured in vitro at 41 °C for 12 h and then at 39 °C | Reduced cleavage and blastocyst formation rates [82] |
| Ovine oocytes cultured in vitro at 41 °C | Altered the expression patterns of interleukin (IL-6) and its receptor [85] |
| Caprine oocytes cultured in vitro at 41 °C | Reduced maturation rates and blastocyst rates [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, D.R.; Azevedo, V.A.N.; Ribeiro, R.P.; Ximenes, G.d.O.; Silva, A.d.A.; Barbalho, E.C.; Barrozo, L.G.; Chaves, S.C.; Castro, M.S.M.; Marcelino, E.C.; et al. Reproductive Challenges in Ruminants Under Heat Stress: A Review of Follicular, Oocyte, and Embryonic Responses. Animals 2025, 15, 2296. https://doi.org/10.3390/ani15152296
Nascimento DR, Azevedo VAN, Ribeiro RP, Ximenes GdO, Silva AdA, Barbalho EC, Barrozo LG, Chaves SC, Castro MSM, Marcelino EC, et al. Reproductive Challenges in Ruminants Under Heat Stress: A Review of Follicular, Oocyte, and Embryonic Responses. Animals. 2025; 15(15):2296. https://doi.org/10.3390/ani15152296
Chicago/Turabian StyleNascimento, Danisvânia Ripardo, Venância Antonia Nunes Azevedo, Regislane Pinto Ribeiro, Gabrielle de Oliveira Ximenes, Andreza de Aguiar Silva, Efigênia Cordeiro Barbalho, Laryssa Gondim Barrozo, Sueline Cavalcante Chaves, Maria Samires Martins Castro, Erica Costa Marcelino, and et al. 2025. "Reproductive Challenges in Ruminants Under Heat Stress: A Review of Follicular, Oocyte, and Embryonic Responses" Animals 15, no. 15: 2296. https://doi.org/10.3390/ani15152296
APA StyleNascimento, D. R., Azevedo, V. A. N., Ribeiro, R. P., Ximenes, G. d. O., Silva, A. d. A., Barbalho, E. C., Barrozo, L. G., Chaves, S. C., Castro, M. S. M., Marcelino, E. C., Vaz da Silva, L. R. C., Batista, A. M., & Silva, J. R. V. (2025). Reproductive Challenges in Ruminants Under Heat Stress: A Review of Follicular, Oocyte, and Embryonic Responses. Animals, 15(15), 2296. https://doi.org/10.3390/ani15152296








