Spatiotemporal Activity Patterns of Sympatric Rodents and Their Predators in a Temperate Desert-Steppe Ecosystem
Simple Summary
Abstract
1. Introduction
- Quantify the daily and seasonal activity rhythms of each species;
- Assess the temporal and spatial overlap between rodents and predators;
- Evaluate the potential implications of activity synchrony for natural rodent control in desert-steppe habitats.
2. Materials and Methods
2.1. Study Area
2.2. Infrared Camera Deployment and Maintenance
2.3. Species Identification
2.4. Calculation of Activity Intensity
2.5. Temporal Overlap Analysis
2.6. Spatial Overlap Analysis
3. Results
3.1. Species Detection and Data Overview
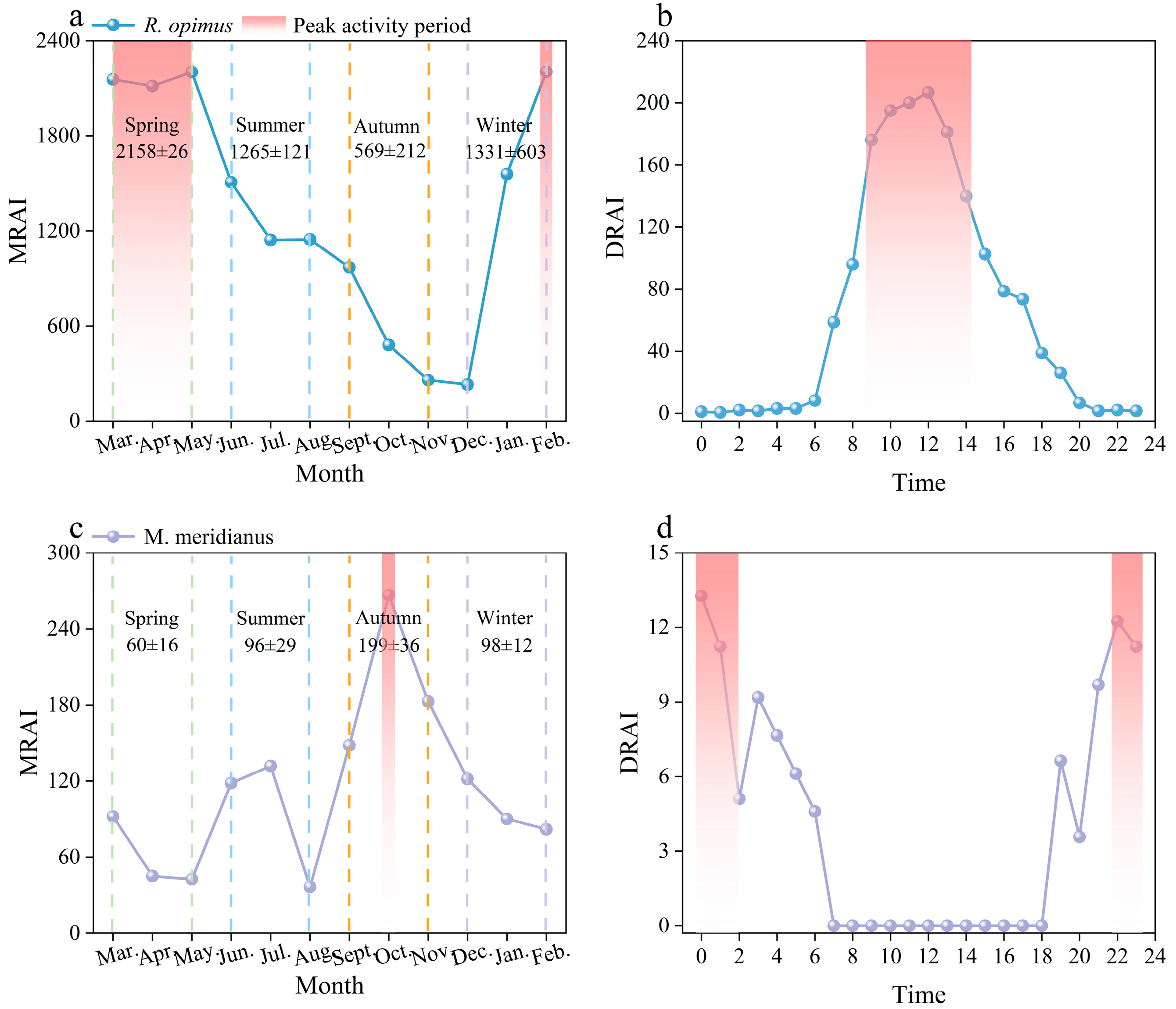
3.2. Daily and Monthly Activity Rhythms of R. opimus and M. meridianus
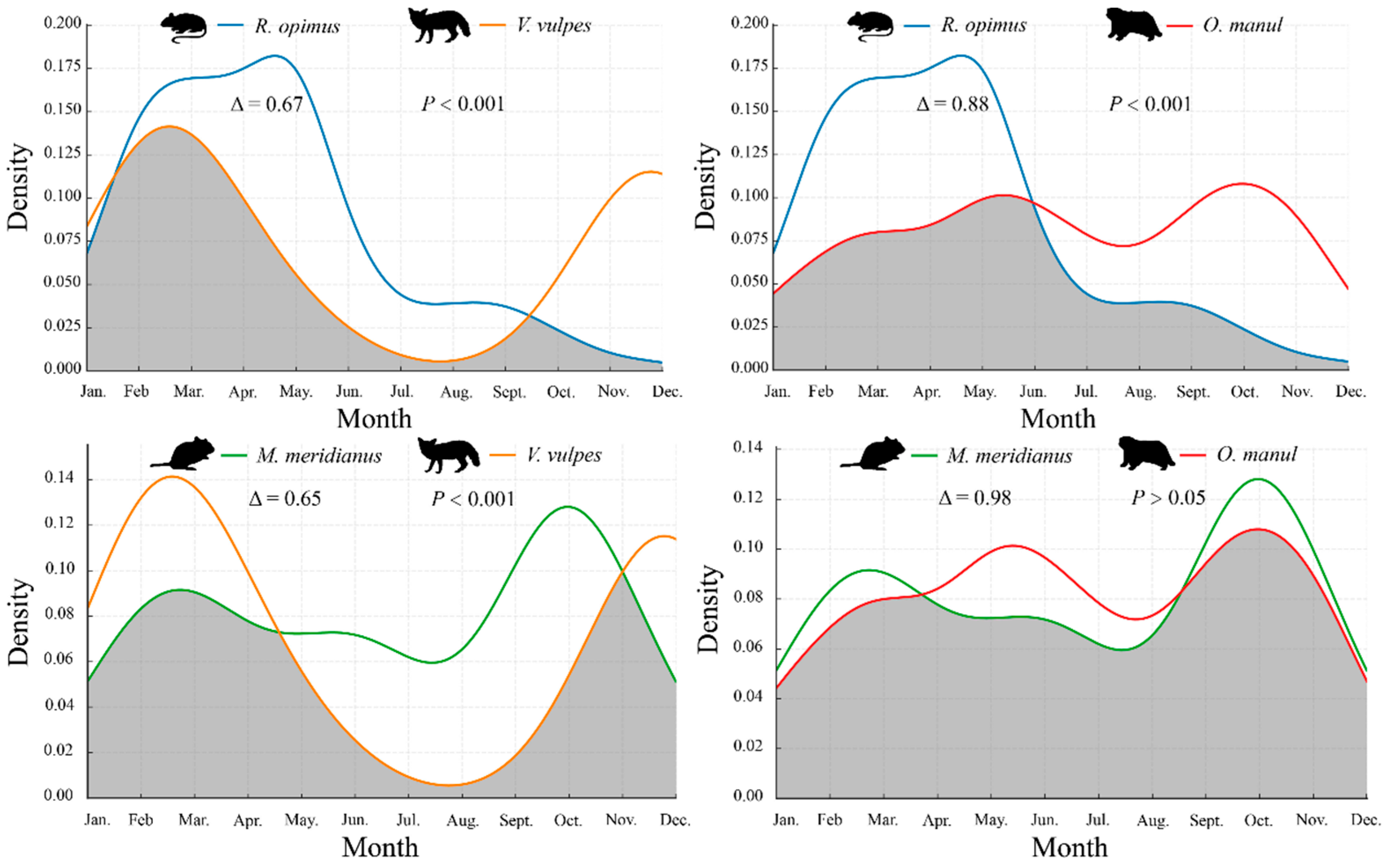
3.3. Temporal Overlap Between Rodents and Their Predators
3.4. Spatial Distribution and Overlap Between Rodents and Their Predators
4. Discussion
4.1. Differences in Activity Rhythms of Rodents
4.2. Spatiotemporal Interactions with Predators
4.3. Ecological Implications and Management Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IP | Independent Photographic Event |
| DRAI | Daily Relative Activity Index |
| MRAI | Monthly Relative Activity Index |
| OI | Spatial Overlap Index |
| Δ | Temporal Overlap Coefficient |
References
- Allada, R.; White, N.E.; So, W.V.; Hall, J.C.; Rosbash, M. A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell 1998, 93, 791–804. [Google Scholar] [CrossRef]
- Nie, Y.; Speakman, J.R.; Wu, Q.; Zhang, C.; Hu, Y.; Xia, M.; Yan, L.; Hambly, C.; Wang, L.; Wei, W.; et al. Exceptionally low daily energy expenditure in the bamboo-eating giant panda. Science 2015, 349, 171–174. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, J.; Hull, V.; Huang, J.; Liu, D.; Zhou, J.; Sun, M.; Zhang, H. Comparative activity patterns of wild giant pandas and livestock. Acta Ecol. Sin. 2019, 39, 1071–1081. [Google Scholar] [CrossRef]
- Probst, R.; Probst, R. High frequency of Apodemus mice boosts inverse activity pattern of bank voles, Clethrionomys glareolus, through non-aggressive intraguild competition. Animals 2023, 13, 981. [Google Scholar] [CrossRef]
- Kronfeld-Schor, N.; Bloch, G.; Schwartz, W.J. Animal clocks: When science meets nature. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131354. [Google Scholar] [CrossRef]
- Kronfeld-Schor, N.; Dayan, T. Partitioning of time as an ecological resource. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 153–181. [Google Scholar] [CrossRef]
- Dominoni, D.M.; Åkesson, S.; Klaassen, R.; Spoelstra, K.; Bulla, M. Methods in field chronobiology. Philos. Trans. R. Soc. B 2017, 372, 20160247. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Santos, L.G.R.; Zucco, C.A.; Agostinelli, C. Using conditional circular kernel density functions to test hypotheses on animal circadian activity. Anim. Behav. 2013, 85, 269–280. [Google Scholar] [CrossRef]
- Su, X.; Li, X.; Sun, H.; Song, Z. Analysis of activity rhythm and behavior pattern for plateau pika in degraded alpine meadow. Discov. Appl. Sci. 2024, 6, 226. [Google Scholar] [CrossRef]
- Zhao, L.J.; Liu, M.Z.; Luo, C.P. Daily activity rhythm of Ithaginis cruentus in the Wanglang National Nature Reserve. Sichuan J. Zool. 2020, 39, 121–128. [Google Scholar] [CrossRef]
- Daan, S.; Aschoff, J. Circadian Contributions to Survival. In Vertebrate Circadian Systems: Structure and Physiology; Aschoff, J., Daan, S., Groos, G.A., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 305–321. [Google Scholar] [CrossRef]
- Wei, F.; Yang, Q.; Wu, Y.; Jiang, X.; Liu, S.; Li, B.; Yang, G.; Li, M.; Zhou, J.; Li, S. Catalogue of Mammals in China (2021). Acta Theriol. Sin. 2021, 41, 487–501. [Google Scholar] [CrossRef]
- Zhou, L.-Z.; Ma, Y. Distribution Patterns of Rodent Diversity in Arid Regions of West China. Biodivers. Sci. 2002, 10, 44–48. [Google Scholar] [CrossRef]
- Zhou, L.; Ma, Y.; Li, D.L. Spatial Distribution Patterns of Chinese Gerbils (Gerbillinae) in Relation to Environmental Factors. Acta Zool. Sin. 2001, 47, 616–624. [Google Scholar]
- Flowerdew, J.R.; Shore, R.F.; Poulton, S.M.C.; Sparks, T.H. Live Trapping to Monitor Small Mammals in Britain. Mammal Rev. 2004, 34, 31–50. [Google Scholar] [CrossRef]
- Davidson, A.D.; Detling, J.K.; Brown, J.H. Ecological Roles and Conservation Challenges of Social, Burrowing, Herbivorous Mammals in the World’s Grasslands. Front. Ecol. Environ. 2012, 10, 477–486. [Google Scholar] [CrossRef]
- Sunyer, P.; Muñoz, A.; Bonal, R.; Espelta, J.M. The Ecology of Seed Dispersal by Small Rodents: A Role for Predator and Conspecific Scents. Funct. Ecol. 2013, 27, 1313–1321. [Google Scholar] [CrossRef]
- Kausrud, K.L.; Viljugrein, H.; Frigessi, A.; Begon, M.; Davis, S.; Leirs, H.; Dubyanskiy, V.; Stenseth, N.C. Climatically Driven Synchrony of Gerbil Populations Allows Large-Scale Plague Outbreaks. Proc. R. Soc. B Biol. Sci. 2007, 274, 1963–1969. [Google Scholar] [CrossRef]
- Wen, X.; Cheng, X.; Dong, Y.; Wang, Q.; Lin, X. Analysis of the Activity Rhythms of the Great Gerbil (Rhombomys opimus) and Its Predators Based on Infrared Camera Technology. Glob. Ecol. Conserv. 2020, 24, e01337. [Google Scholar] [CrossRef]
- Hua, L.M.; Chai, S.Q. Rodent Pest Control on Grasslands in China: Current State, Problems and Prospects. J. Plant Prot. 2022, 49, 415–423. [Google Scholar] [CrossRef]
- Kuhn, K.M.; Vander Wall, S.B. Linking Summer Foraging to Winter Survival in Yellow Pine Chipmunks (Tamias amoenus). Oecologia 2008, 157, 349–360. [Google Scholar] [CrossRef]
- Smale, L.; Lee, T.; Nuñez, A.A. Mammalian Diurnality: Some Facts and Gaps. J. Biol. Rhythm. 2003, 18, 356–366. [Google Scholar] [CrossRef]
- Rowcliffe, J.M.; Carbone, C. Surveys Using Camera Traps: Are We Looking to a Brighter Future? Anim. Conserv. 2008, 11, 185–186. [Google Scholar] [CrossRef]
- Cappelle, N.; Després-Einspenner, M.; Howe, E.J.; Boesch, C.; Kühl, H.S. Validating Camera Trap Distance Sampling for Chimpanzees. Am. J. Primatol. 2019, 81, e22962. [Google Scholar] [CrossRef] [PubMed]
- Ferreguetti, Á.C.; Tomás, W.M.; Bergallo, H.G. Density, Occupancy, and Activity Pattern of Two Sympatric Deer (Mazama spp.) in the Atlantic Forest, Brazil. J. Mammal. 2015, 96, 1245–1254. [Google Scholar] [CrossRef]
- Gerber, B.D.; Karpanty, S.M.; Randrianantenaina, J. Activity Patterns of Carnivores in the Rain Forests of Madagascar: Implications for Species Coexistence. J. Mammal. 2012, 93, 667–676. [Google Scholar] [CrossRef]
- Srbek-Araujo, A.C.; Silveira, L.F.; Chiarello, A.G. The Red-Billed Curassow (Crax blumenbachii): Social Organization, and Daily Activity Patterns. Wilson J. Ornithol. 2012, 124, 321–327. [Google Scholar] [CrossRef]
- Oliveira-Santos, L.G.R.; Tortato, M.A.; Graipel, M.E. Activity Pattern of Atlantic Forest Small Arboreal Mammals as Revealed by Camera Traps. J. Trop. Ecol. 2008, 24, 563–567. [Google Scholar] [CrossRef]
- Galetti, M.; Camargo, H.; Siqueira, T.; Keuroghlian, A.; Donatti, C.I.; Jorge, M.L.S.P.; Pedrosa, F.; Kanda, C.Z.; Ribeiro, M.C. Diet Overlap and Foraging Activity between Feral Pigs and Native Peccaries in the Pantanal. PLoS ONE 2015, 10, e0141459. [Google Scholar] [CrossRef]
- Tang, X.; Tang, S.; Li, X.; Menghe, D.; Bao, W.; Xiang, C.; Gao, F.; Bao, W. A Study of Population Size and Activity Patterns and Their Relationship to the Prey Species of the Eurasian Lynx Using a Camera Trapping Approach. Animals 2019, 9, 864. [Google Scholar] [CrossRef]
- Smith, A.T.; Yan, X. Distribution and Identification of Chinese Mammals. Chin. Mammal. J. 2013, 1, 1–280, [Adapted from: Princeton University Press]. [Google Scholar]
- MacKinnon, J.R.; Phillipps, K. Illustrated Guide to the Birds of China. Chin. Avian J. 2000, 1, 1–400, [Adapted from: Oxford University Press]. [Google Scholar]
- Zheng, G. Checklist on the Classification and Distribution of the Birds of China. Avian Taxon. Bull. China 2005, 1, 1–350, [Adapted from: Geological Publishing House]. [Google Scholar]
- Zhang, Y.-Y.; Zhang, Z.-W.; Dong, L.; Ding, P.; Ding, C.-Q.; Ma, Z.-J.; Zheng, G.-M. Assessment of Red List of Birds in China. Biodivers. Sci. 2016, 24, 568. [Google Scholar] [CrossRef]
- Chen, L.J.; Shu, Z.F.; Xiao, Z.S. Application of Camera-Trapping Data to Study Daily Activity Patterns of Galliformes in Guangdong Chebaling National Nature Reserve. Sci. Silvae Sin. 2019, 55, 10–18. [Google Scholar] [CrossRef]
- Azevedo, F.C.; Lemos, F.G.; Freitas-Junior, M.C.; Rocha, D.G.; Azevedo, F.C.C. Puma Activity Patterns and Temporal Overlap with Prey in a Human-modified Landscape at Southeastern Brazil. J. Zool. 2018, 305, 246–255. [Google Scholar] [CrossRef]
- Guo, Q.; Zuo, Y.; Lin, W.; Xiao, X.; Zhao, X.; Li, Y.; Ying, Z.; Zhou, C.; Xie, X. Niche and Interspecific Association of Juvenile Tachypleus tridentatus in the Beibu Gulf. Acta Oceanol. Sin. 2022, 44, 109–118. [Google Scholar] [CrossRef]
- Namgail, T.; Fox, J.L.; Bhatnagar, Y.V. Habitat Segregation between Sympatric Tibetan Argali (Ovis ammon hodgsoni) and Blue Sheep (Pseudois nayaur) in the Indian Trans-Himalaya. J. Zool. 2004, 262, 57–63. [Google Scholar] [CrossRef]
- Qiao, H.H.; Liu, W.; Yang, W.K.; Xü, W.X. Behavioral Ecology of Rhombomys opimus: A Review. Chin. J. Ecol. 2011, 30, 603–610. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, X.; Wang, C. Demography and Reproduction of Meriones meridianus in Inner Mongolia. Endem. Dis. Bull. 2006, 21, 5–10. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, C.M.; He, M.X.; Chen, L.X.; He, X.C.; Ran, J.H. Habitat Suitability Assessment and Daily Activity Patterns of Otocolobus manul in the Gongga Mountain National Nature Reserve. Sichuan J. Zool. 2019, 38, 320–327. [Google Scholar] [CrossRef]
- Murdoch, J.D.; Munkhzul, T.; Reading, R.P. Pallas’s Cat Ecology and Conservation in the Semi-Desert Steppes of Mongolia. Cat News 2006, 45, 18–19. [Google Scholar]
- Shi, X.H.; Hu, Q.U.; Feng, X.; Jin, S.H.; Cheng, Y.; Zhang, J.; Yao, M.A.; Li, S.L. Spatiotemporal Relationships between Snow Leopard (Panthera uncia) and Red Fox (Vulpes vulpes) in Qionglai Mountains, Sichuan Province. Acta Theriol. Sin. 2021, 41, 115–123. [Google Scholar] [CrossRef]
- Li, X.; Yuan, S.; Li, L.; Wang, J.; Ma, J.; Ji, Y.; Lu, X. Influence of Grazing on the Activity Pattern and Temporal Niche of Two Dominant Rodent Species in Alxa Desert. Front. Ecol. Evol. 2023, 10, 1105729. [Google Scholar] [CrossRef]
- Colella, J.P.; Blumstein, D.M.; MacManes, M.D. Disentangling Environmental Drivers of Circadian Metabolism in Desert-Adapted Mice. J. Exp. Biol. 2021, 224, jeb242529. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Dou, H.; Liu, S.; Wang, M. Red Fox Habitat Selection and Landscape Feature Analysis in the Dalai Lake Natural Reserve in Inner Mongolia. Acta Ecol. Sin. 2012, 32, 2342–2450. [Google Scholar] [CrossRef]
- Burt, S.A.; Lipman, S.A. What Do They Know? Comparing Public Knowledge and Opinions about Rodent Management to the Expectations of Pest Controllers. Animals 2021, 11, 3429. [Google Scholar] [CrossRef]
- Kotler, B.P.; Ayal, Y.; Subach, A. Effects of Predatory Risk and Resource Renewal on the Timing of Foraging Activity in a Gerbil Community. Oecologia 1994, 100, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Spencer, E.E.; Crowther, M.S.; Dickman, C.R. Risky Business: Do Native Rodents Use Habitat and Odor Cues to Manage Predation Risk in Australian Deserts? PLoS ONE 2014, 9, e90566. [Google Scholar] [CrossRef]



| Species | Total_Images | Independent_Events |
|---|---|---|
| R. opimus | 25,215 | 3143 |
| M. meridianus | 754 | 197 |
| V. vulpes | 84 | 29 |
| O. manul | 159 | 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, C.; Ma, Y.; Fan, Y.; Zhi, X.; Hua, L. Spatiotemporal Activity Patterns of Sympatric Rodents and Their Predators in a Temperate Desert-Steppe Ecosystem. Animals 2025, 15, 2290. https://doi.org/10.3390/ani15152290
Wei C, Ma Y, Fan Y, Zhi X, Hua L. Spatiotemporal Activity Patterns of Sympatric Rodents and Their Predators in a Temperate Desert-Steppe Ecosystem. Animals. 2025; 15(15):2290. https://doi.org/10.3390/ani15152290
Chicago/Turabian StyleWei, Caibo, Yijie Ma, Yuquan Fan, Xiaoliang Zhi, and Limin Hua. 2025. "Spatiotemporal Activity Patterns of Sympatric Rodents and Their Predators in a Temperate Desert-Steppe Ecosystem" Animals 15, no. 15: 2290. https://doi.org/10.3390/ani15152290
APA StyleWei, C., Ma, Y., Fan, Y., Zhi, X., & Hua, L. (2025). Spatiotemporal Activity Patterns of Sympatric Rodents and Their Predators in a Temperate Desert-Steppe Ecosystem. Animals, 15(15), 2290. https://doi.org/10.3390/ani15152290





