The Correlation Between Body Pain Indicators and the Facial Expression Scale in Sows During Farrowing and Pre-Weaning: The Effects of Parity, the Farrowing Moment, and Suckling Events
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing, and General Management
2.2. Experimental Procedure
2.3. Images, Selection, and Evaluation
- Expulsion of the piglets (indicative of severe pain; n = 41 images). One image was chosen within the 30 s prior to each piglet expulsion.
- Inter-expulsion moment, described as the interval time between the delivery of two piglets (indicative of moderate pain; n = 43 images). One image was chosen at each interval.
- Pre-weaning (indicative of pain-free; n = 38 images). Images were chosen every 15–20 min.
2.4. Video Analysis
2.5. Statistical Analysis
3. Results
3.1. Incidence of Body Pain Indicators
3.2. Facial Action Units
3.3. Correlation Between Body Pain Indicators and Facial Expressions
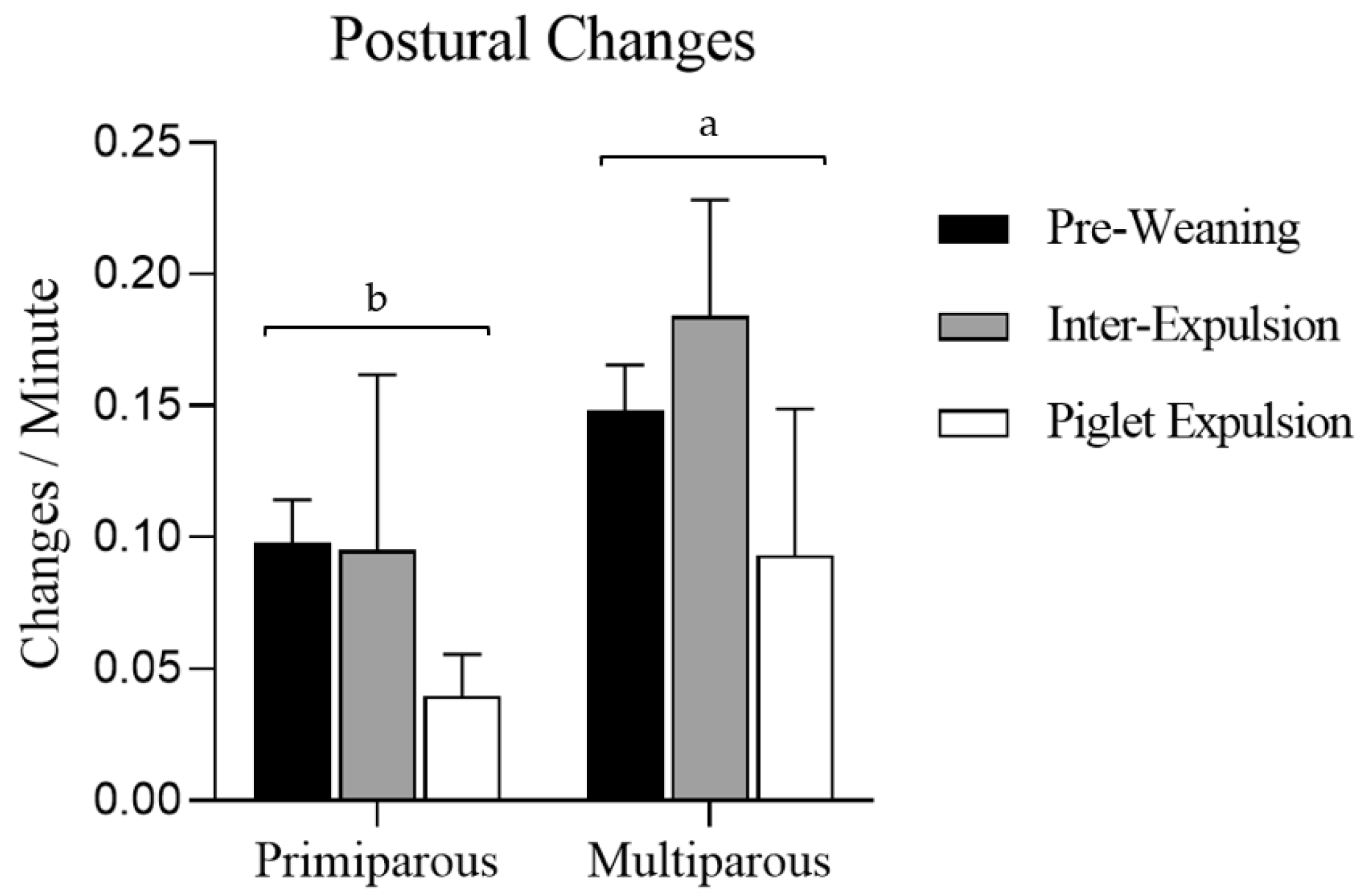
3.4. Activity During Farrowing
4. Discussion
4.1. Incidence of Body Pain Indicators Considering Parturition, Effect of Suckling Events, and Farrowing Moment
4.2. Facial Expressions and Their Correlation with the Body Pain Indicators
4.3. Sows’ Postures, Postural Changes, and Time Spent in Each Posture
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BPI | Body pain indicator |
| FAU | Facial Action Unit |
| BCS | Body Condition Score |
| TFI | Total Facial Index |
| GLMs | General lineal models |
| L.Lat | Lying lateral |
| L.St | Lying sternal |
| Sit | Sitting |
| St | Standing |
References
- McLennan, K.M. Why Pain Is Still a Welfare Issue for Farm Animals, and How Facial Expression Could Be the Answer. Agriculture 2018, 8, 127. [Google Scholar] [CrossRef]
- Mogil, J.S.; Pang, D.S.J.; Silva Dutra, G.G.; Chambers, C.T. The Development and Use of Facial Grimace Scales for Pain Measurement in Animals. Neurosci. Biobehav. Rev. 2020, 116, 480–493. [Google Scholar] [CrossRef]
- Di Giminiani, P.; Brierley, V.L.M.H.; Scollo, A.; Gottardo, F.; Malcolm, E.M.; Edwards, S.A.; Leach, M.C. The Assessment of Facial Expressions in Piglets Undergoing Tail Docking and Castration: Toward the Development of the Piglet Grimace Scale. Front. Vet. Sci. 2016, 3, 100. [Google Scholar] [CrossRef] [PubMed]
- Viscardi, A.V.; Hunniford, M.; Lawlis, P.; Leach, M.; Turner, P.V. Development of a Piglet Grimace Scale to Evaluate Piglet Pain Using Facial Expressions Following Castration and Tail Docking: A Pilot Study. Front. Vet. Sci. 2017, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, S.; McLean, K.A.; Chirnside, J.; Deans, L.A.; Calvert, S.K.; Molony, V.; Lawrence, A.B. Opioid-Mediated Changes in Nociceptive Threshold during Pregnancy and Parturition in the Sow. Pain 1997, 72, 153–159. [Google Scholar] [CrossRef]
- Mainau, E.; Manteca, X. Pain and Discomfort Caused by Parturition in Cows and Sows. Appl. Anim. Behav. Sci. 2011, 135, 241–251. [Google Scholar] [CrossRef]
- Martínez-Burnes, J.; Muns, R.; Barrios-García, H.; Villanueva-García, D.; Domínguez-Oliva, A.; Mota-Rojas, D. Parturition in Mammals: Animal Models, Pain and Distress. Animals 2021, 11, 2960. [Google Scholar] [CrossRef]
- Mainau, E.; Ruiz-de-la-Torre, J.L.; Dalmau, A.; Salleras, J.M.; Manteca, X. Effects of Meloxicam (Metacam®) on Post-Farrowing Sow Behaviour and Piglet Performance. Animal 2012, 6, 494–501. [Google Scholar] [CrossRef]
- Viitasaari, E.; Hänninen, L.; Heinonen, M.; Raekallio, M.; Orro, T.; Peltoniemi, O.; Valros, A. Effects of Post-Partum Administration of Ketoprofen on Sow Health and Piglet Growth. Vet. J. 2013, 198, 153–157. [Google Scholar] [CrossRef]
- Mainau, E.; Temple, D.; Manteca, X. Experimental Study on the Effect of Oral Meloxicam Administration in Sows on Pre-Weaning Mortality and Growth and Immunoglobulin G Transfer to Piglets. Prev. Vet. Med. 2016, 126, 48–53. [Google Scholar] [CrossRef]
- Navarro, E.; Mainau, E.; de Miguel, R.; Temple, D.; Salas, M.; Manteca, X. Oral Meloxicam Administration in Sows at Farrowing and Its Effects on Piglet Immunity Transfer and Growth. Front. Vet. Sci. 2021, 8, 574250. [Google Scholar] [CrossRef]
- Kuller, W.; Sietsma, S.; Hendriksen, S.; Sperling, D. Use of Paracetamol in Sows around Farrowing: Effect on Health and Condition of the Sow, Piglet Mortality, Piglet Weight and Piglet Weight Gain. Porcine Health Manag. 2021, 7, 46. [Google Scholar] [CrossRef]
- Boonprakob, R.; Vimolmangkang, S.; Tummaruk, P. Impacts of Supplementing Cannabis Sativa Byproducts during the Transition Period on Behaviour, Feed Consumption, Constipation Levels, Colostrum Production and Piglet Performance in Hyperprolific Sows. Theriogenology 2024, 215, 272–280. [Google Scholar] [CrossRef]
- Weary, D.M.; Niel, L.; Flower, F.C.; Fraser, D. Identifying and Preventing Pain in Animals. Appl. Anim. Behav. Sci. 2006, 100, 64–76. [Google Scholar] [CrossRef]
- Hansen, B. Through a Glass Darkly: Using Behavior to Assess Pain. Semin. Vet. Med. Surg. Small Anim. 1997, 12, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Viñuela-Fernández, I.; Jones, E.; Welsh, E.M.; Fleetwood-Walker, S.M. Pain Mechanisms and Their Implication for the Management of Pain in Farm and Companion Animals. Vet. J. 2007, 174, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Ison, S.H.; Clutton, R.E.; Di Giminiani, P.; Rutherford, K.M.D. A Review of Pain Assessment in Pigs. Front. Vet. Sci. 2016, 3, 108. [Google Scholar] [CrossRef] [PubMed]
- Ison, S.H.; Jarvis, S.; Rutherford, K.M.D. The Identification of Potential Behavioural Indicators of Pain in Periparturient Sows. Res. Vet. Sci. 2016, 109, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.; Vulin, A.; Génin, S.; Sales, P.; Prunier, A. Assessment of Pain Induced by Castration in Piglets: Behavioral and Physiological Responses over the Subsequent 5 Days. Appl. Anim. Behav. Sci. 2003, 82, 201–218. [Google Scholar] [CrossRef]
- Noonan, G.J.; Rand, J.S.; Priest, J.; Ainscow, J.; Blackshaw, J.K. Behavioural Observations of Piglets Undergoing Tail Docking, Teeth Clipping and Ear Notching. Appl. Anim. Behav. Sci. 1994, 39, 203–213. [Google Scholar] [CrossRef]
- Mainau, E.; Dalmau, A.; Ruiz-de-la-Torre, J.L.; Manteca, X. A Behavioural Scale to Measure Ease of Farrowing in Sows. Theriogenology 2010, 74, 1279–1287. [Google Scholar] [CrossRef]
- Wischner, D.; Kemper, N.; Stamer, E.; Hellbruegge, B.; Presuhn, U.; Krieter, J. Characterisation of Sows’ Postures and Posture Changes with Regard to Crushing Piglets. Appl. Anim. Behav. Sci. 2009, 119, 49–55. [Google Scholar] [CrossRef]
- Zheng, C.; Yang, X.; Zhu, X.; Chen, C.; Wang, L.; Tu, S.; Yang, A.; Xue, Y. Automatic Posture Change Analysis of Lactating Sows by Action Localisation and Tube Optimisation from Untrimmed Depth Videos. Biosyst. Eng. 2020, 194, 227–250. [Google Scholar] [CrossRef]
- Wischner, D.; Kemper, N.; Stamer, E.; Hellbrügge, B.; Presuhn, U.; Krieter, J. Pre-Lying Behaviour Patterns in Confined Sows and Their Effects on Crushing of Piglets. Appl. Anim. Behav. Sci. 2010, 122, 21–27. [Google Scholar] [CrossRef]
- Navarro, E.; Mainau, E.; Manteca, X. Development of a Facial Expression Scale Using Farrowing as a Model of Pain in Sows. Animals 2020, 10, 2113. [Google Scholar] [CrossRef]
- Tsai, Y.-J.; Huang, Y.-C.; Lin, E.-C.; Lai, S.-C.; Hong, X.-C.; Tsai, J.; Chiang, C.-E.; Kuo, Y.-F. Monitoring the Lactation-Related Behaviors of Sows and Their Piglets in Farrowing Crates Using Deep Learning. Front. Anim. Sci. 2024, 5, 1431285. [Google Scholar] [CrossRef]
- Hansen, M.F.; Baxter, E.M.; Rutherford, K.M.D.; Futro, A.; Smith, M.L.; Smith, L.N. Towards Facial Expression Recognition for On-Farm Welfare Assessment in Pigs. Agriculture 2021, 11, 847. [Google Scholar] [CrossRef]
- Yunas, S.; Shahbaz, A.; Baxter, E.; Farish, M.; Rutherford, K.; Hansen, M.; Smith, M.; Smith, L. Deep Learning-Based Classification of Stress in Sows Using Facial Images. In Proceedings of the 18th International Joint Conference on Biomedical Engineering Systems and Technologies, Porto, Portugal, 20–22 February 2025; SCITEPRESS—Science and Technology Publications: Setúbal, Portugal, 2025; pp. 390–396. [Google Scholar]
- Borges, V.F.; Bernardi, M.L.; Bortolozzo, F.P.; Wentz, I. Risk Factors for Stillbirth and Foetal Mummification in Four Brazilian Swine Herds. Prev. Vet. Med. 2005, 70, 165–176. [Google Scholar] [CrossRef]
- Oliviero, C.; Heinonen, M.; Valros, A.; Peltoniemi, O. Environmental and Sow-Related Factors Affecting the Duration of Farrowing. Anim. Reprod. Sci. 2010, 119, 85–91. [Google Scholar] [CrossRef]
- Ison, S.H.; Jarvis, S.; Hall, S.A.; Ashworth, C.J.; Rutherford, K.M.D. Periparturient Behavior and Physiology: Further Insight into the Farrowing Process for Primiparous and Multiparous Sows. Front. Vet. Sci. 2018, 5, 122. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.; Hatze, B.; Lomax, S.; Bathgate, R. Defining “Normal” in Pig Parturition. Animals 2022, 12, 2754. [Google Scholar] [CrossRef]
- Navarro, E. Indicadores de Dolor En Cerdas Alrededor Del Parto. Efectos Del Meloxicam Oral Sobre La Transferencia Inmunitaria, El Bienestar y La Productividad En Cerdas y Lechones. Ph.D. Thesis, Universidad Autónoma de Barcelona, Bellaterra, Spain, 2022. [Google Scholar]
- Blavi, L.; Solà-Oriol, D.; Llonch, P.; López-Vergé, S.; Martín-Orúe, S.M.; Pérez, J.F. Management and Feeding Strategies in Early Life to Increase Piglet Performance and Welfare around Weaning: A Review. Animals 2021, 11, 302. [Google Scholar] [CrossRef]
- Hansen, C.F.; Hales, J.; Weber, P.M.; Edwards, S.A.; Moustsen, V.A. Confinement of Sows 24 h before Expected Farrowing Affects the Performance of Nest Building Behaviours but Not Progress of Parturition. Appl. Anim. Behav. Sci. 2017, 188, 1–8. [Google Scholar] [CrossRef]
- Parsons, R.L.; Millman, S.T.; Coetzee, J.F.; Karriker, L.A.; Mohling, C.M.; Pairis-Garcia, M.D.; Stalder, K.J.; Johnson, A.K. Sow Behavioral Responses to Transient, Chemically Induced Synovitis Lameness. Acta Agric. Scand. A Anim. Sci. 2015, 65, 122–125. [Google Scholar] [CrossRef]
- Baysinger, A.; Webb, S.R.; Brown, J.; Coetzee, J.F.; Crawford, S.; DeDecker, A.; Karriker, L.A.; Pairis-Garcia, M.; Sutherland, M.A.; Viscardi, A.V. Proposed Multidimensional Pain Outcome Methodology to Demonstrate Analgesic Drug Efficacy and Facilitate Future Drug Approval for Piglet Castration. Anim. Health Res. Rev. 2021, 22, 163–176. [Google Scholar] [CrossRef]
- Adi, Y.K.; Boonprakob, R.; Kirkwood, R.N.; Tummaruk, P. Factors Associated with Farrowing Duration in Hyperprolific Sows in a Free Farrowing System under Tropical Conditions. Animals 2022, 12, 2943. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Björkman, S.; Pöytäkangas, M.; Peltoniemi, O. The Effects of Ovarian Biopsy and Blood Sampling Methods on Salivary Cortisol and Behaviour in Sows. Res. Vet. Sci. 2017, 114, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Pietruszka, A.; Der, A.; Matysiak, B. Analysis of Gestation Length and Its Influence on the Reproductive Performance of Crossbred Sows Kept on a Large-Scale Pig Farm. Rocz. Nauk. Pol. Tow. Zootech. 2020, 16, 29–36. [Google Scholar] [CrossRef]
- Monteiro, M.S.; Muro, B.B.D.; Poor, A.P.; Leal, D.F.; Carnevale, R.F.; Shiroma, M.P.; Almond, G.W.; Garbossa, C.A.P.; Moreno, A.M.; Viana, C.H.C. Effects of Farrowing Induction with Prostaglandins on Farrowing Traits and Piglet Performance: A Systematic Review and Meta-Analysis. Theriogenology 2022, 180, 1–16. [Google Scholar] [CrossRef]
- Fu, L.; Zhou, B.; Li, H.; Liang, T.; Chu, Q.; Schinckel, A.P.; Li, Y.; Xu, F. Effects of Tail Docking and/or Teeth Clipping on Behavior, Lesions, and Physiological Indicators of Sows and Their Piglets. Anim. Sci. J. 2019, 90, 1320–1332. [Google Scholar] [CrossRef]
- D’Eath, R.B.; Arnott, G.; Turner, S.P.; Jensen, T.; Lahrmann, H.P.; Busch, M.E.; Niemi, J.K.; Lawrence, A.B.; Sandøe, P. Injurious Tail Biting in Pigs: How Can It Be Controlled in Existing Systems without Tail Docking? Animal 2014, 8, 1479–1497. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, J.; Bergeron, R.; D’Allaire, S.; Meunier-Salaün, M.-C.; Devillers, N. Assessment of Lameness in Sows Using Gait, Footprints, Postural Behaviour and Foot Lesion Analysis. Animal 2013, 7, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Melišová, M.; Illmann, G.; Chaloupková, H.; Bozděchová, B. Sow Postural Changes, Responsiveness to Piglet Screams, and Their Impact on Piglet Mortality in Pens and Crates1,2. J. Anim. Sci. 2014, 92, 3064–3072. [Google Scholar] [CrossRef] [PubMed]
- Nicolaisen, T.; Lühken, E.; Volkmann, N.; Rohn, K.; Kemper, N.; Fels, M. The Effect of Sows’ and Piglets’ Behaviour on Piglet Crushing Patterns in Two Different Farrowing Pen Systems. Animals 2019, 9, 538. [Google Scholar] [CrossRef]



| Behaviours | Description |
|---|---|
| Body Pain Indicators (BPIs) | |
| Leg | The back leg pushes towards the abdomen [18] |
| Paw | Front leg is strongly pulled forward [18] |
| Arch | Back arching in lateral lying position, one or both sets of legs are pushed away from the body and/or inwards towards the centre [18] |
| Tremble | Visible shaking of the sow’s body [18] |
| Tail tickling | Tail is moving intensely in all directions [18] |
| Postures | |
| Lying lateral (L.Lat) | Lying on the side with the udder exposed [35] |
| Lying sternal (L.St) | Lying on the belly, with the front legs under the sow [35] |
| Standing (St) | An upright posture on extended legs, with all four feet contacting the ground [36] |
| Sitting (Sit) | The posterior end of the sow’s body in contact with/supported by the ground. The anterior of the body supported by the two front feet/legs [36] |
| Postural change | Transitions from lying to sitting or standing and reversed |
| Suckling events | |
| Piglets suckling | If one or more piglets are in contact with the udder or teat with their nose or mouth. Vigorous and rhythmic up and down head movements or suckling movements (Yes or No) [37] |
| Primiparous (n = 5) | Multiparous (n = 5) | p-Value | |||
|---|---|---|---|---|---|
| Items | Mean | SE | Mean | SE | |
| Parity | 1.0 | 0.00 | 2.8 | 0.2 | |
| Total duration of farrowing (h) | 3.1 | 0.34 | 3.2 | 0.44 | 0.764 |
| Total piglets born per litter | 15.8 | 0.58 | 18.6 | 2.32 | 0.275 |
| Born alive per litter | 14.6 | 0.24 | 16.8 | 2.18 | 0.345 |
| Stillborns per litter | 1.0 | 0.45 | 1.4 | 0.75 | 0.659 |
| Mummified foetuses per litter | 0.2 | 0.20 | 0.4 | 0.40 | 0.667 |
| Cross-fostered piglets per litter | 14.2 | 0.20 | 14.8 | 0.20 | 0.701 |
| Facial Action Units | Body Pain Indicators | ||||||
|---|---|---|---|---|---|---|---|
| Tremble | Leg | Arch | Tail | Paw | Total BPI | Total BPI Without Tremble | |
| Tension above eyes | 0.141 | 0.520 *** | 0.737 *** | 0.383 *** | 0.591 *** | 0.771 *** | 0.799 *** |
| Snout angle | 0.141 | 0.445 *** | 0.709 *** | 0.311 *** | 0.590 *** | 0.718 *** | 0.746 *** |
| Neck tension | 0.108 | 0.397 *** | 0.578 *** | 0.252 ** | 0.449 *** | 0.568 *** | 0.596 *** |
| Temporal tension and ear position | 0.060 | 0.367 *** | 0.543 *** | 0.246 ** | 0.396 *** | 0.515 *** | 0.573 *** |
| Cheek tension | 0.151 | 0.508 *** | 0.622 *** | 0.319 ** | 0.532 *** | 0.711 *** | 0.735 *** |
| Total Facial Index 1 | 0.116 | 0.529 *** | 0.704 *** | 0.332 ** | 0.558 *** | 0.722 *** | 0.763 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, E.; Guevara, R.D.; Mainau, E.; de Miguel, R.; Manteca, X. The Correlation Between Body Pain Indicators and the Facial Expression Scale in Sows During Farrowing and Pre-Weaning: The Effects of Parity, the Farrowing Moment, and Suckling Events. Animals 2025, 15, 2225. https://doi.org/10.3390/ani15152225
Navarro E, Guevara RD, Mainau E, de Miguel R, Manteca X. The Correlation Between Body Pain Indicators and the Facial Expression Scale in Sows During Farrowing and Pre-Weaning: The Effects of Parity, the Farrowing Moment, and Suckling Events. Animals. 2025; 15(15):2225. https://doi.org/10.3390/ani15152225
Chicago/Turabian StyleNavarro, Elena, Raúl David Guevara, Eva Mainau, Ricardo de Miguel, and Xavier Manteca. 2025. "The Correlation Between Body Pain Indicators and the Facial Expression Scale in Sows During Farrowing and Pre-Weaning: The Effects of Parity, the Farrowing Moment, and Suckling Events" Animals 15, no. 15: 2225. https://doi.org/10.3390/ani15152225
APA StyleNavarro, E., Guevara, R. D., Mainau, E., de Miguel, R., & Manteca, X. (2025). The Correlation Between Body Pain Indicators and the Facial Expression Scale in Sows During Farrowing and Pre-Weaning: The Effects of Parity, the Farrowing Moment, and Suckling Events. Animals, 15(15), 2225. https://doi.org/10.3390/ani15152225





