Phytochemicals and Monensin in Dairy Cows: Impact on Productive Performance and Ruminal Fermentation Profile
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cows, Experimental Design, and Management
2.2. Sampling and Chemical Analysis
2.3. Dry Matter Intake
2.4. Milk Yield, Composition, and Feed Use-Efficiency
2.5. Ruminal Fermentation Characteristics and Protozoa Concentration
2.6. Statistical Analysis
3. Results
3.1. Dry Matter Intake and Body Condition Score
3.2. Milk Yield and Composition
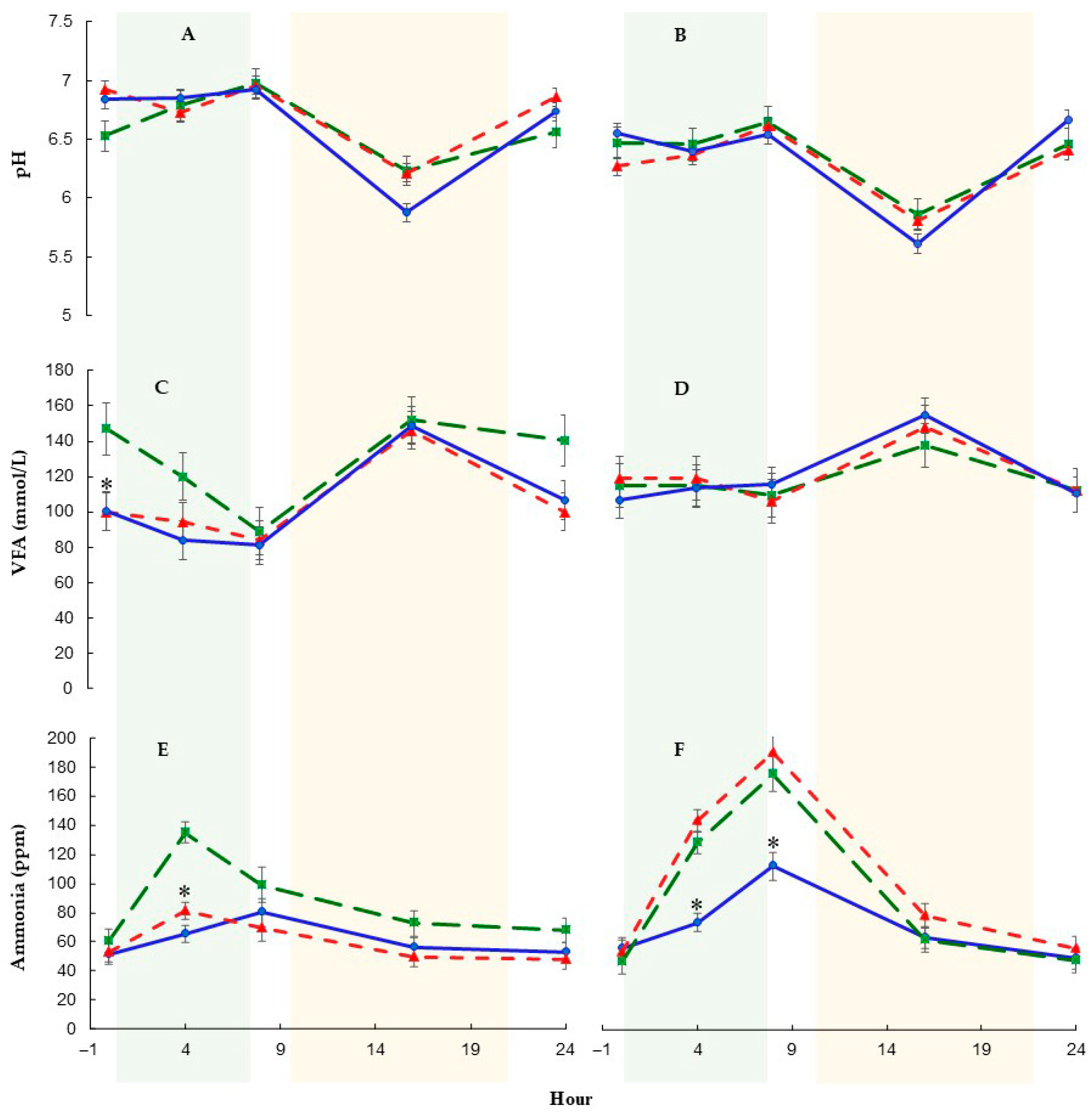
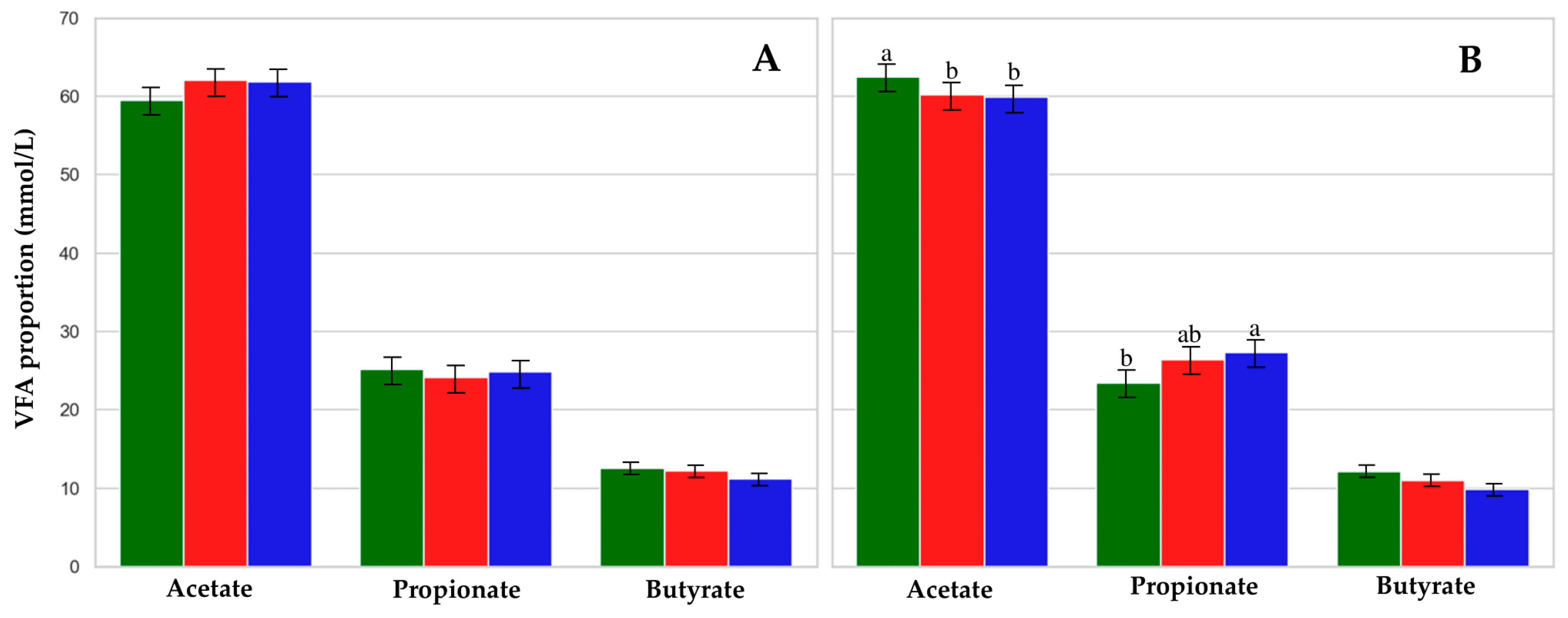
3.3. Rumen Fermentation Characteristics and Protozoa
4. Discussion
4.1. Productive Performance
4.2. Rumen Fermentation Profile
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTL | Control treatment |
| MON | Monensin treatment |
| PHY | Phytochemical treatment |
| TMR | Total mixed ration |
| BCVFA | Branched-chain volatile fatty acid |
| MY | Milk yield |
| DMI | Dry matte intake |
| VFA | Volatile fatty acid |
| NH3-N | Ammonia |
| BCS | Body condition score |
| BW | Body weight |
| NASEM | National Academies of Sciences, Engineering, and Medicine |
| DIM | Days in milk |
| DM | Dry matter |
| OM | Organic matter |
| NDF | Neutral detergent fiber |
| ADF | Acid detergent fiber |
| NEL | Net energy of lactation |
| DE | Digestible energy |
| OMD | Organic matter digestibility |
| ME | Metabolizable energy |
| TMS | Total milk solids |
| ECM | Energy-corrected milk |
| NRC | National Research Council |
| C2 | Acetate |
| C3 | Propionate |
| C4 | Butyrate |
| C2 + C4:C3 | Ketogenic/glucogenic ratio |
References
- Kholif, A.E.; Olafadehan, O.A. Essential oils and phytogenic feed additives in ruminant diet: Chemistry, ruminal microbiota and fermentation, feed utilization, and productive performance. Phytochem. Rev. 2021, 20, 1087–1108. [Google Scholar] [CrossRef]
- Firkins, J.L. Invited review: Advances in rumen efficiency. Appl. Anim. Sci. 2021, 37, 388–403. [Google Scholar] [CrossRef]
- Appuhamy, J.A.; Strathe, A.B.; Jayasundara, S.; Wagner-Riddle, C.; Dijkstra, J.; France, J.; Kebreab, E. Anti-methanogenic effects of monensin in dairy and beef cattle: A meta-analysis. J. Dairy Sci. 2013, 96, 5161–5173. [Google Scholar] [CrossRef] [PubMed]
- Rezaei Ahvanooei, M.R.; Norouzian, M.A.; Piray, A.H.; Vahmani, P.; Ghaffari, M.H. Effects of monensin supplementation on lactation performance of dairy cows: A systematic review and dose-response meta-analysis. Sci. Rep. 2023, 13, 568. [Google Scholar] [CrossRef] [PubMed]
- Official Journal of the European Union. Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Off. J. Eur. Union. 2003, L268, 29–43. [Google Scholar]
- Olynk, N.J. Assessing changing consumer preferences for livestock production processes. Anim. Front. 2012, 2, 32–37. [Google Scholar] [CrossRef]
- Benchaar, C.; Calsamiglia, S.; Chaves, A.V.; Fraser, G.R.; Colombatto, D.; McAllister, T.A.; Beauchemin, K.A. A review of plant-derived essential oils in ruminant nutrition and production. Anim. Feed Sci. Technol. 2008, 145, 209–228. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yun, W.; Lee, J.H.; Lee, C.H.; Kwak, W.K.; Cho, J.H. Effects of essential oil (blended and single essential oils) on anti-biofilm formation of Salmonella and Escherichia coli. J. Anim. Sci. Technol. 2017, 59, 4. [Google Scholar] [CrossRef]
- Cardozo, P.W.; Calsamiglia, S.; Ferret, A.; Kamel, C. Effects of natural plant extracts on ruminal protein degradation and fermentation profiles in continuous culture. J. Anim. Sci. 2004, 82, 3230–3236. [Google Scholar] [CrossRef]
- Khiaosa-ard, R.; Zebeli, Q. Meta-analysis of the effects of essential oils and their bioactive compounds on rumen fermentation characteristics and feed efficiency in ruminants. J. Anim. Sci. 2013, 91, 1819–1830. [Google Scholar] [CrossRef]
- Bodas, R.; Prieto, N.; García-González, R.; Andrés, S.; Giráldez, F.J.; López, S. Manipulation of rumen fermentation and methane production with plant secondary metabolites. Anim. Feed Sci. Technol. 2012, 176, 78–93. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef]
- Silva, R.B.D.; Pereira, M.N.; Araujo, R.C.D.; Silva, W.D.R.; Pereira, R.A.N. A blend of essential oils improved feed efficiency and affected ruminal and systemic variables of dairy cows. Transl. Anim. Sci. 2020, 4, 182–193. [Google Scholar] [CrossRef]
- Cantet, J.M.; Yu, Z.; Tucker, H.A.; Ríus, A.G. A cinnamaldehyde feed additive improved feed use-efficiency in lactating dairy cows. Livest. Sci. 2023, 272, 105236. [Google Scholar] [CrossRef]
- Kung, L.; Williams, P.; Schmidt, R.J.; Hu, W. A blend of essential plant oils used as an additive to alter silage fermentation or used as a feed additive for lactating dairy cows. J. Dairy Sci. 2008, 91, 4793–4800. [Google Scholar] [CrossRef]
- Tassoul, M.D.; Shaver, R.D. Effect of a mixture of supplemental dietary plant essential oils on performance of periparturient and early lactation dairy cows. J. Dairy Sci. 2009, 92, 1734–1740. [Google Scholar] [CrossRef]
- Franz, C.; Baser, K.; Windisch, W. Essential oils and aromatic plants in animal feeding—A European perspective. A review. Flavour. Fragr. J. 2010, 25, 327–340. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Cooke, R.F.; Firmino, F.C.; Moura, M.K.R.; Angeli, B.F.; Ferreira, H.A.O.; Brandão, A.P.; Gex-Fabry, M.; Ostrensky, A.; Vasconcelos, J.L.M. Productive and physiological responses of lactating dairy cows supplemented with phytogenic feed ingredients. Transl. Anim. Sci. 2019, 3, 1133–1142. [Google Scholar] [CrossRef]
- Fariña, S.R.; Chilibroste, P. Opportunities and challenges for the growth of milk production from pasture: The case of farm systems in Uruguay. Agric. Syst. 2019, 176, 102631. [Google Scholar] [CrossRef]
- Edmonson, A.J.; Lean, I.J.; Weaver, L.D.; Farver, T.; Webster, G. A body condition scoring chart for Holstein dairy cows. J. Dairy Sci. 1989, 72, 68–78. [Google Scholar] [CrossRef]
- Pons, M.V.; Adrien, M.L.; Mattiauda, D.A.; Breijo, M.A.; Meikle, A.; Chilibroste, P.; Damián, J.P. Welfare of dairy cows in mixed feeding systems under two different conditions of confinement: Behavioral, biochemical, and physiological indicators. Appl. Anim. Behav. Sci. 2023, 265, 105995. [Google Scholar] [CrossRef]
- NASEM (National Academies of Sciences, Engineering, and Medicine). Nutrient Requirements of Dairy Cattle, 8th ed.; The National Academies Press: Washington, DC, USA, 2021. [Google Scholar] [CrossRef]
- Haydock, K.P.; Shaw, N.H. The comparative yield method for estimating dry matter yield of pasture. Aust. J. Agric. Anim. Husb. 1975, 15, 663–670. [Google Scholar] [CrossRef]
- Coates, D.B.; Penning, P.D. Measuring animal performance. In Field and Laboratory Methods for Grassland and Animal Production Research; Whitehead, D.C., Ed.; CABI: Wallingford, CT, USA, 2000; pp. 353–402. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 15th ed.; AOAC International: Arlington, VA, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Méndez, M.N.; Grille, L.; Mendina, G.R.; Robinson, P.H.; Adrien, M.D.L.; Meikle, A.; Chilibroste, P. Performance of autumn and spring calving Holstein dairy cows with different levels of environmental exposure and feeding strategies. Animals 2023, 13, 1211. [Google Scholar] [CrossRef]
- Rohweder, D.W. Estimating forage hay quality. In Proceedings of the National Alfalfa Hay Quality Testing Workshop, Chicago, IL, USA, 22–23 March 1984; National Alfalfa Hay Quality Committee: Chicago, IL, USA, 1984; pp. 31–37. [Google Scholar]
- NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academies Press: Washington, DC, USA, 2001; ISBN 978-0309069977. [Google Scholar]
- Mattiauda, D.A.; Tamminga, S.; Gibb, M.J.; Soca, P.; Bentancur, O.; Chilibroste, P. Restricting access time at pasture and time of grazing allocation for Holstein dairy cows: Ingestive behaviour, dry matter intake, and milk production. Livest. Sci. 2013, 152, 53–62. [Google Scholar] [CrossRef]
- Chaney, A.L.; Marbach, E.P. Modified reagents for determination of urea and ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef]
- Dehority, B.A. Evaluation of subsampling and fixation procedures used for counting rumen protozoa. Appl. Environ. Microbiol. 1984, 48, 182–185. [Google Scholar] [CrossRef]
- D’Agosto, M.; Carneiro, M.E. Evaluation of Lugol solution used for counting rumen ciliates. Rev. Bras. Zool. 1999, 16, 725–729. [Google Scholar] [CrossRef]
- Benchaar, C. Diet supplementation with thyme oil and its main component thymol failed to favorably alter rumen fermentation, improve nutrient utilization, or enhance milk production in dairy cows. J. Dairy Sci. 2021, 104, 324–336. [Google Scholar] [CrossRef]
- Kholif, A.E.; Hassan, A.A.; El Ashry, G.M.; Bakr, M.H.; El-Zaiat, H.M.; Olafadehan, O.A.; Matloup, O.H.; Sallam, S.M.A. Phytogenic feed additives mixture enhances the lactational performance, feed utilization, and ruminal fermentation of Friesian cows. Anim. Biotechnol. 2021, 32, 708–718. [Google Scholar] [CrossRef]
- Eicher, S.D.; Schutz, M.; Kearney, F.; Willard, S.; Bowers, S.; Gandy, S.; Graves, K. Prepartum milking effects on parlour behaviour, endocrine, and immune responses in Holstein heifers. J. Dairy Res. 2007, 74, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Chilibroste, P.; Mattiauda, D.A.; Bentancur, O.; Soca, P.; Meikle, A. Effect of herbage allowance on grazing behavior and productive performance of early lactation primiparous Holstein cows. Anim. Feed Sci. Technol. 2012, 173, 201–209. [Google Scholar] [CrossRef]
- Meikle, A.; de Brun, V.; Carriquiry, M.; Soca, P.; Sosa, C.; Adrien, M.L.; Chilibroste, P.; Abecia, J.A. Influences of nutrition and metabolism on reproduction of the female ruminant. Anim. Reprod. 2018, 15 (Suppl. S1), 899–911. [Google Scholar] [CrossRef]
- Comesaña, M. (Universidad de la República, Facultad de Veterinaria, Paysandú, Uruguay). Personal communication, 2022. Manuscript in Preparation.
- Tedeschi, L.O.; Fox, D.G.; Tylutki, T.P. Potential environmental benefits of ionophores in ruminant diets. J. Environ. Qual. 2003, 32, 1591–1602. [Google Scholar] [CrossRef]
- Duffield, T.F.; Rabiee, A.R.; Lean, I.J. A meta-analysis of the impact of monensin in lactating dairy cattle. Part 2. Production effects. J. Dairy Sci. 2008, 91, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Yasui, T.; Ryan, C.M.; Mechor, G.D.; Overton, T.R. Performance of early-lactation dairy cows as affected by dietary starch and monensin supplementation. J. Dairy Sci. 2015, 98, 3335–3350. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J. Nutritional Ecology of the Ruminant; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Newbold, C.J.; De La Fuente, G.; Belanche, A.; Ramos-Morales, E.; McEwan, N.R. The role of ciliate protozoa in the rumen. Front. Microbiol. 2015, 6, 1313. [Google Scholar] [CrossRef]
- Dorao, C.; Cáceres, A.; Trujillo, A.; Casal, A.; Mattiauda, D.A.; Dall-Orsolleta, A.C.; Arturto-Schaan, M.; Bruni, M.A. (Universidad de la República, Facultad de Agronomía, Paysandú, Uruguay). Personal communication, 2022. Manuscript in Preparation.
- Hristov, A.N.; Ropp, J.K.; Grandeen, K.L.; Abedi, S.; Etter, R.P.; Melgar, A.; Foley, A.E. Effect of carbohydrate source on ammonia utilization in lactating dairy cows. J. Anim. Sci. 2005, 83, 408–421. [Google Scholar] [CrossRef]
- Reynolds, C.K.; Aikman, P.C.; Lupoli, B.; Humphries, D.J.; Beever, D.E. Splanchnic metabolism of dairy cows during the transition from late gestation through early lactation. J. Dairy Sci. 2003, 86, 1201–1217. [Google Scholar] [CrossRef]
- Larsen, M.; Kristensen, N.B. Precursors for liver gluconeogenesis in periparturient dairy cows. Animal 2013, 7, 1640–1650. [Google Scholar] [CrossRef]
- Phipps, R.H.; Wilkinson, J.I.D.; Jonker, L.J.; Tarrant, M.; Jones, A.K.; Hodge, A. Effect of monensin on milk production of Holstein-Friesian dairy cows. J. Dairy Sci. 2000, 83, 2789–2794. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, R.; Albrecht, G.L.; Tedeschi, L.O.; Jarvis, G.; Russell, J.B.; Fox, D.G. Effect of monensin on the performance and nitrogen utilization of lactating dairy cows consuming fresh forage. J. Dairy Sci. 2001, 84, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Elcoso, G.; Zweifel, B.; Bach, A. Effects of a blend of essential oils on milk yield and feed efficiency of lactating dairy cows. Appl. Anim. Sci. 2019, 35, 304–311. [Google Scholar] [CrossRef]
- Silvestre, T.; Martins, L.F.; Cueva, S.F.; Wasson, D.E.; Stepanchenko, N.; Räisänen, S.E.; Sommai, S.; Hile, M.L.; Hristov, A.N. Lactational performance, rumen fermentation, nutrient use efficiency, enteric methane emissions, and manure greenhouse gas-emitting potential in dairy cows fed a blend of essential oils. J. Dairy Sci. 2023, 106, 7661–7674. [Google Scholar] [CrossRef]
- Benchaar, C.; Petit, H.V.; Berthiaume, R.; Ouellet, D.R.; Chiquette, J.; Chouinard, P.Y. Effects of essential oils on digestion, ruminal fermentation, rumen microbial populations, milk production, and milk composition in dairy cows fed alfalfa silage or corn silage. J. Dairy Sci. 2007, 90, 886–897. [Google Scholar] [CrossRef]
- Tomkins, N.W.; Denman, S.E.; Pilajun, R.; Wanapat, M.; McSweeney, C.S.; Elliott, R. Manipulating rumen fermentation and methanogenesis using an essential oil and monensin in beef cattle fed a tropical grass hay. Anim. Feed Sci. Technol. 2015, 200, 25–34. [Google Scholar] [CrossRef]
- Chaves, A.V.; Dugan, M.E.R.; Stanford, K.; Gibson, L.L.; Bystrom, J.M.; McAllister, T.A.; Van Herk, F.; Benchaar, C. A dose-response of cinnamaldehyde supplementation on intake, ruminal fermentation, blood metabolites, growth performance, and carcass characteristics of growing lambs. Livest. Sci. 2011, 141, 213–220. [Google Scholar] [CrossRef]
- Chapman, C.E.; Ort, S.B.; Aragona, K.M.; Cabral, R.G.; Erickson, P.S. Effect of cinnamaldehyde on feed intake, rumen fermentation, and nutrient digestibility in lactating dairy cows. J. Anim. Sci. 2019, 97, 1819–1827. [Google Scholar] [CrossRef]
- Ando, S.; Nishida, T.; Ishida, M.; Hosoda, K.; Bayaru, E. Effect of peppermint feeding on digestibility, ruminal fermentation, and protozoa. Livest. Prod. Sci. 2003, 82, 245–248. [Google Scholar] [CrossRef]
- Soltan, Y.A.; Natel, A.S.; Araujo, R.C.; Morsy, A.S.; Abdalla, A.L. Progressive adaptation of sheep to a microencapsulated blend of essential oils: Ruminal fermentation, methane emission, nutrient digestibility, and microbial protein synthesis. Anim. Feed Sci. Technol. 2018, 237, 8–18. [Google Scholar] [CrossRef]
- McIntosh, F.M.; Williams, P.; Losa, R.; Wallace, R.J.; Beever, D.A.; Newbold, C.J. Effects of essential oils on ruminal microorganisms and their protein metabolism. Appl. Environ. Microbiol. 2003, 69, 5011–5014. [Google Scholar] [CrossRef] [PubMed]
- Benchaar, C.; Petit, H.V.; Berthiaume, R.; Whyte, T.D.; Chouinard, P.Y. Effects of addition of essential oils and monensin premix on digestion, ruminal fermentation, milk production, and milk composition in dairy cows. J. Dairy Sci. 2006, 89, 4352–4364. [Google Scholar] [CrossRef]
- Bargo, F.; Muller, L.D.; Kolver, E.S.; Delahoy, J.E. Invited review: Production and digestion of supplemented dairy cows on pasture. J. Dairy Sci. 2003, 86, 1–42. [Google Scholar] [CrossRef]
- Owens, F.N.; Basalan, M. Ruminal fermentation. In Rumenology; Millen, D.D., De Beni Arrigoni, M., Lauritano Pacheco, R.D., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 63–102. [Google Scholar] [CrossRef]
- Mezzetti, M.; Piccioli-Cappelli, F.; Bani, P.; Amadori, M.; Calamari, L.; Minuti, A.; Loor, J.J.; Bionaz, M.; Trevisi, E. Monensin controlled-release capsule administered in late pregnancy differentially affects rumination patterns, metabolic status, and cheese-making properties of the milk in primiparous and multiparous cows. Ital. J. Anim. Sci. 2019, 18, 1271–1283. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Busquet, M.; Cardozo, P.W.; Castillejos, L.; Ferret, A. Invited review: Essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef]
- Ishlak, A.; Günal, M.; AbuGhazaleh, A.A. The effects of cinnamaldehyde, monensin, and quebracho condensed tannin on rumen fermentation, biohydrogenation, and bacteria in continuous culture system. Anim. Feed Sci. Technol. 2015, 207, 31–40. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, S.; Li, Y.; Zhang, S.; Qi, X.; Guo, K.; Guo, Y.; Fortina, R. Pilot study of the effects of polyphenols from chestnut involucre on methane production, volatile fatty acids, and ammonia concentration during in vitro rumen fermentation. Animals 2021, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.J.; Garciarena, A.D.; Vieyra, J.M.H.; Beauchemin, K.A.; Colombatto, D. Effects of specific essential oil compounds on the ruminal environment, milk production, and milk composition of lactating dairy cows at pasture. Anim. Feed Sci. Technol. 2013, 186, 20–26. [Google Scholar] [CrossRef]
- Hayes, D.P.; Pfeiffer, D.U.; Williamson, N.B. Effect of intraruminal monensin capsules on reproductive performance and milk production of dairy cows fed pasture. J. Dairy Sci. 1996, 79, 1000–1008. [Google Scholar] [CrossRef]
- Melchior, E.A.; Hales, K.E.; Lindholm-Perry, A.K.; Freetly, H.C.; Wells, J.E.; Hemphill, C.N.; Wickersham, T.A.; Sawyer, J.E.; Myer, P.R. The effects of feeding monensin on rumen microbial communities and methanogenesis in bred heifers fed in a drylot. Livest. Sci. 2018, 212, 131–136. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Ferret, A.; Reynolds, C.K.; Kristensen, N.B.; Van Vuuren, A.M. Strategies for optimizing nitrogen use by ruminants. Animal 2010, 4, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Koser, S.L.; Bequette, B.J.; Donkin, S.S. Effect of propionate on mRNA expression of key genes for gluconeogenesis in liver of dairy cattle. J. Dairy Sci. 2015, 98, 8698–8709. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.; Galindo, C.; Ouellet, D.R.; Maxin, G.; Kristensen, N.B.; Lapierre, H. Abomasal amino acid infusion in postpartum dairy cows: Effect on whole-body, splanchnic, and mammary amino acid metabolism. J. Dairy Sci. 2015, 98, 7944–7961. [Google Scholar] [CrossRef] [PubMed]
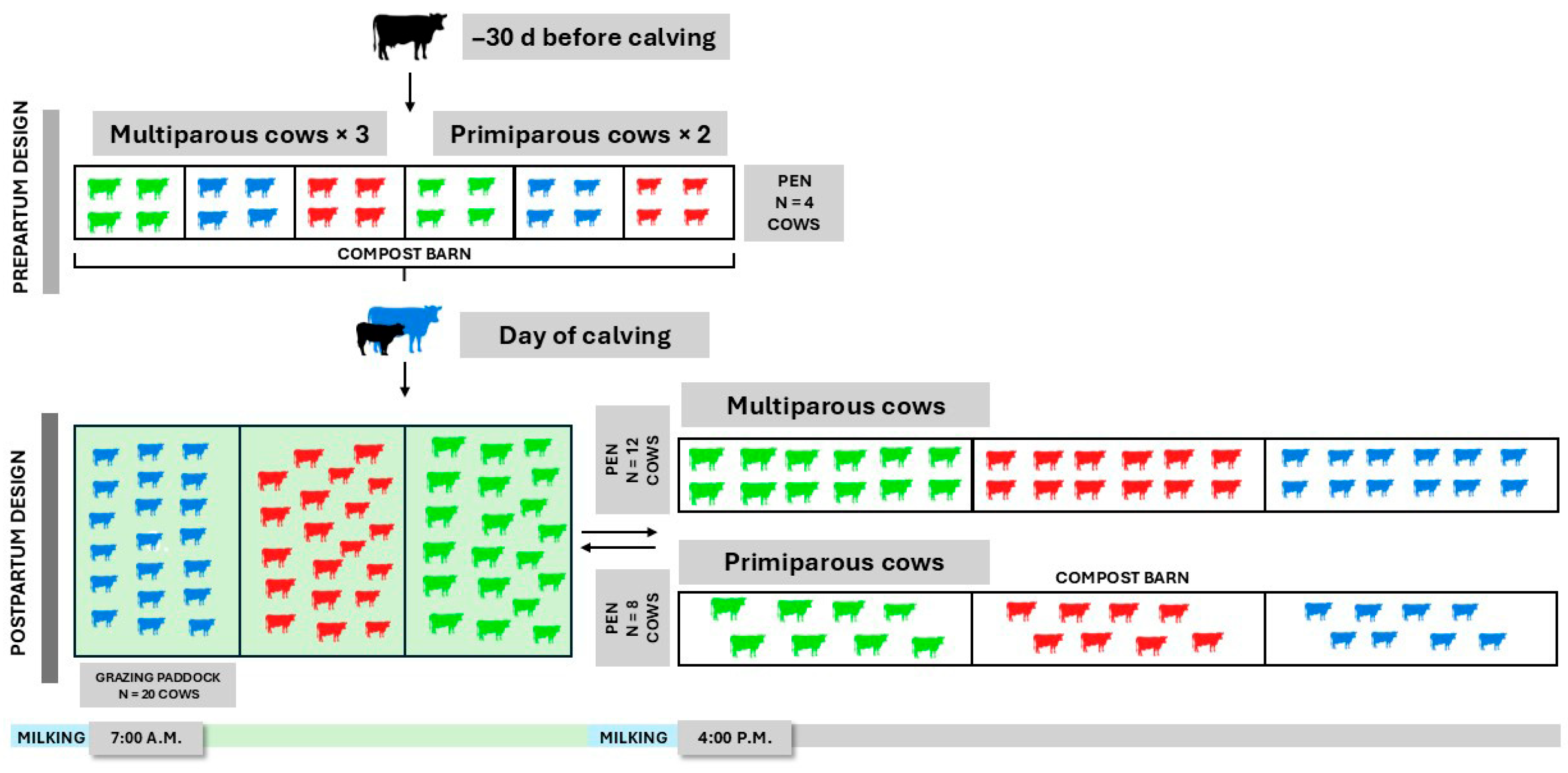
| Ingredient (% of DM) | TMR Prepartum | TMR Postpartum | Pasture 5 |
|---|---|---|---|
| Corn Silage | 38.8 | 30.6 | - |
| Barley Straw | 31.4 | - | - |
| Alfalfa Hay | - | 13.6 | - |
| Soybean Meal | 16.2 | 13.6 | - |
| Grain Corn | 4.37 | 26.4 | - |
| Wheat Bran | 4.42 | - | - |
| Barley grain | - | 7.65 | - |
| Calcium carbonate | 1.36 | 1.69 | - |
| Sodium chloride | - | 0.68 | - |
| Bicarbonate | - | 0.66 | - |
| Magnesium oxide | - | 0.66 | - |
| Monocalcium phosphate | - | 0.66 | - |
| Sulfur | - | 1.74 | - |
| Semitin wheat | - | 1.74 | - |
| Mineral and Vitamin supplement 1 | 3.45 | 0.32 | - |
| Chemical Composition | |||
| Organic Matter | 90.6 ± 1.34 | 91.3 ± 0.44 | 88.1 ± 2.1 |
| Crude Protein | 10.8 ± 1.48 | 12.8 ± 0.96 | 17.4 ± 0.6 |
| NDF 2 | 44.2 ± 2.51 | 26.8 ± 3.44 | 36.8 ± 7.0 |
| ADF 3 | 25.9 ± 1.02 | 14.0 ± 1.50 | 22.4 ± 2.6 |
| Digestible Energy (Mcal/Kg of DM) | 2.86 ± 0.01 | 3.07 ± 0.01 | 2.97 ± 0.09 |
| NEL 4 (Mcal/Kg of DM) | 1.47 ± 0.01 | 1.67 ± 0.01 | 1.61 ± 0.06 |
| Treatment | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | CTL | MON | PHY | SEM | T 5 | BW 6 | DIM 7 | DIM^2 |
| Production | ||||||||
| Milk yield (kg/day) | 26.5 b | 28.5 a | 28.7 a | 0.52 | 0.01 | <0.01 | <0.01 | <0.01 |
| ECM 1 (Mcal/day) | 19.5 | 21.0 | 20.2 | 0.86 | 0.50 | 0.47 | 0.21 | 0.41 |
| TMS 2 (kg/day) | 3.24 | 3.49 | 3.39 | 0.20 | 0.44 | 0.50 | 0.06 | 0.19 |
| Feed efficiency 3 | 1.44 | 1.45 | 1.51 | 0.04 | 0.54 | - | - | - |
| Composition (%) | ||||||||
| Fat | 3.71 | 3.73 | 3.59 | 0.15 | 0.53 | 0.49 | <0.01 | <0.01 |
| Protein | 3.38 | 3.46 | 3.31 | 0.05 | 0.22 | 0.97 | <0.01 | <0.01 |
| Lactose | 4.88 b | 5.00 a | 4.99 a | 0.05 | 0.04 | 0.55 | <0.01 | <0.01 |
| MUN 4 (mg/dL) | 14.2 | 14.5 | 13.0 | 0.99 | 0.54 | 0.69 | 0.14 | 0.29 |
| Yield (kg/d) | ||||||||
| Fat | 0.99 | 1.05 | 1.02 | 0.05 | 0.76 | 0.28 | 0.38 | 0.55 |
| Protein | 0.91 | 0.98 | 0.94 | 0.03 | 0.35 | 0.45 | 0.30 | 0.52 |
| Lactose | 1.30 | 1.43 | 1.42 | 0.04 | 0.11 | 0.49 | <0.01 | <0.01 |
| Treatment | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | CTL | MON | PHY | SEM | T 5 | BW 6 | DIM 7 | DIM^2 |
| Production | ||||||||
| Milk yield (kg/day) | 36.9 | 37.7 | 36.0 | 0.48 | 0.07 | <0.01 | <0.01 | <0.01 |
| ECM 1 (Mcal/day) | 25.9 | 26.3 | 25.5 | 0.55 | 0.50 | <0.01 | 0.18 | 0.26 |
| TMS 2 (kg/day) | 4.31 | 4.41 | 4.28 | 0.13 | 0.55 | <0.01 | <0.05 | 0.10 |
| Feed efficiency 3 | 1.56 | 1.59 | 1.61 | 0.03 | 0.56 | - | - | - |
| Composition (%) | ||||||||
| Fat | 3.68 | 3.54 | 3.44 | 0.09 | 0.19 | 0.61 | <0.01 | <0.01 |
| Protein | 3.41 | 3.31 | 3.33 | 0.04 | 0.29 | 0.78 | <0.01 | <0.01 |
| Lactose | 4.83 | 4.88 | 4.88 | 0.03 | 0.56 | 0.93 | <0.01 | <0.01 |
| MUN 4 (mg/dL) | 13.4 | 15.4 | 13.6 | 0.62 | 0.08 | 0.69 | 0.13 | 0.47 |
| Yield (kg/d) | ||||||||
| Fat | 1.30 | 1.32 | 1.25 | 0.03 | 0.21 | <0.01 | 0.52 | 0.50 |
| Protein | 1.23 | 1.24 | 1.22 | 0.03 | 0.83 | <0.01 | 0.23 | 0.36 |
| Lactose | 1.73 | 1.84 | 1.78 | 0.04 | 0.25 | <0.01 | <0.01 | <0.01 |
| Treatment | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| Item | CTL | MON | PHY | SEM | T | Hour | T × Hour |
| Ruminal pH | 6.50 | 6.53 | 6.66 | 0.06 | 0.18 | <0.01 | 0.83 |
| Ammonia (ppm) | 101 | 101 | 77.3 | 9.70 | 0.14 | 0.20 | 0.36 |
| Total VFA 1 | 91.5 | 94.8 | 103 | 7.95 | 0.57 | 0.33 | 0.99 |
| Acetate (%) | 62.9 | 64.7 | 61.1 | 1.56 | 0.24 | 0.12 | 0.96 |
| Propionate (%) | 23.2 | 23.2 | 22.2 | 0.89 | 0.63 | 0.40 | 0.77 |
| Butyrate (%) | 11.1 | 11.0 | 11.6 | 0.97 | 0.88 | 0.07 | 0.98 |
| Valerate (%) | 1.18 a | 0.96 b | 1.16 ab | 0.07 | 0.05 | 0.02 | 0.99 |
| BCVFA 2 (%) | 1.22 b | 1.30 ab | 1.78 a | 0.15 | 0.04 | 0.10 | 0.99 |
| C2:C3 3 | 2.72 | 2.91 | 2.73 | 0.16 | 0.64 | 0.32 | 0.99 |
| C2 + C4:C3 4 | 3.22 | 3.26 | 3.48 | 0.17 | 0.55 | 0.46 | 0.90 |
| Protozoa 5 | 12.3 a | 6.72 b | 8.23 ab | 1.35 | 0.01 | 0.01 | 0.88 |
| Treatment | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | CTL | MON | PHY | SEM | T | Hour | Per 6 | T × Per | T × Hour |
| Ruminal pH | 6.49 | 6.51 | 6.49 | 0.03 | 0.90 | <0.01 | <0.01 | 0.04 | 0.01 |
| Ammonia (ppm) | 89.4 a | 82.0 a | 65.8 b | 2.59 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Total VFA 1 | 121 | 110 | 109 | 3.85 | 0.09 | <0.01 | 0.95 | 0.02 | 0.91 |
| Acetate (%) | 62.0 | 61.1 | 60.9 | 0.54 | 0.95 | <0.01 | 0.70 | 0.01 | 0.99 |
| Propionate (%) | 24.3 | 25.2 | 26.1 | 0.55 | 0.08 | 0.20 | 0.14 | 0.02 | 0.99 |
| Butyrate (%) | 12.3 a | 11.6 a | 10.5 b | 0.28 | 0.01 | <0.01 | 0.01 | 0.50 | 0.61 |
| Valerate (%) | 1.56 a | 1.18 b | 1.42 a | 0.07 | 0.01 | 0.24 | 0.14 | 0.05 | 0.69 |
| BCVFA 2 (%) | 1.15 a | 0.93 b | 0.93 b | 0.04 | 0.01 | <0.01 | 0.06 | 0.36 | 0.93 |
| C2:C3 3 | 2.57 | 2.45 | 2.38 | 0.07 | 0.24 | 0.04 | 0.48 | 0.02 | 0.99 |
| C2 + C4:C3 4 | 3.06 | 2.91 | 2.78 | 0.09 | 0.09 | 0.40 | 0.61 | 0.03 | 0.98 |
| Protozoa 5 | 11.6 a | 7.12 b | 4.03 b | 1.10 | <0.01 | 0.62 | 0.02 | 0.17 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Chappe, L.; Bruni, M.A.; Dall-Orsoletta, A.C.; Chilibroste, P.; Meikle, A.; Adrien, M.L.; Casal, A.; Damián, J.P.; Naya, H.; Arturo-Schaan, M.; et al. Phytochemicals and Monensin in Dairy Cows: Impact on Productive Performance and Ruminal Fermentation Profile. Animals 2025, 15, 2172. https://doi.org/10.3390/ani15152172
Gonzalez-Chappe L, Bruni MA, Dall-Orsoletta AC, Chilibroste P, Meikle A, Adrien ML, Casal A, Damián JP, Naya H, Arturo-Schaan M, et al. Phytochemicals and Monensin in Dairy Cows: Impact on Productive Performance and Ruminal Fermentation Profile. Animals. 2025; 15(15):2172. https://doi.org/10.3390/ani15152172
Chicago/Turabian StyleGonzalez-Chappe, Lucas, Maria A. Bruni, Aline C. Dall-Orsoletta, Pablo Chilibroste, Ana Meikle, Maria L. Adrien, Alberto Casal, Juan P. Damián, Hugo Naya, Marisela Arturo-Schaan, and et al. 2025. "Phytochemicals and Monensin in Dairy Cows: Impact on Productive Performance and Ruminal Fermentation Profile" Animals 15, no. 15: 2172. https://doi.org/10.3390/ani15152172
APA StyleGonzalez-Chappe, L., Bruni, M. A., Dall-Orsoletta, A. C., Chilibroste, P., Meikle, A., Adrien, M. L., Casal, A., Damián, J. P., Naya, H., Arturo-Schaan, M., & Mattiauda, D. A. (2025). Phytochemicals and Monensin in Dairy Cows: Impact on Productive Performance and Ruminal Fermentation Profile. Animals, 15(15), 2172. https://doi.org/10.3390/ani15152172






