High Temperature and Ethinylestradiol May Reduce Body Growth, Liver and Hepatocyte Volumes and Lipid Droplets in Adult Male Guppies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Husbandry
2.2. Exposure and Sampling
2.3. Histological Processing, Routine Staining, and Glycogen Detection in Hepatocytes
2.4. Qualitative and Semiquantitative Analysis of the Hepatocytes
2.5. Lipid Droplets Visualization in Hepatocytes
2.6. Stereological Analysis and Derived Quantitative Parameters
2.7. EE2 Quantification in Water
2.8. Statistical Analysis
3. Results
3.1. EE2 Concentrations in the Water
3.2. Survival and Body Biometry
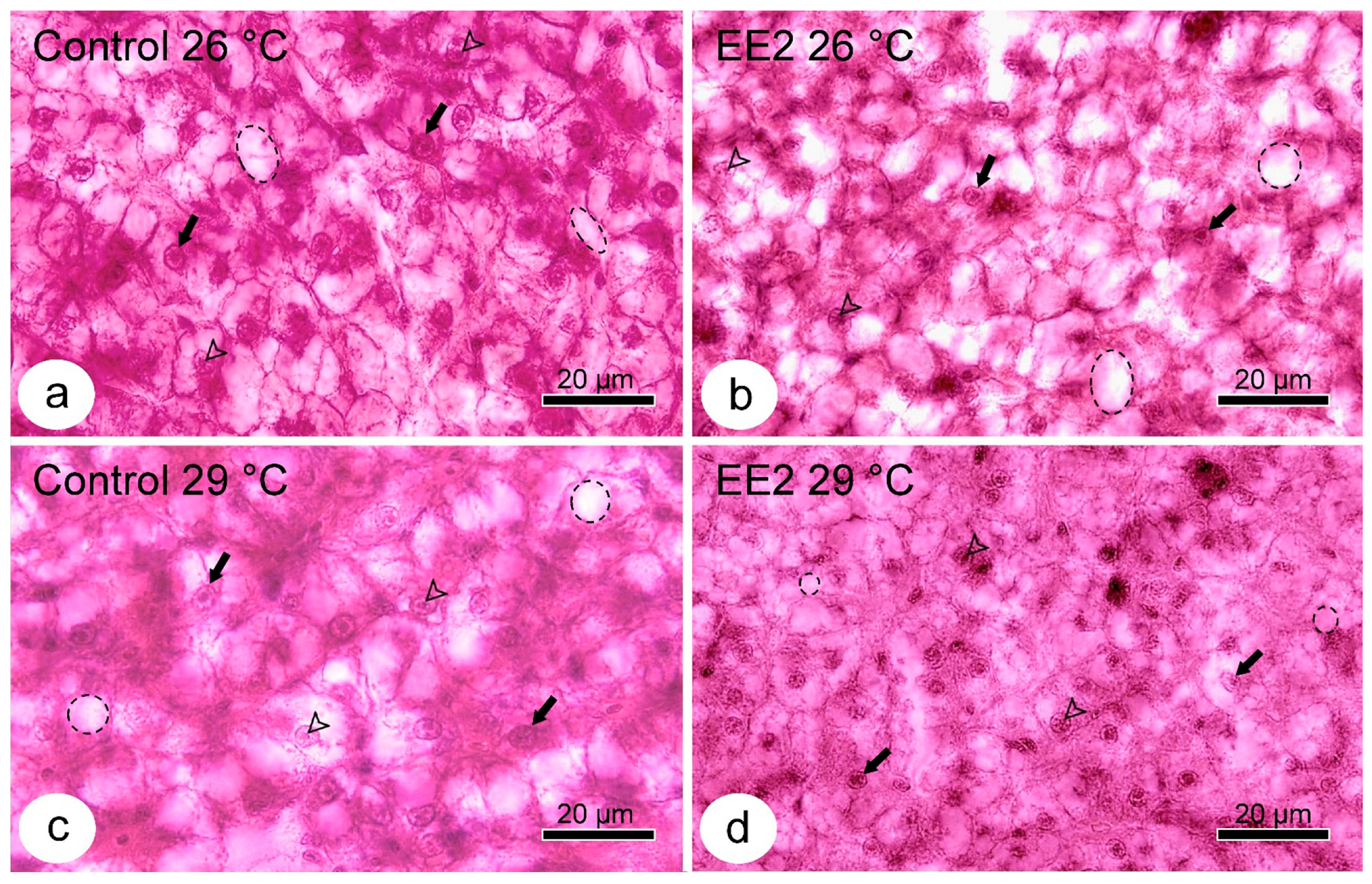
3.3. Qualitative and Semiquantitative Histological Analysis
3.4. PAS Staining
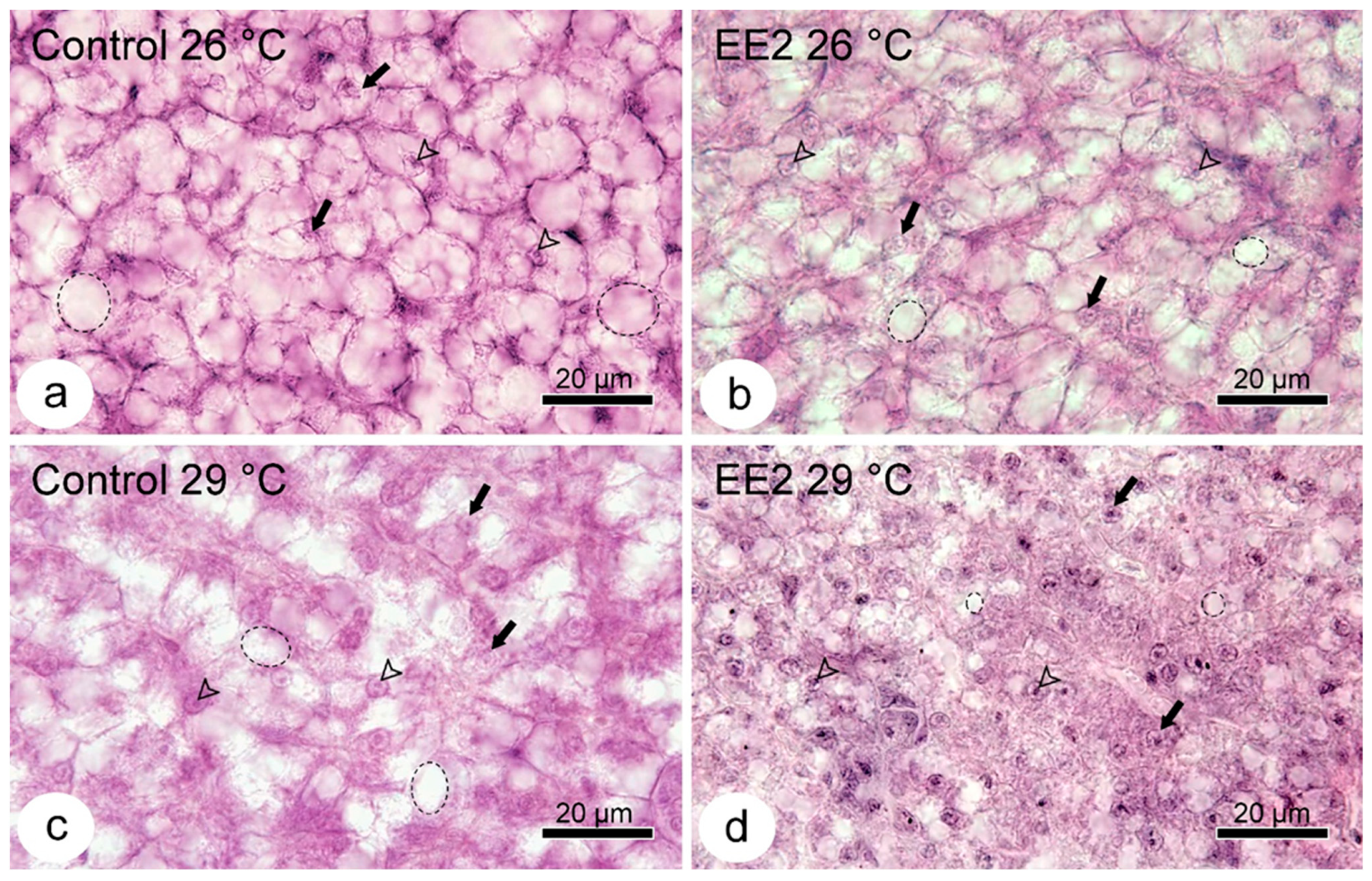
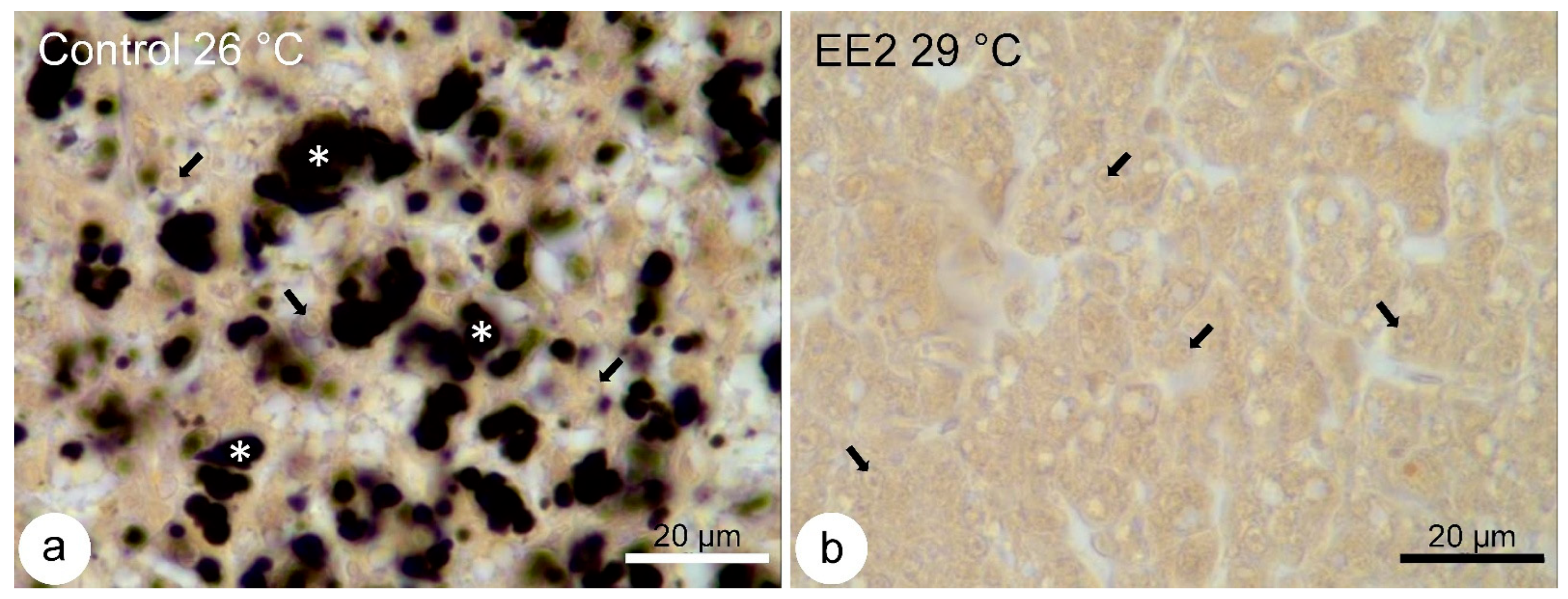
3.5. Lipid Droplets Staining
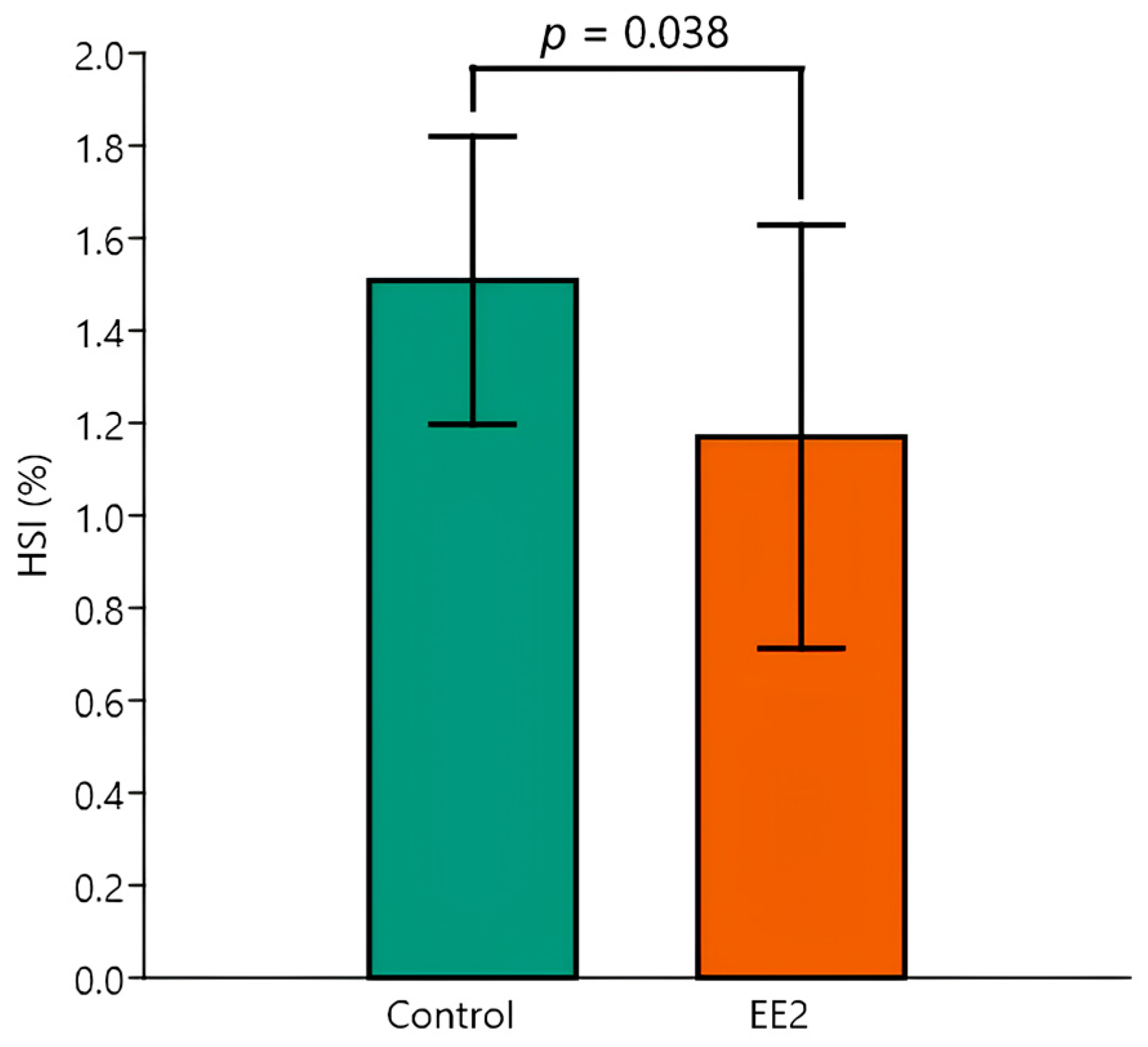
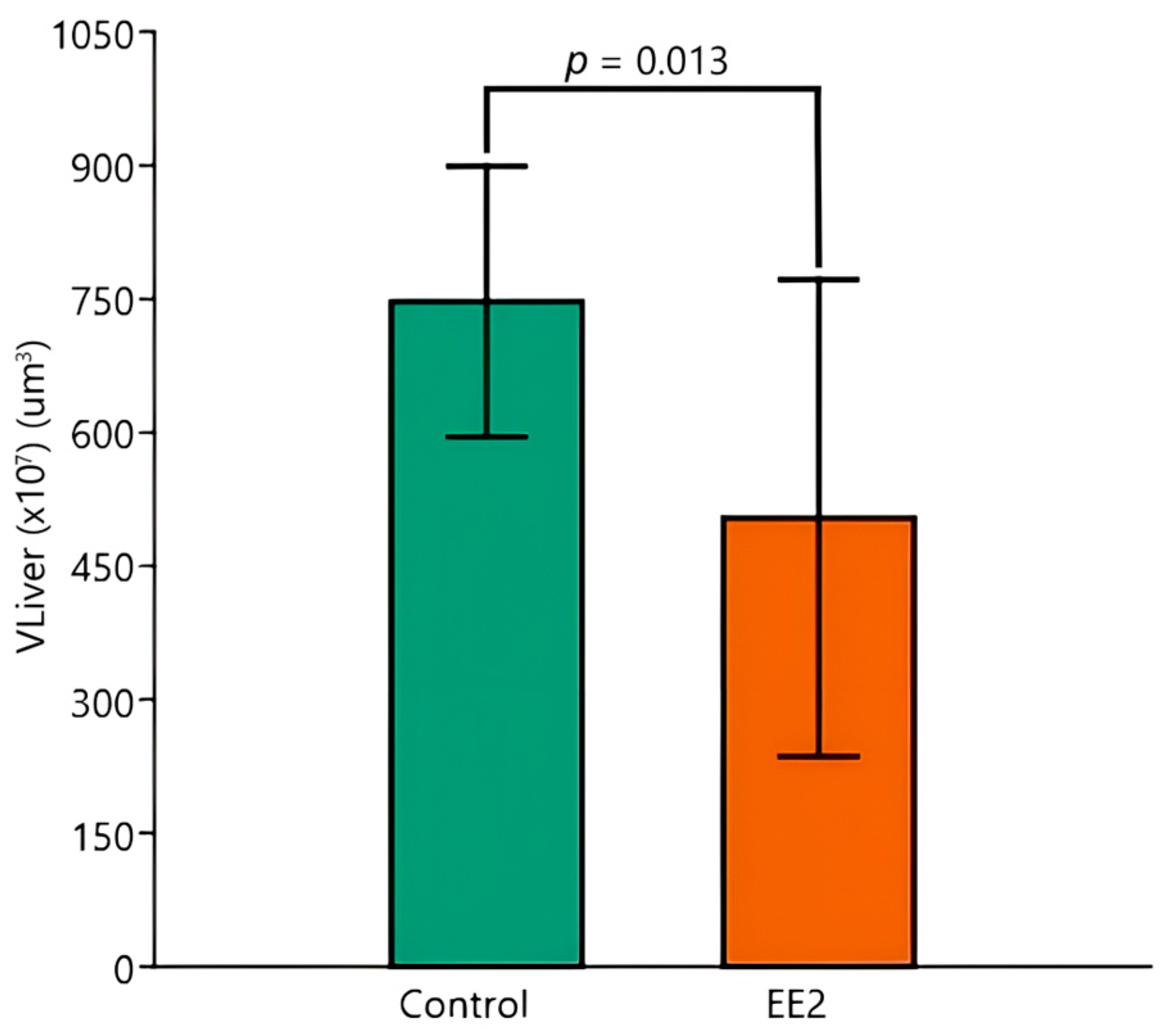
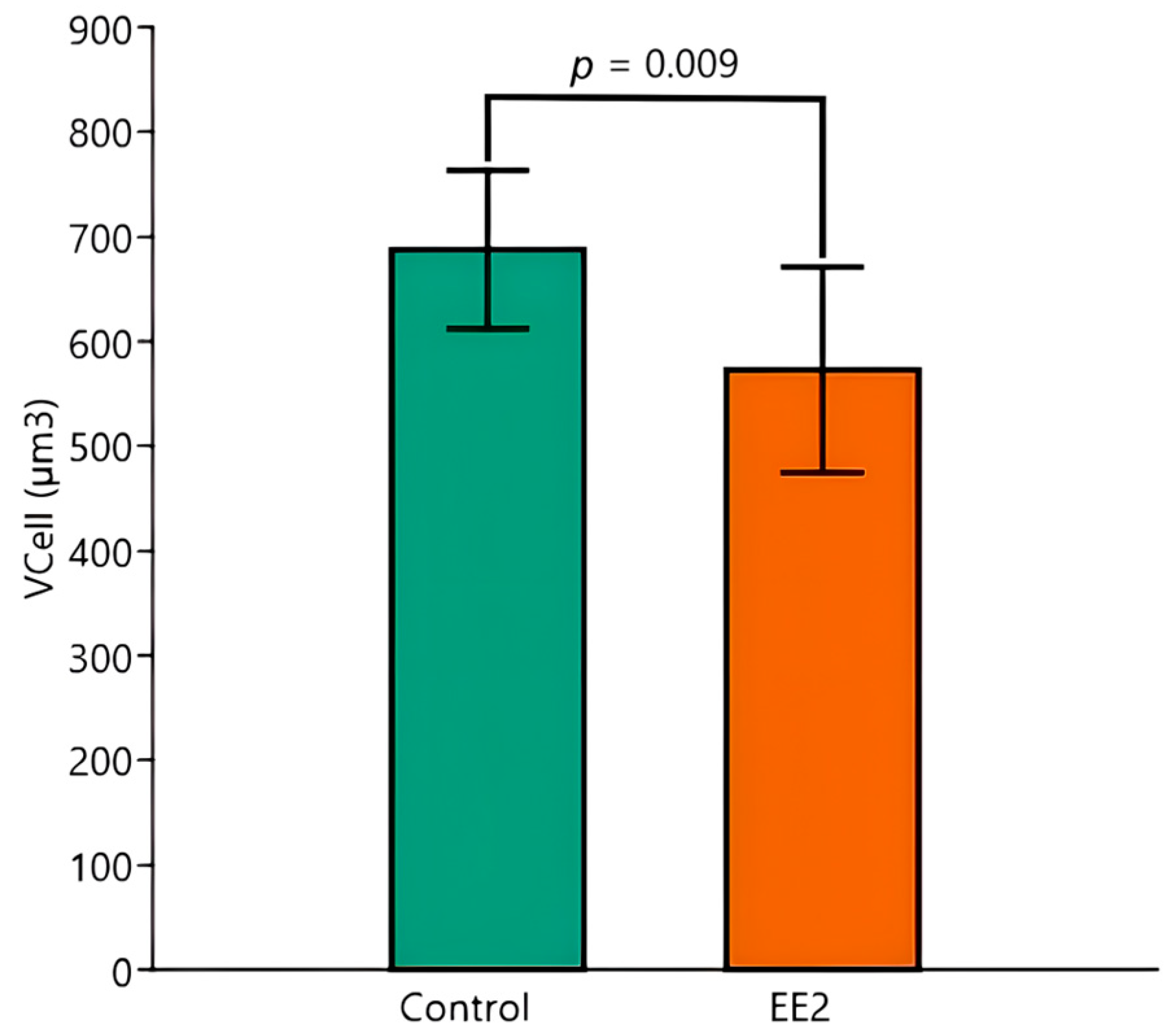
3.6. HSI and Stereological Parameters
3.7. Testis Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gabric, A.J. The Climate Change Crisis: A review of its causes and possible responses. Atmosphere 2023, 14, 1081. [Google Scholar] [CrossRef]
- Ficke, A.D.; Myrick, C.A.; Hansen, L.J. Potential impacts of global climate change on freshwater fisheries. Rev. Fish Biol. Fish. 2007, 17, 581–613. [Google Scholar] [CrossRef]
- Deutsch, C.; Penn, J.L.; Verberk, W.C.E.P.; Inomura, K.; Endress, M.G.; Payne, J.L. Impact of warming on aquatic body sizes explained by metabolic scaling from microbes to macrofauna. Proc. Natl. Acad. Sci. USA 2022, 119, e2201345119. [Google Scholar] [CrossRef] [PubMed]
- Neuheimer, A.B.; Thresher, R.E.; Lyle, J.M.; Semmens, J.M. Tolerance limit for fish growth exceeded by warming waters. Nat. Clim. Change 2011, 1, 110–113. [Google Scholar] [CrossRef]
- Little, A.G.; Loughland, I.; Seebacher, F. What do warming waters mean for fish physiology and fisheries? J. Fish Biol. 2020, 97, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Overgaard, J. The heartbreak of adapting to global warming. Science 2007, 315, 49–50. [Google Scholar] [CrossRef] [PubMed]
- Ern, R.; Andreassen, A.H.; Jutfelt, F. Physiological mechanisms of acute upper thermal tolerance in fish. Physiology 2023, 38, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Woodward, G.; Perkins, D.M.; Brown, L.E. Climate change and freshwater ecosystems: Impacts across multiple levels of organization. Philos. Trans. R. Soc. B 2010, 365, 2093–2106. [Google Scholar] [CrossRef] [PubMed]
- Basova, M.; Krasheninnikova, S.; Parrino, V. Intra-decadal (2012–2021) dynamics of spatial ichthyoplankton distribution in sevastopol bay (Black Sea) affected by hydrometeorological factors. Animals 2022, 12, 3317. [Google Scholar] [CrossRef] [PubMed]
- Lotze, H.K.; Bryndum-Buchholz, A.; Boyce, D.G. Chapter 8: Effects of Climate Change on Food Production (Fishing). In The Impacts of Climate Change: A Comprehensive Study of Physical, Biophysical, Social and Political Issues; Letcher, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 205–231. [Google Scholar] [CrossRef]
- Predragovic, M.; Cvitanovic, C.; Karcher, D.B.; Tietbohl, M.D.; Sumaila, U.R.; Horta e Costa, B. A systematic literature review of climate change research on Europe’s threatened commercial fish species. Ocean Coast. Manag. 2023, 242, 106719. [Google Scholar] [CrossRef]
- Liu, B.; Xu, P.; Brown, P.B.; Xie, J.; Ge, X.; Miao, L.; Pan, L. The effect of hyperthermia on liver histology, oxidative stress and disease resistance of the Wuchang bream, Megalobrama amblycephala. Fish Shellfish. Immunol. 2016, 52, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Nuez-Ortín, W.G.; Carter, C.G.; Nichols, P.D.; Cooke, I.R.; Wilson, R. Liver proteome response of pre-harvest Atlantic salmon following exposure to elevated temperature. BMC Genom. 2018, 19, 133. [Google Scholar] [CrossRef] [PubMed]
- Parrino, V.; Cappello, T.; Costa, G.; Cannavà, C.; SanFilippo, M.; Fasulo, S. Comparative study of haematology of two teleost fish (Mugil cephalus and Carassius auratus) from different environments and feeding habits. Eur. Zool. J. 2018, 85, 193–199. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Proto, A.; Bianco, P.M.; Montano, L.; Motta, O. Endocrine-disrupting compounds: An overview on their occurrence in the aquatic environment and human exposure. Water 2021, 13, 1347. [Google Scholar] [CrossRef]
- Rocha, M.J.; Rocha, E. Estrogenic Compounds in Estuarine and Coastal Water Environments of the Iberian Western Atlantic Coast and Selected Locations Worldwide—Relevancy, Trends and Challenges in View of the EU Water Framework Directive. In Toxicology Studies—Cells, Drugs and Environment; In Tech: London, UK, 2015. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.D.; Bayen, S.; Desrosiers, M.; Muñoz, G.; Sauvé, S.; Yargeau, V. An introduction to the sources, fate, occurrence and effects of endocrine disrupting chemicals released into the environment. Environ. Res. 2022, 207, 112658. [Google Scholar] [CrossRef] [PubMed]
- Lauretta, R.; Sansone, A.; Sansone, M.; Romanelli, F.; Appetecchia, M. Endocrine disrupting chemicals: Effects on endocrine glands. Front. Endocrinol. 2019, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5991, Ethinyl Estradiol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ethinyl-estradiol (accessed on 4 April 2025).
- Tang, Z.; Liu, Z.H.; Wang, H.; Dang, Z.; Liu, Y. A review of 17α-ethynylestradiol (EE2) in surface water across 32 countries: Sources, concentrations, and potential estrogenic. J. Environ. Manag. 2021, 292, 112804. [Google Scholar] [CrossRef] [PubMed]
- Arukwe, A.; Goksøyr, A. Eggshell and egg yolk proteins in fish: Hepatic proteins for the next generation: Oogenetic, population, and evolutionary implications of endocrine disruption. Comp. Hepatol. 2003, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Kinnberg, K.; Bodil, K.; Bjerregaard, P. Effects of octylphenol and 17β-estradiol on the gonads of guppies (Poecilia reticulata) exposed as adults via the water or as embryos via the mother. Comp. Biochem. Physiol. 2003, 134, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, G.; Togan, I.; Severcan, F. 17Beta-estradiol induced compositional, structural and functional changes in rainbow trout liver, revealed by FT-IR spectroscopy: A comparative study with nonylphenol. Aquat. Toxicol. 2006, 77, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Real, M.V.F.; Rocha, M.J.; Gonçalves, J.F.; Rocha, E. Histology and design-based estimation of hepatocellularity and volumes of hepatocytes in control and ethynylestradiol exposed males of platyfish (Xiphophorus maculatus). Tissue Cell 2020, 63, 101327. [Google Scholar] [CrossRef] [PubMed]
- Chaturantabut, S.; Shwartz, A.; Evason, K.J.; Cox, A.G.; Labella, K.; Schepers, A.G.; Yang, S.; Aravena, M.; Houvras, Y.; Mancio-Silva, L.; et al. Estrogen activation of G-protein–coupled estrogen receptor 1 regulates phosphoinositide 3-kinase and mTOR signaling to promote liver growth in zebrafish and proliferation of human hepatocytes. Gastroenterology 2019, 156, 1788. [Google Scholar] [CrossRef] [PubMed]
- Mayol, X.; Neal, G.E.; Davies, R.; Romero, A.; Domingo, J. Ethinyl estradiol-induced cell proliferation in rat liver. Involvement of specific populations of hepatocytes. Carcinogenesis 1992, 13, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- DeCourten, B.M.; Brander, S.M. Combined effects of increased temperature and endocrine disrupting pollutants on sex determination, survival, and development across generations. Sci. Rep. 2017, 7, 9310. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, P.G.; Rodrigues, D.; Madureira, T.V.; Oliveira, N.; Rocha, M.J.; Rocha, E. Warming modulates the effects of the endocrine disruptor progestin levonorgestrel on the zebrafish fitness, ovary maturation kinetics and reproduction success. Environ. Pollut. 2017, 229, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Jacquin, L.; Gandar, A.; Aguirre-Smith, M.; Perrault, A.; Henaff, M.L.; Jong, L.; Jean, S. High temperature aggravates the effects of pesticides in goldfish. Ecotoxicol. Environ. Saf. 2019, 172, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.K.; Peterson, K.N.; Tan, D.; Novak, P.J.; Schoenfuss, H.L.; Ward, J.L. Temperature modulates estrone degradation and biological effects of exposure in fathead minnows. Sci. Total Environ. 2018, 621, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- MacAulay, S.; Masud, N.; Davies-Jones, J.; Ward, B.D.; Cable, J. The impacts of synthetic and cellulose-based fibres and their associated dyes on fish hosts and parasite health. Environ. Sci. Pollut. Res. Int. 2023, 58, 121558–121568. [Google Scholar] [CrossRef] [PubMed]
- Hall, N.A.; Hayton, W.L. Absorption of ethanol by the Common guppy (Lebistes reticulatus). J. Pharm. Sci. 1967, 56, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.C.; Gregório, C.; Uribe-Cruz, C.; Guizzo, R.; Malysz, T.; Faccioni-Heuser, M.C.; Longo, L.; da Silveira, T.R. Chronic exposure to ethanol causes steatosis and inflammation in zebrafish liver. World J. Hepatol. 2017, 9, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kim, S. Low dose of chronic ethanol exposure in adult zebrafish induces hepatic steatosis and injury. Biomed. Pharmacother. 2019, 117, 109179. [Google Scholar] [CrossRef] [PubMed]
- Ricker, W.E. Computation and interpretation of biological statistics of fish populations. Bull. Fish. Res. Board Can. 1975, 191, 1–382. [Google Scholar]
- Zanotelli, V.R.; Neuhauss, S.C.; Ehrengruber, M.U. Long-term exposure to bis(2-ethylhexyl)phthalate (DEHP) inhibits growth of guppy fish (Poecilia reticulata). J. Appl. Toxicol. 2010, 30, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Jordanova, M.; Rocha, M.J.; Rebok, K.; Rocha, E. Variations in the volumes of parenchyma and stroma of the liver and in the cytology of hepatocytes are associated with gonadal stages in female Ohrid trout (Salmo letnica). Ichthyol. Res. 2013, 60, 26–35. [Google Scholar] [CrossRef]
- Gates, L.; Adler, R.R.; Elangbam, C.S. Osmium tetroxide post-fixation and periodic acid-Schiff dual-staining technique to demonstrate intracellular lipid and glycogen in the mouse liver section—A novel method for co-visualization of intracellular contents in paraffin-embedded tissue. J. Histotechnol. 2016, 39, 2–7. [Google Scholar] [CrossRef]
- Lourenço, T.; Rocha, E.; Gonçalves, J.F.; Rocha, M.J.; Madureira, T.V. A proof-of-concept for a hypolipidemic brown trout model. Toxics 2024, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, H.J.G. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J. Microsc. 1986, 143, 3–45. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, H.J.G. The nucleator. J. Microsc. 1988, 151, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, H.; Bagger, P.; Bendtsen, T.; Evans, S.; Korbo, L.; Marcussen, N.; Møller, A.; Nielsen, K.; Nyengaard, J.; Pakkenberg, B. The new stereological tools: Disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 1988, 96, 857–881. [Google Scholar] [CrossRef] [PubMed]
- Marcos, R.; Monteiro, R.A.; Rocha, E. Design-based stereological estimation of hepatocyte number, by combining the smooth optical fractionator and immunocytochemistry with anti-carcinoembryonic antigen polyclonal antibodies. Liver Int. 2006, 26, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Sterio, D. The unbiased estimation of number and sizes of arbitrary particles using the disector. J. Microsc. 1983, 134, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, H.J.G. Notes on the estimation of the numerical density of arbitrary profiles: The edge effect. J. Microsc. 1977, 111, 219–223. [Google Scholar] [CrossRef]
- Calado, A.M.; Rocha, E.; Colaço, A.; Sousa, M.R. Stereologic characterization of bovine (Bos taurus) cumulus-oocyte complexes aspirated from small antral follicles during the diestrous phase 1. Biol. Reprod. 2001, 65, 1383–1391. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rašković, B.; Cruzeiro, C.; Poleksić, V.; Rocha, E. Estimating volumes from common carp hepatocytes using design-based stereology and examining correlations with profile areas: Revisiting a nutritional assay and unveiling guidelines to microscopists. Microsc. Res. Tech. 2019, 82, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, H.J.; Jensen, E.B. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 1987, 147, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Weibel, E.R.; Kistler, G.S.; Scherle, W.F. Practical stereological methods for morphometric cytology. J. Cell Biol. 1966, 30, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Ruedig, E.; Caffrey, E.; Hess, C.; Higley, K. Monte Carlo derived absorbed fractions for a voxelized model of Oncorhynchus mykiss, a rainbow trout. Radiat. Environ. Biophys. 2014, 53, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Kalshabay, Y.; Zholdybay, Z.; Martino, M.D.; Medeubekov, U.; Baiguissova, D.; Ainakulova, A.; Doskhanov, M.; Baimakhanov, B. CT volume analysis in living donor liver transplantation: Accuracy of three different approaches. Insights Imaging 2023, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.J.; Ribeiro, C.; Ribeiro, M. Development and Optimisation of a GC-MS Method for the Evaluation of Oestrogens and Persistent Pollutants in River and Seawater Samples. Int. J. Environ. Anal. Chem. 2011, 91, 1191–1205. [Google Scholar] [CrossRef]
- Rocha, M.J.; Cruzeiro, C.; Rocha, E. Quantification of 17 endocrine disruptor compounds and their spatial and seasonal distribution in the Iberian Ave River and its coastline. Toxicol. Environ. Chem. 2013, 95, 386–399. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar] [CrossRef]
- Liley, N.R.; Seghers, B.H. Factors Affecting the Morphology and Behaviour of Guppies in Trinidad. In Function and Evolution in Behaviour: Essays in Honour of Professor Niko Tinbergen; Baerends, G., Beer, P., Manning, C., Eds.; Oxford Univ. Press: Oxford, UK, 1975; pp. 92–118. ISBN 0198573820. [Google Scholar]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Lannig, G.; Tillmann, A.; Howald, S.; Stapp, L.S. Thermal sensitivity of cell metabolism of different Antarctic fish species mirrors organism temperature tolerance. Polar Biol. 2020, 43, 1887–1898. [Google Scholar] [CrossRef]
- Cleveland, B.M.; Weber, G.M. Effects of steroid treatment on growth, nutrient partitioning, and expression of genes related to growth and nutrient metabolism in adult triploid rainbow trout (Oncorhynchus mykiss). Domest. Anim. Endocrinol. 2016, 56, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Versonnen, B.J.; Janssen, C.R. Xenoestrogenic effects of ethinylestradiol in zebrafish (Danio rerio). Environ. Toxicol. 2004, 19, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Mo, N.; Zhang, M.; Wang, R.; Xia, S.; Meng, F.; Qian, Y.; Li, M. Effects of α-ethinyl estradiol (EE2) and diethylhexyl phthalate (DEHP) on growth performance, antioxidant status and immune response of juvenile yellow catfish Pelteobagrus fulvidraco. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 226, 108615. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.C.; Wolfe, M.J. A brief overview of nonneoplastic hepatic toxicity in fish. Toxicol. Pathol. 2005, 33, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.C.; Wheeler, J.R. A critical review of histopathological findings associated with endocrine and non-endocrine hepatic toxicity in fish models. Aquat. Toxicol. 2018, 197, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.; Rocha, M.J.; Rocha, E. Characterization and spatial relationships of the hepatic vascular–biliary tracts, and their associated pancreocytes and macrophages, in the model fish guppy (Poecilia reticulata): A study of serial sections by light microscopy. Tissue Cell 2018, 50, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Shi, H. Sex hormones and their receptors regulate liver energy homeostasis. Int. J. Endocrinol. 2015, 2015, 294278. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Ma, A.; Huang, Z.; Liu, Z.; Sun, Z.; Zhu, C.; Yang, J.; Li, Y.; Wang, Q.; Qiao, X.; et al. Transcriptome analysis reveals that high temperatures alter modes of lipid metabolism in juvenile turbot (Scophthalmus maximus) liver. Comp. Biochem. Physiol. D Genom. Proteom. 2021, 40, 100887. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.; Prosser, C.; Graham, G. Alterations in liver composition of channel catfish (Ictalurus punctatus) during seasonal acclimatization. Physiol. Zool. 1992, 65, 86–884. [Google Scholar] [CrossRef]
- Tenji, D.; Micic, B.; Sipos, S.; Miljanovic, B.; Teodorovic, I.; Kaisarevic, S. Fish biomarkers from a different perspective: Evidence of adaptive strategy of Abramis brama (L.) to chemical stress. Environ. Sci. Eur. 2020, 32, 1–15. [Google Scholar] [CrossRef]
- Orso, G.; Imperatore, R.; Coccia, E.; Rinaldi, G.; Cicchella, D.; Paolucci, M. A deep survey of fish health for the recognition of useful biomarkers to monitor water pollution. Environments 2023, 10, 219. [Google Scholar] [CrossRef]
- Skillman, A.D.; Nagler, J.J.; Hook, S.E.; Small, J.A.; Schultz, I.R. Dynamics of 17alpha-ethynylestradiol exposure in rainbow trout (Oncorhynchus mykiss): Absorption, tissue distribution, and hepatic gene expression pattern. Environ. Toxicol. Chem. 2006, 25, 2997–3005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhong, H.; Han, Z.; Tang, Z.; Xiao, J.; Guo, Z.; Wang, F.; Luo, Y.; Zhou, Y. Effects of waterborne exposure to 17β-estradiol on hepatic lipid metabolism genes in tilapia (Oreochromis niloticus). Aquac. Rep. 2020, 17, 100382. [Google Scholar] [CrossRef]
- Holloway, A.C.; Melroe, G.T.; Ehrman, M.M.; Reddy, P.K.; Leatherland, J.F.; Sheridan, M.A. Effect of 17β-estradiol on the expression of somatostatin genes in rainbow trout (Oncorhynchus mykiss). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R389–R393. [Google Scholar] [CrossRef] [PubMed]
- Schultz, I.R.; Skillman, A.; Nicolas, J.M.; Cyr, D.G.; Nagler, J.J. Short-term exposure to 17 alpha-ethynylestradiol decreases the fertility of sexually maturing male rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 2003, 22, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.D.; Olsson, P.E.; Mayer, G.D.; Haux, C.; Walsh, P.J.; Burge, E.; Hogstrand, C. Effects of 17 beta-estradiol on levels and distribution of metallothionein and zinc in squirrelfish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R527–R535. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.M.; Iatropoulos, M.J. Alteration of liver cell function and proliferation: Differentiation between adaptation and toxicity. Toxicol. Pathol. 2002, 30, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo-Fernandes, A.M.; Fontaínhas-Fernandes, A.A.; Monteiro, R.A.; Reis-Henriques, M.A.; Rocha, E. Temperature and gender influences on the hepatic stroma (and associated pancreatic acini) of Nile tilapia, Oreochromis niloticus (Teleostei, Cichlidae): A stereological analysis by light microscopy. J. Morphol. 2006, 267, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Malhi, H.; Guicciardi, M.E.; Gores, G.J. Hepatocyte death: A clear and present danger. Physiol. Rev. 2010, 90, 1165. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.M.; Callstrom, M.R.; Butters, K.A.; Knudsen, B.; Grande, J.P.; Roberts, L.R.; Woodrum, D.A. Heat stress induced cell death mechanisms in hepatocytes and hepatocellular carcinoma: In vitro and in vivo study. Lasers Surg. Med. 2014, 46, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Jevtić, P.; Levy, D.L. Mechanisms of nuclear size regulation in model systems and cancer. Adv. Exp. Med. Biol. 2014, 773, 537–569. [Google Scholar] [CrossRef] [PubMed]
- Petreaca, M.; Martins-Green, M. Cell–ECM Interactions in Repair and Regeneration. In Handbook of Stem Cells, 2nd ed.; Lanza, R., Atala, A., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 191–226. [Google Scholar] [CrossRef]
- Nieminen, P.; Mustonen, A.M.; Hyvärinen, H. Fasting reduces hepatocyte size in the rat liver. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 145, 310–316. [Google Scholar] [CrossRef]
- Dunkelberg, J.C.; Feranchak, A.P.; Fitz, J.G. Liver cell volume regulation: Size matters. Hepatology 2001, 33, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Molnar, F.; Daoust, R. Nucleocytoplasmic ratios in different populations of rat liver parenchymal cells during azo dye carcinogenesis. Cancer Res. 1965, 25, 1213–1218. [Google Scholar] [PubMed]
- Balachandra, S.; Sarkar, S.; Amodeo, A.A. The nuclear-to-cytoplasmic ratio: Coupling DNA content to cell size, cell cycle, and biosynthetic capacity. Annu. Rev. Genet. 2022, 56, 165. [Google Scholar] [CrossRef] [PubMed]
- Goto, R.; Kawamura, N.; Watanabe, M.; Ganchiku, Y.; Nagatsu, A.; Okada, K.; Ito, Y.M.; Kamiyama, T.; Shimamura, T.; Taketomi, A. Long-term risk of a fatty liver in liver donors. Ann. Gastroenterol. Surg. 2023, 7, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Kinnberg, K.; Toft, G. Effects of estrogenic and antiandrogenic compounds on the testis structure of the adult guppy (Poecilia reticulata). Ecotoxicol. Environ. Saf. 2003, 54, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.; Baatrup, E. Quantitative studies on the effects of environmental estrogens on the testis of the guppy, Poecilia reticulata. Aquat. Toxicol. 2006, 80, 140–148. [Google Scholar] [CrossRef] [PubMed]
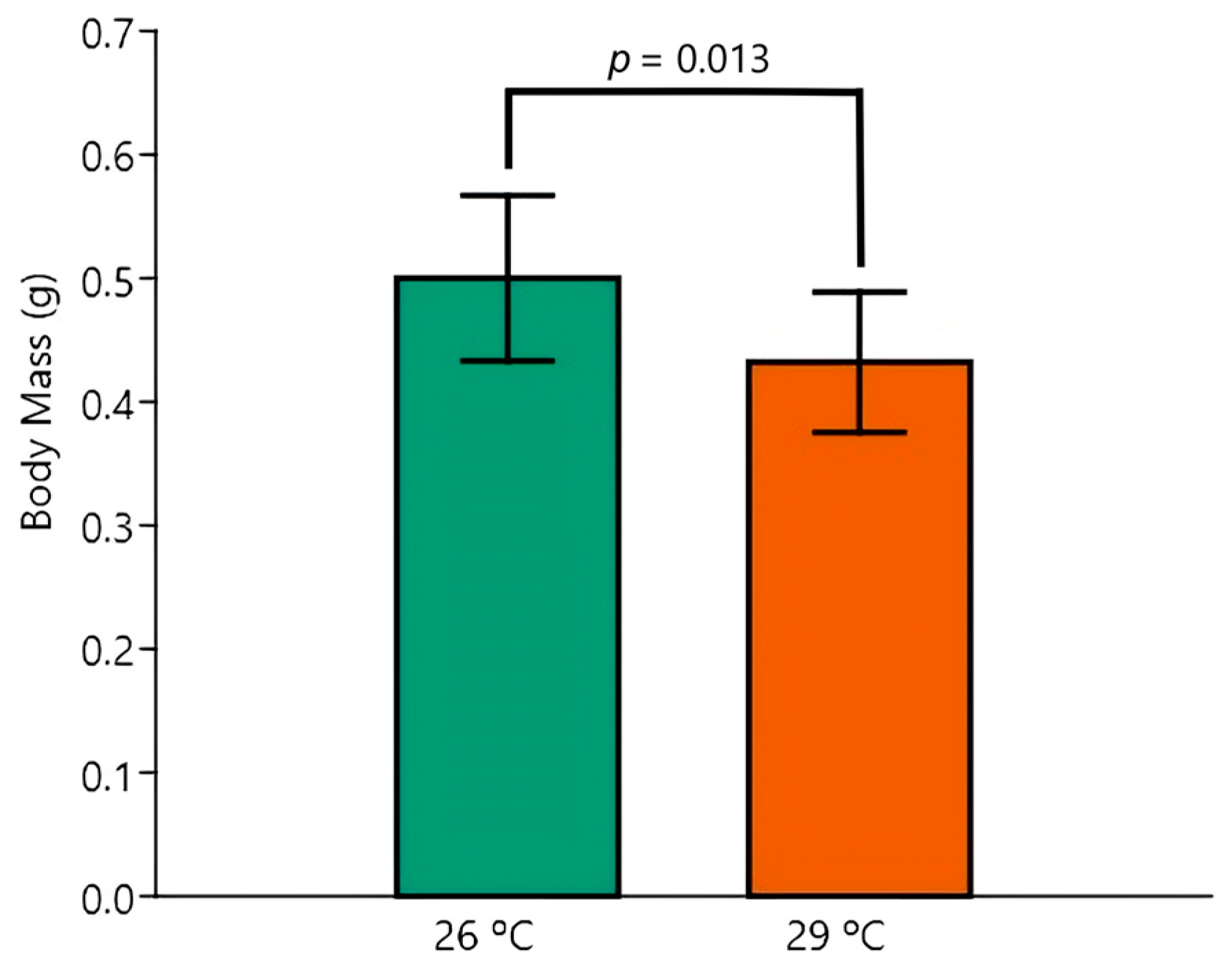
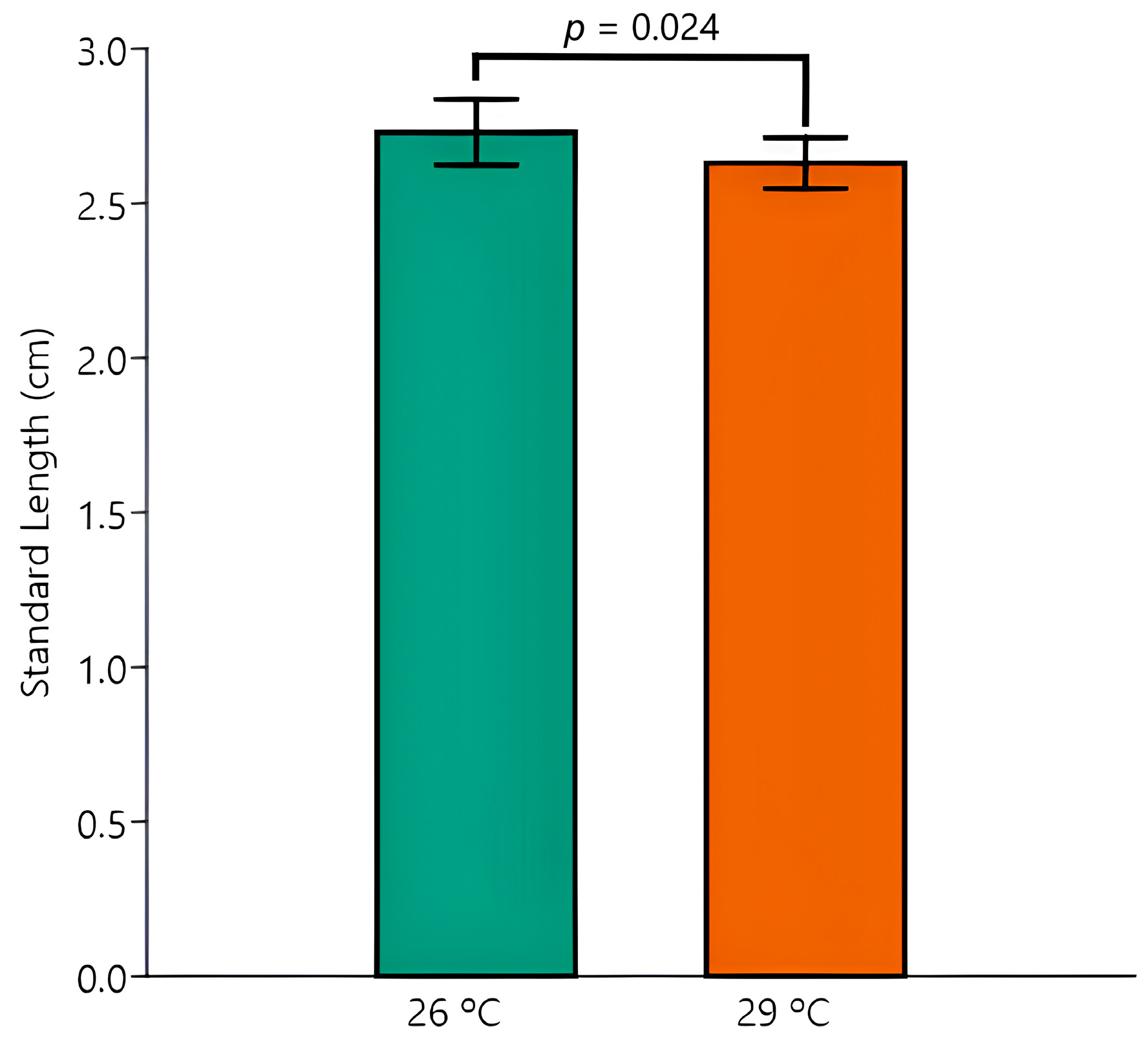

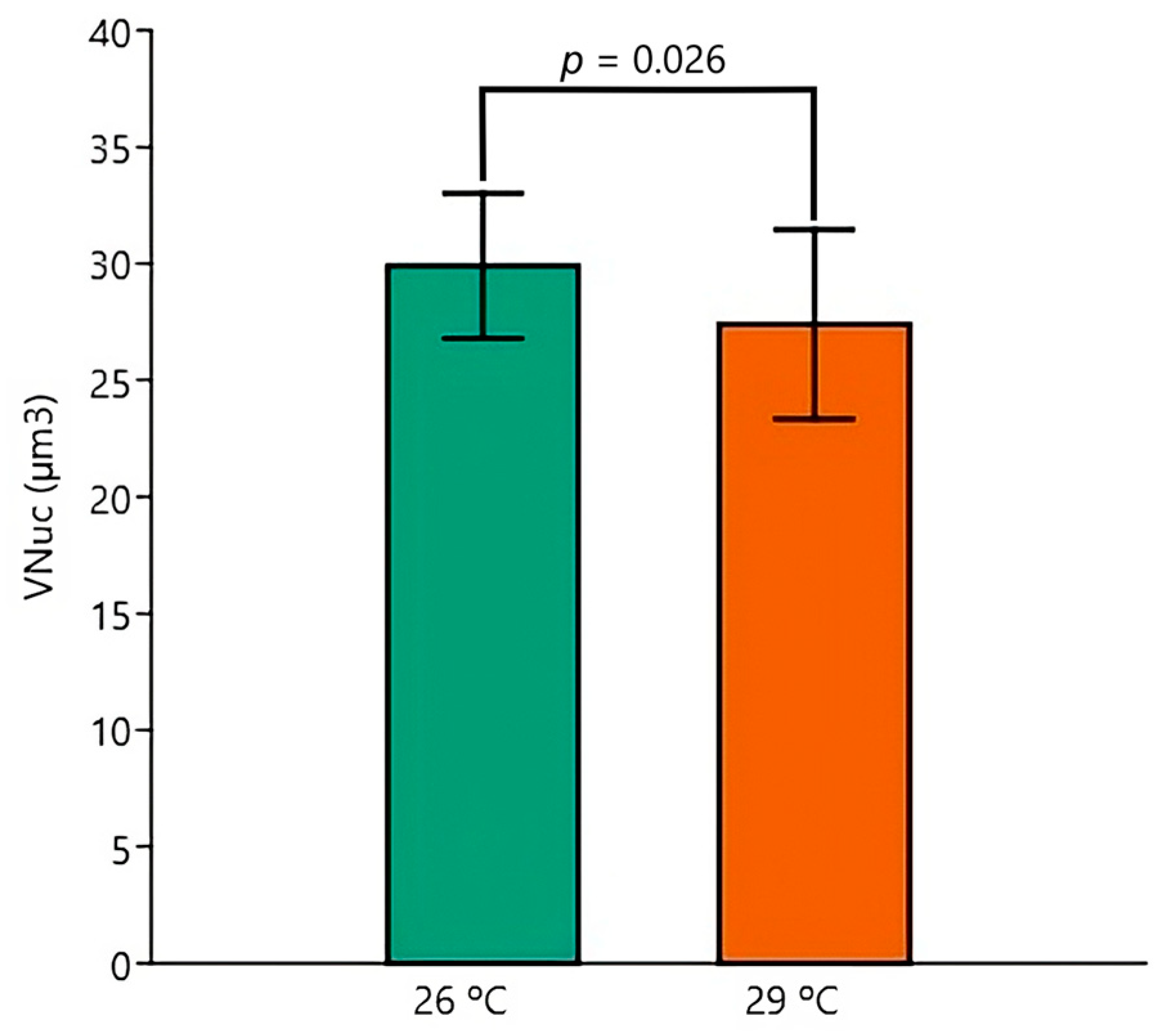
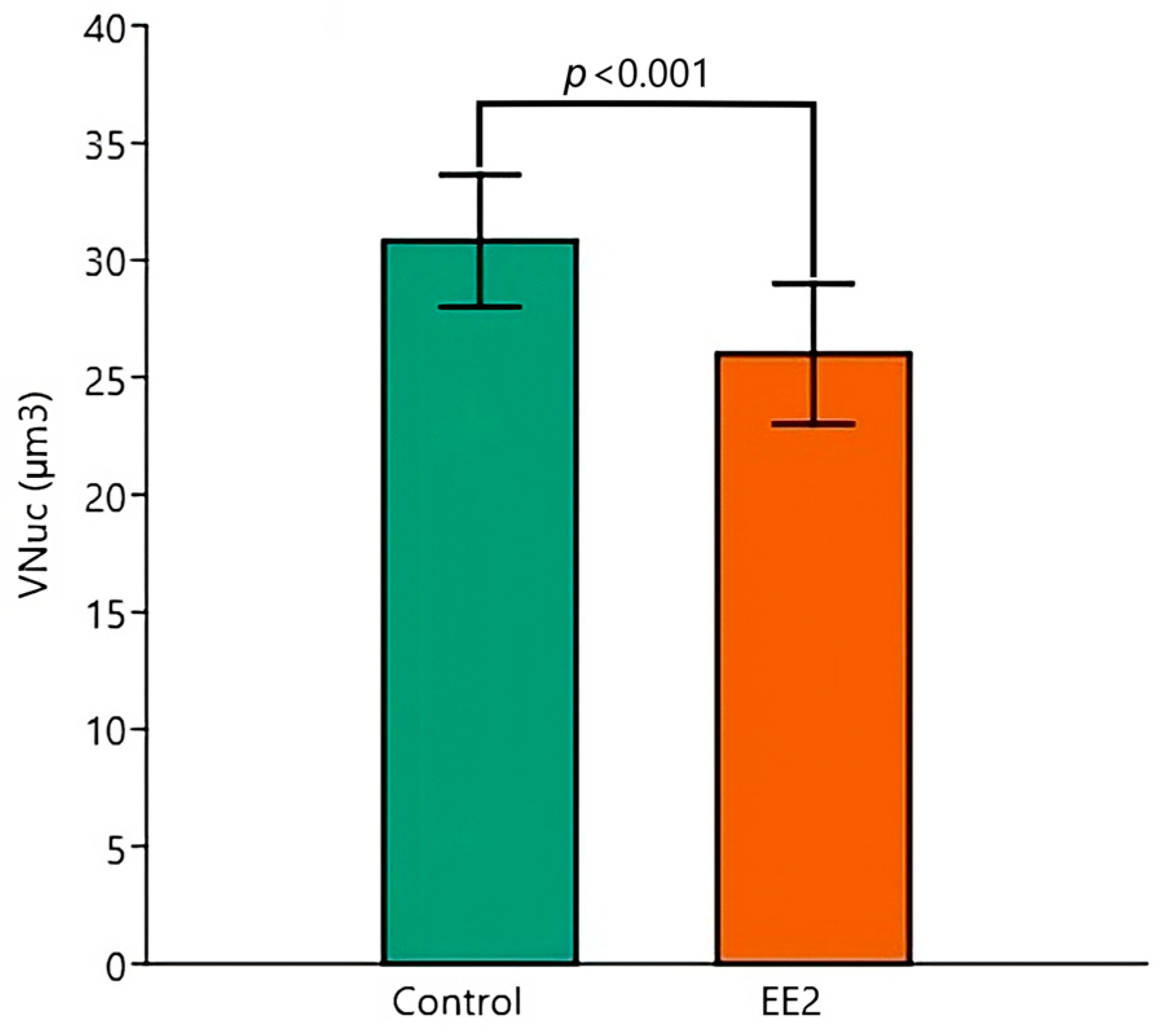

| BM | Control 26 °C (n = 5) | Control 29 °C (n = 5) | EE2 26 °C (n = 5) | EE2 29 °C (n = 5) |
|---|---|---|---|---|
| Mean (g) | 0.53 | 0.47 | 0.47 | 0.39 |
| SD (g) | 0.06 | 0.04 | 0.07 | 0.05 |
| CV (%) | 10.8 | 8.5 | 14.9 | 11.9 |
| TL | ||||
| Mean (cm) | 3.8 | 3.7 | 3.7 | 3.5 |
| SD (cm) | 0.2 | 0.2 | 0.1 | 0.3 |
| CV (%) | 6.2 | 5.9 | 2.4 | 7.4 |
| SL | ||||
| Mean (cm) | 2.8 | 2.7 | 2.7 | 2.6 |
| SD (cm) | 0.1 | 0.1 | 0.1 | 0.0 |
| CV (%) | 3.2 | 3.1 | 2.1 | 1.7 |
| K | ||||
| Mean (100× g/cm3) | 1.0 | 0.9 | 1.0 | 0.9 |
| SD (100× g/cm3) | 0.1 | 0.1 | 0.1 | 0.2 |
| CV (%) | 10.1 | 14.6 | 15.0 | 23.1 |
| Vacuolation Grade | Control 26 °C (n = 5) | Control 29 °C (n = 5) | EE2 26 °C (n = 5) | EE2 29 °C (n = 5) |
|---|---|---|---|---|
| Median | 2 | 2 | 2 | 1 |
| Mode | 2 | 2 | 2 | 1 |
| Minimum | 1 | 2 | 0 | 1 |
| Maximum | 3 | 3 | 2 | 2 |
| HSI | Control 26 °C (n = 5) | Control 29 °C (n = 5) | EE2 26 °C (n = 5) | EE2 29 °C (n = 5) |
|---|---|---|---|---|
| Mean (%) | 1.4 | 1.6 | 1.4 | 1.0 |
| SD (%) | 0.4 | 0.2 | 0.5 | 0.3 |
| CV (%) | 27.3 | 11.4 | 33.7 | 35.2 |
| VLiver (×107) | Control 26 °C (n = 5) | Control 29 °C (n = 5) | EE2 26 °C (n = 5) | EE2 29 °C (n = 5) |
|---|---|---|---|---|
| Mean (µm3) | 753.9 | 762.9 | 657.0 | 375.9 |
| SD (µm3) | 221.6 | 70.0 | 288.0 | 118.6 |
| CV (%) | 29.4 | 9.2 | 43.8 | 31.6 |
| VCell | ||||
| Mean (µm3) | 704.3 | 677.2 | 632.9 | 528.7 |
| SD (µm3) | 106.5 | 46.1 | 99.5 | 64.0 |
| CV (%) | 15.1 | 6.8 | 15.7 | 12.1 |
| VNuc | ||||
| Mean (µm3) | 31.8 | 30.9 | 28.2 | 24.0 |
| SD (µm3) | 2.8 | 2.1 | 2.2 | 1.9 |
| CV (%) | 8.7 | 6.8 | 7.6 | 7.9 |
| NCell (×104) | ||||
| Mean | 33.7 | 43.7 | 36.8 | 26.8 |
| SD | 9.0 | 15.1 | 11.2 | 6.0 |
| CV (%) | 26.7 | 34.7 | 30.5 | 22.4 |
| NV (Cell, Liver) (×103) | Control 26 °C (n = 5) | Control 29 °C (n = 5) | EE2 26 °C (n = 5) | EE2 29 °C (n = 5) |
|---|---|---|---|---|
| Mean (mm−3) | 47.1 | 56.8 | 65.0 | 74.9 |
| SD (mm−3) | 15.4 | 17.1 | 38.2 | 19.7 |
| CV (%) | 32.8 | 30.1 | 58.9 | 26.3 |
| NV (Cell, Par) (×105) | ||||
| Mean (mm−3) | 11.0 | 10.8 | 11.9 | 15.1 |
| SD (mm−3) | 3.3 | 2.4 | 2.8 | 3.2 |
| CV (%) | 29.9 | 22.6 | 23.5 | 20.9 |
| VV (Nuc, Cell) | ||||
| Mean (%) | 4.6 | 4.6 | 4.5 | 4.6 |
| SD (%) | 0.6 | 0.2 | 0.6 | 0.6 |
| CV (%) | 12.1 | 5.1 | 13.6 | 14.0 |
| VV (Nuc, Cyto) | ||||
| Mean (%) | 4.8 | 4.8 | 4.7 | 4.8 |
| SD (%) | 0.6 | 0.3 | 0.7 | 0.7 |
| CV (%) | 12.1 | 5.4 | 14.3 | 14.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilaça, M.; Tantiwisawaruji, S.; Rocha, M.J.; Rocha, E. High Temperature and Ethinylestradiol May Reduce Body Growth, Liver and Hepatocyte Volumes and Lipid Droplets in Adult Male Guppies. Animals 2025, 15, 2152. https://doi.org/10.3390/ani15142152
Vilaça M, Tantiwisawaruji S, Rocha MJ, Rocha E. High Temperature and Ethinylestradiol May Reduce Body Growth, Liver and Hepatocyte Volumes and Lipid Droplets in Adult Male Guppies. Animals. 2025; 15(14):2152. https://doi.org/10.3390/ani15142152
Chicago/Turabian StyleVilaça, Margarida, Sukanlaya Tantiwisawaruji, Maria João Rocha, and Eduardo Rocha. 2025. "High Temperature and Ethinylestradiol May Reduce Body Growth, Liver and Hepatocyte Volumes and Lipid Droplets in Adult Male Guppies" Animals 15, no. 14: 2152. https://doi.org/10.3390/ani15142152
APA StyleVilaça, M., Tantiwisawaruji, S., Rocha, M. J., & Rocha, E. (2025). High Temperature and Ethinylestradiol May Reduce Body Growth, Liver and Hepatocyte Volumes and Lipid Droplets in Adult Male Guppies. Animals, 15(14), 2152. https://doi.org/10.3390/ani15142152









