The Biology of Demodecid Mites (Trombidiformes: Demodecidae) and Their Parasitism in the Eurasian Beaver Castor fiber Linnaeus, 1758, with a Description of a New Species †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
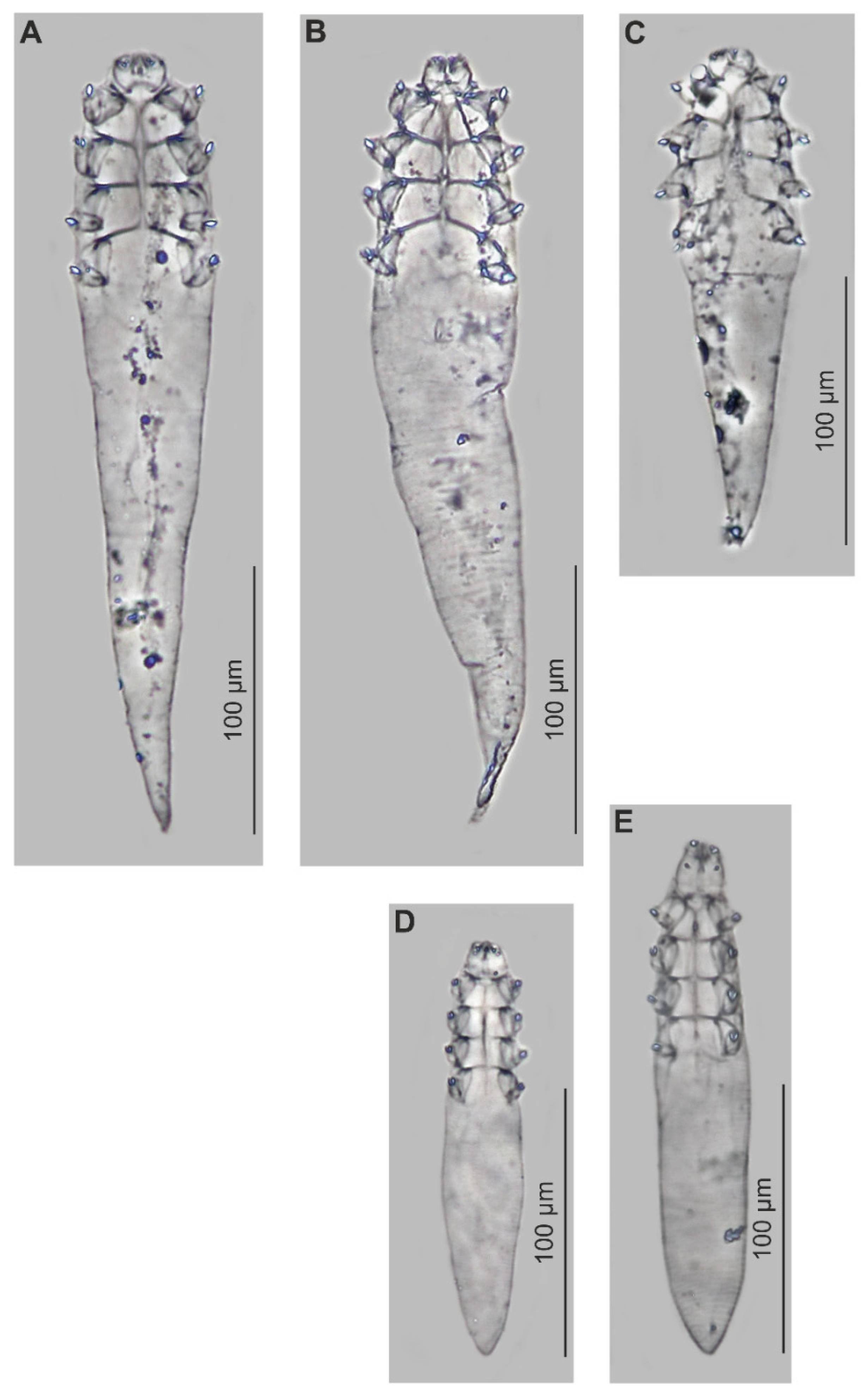
3.1. Systematics (Table 1, Figure 1 and Figure 2)
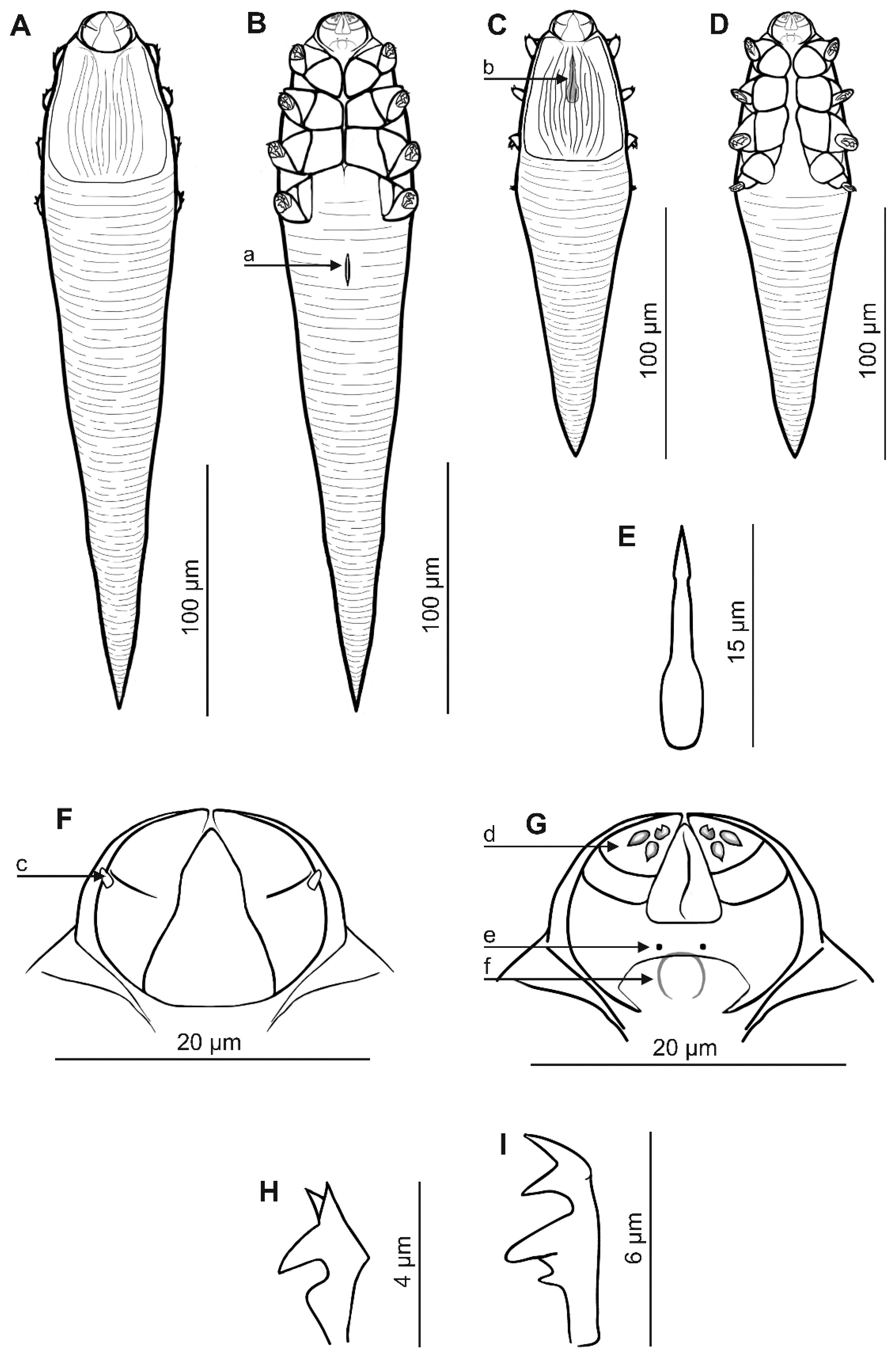
| Feature/Species | Demodex ovaportans sp. nov. | Demodex castoris | ||
|---|---|---|---|---|
| Source | Present Study | Izdebska et al. [8] and Authors’ Unpublished Data | ||
| Sex Sample Size | Males (n = 6) | Females (n = 69) | Males (n = 16) | Females (n = 27) |
| Length of gnathosoma | 13 (11–13) ± 1 | 14 (13–17) ± 1 | 18 (15–23) ± 2 | 19 (16–22) ± 2 |
| Width of gnathosoma (at base) | 19 (18–20) ± 1 | 22 (20–25) ± 1 | 18 (14–24) ± 2 | 18 (14–22) ± 2 |
| Length of podosoma | 60 (55–65) ± 3 | 72 (63–80) ± 3 | 49 (45–63) ± 4 | 60 (50–68) ± 4 |
| Width of podosoma | 41 (38–46) ± 3 | 50 (43–60) ± 3 | 28 (21–33) ± 3 | 27 (22–35) ± 3 |
| Length of opisthosoma | 107 (75–147) ± 25 | 195 (158–232) ± 16 | 85 (70–100) ± 8 | 106 (90–127) ± 9 |
| Width of opisthosoma | 36 (33–39) ± 3 | 46 (28–53) ± 4 | 26 (22–33) ± 3 | 32 (24–38) ± 3 |
| Aedeagus length | 15 (13–17) ± 1 | - | 21 (20–26) ± 2 | - |
| Vulva length | - | 11 (9–16) ± 1 | - | 11 (9–16) ± 2 |
| Body total length | 179 (145–218) ± 24 | 281 (243–318) ± 17 | 152 (134–168) ± 10 | 185 (164–209) ± 11 |
| Body length to width ratio | 4.4 (3.8–5.5) ± 0.7 | 5.6 (4.8–6.8) ± 0.4 | 5.5 (4.7–7.3) ± 0.8 | 5.8 (4.5–7.4) ± 1 |
| Opisthosoma length to body length ratio (%) | 59 (52–67) ± 6 | 69 (65–74) ± 2 | 56 (49–60) ± 3 | 57 (52–61) ± 0.02 |
3.2. Infestation and Biological Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, D.E.; Reeder, D.M. (Eds.) Mammals Species of the World: A Taxonomic and Geographic Reference, 3rd ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 2005; Available online: http://www.departments.bucknell.edu/biology/resources/msw3/ (accessed on 12 June 2025).
- Rybczynski, N. Castorid phylogenetics: Implications for the evolution of swimming and tree-exploitation in beavers. J. Mammal. Evol. 2007, 14, 1–35. [Google Scholar] [CrossRef]
- Gabryś, G.; Ważna, A. Subspecies of the European beaver Castor fiber Linnaeus, 1758. Acta Theriol. 2003, 48, 433–439. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L.; Kozina, P.; Skrzypczak, M. Parasitic arthropods of mammals and their adaptations for living in the hosts in aquatic environment. In Arthropods. In the Contemporary World; Buczek, A., Błaszak, C., Eds.; Koliber: Lublin, Poland, 2015; pp. 13–25. [Google Scholar]
- Rosell, F.; Orsolya Bozsér, O.; Peter Collen, P.; Howard Parker, H. Ecological impact of beavers Castor fiber and Castor canadensis and their ability to modify ecosystems. Mammal. Rev. 2005, 35, 248–276. [Google Scholar] [CrossRef]
- Ciechanowski, M.; Kubic, W.; Rynkiewicz, A.; Zwolicki, A. Reintroduction of beavers Castor fiber may improve habitat quality for vespertilionid bats foraging in small river valleys. Eur. J. Wildl. Res. 2011, 57, 737–747. [Google Scholar] [CrossRef]
- Brazier, R.E.; Puttock, A.; Graham, H.A.; Auster, R.E.; Davies, K.H.; Brown, C.M.L. Beaver: Nature’s ecosystem engineers. WIREs Water 2021, 8, e1494. [Google Scholar] [CrossRef] [PubMed]
- Izdebska, J.N.; Fryderyk, S.; Rolbiecki, L. Demodex castoris sp. nov. (Acari: Demodecidae) parasitizing Castor fiber (Rodentia), and other parasitic arthropods associated with Castor spp. Dis. Aquat. Organ. 2016, 118, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bochkov, A.V. A review of mammal-associated Psoroptidia (Acariformes: Astigmata). Acarina 2010, 18, 99–260. [Google Scholar]
- Pushkin, S.V. A new record of the parasitic beaver beetle (Platypsyllus castoris) (Coleoptera: Leiodidae) from Stavropol Territory (Russia). Entomol. Appl. Sci. Lett. 2014, 1, 1–3. [Google Scholar]
- Åhlen, P.-A.; Sjöberg, G.; Stéen, M. Parasitic fauna of Eurasian beavers (Castor fiber) in Sweden (1997–1998). Acta Vet. Scand. 2021, 63, 23. [Google Scholar] [CrossRef] [PubMed]
- Peck, S.B. Distribution and biology of the ectoparasitic beaver beetle Platypsyllus castoris Ritsema in North America (Coleoptera: Leiodidae: Platypsyllinae). Insecta Mundi 2006, 20, 85–94. [Google Scholar]
- Winter, G. Untersuchungen zur Morphologie des Biberkäfers Platypsyllus castoris Ritsema 1869 (Coleoptera), eines extrem gut angepaßten Vertreters des Lebensformtyps fellbewohnender Insekten. Zool. Jb. Anat. 1979, 101, 456–471. [Google Scholar]
- Fain, A.; Whitaker, J.O.; Smith, M.A. Fur mites of the genus Schizocarpus Trouessart, 1896 (Acari, Chirodiscidae) parasitic on the American beaver Castor canadensis in Indiana, U.S.A. Bull. Ann. Soc. R. Belg. D’entomol. 1984, 120, 211–239. [Google Scholar]
- Fain, A.; Whitaker, J.O. Mites of the genus Schizocarpus Trouessart, 1896 (Acari, Chirodiscidae) from Alaska and Indiana, USA. Acarologia 1988, 29, 395–409. [Google Scholar]
- Whitaker, J.O.; Fain, A.; Jones, G.S. Ectoparasites from beavers from Massachusetts and Maine. Int. J. Acarol. 1989, 15, 153–162. [Google Scholar] [CrossRef]
- Whitaker, J.O.; Ruckdeschel, C.; Bochkov, A.V. Species of the genus Schizocarpus Trouessart, 1896 (Acari: Chirodiscidae) from Florida and Georgia beavers. Biol. Sci. 2009, 72, 18–21. [Google Scholar]
- Izdebska, J.N.; Rolbiecki, L. The biodiversity of demodecid Mites (Acariformes: Prostigmata), specific parasites of mammals with a global checklist and a new finding for Demodex sciurinus. Diversity 2020, 12, 261. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L. A new genus and species of demodecid mites from the tongue of a house mouse Mus musculus: Description of adult and immature stages with data on parasitism. Med. Vet. Entomol. 2016, 30, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Izdebska, J.N.; Rolbiecki, L.; Rehbein, S. Morphological and ontogenetic characteristics of Miridex putorii (Acariformes: Demodecidae), a new genus and species of skin mite specific to the European polecat Mustela putorius. Int. J. Parasitol. Parasit. Wildl. 2022, 18, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Desch, C.E. A new species of Demodex Owen, 1843 (Acari: Demodecidae) from the meibomian glands of the vampire bat Desmodus rotundus (E. Geoffroy, 1810) (Chiroptera: Phyllostomidae: Desmodontinae) from Surinam. Int. J. Acarol. 1994, 20, 39–43. [Google Scholar] [CrossRef]
- Izdebska, J.N. Demodex spp. (Acari: Demodecidae) in brown rat (Rodentia: Muridae) in Poland. Wiad. Parazytol. 2004, 50, 333–335. [Google Scholar] [PubMed]
- Zhang, Z.-Q. Repositories for mite and tick specimens: Acronyms and their nomenclature. Syst. Appl. Acarol. 2018, 23, 2432–2446. [Google Scholar] [CrossRef]
- Nutting, W.B. Hair follicle mites (Demodex spp.) of medical and veterinary concern. Cornell Vet. 1976, 66, 214–231. [Google Scholar] [PubMed]
- Bochkov, A.V. New observations on phylogeny of cheyletoid mites (Acari: Prostigmata: Cheyletoidea). Proc. Zool. Inst. RAS 2008, 312, 54–73. [Google Scholar] [CrossRef]
- Taxonomic Information System (ITIS). Available online: http://www.itis.gov (accessed on 12 June 2025).
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 68, 131–133. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L.; Fryderyk, S.; Mierzyński, Ł. Adult and immature stages of the new species of the genus Demodex (Acariformes: Demodecidae) with data on parasitism, topography, and topical specificity of demodecid mites in the yellow-necked mouse, Apodemus flavicollis (Rodentia: Muridae). J. Parasitol. 2017, 103, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Cierocka, K.; Izdebska, J.N.; Rolbiecki, L. The co-occurrence of Demodecidae and Psorergatidae (Acariformes: Prostigmata) in the yellow-necked field mouse Apodemus flavicollis (Rodentia: Muridae) with a description of two new species and a new host record. Diversity 2024, 16, 550. [Google Scholar] [CrossRef]
- Izdebska, J.N.; Rolbiecki, L. A new species of the genus Demodex Owen, 1843 (Acari: Demodecidae) from the ear canals of the house mouse Mus musculus L. (Rodentia: Muridae). Syst. Parasitol. 2015, 91, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Izdebska, J.N.; Rolbiecki, L. Two new species of Demodex (Acari: Demodecidae) with a redescription of Demodex musculi and data on parasitism in Mus musculus (Rodentia: Muridae). J. Med. Entomol. 2015, 52, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Izdebska, J.N.; Rolbiecki, L.; Fryderyk, S. A new species of Demodex (Acari: Demodecidae) from the skin of the vibrissal area of the house mouse Mus musculus (Rodentia: Muridae), with data on parasitism. Syst. Appl. Acarol. 2016, 21, 1031–1039. [Google Scholar] [CrossRef]
- Boczek, J. Biologia i ekologia sierposza rozkruszkowca (Cheyletus eruditus) (Schrank 1781)—(Acarina, Cheyletidae). Pr. Nauk. Inst. Ochr. Rośl. 1959, 1, 175–230. [Google Scholar]
- Walter, D.E.; Lindquist, E.E.; Smith, I.M.; Cook, D.R. Order Trombidiformes. In A Manual of Acarology, 3rd ed.; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 233–420. [Google Scholar]

 Demodex ovaportans sp. nov.,
Demodex ovaportans sp. nov.,  Demodex castoris.
Demodex castoris.
 Demodex ovaportans sp. nov.,
Demodex ovaportans sp. nov.,  Demodex castoris.
Demodex castoris.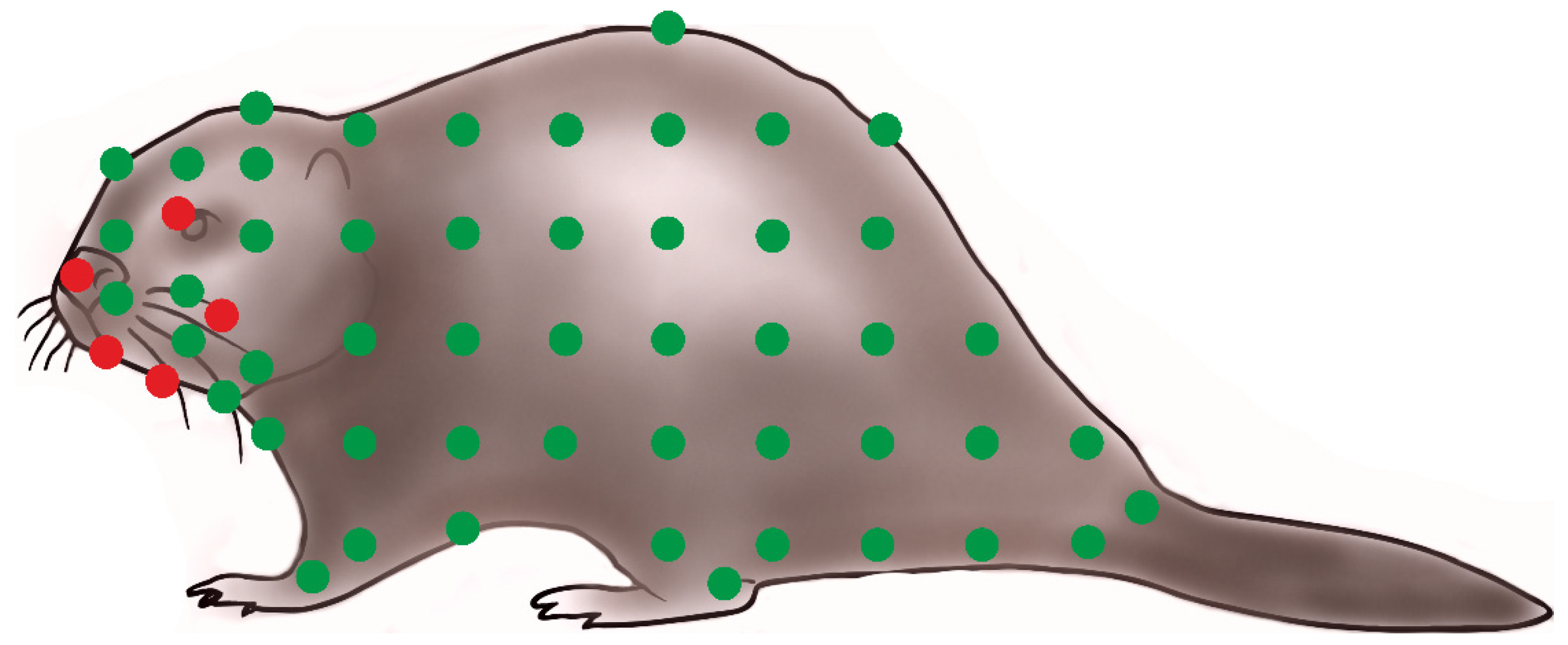
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rolbiecki, L.; Izdebska, J.N.; Dzido, J.; Fryderyk, S. The Biology of Demodecid Mites (Trombidiformes: Demodecidae) and Their Parasitism in the Eurasian Beaver Castor fiber Linnaeus, 1758, with a Description of a New Species. Animals 2025, 15, 2136. https://doi.org/10.3390/ani15142136
Rolbiecki L, Izdebska JN, Dzido J, Fryderyk S. The Biology of Demodecid Mites (Trombidiformes: Demodecidae) and Their Parasitism in the Eurasian Beaver Castor fiber Linnaeus, 1758, with a Description of a New Species. Animals. 2025; 15(14):2136. https://doi.org/10.3390/ani15142136
Chicago/Turabian StyleRolbiecki, Leszek, Joanna N. Izdebska, Joanna Dzido, and Sławomira Fryderyk. 2025. "The Biology of Demodecid Mites (Trombidiformes: Demodecidae) and Their Parasitism in the Eurasian Beaver Castor fiber Linnaeus, 1758, with a Description of a New Species" Animals 15, no. 14: 2136. https://doi.org/10.3390/ani15142136
APA StyleRolbiecki, L., Izdebska, J. N., Dzido, J., & Fryderyk, S. (2025). The Biology of Demodecid Mites (Trombidiformes: Demodecidae) and Their Parasitism in the Eurasian Beaver Castor fiber Linnaeus, 1758, with a Description of a New Species. Animals, 15(14), 2136. https://doi.org/10.3390/ani15142136









