1. Introduction
The skin is the largest organ of mammals, accounting for about 15% of body weight and serving critical roles in homeostasis, protection, thermoregulation, sensory perception, and immune defense [
1,
2]. Its multilayered structure—epidermis, dermis, and hypodermis—provides a robust barrier while enabling regeneration after injury [
3,
4]. In veterinary medicine, efficient wound healing is essential for post-surgical recovery, minimizing infection risks, and improving patient comfort and prognosis [
5].
Wound healing is a dynamic process with three overlapping phases: inflammation, proliferation, and remodeling [
5,
6]. Each stage is coordinated through interactions between inflammatory cells, fibroblasts, endothelial cells, cytokines, and extracellular matrix (ECM) components [
7,
8]. Factors such as tissue oxygenation, vascularization, pH, comorbidities, and local immune status influence the wound healing process [
9,
10,
11]. Notably, maintaining a slightly acidic skin pH helps to prevent pathogen colonization and supports enzyme activity essential for epidermal renewal [
12].
In recent years, photobiomodulation therapy (PBMT), particularly using class IV therapeutic lasers, has gained recognition as a promising tool in regenerative medicine. Class IV lasers emit high-power infrared light (typically in the 780–980 nm range), allowing deeper tissue penetration and direct stimulation of mitochondrial cytochrome C oxidase. This mechanism enhances ATP production, promotes cellular proliferation, stimulates collagen synthesis, and induces angiogenesis [
5,
13,
14]. These effects contribute to all stages of wound healing: they reduce inflammation and oxidative stress during the inflammatory phase, support fibroblast and keratinocyte activity during the proliferative phase, and facilitate organized ECM remodeling during the final maturation phase [
6,
15,
16,
17].
Although the literature on PBMT has grown steadily in both human and veterinary contexts, controlled studies specifically investigating its application in companion animals—especially cats—remain limited. Furthermore, there is a lack of intra-individual controlled studies that evaluate differential healing responses within the same animal and in the same surgical wound [
7,
9,
10,
11].
Therefore, the present study aimed to evaluate the effect of class IV laser therapy on the healing of surgical wounds in dogs (Canis familiaris) and cats (Felis catus). Using an intra-individual split-wound design, this preliminary study investigates differences in key healing parameters—such as skin thickness, skin color, presence of hematoma, regional temperature, skin elasticity, and presence of fluids—over an 8-day postoperative period. This work contributes to the growing evidence base for PBMT in small animal practice and may support the development of standardized protocols for its clinical use.
2. Materials and Methods
2.1. Study Design and Animal Selection
This prospective intra-individual controlled study included a total of 49 animals (n = 49), comprising 25 dogs (Canis familiaris) and 24 cats (Felis catus) of both sexes (27 females and 22 males), various ages, and breeds. All patients underwent soft tissue and orthopedic procedures performed under aseptic conditions using a CO2 surgical laser technique, ensuring precise tissue incision, reduced intraoperative bleeding, and minimal collateral tissue trauma.
Each surgical incision was systematically divided into two equal zones: a cranial/proximal Laser-treated Zone (LZ) and a caudal/distal Control Zone (CZ) without therapeutic laser application. The cranial/proximal segment was consistently designated for laser therapy. This standardized approach accounted for anatomical and physiological factors (e.g., typical blood flow direction) and practical considerations, such as patient positioning and consistent reproducibility, across cases. (Note: for vertical or limb incisions, the terms proximal/distal are used instead of cranial/caudal to maintain anatomical accuracy).
To ensure the feasibility of the split-wound approach and meaningful intra-individual comparison, only surgical incisions measuring at least 5 cm in length were included, providing a minimum of 2.5 cm for each zone (LZ and CZ). The depth and anatomical location of the incisions varied, depending on the specific surgical indication; however, the intra-individual design ensured that each patient served as its own control, effectively minimizing the impact of local tissue variability [
7,
9,
18].
Animals with any current or previous oncological disease, metabolic or endocrine disorders (e.g., diabetes mellitus, hypothyroidism, Cushing’s syndrome), or any other systemic condition that could affect wound healing were excluded. Cases where complete postoperative follow-up was not feasible were also excluded.
Written informed consent was obtained from all animal owners. The study protocol was approved by the institutional ethics committee (Ref. 015/2022).
2.2. Laser Treatment Protocol
Laser therapy was performed using a class IV diode laser system (Doctor Vet Therapy Laser, LAMBDA S.p.A., Vicenza, Italy) emitting a combination of wavelengths (660, 808, and 915 nm) in continuous wave (CW) and pulse modes. Irradiation was applied immediately postoperatively only once. The energy dose was adjusted according to the estimated wound area as follows:
- ▪
Post-op S: 5 cm2 area; 25 s; 2 W output; total energy 50 J; dose per area: 10 J/cm2.
- ▪
Post-op M: 25 cm2 area; 2 min 5 s; 2 W output; total energy 250 J; dose per area: 10 J/cm2.
- ▪
Post-op L: 50 cm2 area; 4 min 10 s; 2 W output; total energy 500 J; dose per area: 10 J/cm2.
Frequencies used included CW, 1, 2, 10, and 25 kHz, with distinct purposes [
17,
19] as follows:
- ▪
1 kHz: epithelialization;
- ▪
2 kHz: fibroblast stimulation;
- ▪
10 kHz: infection control;
- ▪
25 kHz: antimicrobial effect.
These frequency settings are based on previous validated protocols [
10,
12]. The laser beam was applied at least two passes to ensure homogeneous energy distribution over the treatment area.
2.3. Evaluation Parameters and Timeline
The surgical incision of each patient was divided into two equal parts as follows:
- ▪
Laser Zone (LZ): cranial/proximal segment receiving class IV laser application;
- ▪
Control Zone (CZ): caudal/distal segment without laser application.
Three postoperative timepoints were used for evaluation as follows:
- ▪
T0: immediately after surgery;
- ▪
T1: 48 h post-surgery;
- ▪
T2: 8 days post-surgery.
Wound healing was assessed using a validated scoring system adapted from Vitor & Carreira (2015) [
18], including the following clinical parameters: skin thickness, skin color, presence of hematoma, regional temperature, skin elasticity, and presence of fluids. Regional temperature was measured using a non-contact infrared thermometer. To enhance reproducibility and minimize observer bias, standardized photographic monitoring was performed at each evaluation point under controlled lighting, distance, and scale. All assessments were performed by the same investigator to minimize variability and ensure consistency.
2.4. Statistical Analysis
Data was recorded in Microsoft Excel and analysed using IBM SPSS Statistics version 29 (Windows). Descriptive statistics included means, standard deviations, and frequencies. The Shapiro–Wilk test was used to check normality, and Levene’s test was used for homogeneity of variance. Inferential statistics comprised the following:
- ▪
Repeated measures ANOVA for intra-group comparisons over time (normally distributed data);
- ▪
Student’s t-test for independent samples where assumptions of normality and homogeneity were met;
- ▪
Mann–Whitney U test for non-normally distributed data;
- ▪
Fisher’s exact test for categorical associations with low-frequency outcomes;
- ▪
Cochran’s Q test for repeated binary variables across timepoints.
Categorical outcomes were coded as binary (dummy) variables. A p-value ≤ 0.05 was considered statistically significant.
4. Discussion
This study evaluated the therapeutic effects of class IV laser therapy on the healing process of post-surgical wounds in dogs and cats. The sample included 49 animals of different ages, sexes, body weights, and body condition scores, allowing for a broad assessment of the clinical effects of photobiomodulation (PBMT) using a class IV therapeutic laser.
The intra-individual split-wound design isolated the independent variable (laser treatment) while keeping other intrinsic and extrinsic variables constant. This approach minimized confounding factors, reduced bias, and improved internal validity and statistical robustness, consistent with other studies supporting intra-individual models as the gold standard for clinical comparisons [
1,
2,
3,
4,
7,
16,
17,
22].
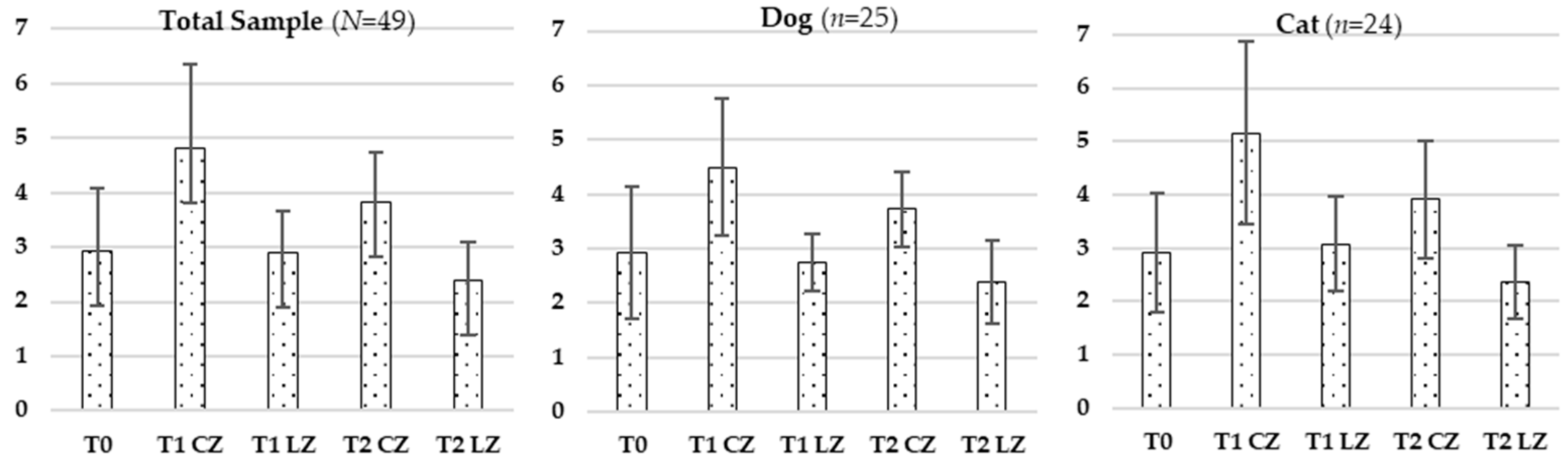
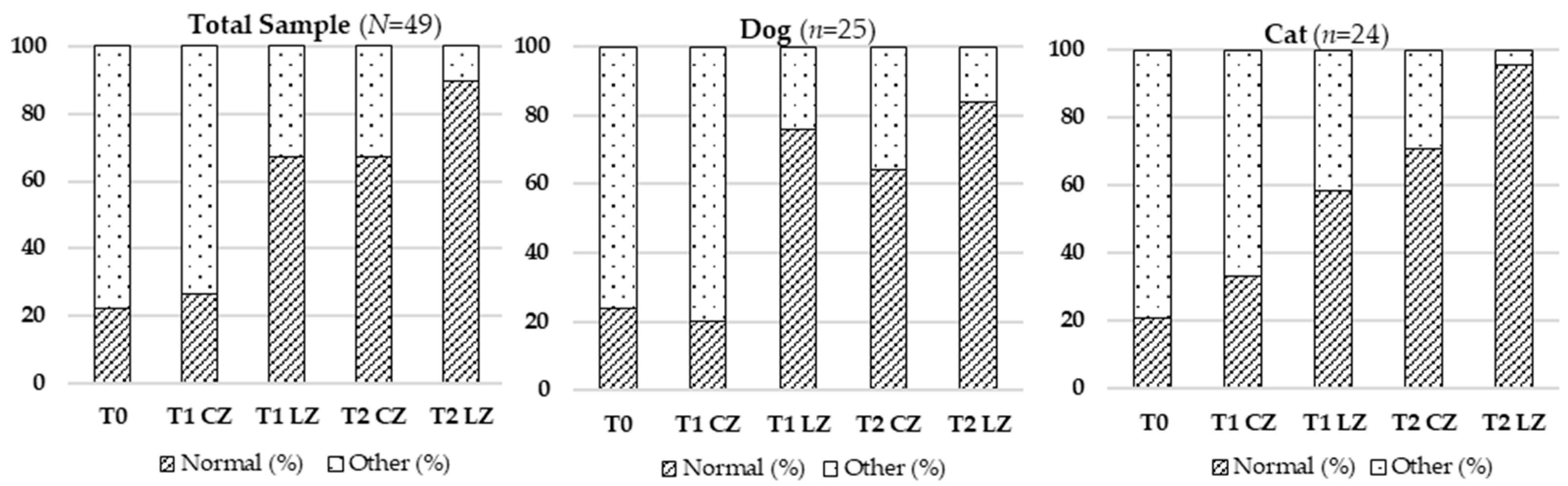
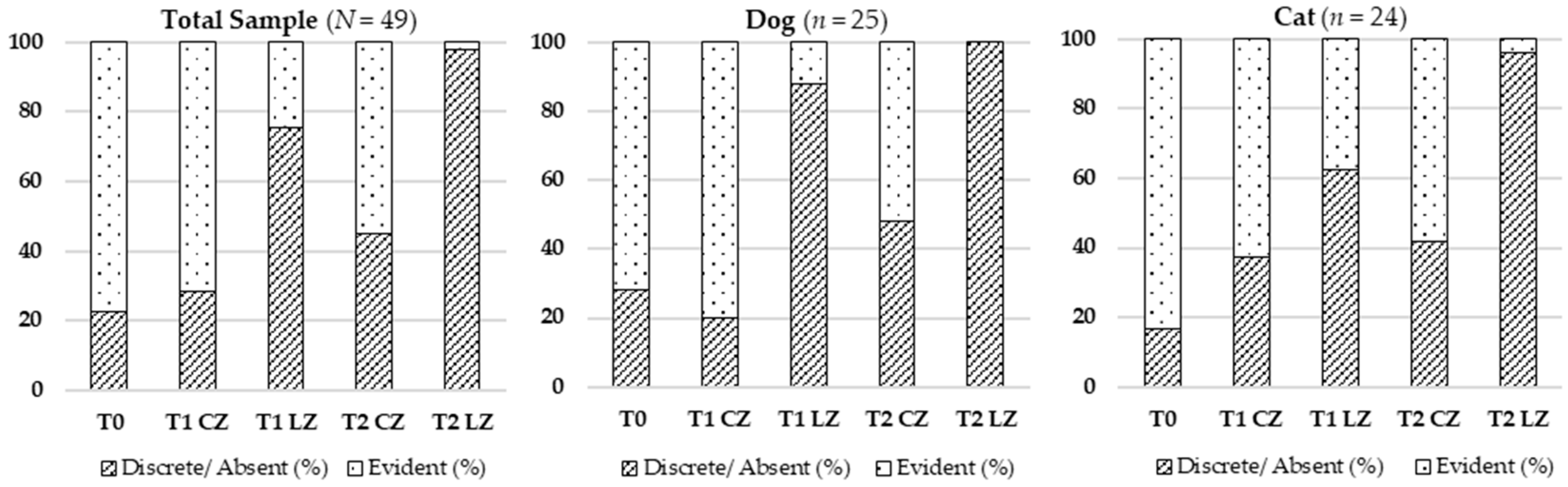
Skin thickness decreased significantly in the Laser Zones (LZs) compared to Control Zones (CZs) at all timepoints, suggesting reduced local inflammation and lower extracellular matrix (ECM) density. During the inflammatory phase, vasodilation and increased vascular permeability enable immune cell infiltration and protein extravasation, leading to localized swelling. Transition to the proliferative phase involves fibroblast proliferation, ECM remodeling, and granulation tissue formation. Class IV laser therapy accelerates this process by modulating pro-inflammatory cytokines (e.g., TNF-α, IL-1β) and enhancing anti-inflammatory mediators, like IL-10 [
5,
18]. It also stimulates cytochrome C oxidase in mitochondria, boosting ATP production and cellular metabolism. This bioenergetic effect promotes fibroblast activity, type III collagen synthesis, and myofibroblast differentiation, supporting wound contraction and tissue remodeling [
5,
6]. Growth factors such as TGF-β, FGF, and IGF are upregulated in response to laser exposure, contributing to the observed reduction in skin thickness as an indicator of faster healing.
No significant differences in skin thickness were observed between dogs and cats or across different ages and sexes, suggesting that the laser consistently modulated healing mechanisms regardless of physiological profile. Estrogens typically promote tissue regeneration by enhancing epidermal thickness, vascularization, and collagen deposition, whereas androgens can counteract these effects [
8,
21]. The consistent outcomes here indicate that the laser’s effects may override baseline hormonal differences.
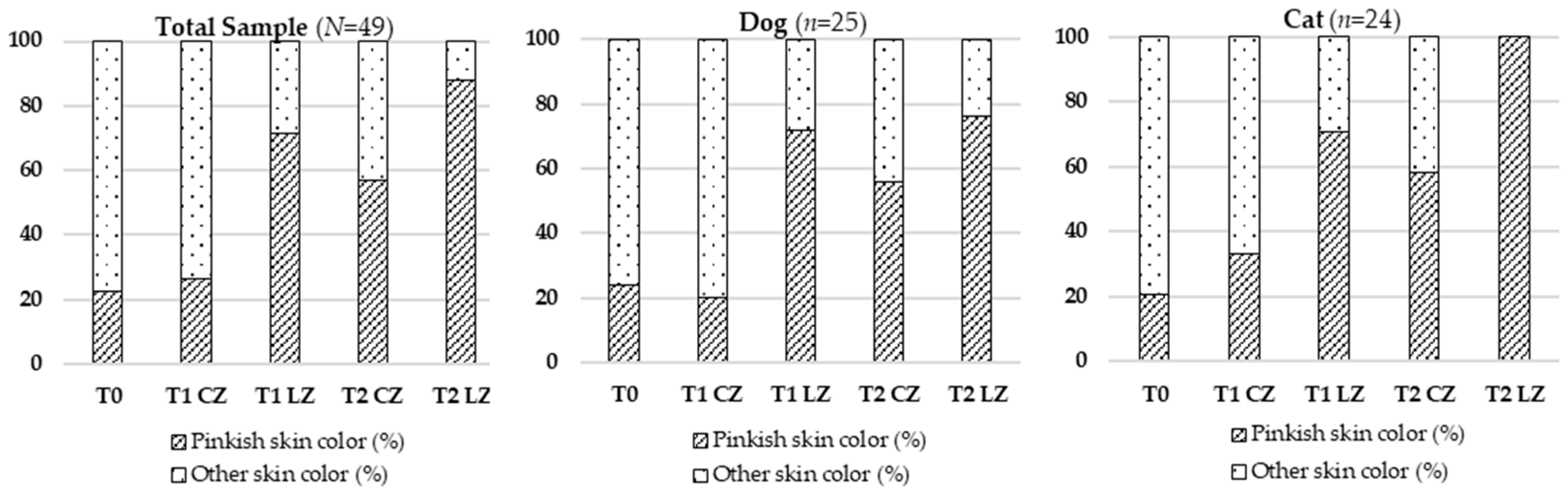
Skin color, an indicator of vascular perfusion and oxygenation, appeared as a more vivid pinkish hue in Lazer Zones (LZs), especially at T2, aligning with the peak of the proliferative phase characterized by angiogenesis driven by VEGF, FGF, and TGF-β [
9,
23]. The laser likely promoted VEGF expression through increased ATP production and mitochondrial activation, resulting in improved microvascular density and tissue oxygenation. This effect was more evident in cats, possibly due to their thinner epidermis, reduced subcutaneous fat, and more superficial vasculature, enhancing light absorption [
10].
In Control Zones (CZs), females showed more pronounced pinkish skin color than males, likely due to estrogen-mediated vasodilation and angiogenesis [
8]. However, this difference was not evident in Laser Zones (LZs), suggesting that the laser-induced vascular response compensated for hormonal variations. These hypotheses are supported by evidence on ROS and mitochondrial pathways involved in VEGF upregulation and vascular remodeling [
10,
16,
17,
19].
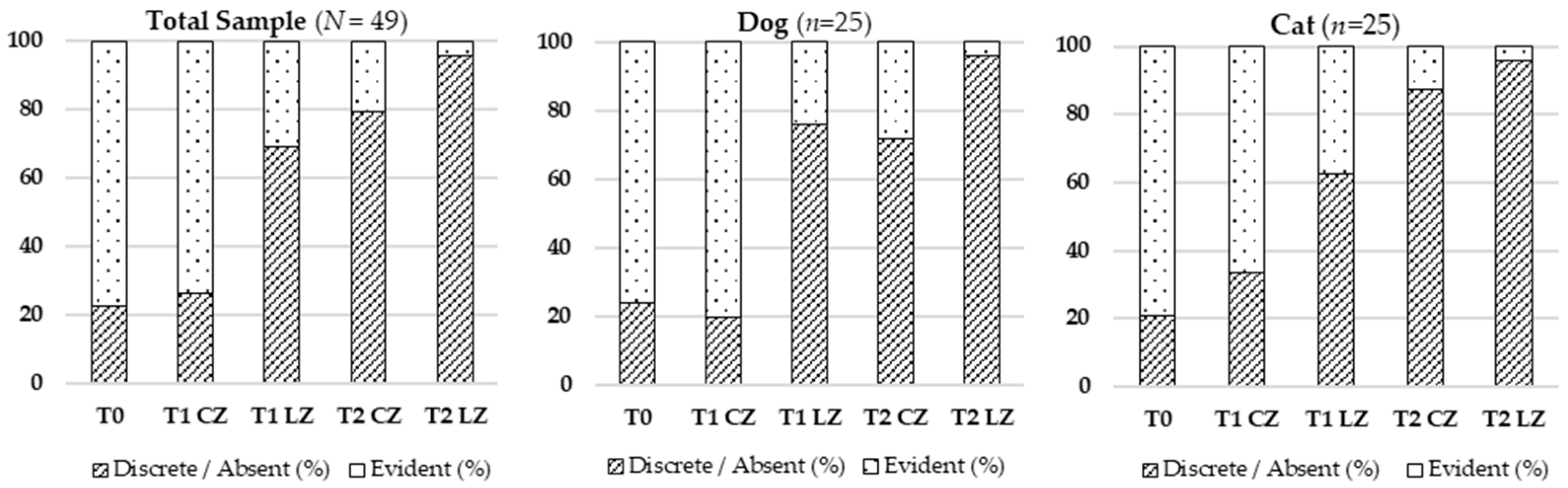
Hematoma resolution occurred faster in LZs, reflecting improved inflammation control and vascular repair. Class IV laser therapy is thought to enhance lymphatic drainage, nitric oxide release, and endothelial stabilization [
11,
13,
17,
19,
23]. ROS signalling and cytochrome C oxidase activation reduce capillary permeability and stimulate macrophage activity, facilitating erythrocyte phagocytosis and the degradation of extravascular hemoglobin [
4,
6,
13,
17,
19].
It is important to note that lymphatic flow is inherently continuous across the wound area, even when divided anatomically into treated and Control Zones. This means that drainage in one segment may partially influence the adjacent zone—a recognized limitation of the split wound model. Nevertheless, the localized bio-stimulatory effect of laser therapy can modulate lymphatic activity within the treated zone (LZ) and promote more efficient clearance of interstitial fluids.
Additionally, animals with higher body condition scores showed prolonged hematoma presence in CZs, likely because excess subcutaneous fat can compress lymphatic vessels, reduce drainage efficiency, and contribute to low-grade inflammation. This mechanism explains why fluid and hematoma persistence were more evident in the CZs of overweight animals. In contrast, the laser’s stimulation of endothelial stability and lymphatic flow appeared to offset this disadvantage, resulting in faster resolution of hematoma in LZs.
Skin temperature increased significantly over time in LZs, consistent with metabolic activation induced by laser therapy. Enhanced mitochondrial function increases ATP production, vasodilation, and local perfusion—key factors in active tissue repair [
4,
6,
14]. This effect was uniform across species and unaffected by sex, age, or body condition score.
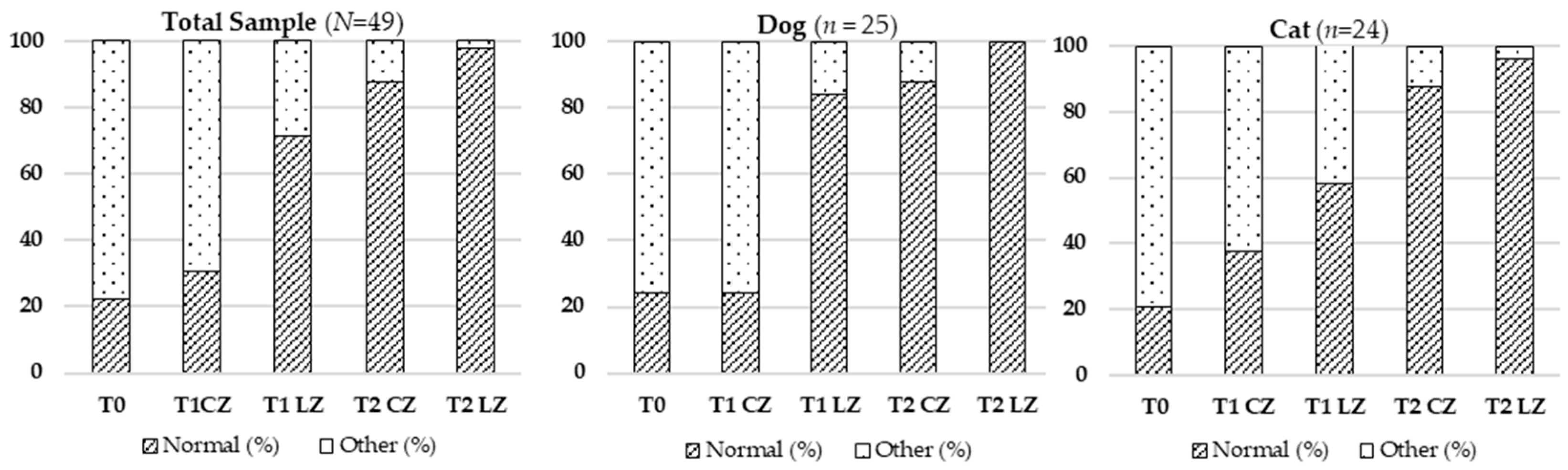
Skin elasticity, reflecting ECM integrity, improved significantly in LZs. This improvement was likely due to increased synthesis of collagen types I and III, elastin, and fibronectin driven by elevated ATP levels and fibroblast activity [
15,
17,
19]. Laser therapy also modulates MMPs and TIMPs, ensuring balanced ECM turnover [
14,
16]. While sex hormones can affect dermal elasticity [
8,
21], the laser’s bio-stimulating effect appeared to offset these variations, resulting in more uniform tissue quality.
The presence of fluids, including lymphatic and serosanguinous exudate, decreased more rapidly in LZs. This outcome can be explained by enhanced endothelial and lymphatic function via cytochrome C oxidase activation and improved ATP-driven ion transport [
4,
6,
17,
24]. Aquaporin regulation and reduced histamine-mediated capillary permeability [
12,
15,
23] also contributed to faster fluid resolution. Again, the continuous nature of lymphatic circulation across the wound zones must be acknowledged as a possible confounding factor when interpreting intra-wound comparisons.
Given the preliminary nature of this study, several limitations should be acknowledged. First, the follow-up period was relatively short and focused on early healing phases, limiting conclusions about long-term outcomes. Second, although standardized photographic monitoring was implemented, advanced imaging methods such as elastography, digital thermography, or histological analysis were not performed. Third, the absence of blinded outcome assessors could result in observer bias. Fourth, despite excluding animals with obvious oncological history or severe systemic disease, undetected comorbidities, like diabetes or hypothyroidism, may have influenced healing responses. Fifth, only a single laser application was used immediately post-surgery, which does not fully represent multi-session protocols common in clinical practice. Finally, the inclusion of various surgical procedures—both soft tissue and orthopedic—performed with the CO2 laser technique naturally introduced variability in wound depth, anatomical location, and healing characteristics.