Identification of Simultaneous Occurrence of Amphibian Chytrid Fungi and Ranavirus in South Korea
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Selections
2.2. Species Selection
2.3. Sample Collection
2.4. Molecular Detection of Bd and RV
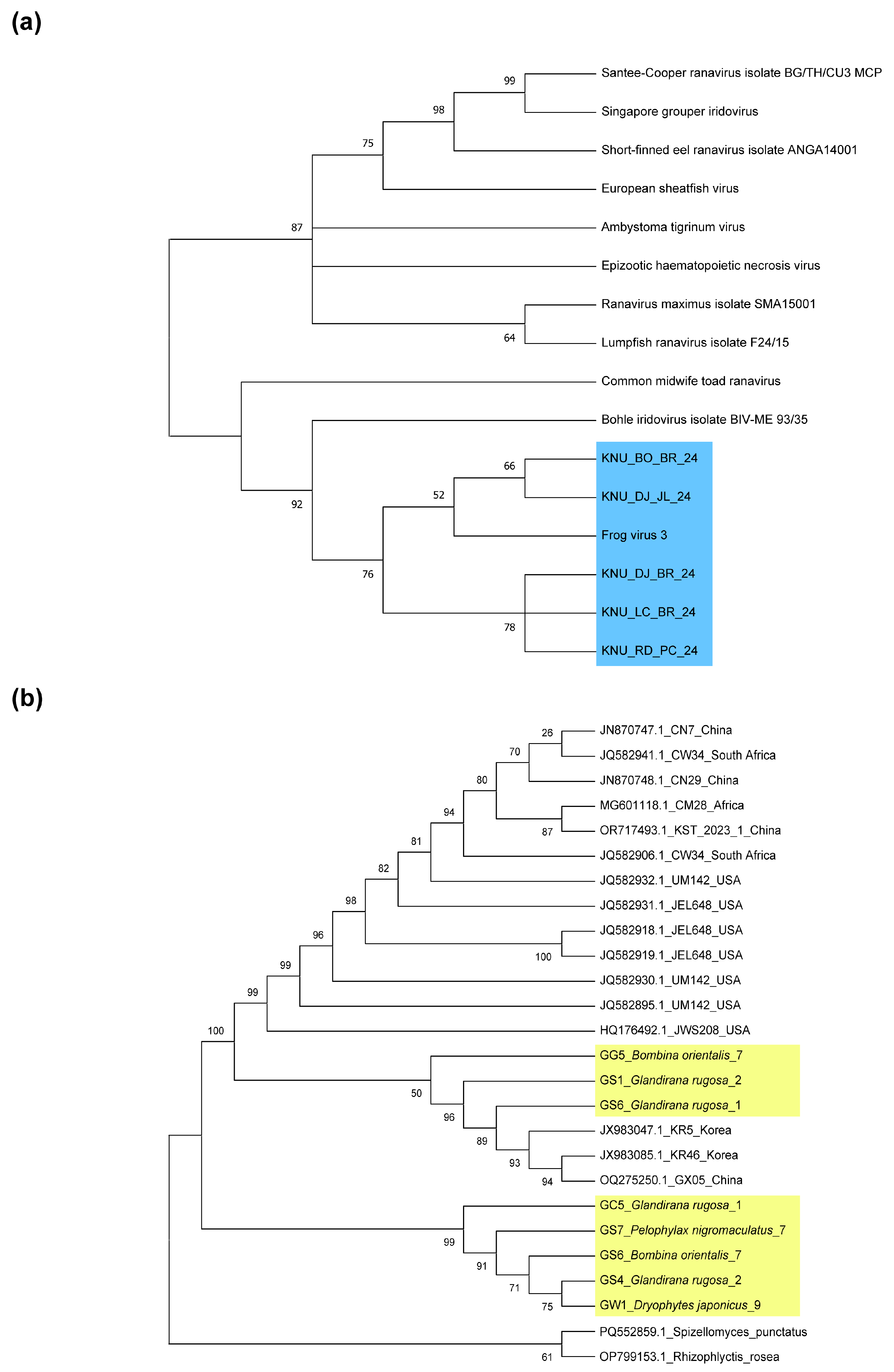
2.5. Lineage Verification of Bd and RV
2.6. Statistical Analysis
3. Results
3.1. Phylogenetic Analysis of Bd and RV Pathogens
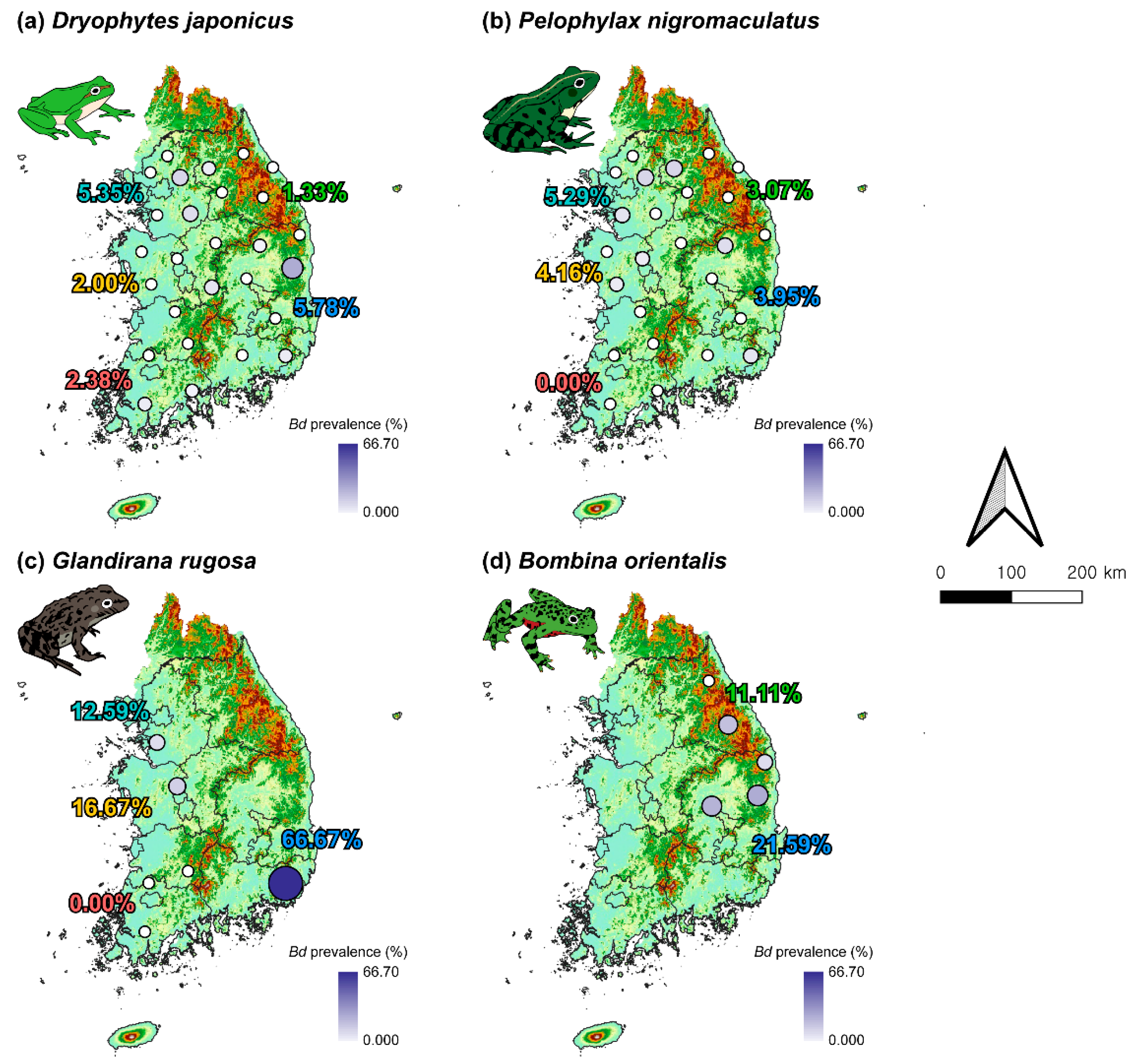
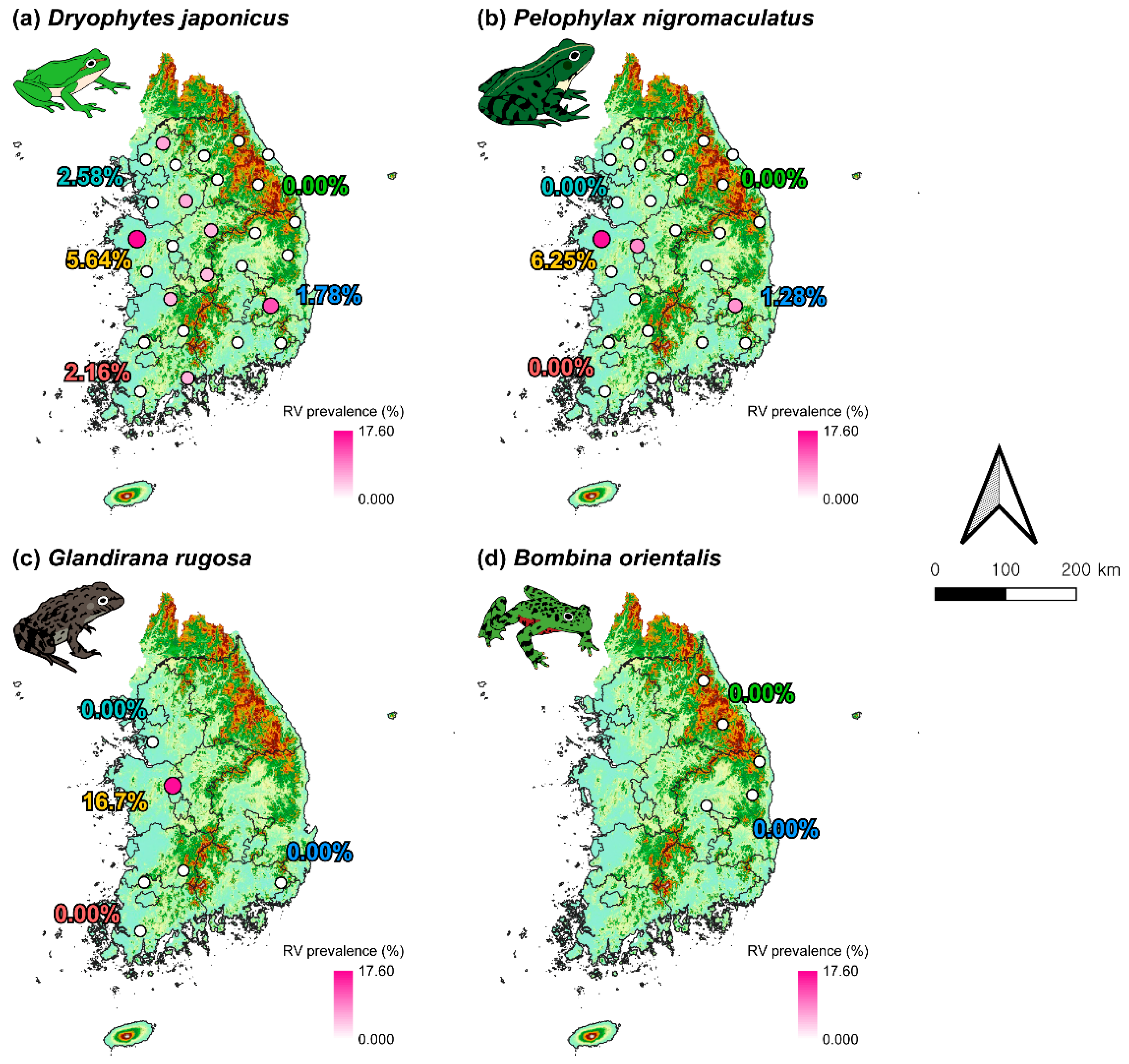
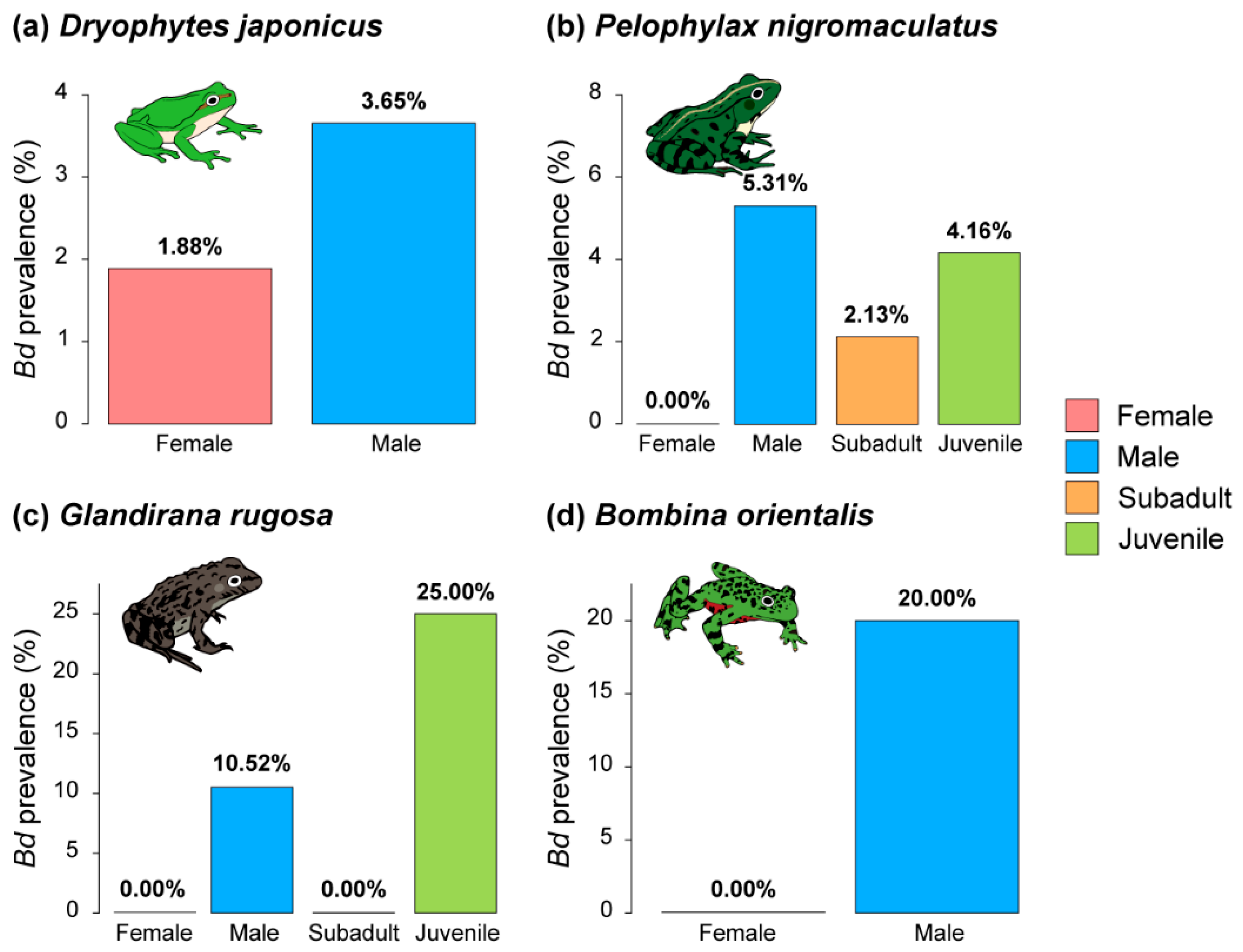
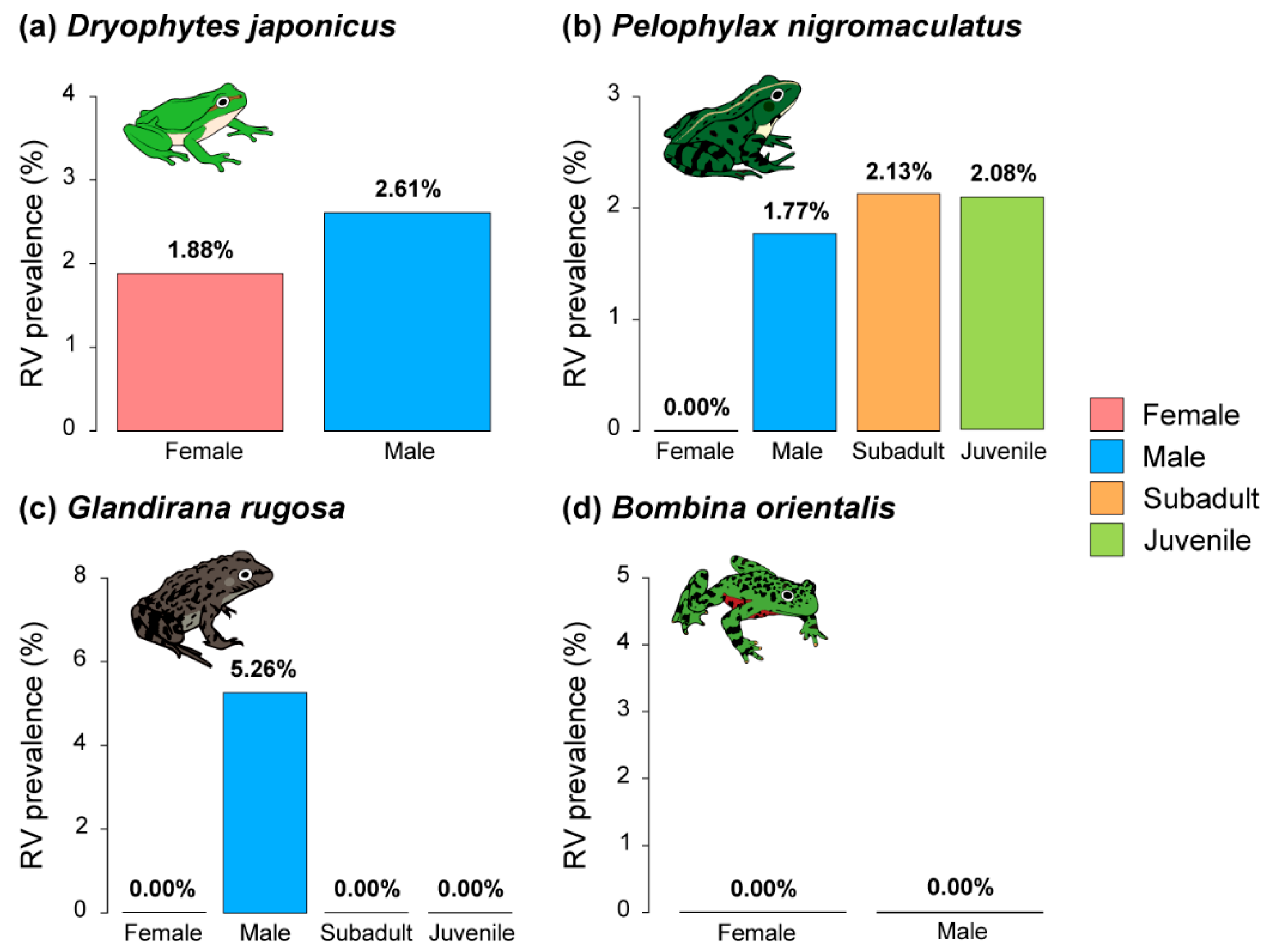
3.2. Prevalence of Bd and RV by Species
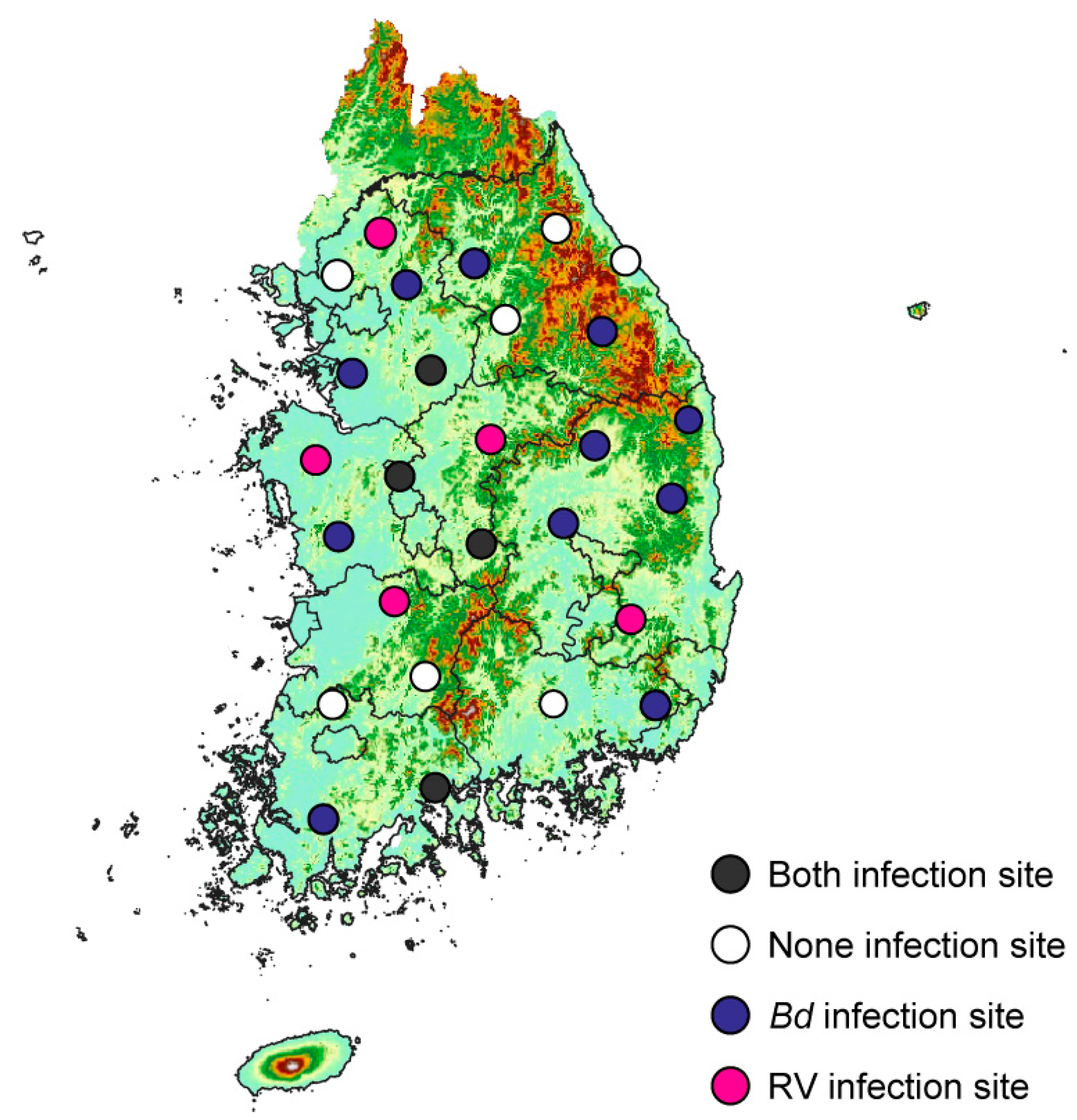
3.3. Distribution of Bd Throughout the Study Area
3.4. Distribution of RVs Throughout the Study Area
3.5. Pathogen Prevalences by Difference in Sex and Life History
3.6. Co-Occurrence of Bd and RV in the Study Area
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luedtke, J.A.; Chanson, J.; Neam, K.; Hobin, L.; Maciel, A.O.; Catenazzi, A.; Borzée, A.; Hamidy, A.; Aowphol, A.; Jean, A. Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 2023, 622, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.J.; Miller, D.L.; Hoverman, J.T. Ecology and pathology of amphibian ranaviruses. Dis. Aquat. Organ. 2009, 87, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.A.; Skerratt, L.F.; Speare, R.; McCALLUM, H. Impact and dynamics of disease in species threatened by the amphibian chytrid fungus, Batrachochytrium dendrobatidis. Conserv. Biol. 2009, 23, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.B. An aquatic disease on a terrestrial salamander: Individual and population level effects of the amphibian chytrid fungus, Batrachochytrium dendrobatidis, on Batrachoseps attenuatus (Plethodontidae). Copeia 2009, 2009, 653–660. [Google Scholar] [CrossRef]
- Price, S.J.; Garner, T.W.; Nichols, R.A.; Balloux, F.; Ayres, C.; de Alba, A.M.-C.; Bosch, J. Collapse of amphibian communities due to an introduced Ranavirus. Curr. Biol. 2014, 24, 2586–2591. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Garner, T.W. Chytrid fungi and global amphibian declines. Nat. Rev. Microbiol. 2020, 18, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.R.; Voyles, J.; Cook, D.I.; Dinudom, A. Frog skin epithelium: Electrolyte transport and chytridiomycosis. Int. J. Biochem. Cell Biol. 2012, 44, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Docherty, D.E.; Meteyer, C.U.; Wang, J.; Mao, J.; Case, S.T.; Chinchar, V.G. Diagnostic and molecular evaluation of three iridovirus–associated salamander mortality events. J. Wildl. Dis. 2003, 39, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Scheele, B.C.; Pasmans, F.; Skerratt, L.F.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; Monsalve-Carcaño, C.; Price, S.J.; Bielby, J. Single infection with Batrachochytrium dendrobatidis or Ranavirus does not increase probability of co–infection in a montane community of amphibians. Sci. Rep. 2020, 10, 21115. [Google Scholar] [CrossRef] [PubMed]
- Bataille, A.; Fong, J.J.; Cha, M.; Wogan, G.O.; Baek, H.J.; Lee, H.; Min, M.S.; Waldman, B. Genetic evidence for a high diversity and wide distribution of endemic strains of the pathogenic chytrid fungus Batrachochytrium dendrobatidis in wild Asian amphibians. Mol. Ecol. 2013, 22, 4196–4209. [Google Scholar] [CrossRef] [PubMed]
- Roh, N.; Park, J.; Kim, J.; Kwon, H.; Park, D. Prevalence of Ranavirus infection in three anuran species across South Korea. Viruses 2022, 14, 1073. [Google Scholar] [CrossRef] [PubMed]
- Berger, L.; Speare, R.; Hines, H.; Marantelli, G.; Hyatt, A.; McDonald, K.; Skerratt, L.; Olsen, V.; Clarke, J.; Gillespie, G. Effect of season and temperature on mortality in amphibians due to chytridiomycosis. Aust. Vet. J. 2004, 82, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Hill, R.D.; Brenes, R.; Chaney, J.C.; Wilkes, R.P.; Grayfer, L.; Miller, D.L.; Gray, M.J. Water temperature affects susceptibility to ranavirus. EcoHealth 2016, 13, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Knapp, R.A.; Briggs, C.J.; Smith, T.C.; Maurer, J.R. Nowhere to hide: Impact of a temperature-sensitive amphibian pathogen along an elevation gradient in the temperate zone. Ecosphere 2011, 2, 1–26. [Google Scholar] [CrossRef]
- Cortazar-Chinarro, M.; Richter-Boix, A.; Rödin-Mörch, P.; Halvarsson, P.; Logue, J.; Laurila, A.; Höglund, J. Association between the skin microbiome and MHC class II diversity in an amphibian. Mol. Ecol. 2024, 33, e17198. [Google Scholar] [CrossRef] [PubMed]
- Lau, Q.; Igawa, T.; Kosch, T.A.; Dharmayanthi, A.B.; Berger, L.; Skerratt, L.F.; Satta, Y. Conserved evolution of MHC supertypes among Japanese frogs suggests selection for Bd resistance. Animals 2023, 13, 2121. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.; George, E.; Andino, F.D.J.; Chen, G. Waterborne infectivity of the Ranavirus frog virus 3 in Xenopus laevis. Virology 2011, 417, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, J.; de Carvalho-e-Silva, S.P.; James, T.Y.; Toledo, L.F. Amphibian chytrid infection is influenced by rainfall seasonality and water availability. Dis. Aquat. Org. 2018, 127, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Brunner, J.L.; Storfer, A.; Gray, M.J.; Hoverman, J.T. Ranavirus ecology and evolution: From epidemiology to extinction. In Ranaviruses: Lethal Pathogens of Ectothermic Vertebrates; Springer International Publishing: Cham, Switzerland, 2015; pp. 71–104. [Google Scholar]
- Dendrobatidis, B. No Safe Space: Prevalence and Distribution of. Herpetol. Conserv. Biol. 2018, 13, 373–382. [Google Scholar]
- Gould, J.; Valdez, J.; Stockwell, M.; Clulow, S.; Mahony, M. Mosquitoes as a potential vector for the transmission of the amphibian chytrid fungus. Preprints 2019, 29, 36–42. [Google Scholar] [CrossRef]
- Brunner, J.L.; Yarber, C.M. Evaluating the importance of environmental persistence for Ranavirus transmission and epidemiology. Adv. Virus Res. 2018, 101, 129–148. [Google Scholar] [PubMed]
- Park, J.-K.; Do, Y. Developmental temperature modulates microplastics impact on amphibian life history without affecting ontogenetic microplastic transfer. J. Hazard. Mater. 2024, 477, 135325. [Google Scholar] [CrossRef] [PubMed]
- Hoverman, J.T.; Gray, M.J.; Haislip, N.A.; Miller, D.L. Phylogeny, life history, and ecology contribute to differences in amphibian susceptibility to ranaviruses. EcoHealth 2011, 8, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Haislip, N.A.; Gray, M.J.; Hoverman, J.T.; Miller, D.L. Development and disease: How susceptibility to an emerging pathogen changes through anuran development. PLoS ONE 2011, 6, e22307. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.J.; Gregory Chinchar, V. Introduction: History and Future of Ranaviruses. In Ranaviruses; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–7. [Google Scholar]
- Warne, R.W.; Crespi, E.J.; Brunner, J.L. Escape from the pond: Stress and developmental responses to ranavirus infection in wood frog tadpoles. Funct. Ecol. 2011, 25, 139–146. [Google Scholar] [CrossRef]
- Green, D.E.; CoNVerSe, K.A.; SCHrADer, A.K. Epizootiology of sixty-four amphibian morbidity and mortality events in the USA, 1996–2001. Ann. N. Y. Acad. Sci. 2002, 969, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Thekkiniath, J.C.; Zabet-Moghaddam, M.; San Francisco, S.K.; San Francisco, M.J. A novel subtilisin–like serine protease of Batrachochytrium dendrobatidis is induced by thyroid hormone and degrades antimicrobial peptides. Fungal Biol. 2013, 117, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.J.; Kupferberg, S.J.; Wilber, M.Q.; Pessier, A.P.; Grefsrud, M.; Bobzien, S.; Vredenburg, V.T.; Briggs, C.J. Extreme drought, host density, sex, and bullfrogs influence fungal pathogen infection in a declining lotic amphibian. Ecosphere 2017, 8, e01740. [Google Scholar] [CrossRef]
- Ryser, J. Weight loss, reproductive output, and the cost of reproduction in the common frog, Rana temporaria. Oecologia 1989, 78, 264–268. [Google Scholar] [CrossRef]
- Bevier, C.R. Utilization of energy substrates during calling activity in tropical frogs. Behav. Ecol. Sociobiol. 1997, 41, 343–352. [Google Scholar] [CrossRef]
- Piotrowski, J.S.; Annis, S.L.; Longcore, J.E. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 2004, 96, 9–15. [Google Scholar] [CrossRef] [PubMed]
- La Fauce, K.; Ariel, E.; Munns, S.; Rush, C.; Owens, L. Influence of temperature and exposure time on the infectivity of Bohle iridovirus, a ranavirus. Aquaculture 2012, 354, 64–67. [Google Scholar] [CrossRef]
- AmphibiaWeb. Hyla japonica: Japanese Tree Frog. Available online: https://amphibiaweb.org/species/832 (accessed on 23 December 2024).
- AmphibiawWeb. Bombina orientalis: Oriental Fire-Bellied Toad. Available online: https://amphibiaweb.org/species/2045 (accessed on 23 December 2024).
- AmphibiaWeb. Pelophylax nigromaculatus: Dark-Spotted Frog. Available online: https://amphibiaweb.org/species/5109 (accessed on 23 December 2024).
- AmphibiaWeb. Glandirana rugosa: Wrinkled Frog. Available online: https://amphibiaweb.org/species/5138 (accessed on 23 December 2024).
- Boyle, A.H.D.; Olsen, V.; Boyle, D.; Berger, L.; Obendorf, D.; Dalton, A.; Kriger, K.; Hero, M.; Hines, H.; Phillott, R. Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis. Aquat. Org. 2007, 73, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Archard, G.A.; Goldsmith, A. Euthanasia methods, corticosterone and haematocrit levels in Xenopus laevis: Evidence for differences in stress? Anim. Welf. 2010, 19, 85–92. [Google Scholar] [CrossRef]
- Balko, J.A.; Posner, L.P.; Chinnadurai, S.K. Immersion in tricaine methanesulfonate (MS–222) is not sufficient for euthanasia of smokey jungle frogs (Leptodactylus pentadactylus). J. Zoo Wildl. Med. 2019, 50, 89–95. [Google Scholar] [PubMed]
- Navarro, K.; Jampachaisri, K.; Chu, D.; Pacharinsak, C. Bupivacaine as a euthanasia agent for African Clawed Frogs (Xenopus laevis). PLoS ONE 2022, 17, e0279331. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Grajal-Puche, A.; Roh, N.-H.; Park, I.-K.; Ra, N.-Y.; Park, D. First detection of ranavirus in a wild population of Dybowski’s brown frog (Rana dybowskii) in South Korea. J. Ecol. Environ. 2021, 45, 2. [Google Scholar] [CrossRef]
- Boyle, D.G.; Boyle, D.; Olsen, V.; Morgan, J.; Hyatt, A. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real–time Taqman PCR assay. Dis. Aquat. Org. 2004, 60, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, J.P.; Forstner, M.R.; O’Donnell, L.; Hahn, D. Detection of Batrachochytrium dendrobatidis in endemic salamander species from Central Texas. EcoHealth 2009, 6, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Annis, S.L.; Dastoor, F.P.; Ziel, H.; Daszak, P.; Longcore, J.E. A DNA–based assay identifies Batrachochytrium dendrobatidis in amphibians. J. Wildl. Dis. 2004, 40, 420–428. [Google Scholar] [CrossRef] [PubMed]
- WOAH—World Organisation for Animal Health. Manual of Diagnostic Tests for Aquatic Animals—Chapter 2. 1. 1. Infection with Batrachochytrium dendrobatidis. 2019. Available online: https://www.woah.org/en/disease/chytridiomycosis-batrachochytrium-dendrobatidis/ (accessed on 27 November 2024).
- Stilwell, N.K.; Whittington, R.J.; Hick, P.M.; Becker, J.A.; Ariel, E.; Van Beurden, S.; Vendramin, N.; Olesen, N.J.; Waltzek, T.B. Partial validation of a TaqMan real–time quantitative PCR for the detection of ranaviruses. Dis. Aquat. Org. 2018, 128, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.G.; Barkman, T.J.; Chinchar, V.G.; Essani, K. Comparative genomic analyses of frog virus 3, type species of the genus Ranavirus (family Iridoviridae). Virology 2004, 323, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Karwacki, E.E.; Atkinson, M.S.; Ossiboff, R.J.; Savage, A.E. Novel quantitative PCR assay specific for the emerging Perkinsea amphibian pathogen reveals seasonal infection dynamics. Dis. Aquat. Org. 2018, 129, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Goka, K.; Yokoyama, J.; Une, Y.; Kuroki, T.; Suzuki, K.; Nakahara, M.; Kobayashi, A.; Inaba, S.; Mizutani, T.; Hyatt, A.D. Amphibian chytridiomycosis in Japan: Distribution, haplotypes and possible route of entry into Japan. Mol. Ecol. 2009, 18, 4757–4774. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- WOAH—World Organisation for Animal Health. Manual of Diagnostic Tests for Aquatic Animals—Chapter 2. 1. 3. Infection with Ranavirus. 2021. Available online: https://www.woah.org/en/document/ranaviruses-infection-with/ (accessed on 27 November 2024).
- Lee, J.-E.; Park, J.-K.; Do, Y. Gut microbiome diversity and function during hibernation and spring emergence in an aquatic frog. PLoS ONE 2024, 19, e0298245. [Google Scholar] [CrossRef] [PubMed]
- PARK, J.K.; Do, Y. The difference and variation of gut bacterial community and host physiology can support adaptation during and after overwintering in frog population. Integr. Zool. 2024, 19, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004, 26, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Kelehear, C.; Brown, G.P.; Shine, R. Size and sex matter: Infection dynamics of an invading parasite (the pentastome Raillietiella frenatus) in an invading host (the cane toad Rhinella marina). Parasitology 2012, 139, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Titon, S.C.M.; de Assis, V.R.; Junior, B.T.; Barsotti, A.M.G.; Flanagan, S.P.; Gomes, F.R. Calling rate, corticosterone plasma levels and immunocompetence of Hypsiboas albopunctatus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 201, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Halfwerk, W.; Blaas, M.; Kramer, L.; Hijner, N.; Trillo, P.A.; Bernal, X.E.; Page, R.A.; Goutte, S.; Ryan, M.J.; Ellers, J. Adaptive changes in sexual signalling in response to urbanization. Nat. Ecol. Evol. 2019, 3, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Toledo, L.F.; Ruggeri, J.; Leite Ferraz de Campos, L.; Martins, M.; Neckel-Oliveira, S.; Breviglieri, C.P.B. Midges not only sucks, but may carry lethal pathogens to wild amphibians. Biotropica 2021, 53, 722–725. [Google Scholar] [CrossRef]
- Reinhold, J.M.; Halbert, E.; Roark, M.; Smith, S.N.; Stroh, K.M.; Siler, C.D.; McLeod, D.S.; Lahondère, C. The role of Culex territans mosquitoes in the transmission of Batrachochytrium dendrobatidis to amphibian hosts. Parasites Vectors 2023, 16, 424. [Google Scholar] [CrossRef] [PubMed]
- Gittins, S. The breeding migration of the Common toad (Bufo bufo) to a pond in mid-Wales. J. Zool. 1983, 199, 555–562. [Google Scholar] [CrossRef]
- Cullen, B.; Owens, L. Experimental challenge and clinical cases of Bohle iridovirus (BIV) in native Australian anurans. Dis. Aquat. Org. 2002, 49, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Fellers, G.M.; Cole, R.A.; Reinitz, D.M.; Kleeman, P.M. Amphibian chytrid fungus (Batrachochytrium dendrobatidis) in coastal and montane California, USA anurans. Herpetol. Conserv. Biol. 2011, 6, 383–394. [Google Scholar]
- Gründler, M.C.; Toledo, L.F.; Parra-Olea, G.; Haddad, C.F.; Giasson, L.O.; Sawaya, R.J.; Prado, C.P.; Araujo, O.G.; Zara, F.J.; Centeno, F.C. Interaction between breeding habitat and elevation affects prevalence but not infection intensity of Batrachochytrium dendrobatidis in Brazilian anuran assemblages. Dis. Aquat. Org. 2012, 97, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Ariel, E.; Nicolajsen, N.; Christophersen, M.-B.; Holopainen, R.; Tapiovaara, H.; Jensen, B.B. Propagation and isolation of ranaviruses in cell culture. Aquaculture 2009, 294, 159–164. [Google Scholar] [CrossRef]
- Roh, N.-H.; Kim, J.; Park, J.; Park, D. High ranavirus infection rates at low and extreme temperatures in the tadpoles of Japanese treefrogs (Dryophytes japonicus) that breed in rice paddies in the summer. J. Ecol. Environ. 2023, 47, 04. [Google Scholar] [CrossRef]
- Zhou, L.; Gratwicke, B.; Wang, J.; Guo, Z.; Zhang, M.; Wang, C.; Hou, M.; Xu, T.; Wu, H.; Jin, T.; et al. Global and endemic Asian lineages of the emerging pathogenic fungus Batrachochytrium dendrobatidis widely infect amphibians in China. Sci. Rep. 2023, 13, 12018. [Google Scholar]
- Herath, J.; Xu, T.; Zhou, L.; Jin, T.; Hou, M.; Zhang, M.; Guo, Z.; Wu, H.; Gao, J. Emerging threat of ranavirus: Prevalence, genetic diversity, and climatic drivers of Ranavirus (Iridoviridae) in ectothermic vertebrates of Asia. Front. Vet. Sci. 2023, 10, 1291872. [Google Scholar] [CrossRef] [PubMed]
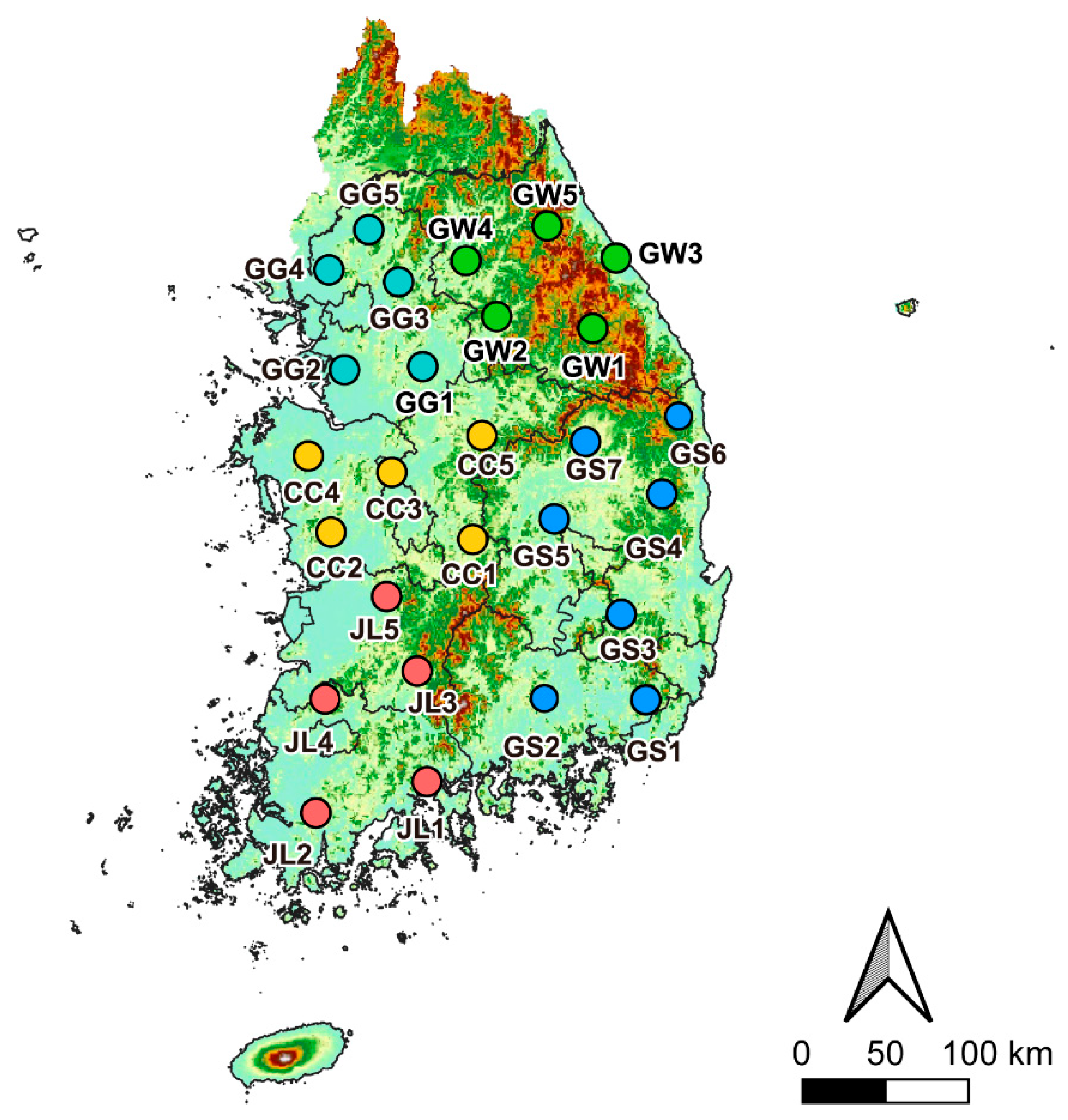

| Sites | Sampling Date | Sampling Temperature/Humidity | Number of Samples | |||
|---|---|---|---|---|---|---|
| Bombinatoridae | Hylidae | Ranidae | Ranidae | |||
| Bombina orientalis | Dryophytes japonicus | Glandirana rugosa | Pelophylax nigromaculatus | |||
| GG1 | May 28 | 18.3 °C/56% | - | 16 | - | 10 |
| GG2 | May 28 | 18.6 °C/62% | - | 21 | 8 | 9 |
| GG3 | May 23 | 20.9 °C/63% | - | 14 | - | 13 |
| GG4 | May 23 | 19.2 °C/78% | - | 15 | - | 10 |
| GG5 | May 23 | 18.8 °C/66% | - | 15 | - | 15 |
| GW1 | May 28 | 11.8 °C/77% | 9 | 14 | - | 9 |
| GW2 | June 03 | 20.6 °C/45% | - | 21 | - | 7 |
| GW3 | June 03 | 15.3 °C/88% | - | 18 | - | 3 |
| GW4 | June 03 | 20.6 °C/32% | - | 15 | - | 13 |
| GW5 | June 03 | 17.3 °C/57% | 15 | 15 | - | 9 |
| CC1 | June 06 | 22.4 °C/57% | - | 20 | - | - |
| CC2 | June 07 | 21.8 °C/57% | - | 14 | - | 12 |
| CC3 | June 07 | 22.5 °C/66% | - | 12 | 6 | 12 |
| CC4 | June 06 | 16.9 °C/61% | - | 17 | - | 6 |
| CC5 | June 06 | 19.1 °C/59% | - | 18 | - | 7 |
| JL1 | May 31 | 22.6 °C/73% | - | 19 | - | 7 |
| JL2 | May 31 | 19.5 °C/71% | - | 15 | 8 | 6 |
| JL3 | May 31 | 20.4 °C/53% | - | 15 | 6 | 7 |
| JL4 | May 31 | 18.6 °C/70% | - | 13 | 7 | 11 |
| JL5 | May 31 | 18.2 °C/69% | - | 18 | - | 11 |
| GS1 | May 31 | 21.5 °C/67% | - | 15 | 3 | 9 |
| GS2 | May 28 | 18.8 °C/64% | - | 20 | - | 2 |
| GS3 | May 28 | 17.6 °C/69% | - | 16 | - | 13 |
| GS4 | May 28 | 17.1 °C/65% | 11 | 14 | - | - |
| GS5 | May 23 | 19.6 °C/54% | 4 | 15 | - | 9 |
| GS6 | May 23 | 23.4 °C/45% | 8 | 12 | - | 8 |
| GS7 | May 23 | 22.3 °C/52% | - | 19 | - | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-E.; Park, Y.J.; Kwon, M.-G.; Oh, Y.-K.; Kim, M.S.; Do, Y. Identification of Simultaneous Occurrence of Amphibian Chytrid Fungi and Ranavirus in South Korea. Animals 2025, 15, 2132. https://doi.org/10.3390/ani15142132
Lee J-E, Park YJ, Kwon M-G, Oh Y-K, Kim MS, Do Y. Identification of Simultaneous Occurrence of Amphibian Chytrid Fungi and Ranavirus in South Korea. Animals. 2025; 15(14):2132. https://doi.org/10.3390/ani15142132
Chicago/Turabian StyleLee, Ji-Eun, Young Jin Park, Mun-Gyeong Kwon, Yun-Kyeong Oh, Min Sun Kim, and Yuno Do. 2025. "Identification of Simultaneous Occurrence of Amphibian Chytrid Fungi and Ranavirus in South Korea" Animals 15, no. 14: 2132. https://doi.org/10.3390/ani15142132
APA StyleLee, J.-E., Park, Y. J., Kwon, M.-G., Oh, Y.-K., Kim, M. S., & Do, Y. (2025). Identification of Simultaneous Occurrence of Amphibian Chytrid Fungi and Ranavirus in South Korea. Animals, 15(14), 2132. https://doi.org/10.3390/ani15142132





