Long-Term Heat Stress and Genetic Responses in Growth Traits of Thai Native Synthetic Chicken Lines
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management
2.2. Data Collection
2.3. Genetic Analysis
3. Results
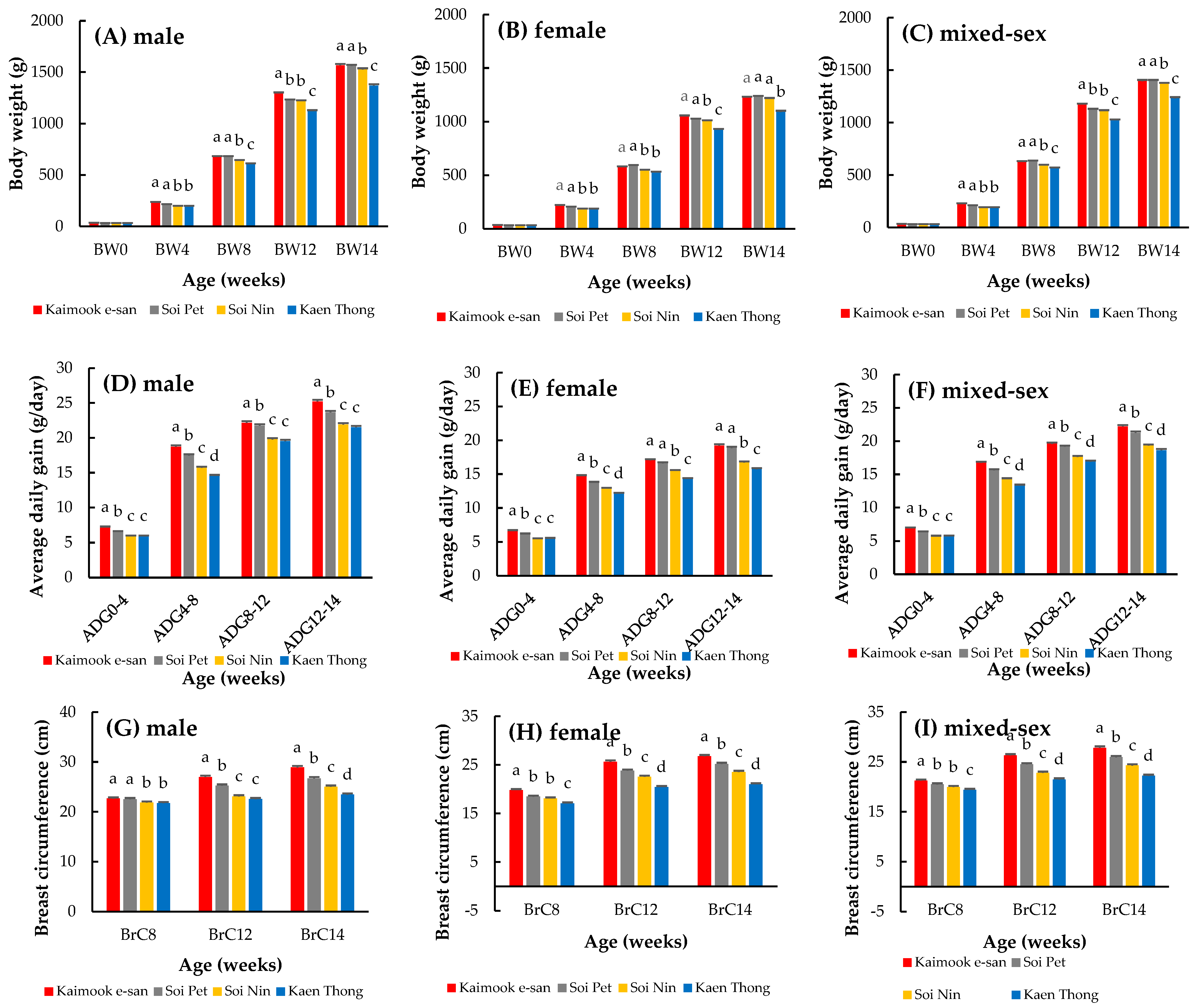
3.1. Comparison of Growth Traits
3.2. Heritability Estimates
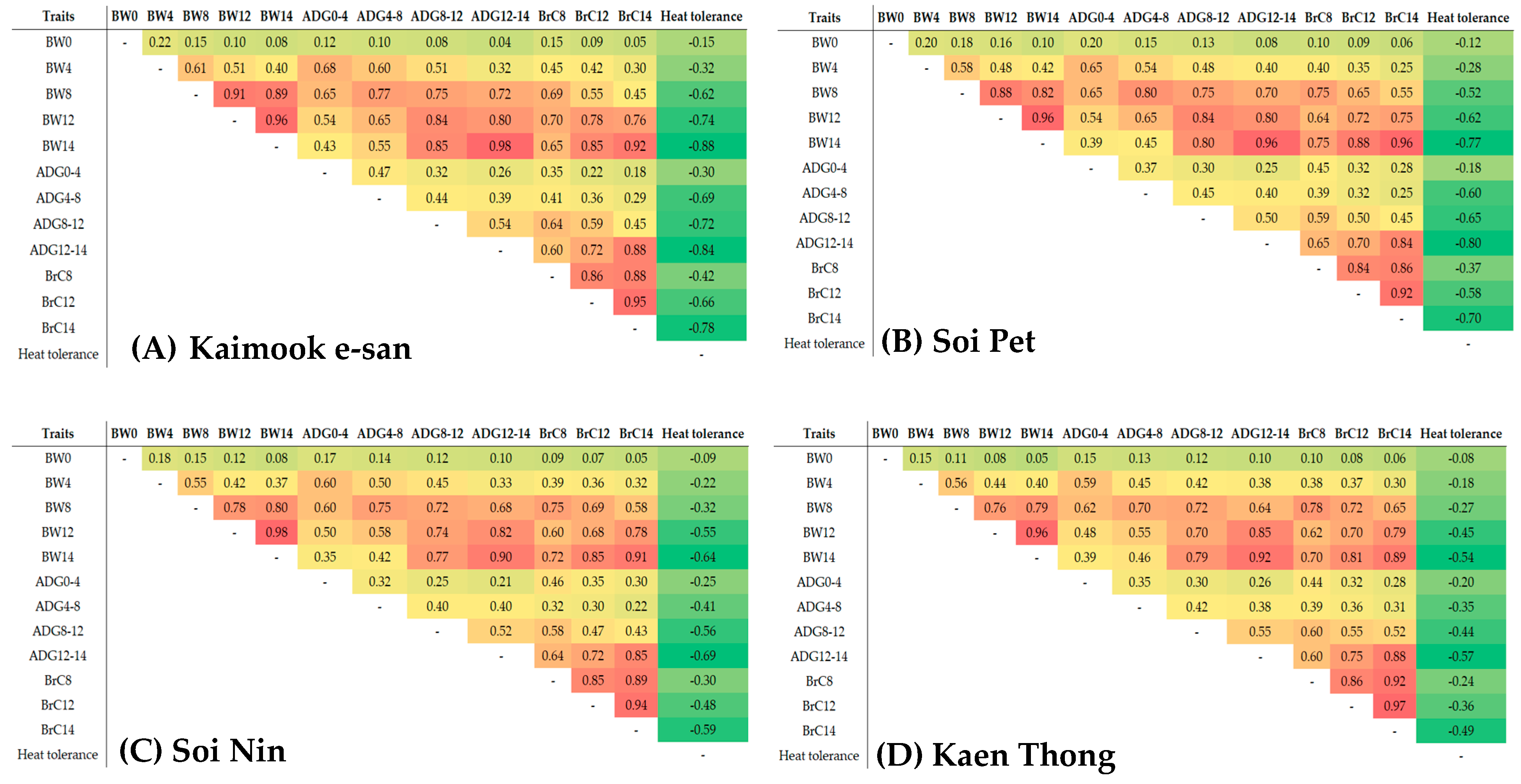
3.3. Genetic Correlation Estimates
3.4. Genetic Progress Estimates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boonkum, W.; Chankitisakul, V.; Kananit, S.; Kenchaiwong, W. Heat stress effects on the genetics of growth traits in Thai native chickens (Pradu Hang Dum). Anim. Biosci. 2024, 37, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Juiputta, J.; Chankitisakul, V.; Boonkum, W. Appropriate genetic approaches for heat tolerance and maintaining good productivity in tropical poultry production: A review. Vet. Sci. 2023, 10, 591. [Google Scholar] [CrossRef] [PubMed]
- Mangan, M.; Siwek, M. Strategies to combat heat stress in poultry production—A review. J. Anim. Physiol. Anim. Nutr. 2024, 108, 576–595. [Google Scholar] [CrossRef] [PubMed]
- Mussa, N.J.; Ratchamak, R.; Ratsiri, T.; Vongpralub, T.; Boonkum, W.; Semaming, Y.; Chankitisakul, V. Lipid profile of sperm cells in Thai native and commercial roosters and its impact on cryopreserved semen quality. Trop. Anim. Health Prod. 2021, 53, 321. [Google Scholar] [CrossRef] [PubMed]
- Chankitisakul, V.; Boonkum, W.; Kaewkanha, T.; Pimprasert, M.; Ratchamak, R.; Authaida, S.; Thananurak, P. Fertilizing ability and survivability of rooster sperm diluted with a novel semen extender supplemented with serine for practical use on smallholder farms. Poult. Sci. 2022, 1, 102188. [Google Scholar] [CrossRef] [PubMed]
- Pimprasert, M.; Kheawkanha, T.; Boonkum, W.; Chankitisakul, V. Influence of semen collection frequency and seasonal variations on fresh and frozen semen quality in Thai native roosters. Animals 2023, 13, 573. [Google Scholar] [CrossRef] [PubMed]
- Fatai, R.B.; Akinyemi, M.O.; Osaiyuwu, O.H.; Ewuola, K.M.; Salako, A.E. Haematological responses of Nigerian locally adapted chickens with different heat shock protein 70 genotypes to acute heat stress. In Proceedings of the 49th Annual Conference of the Nigerian Society for Animal Production, Ibadan, Nigeria, 24–27 March 2024; University of Ibadan: Ibadan, Nigeria, 2024. [Google Scholar]
- Nawaz, A.H.; Lin, S.; Wang, F.; Zheng, J.; Sun, J.; Zhang, W.; Jiao, Z.; Zhu, Z.; An, L.; Zhang, L. Investigating the heat tolerance and production performance in local chicken breed having normal and dwarf size. Animal 2023, 17, 100707. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, R.D.; Oliveira, C.H.; Calderano, A.A.; Ferreira, R.S.; Dias, K.M.M.; Almeida, B.F.; Aleixo, P.E.; Albino, L.F.T. Effect of phytase and protease combination on performance, metabolizable energy, and amino acid digestibility of broilers fed nutrient-restricted diets. Rev. Bras. Zootec. 2022, 51, e20210211. [Google Scholar] [CrossRef]
- Strifler, P.; Horváth, B.; Such, N.; Farkas, V.; Wágner, L.; Dublecz, K.; Pál, L. Effects of feeding low protein diets with different energy-to-protein ratios on performance, carcass characteristics, and nitrogen excretion of broilers. Animals 2023, 13, 1476. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdullatif, A.; Azzam, M.M. Effects of hot arid environments on the production performance, carcass traits, and fatty acids composition of breast meat in broiler chickens. Life 2023, 13, 1239. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-C.; Pan, Z.-Y.; Zhao, Y.; Guo, Y.; Qiu, S.-J.; Balasubramanian, B.; Jha, R. Effects of heat stress on production performance, redox status, intestinal morphology and barrier-related gene expression, cecal microbiome, and metabolome in indigenous broiler chickens. Front. Physiol. 2022, 13, 890520. [Google Scholar] [CrossRef] [PubMed]
- Kpomasse, C.C.; Kouame, Y.A.E.; N’nanle, O.; Houndonougbo, F.M.; Tona, K.; Oke, O.E. The productivity and resilience of the indigenous chickens in the tropical environments: Improvement and future perspectives. J. Appl. Anim. Res. 2023, 51, 456–469. [Google Scholar] [CrossRef]
- Xu, N.-Y.; Liu, Z.-Y.; Yang, Q.-M.; Bian, P.-P.; Li, M.; Zhao, X. Genomic analyses for selective signatures and genes involved in hot adaptation among indigenous chickens from different tropical climate regions. Front. Genet. 2022, 13, 906447. [Google Scholar] [CrossRef] [PubMed]
- Krishanender, D.; Sankhyan, V.; Thakur, Y.P.; Rajesh, K.; Gurdeep, S.S. Estimation of phenotypic trend in performance traits of native chicken germplasm of Himachal Pradesh. J. Anim. Res. 2018, 8, 1071–1074. [Google Scholar] [CrossRef]
- Sheng, Z.; Pettersson, M.E.; Hu, X.; Luo, C.; Qu, H.; Shu, D.; Shen, X.; Carlborg, O.; Li, N. Genetic dissection of growth traits in a Chinese indigenous × commercial broiler chicken cross. BMC Genom. 2013, 14, 151. [Google Scholar] [CrossRef] [PubMed]
- Promwatee, N.; Laopaiboon, B.; Vongpralub, T.; Phasuk, Y.; Kunhareang, S.; Boonkum, W.; Duangjinda, M. Insulin-like growth factor I gene polymorphism associated with growth and carcass traits in Thai synthetic chickens. Genet. Mol. Res. 2013, 12, 4332–4341. [Google Scholar] [CrossRef] [PubMed]
- Chomchuen, K.; Tuntiyasawasdikul, V.; Chankitisakul, V.; Boonkum, W. Genetic evaluation of body weights and egg production traits using a multi-trait animal model and selection index in Thai native synthetic chickens (Kaimook e-san2). Animals 2022, 12, 335. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.H.; Choi, E.S.; Kim, K.G.; Park, B.; Choo, H.J.; Heo, J.M.; Oh, K.S. Development of a new synthetic Korean native chicken breed using the diallel cross-mating test. Korean J. Poult. Sci. 2021, 48, 69–80. [Google Scholar] [CrossRef]
- Vikram, S.; Jain, L.S. Genetic analysis of growth rate in a synthetic broiler strain. Indian J. Anim. Res. 2008, 42, 128–130. [Google Scholar]
- Nawaz, A.H.; Amoah, K.; Leng, Q.Y.; Zheng, J.H.; Zhang, W.L.; Zhang, L. Poultry response to heat stress: Its physiological, metabolic, and genetic implications on meat production and quality including strategies to improve broiler production in a warming world. Front. Vet. Sci. 2021, 8, 699081. [Google Scholar] [CrossRef] [PubMed]
- Qaid, M.M.; Al-Garadi, M.A. Protein and amino acid metabolism in poultry during and after heat stress: A review. Animals 2021, 11, 1167. [Google Scholar] [CrossRef] [PubMed]
- Zampiga, M.; Laghi, L.; Zhu, C.; Mancinelli, A.C.; Mattioli, S.; Sirri, F. Breast muscle and plasma metabolomics profile of broiler chickens exposed to chronic heat stress conditions. Animal 2021, 15, 100275. [Google Scholar] [CrossRef] [PubMed]
- Brugaletta, G.; Teyssier, J.-R.; Rochell, S.J.; Dridi, S.; Sirri, F. A review of heat stress in chickens. Part I: Insights into physiology and gut health. Front. Physiol. 2022, 13, 934381. [Google Scholar] [CrossRef] [PubMed]
- Teyssier, J.-R.; Brugaletta, G.; Sirri, F.; Dridi, S.; Rochell, S.J. A review of heat stress in chickens. Part II: Insights into protein and energy utilization and feeding. Front. Physiol. 2022, 13, 943612. [Google Scholar] [CrossRef] [PubMed]
- Lara, L.J.; Rostagno, M.H. Impact of heat stress on poultry production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef] [PubMed]
- National Oceanic and Atmospheric Administration. Livestock Hot Weather Stress; US Government Printing Office: Washington, DC, USA, 1976.
- Boonkum, W.; Duangjinda, M.; Kananit, S.; Chankitisakul, V.; Kenchaiwong, W. Genetic effect and growth curve parameter estimation under heat stress in slow-growing Thai native chickens. Vet. Sci. 2021, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Misztal, I.; Tsuruta, S.; Lourenco, D.; Masuda, Y.; Aguilar, I.; Legarra, A.; Vitezica, Z. Manual for BLUPF90 Family of Programs. Available online: https://nce.ads.uga.edu/html/projects/programs/docs/blupf90_all8.pdf (accessed on 24 April 2024).
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Longman Group Ltd.: Harlow, UK, 1996. [Google Scholar]
- Hemanth, M.; Venugopal, S.; Devaraj, C.; Shashank, C.G.; Ponnuvel, P.; Mandal, P.K.; Sejian, V. Comparative assessment of growth performance, heat resistance and carcass traits in four poultry genotypes reared in hot-humid tropical environment. J. Anim. Physiol. Anim. Nutr. 2024, 108, 1510–1523. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.O.; Nassar, F.S.; Al Ali, A.M. Challenges of ensuring sustainable poultry meat production and economic resilience under climate change for achieving sustainable food security. Res. World Agric. Econ. 2025, 6, 159–171. [Google Scholar] [CrossRef]
- Knížetová, H.; Hyanek, J.; Hájková, H.; Kníže, B.; ŠIler, R. Growth curves of chickens with different type of performance. J. Anim. Breed. Genet. 1985, 102, 256–270. [Google Scholar] [CrossRef]
- Zhong, C.; Li, X.; Guan, D.; Zhang, B.; Wang, X.; Qu, L.; Zhou, H.; Fang, L.; Sun, C.; Yang, N. Age-dependent genetic architectures of chicken body weight explored by multidimensional GWAS and molQTL analyses. J. Genet. Genom. 2024, 51, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.L.N.; Chatterjee, R.N.; Rajkumar, U.; Niranjan, M.; Rajaravindra, S.K.; Bhattacharya, T.K. Genetic evaluation of short-term selection in synthetic coloured broiler male and female lines-direct and correlated responses. Indian J. Anim. Sci. 2013, 83, 285–289. [Google Scholar]
- Fatai, R.B.; Akinyemi, M.O.; Osaiyuwu, O.H.; Ewuola, K.M.; Bello, S.F.; Salako, A.E. Weight-age relationship in Yoruba and Funaab-alpha chickens using non-linear regression models. Niger. J. Anim. Prod. 2023, 50, 88–95. [Google Scholar]
- Siegel, P.B.; Honaker, C.F. Sexual dimorphism for juvenile body weight in chickens divergently selected for 8-week body weight. Front. Physiol. 2025, 15, 1534334. [Google Scholar] [CrossRef] [PubMed]
- Decuypere, E.; Onagbesan, O.; Swennen, Q.; Buyse, J.; Bruggeman, V. The endocrine and metabolic interface of genotype-nutrition interactions in broilers and broiler breeders. Worlds Poult. Sci. J. 2007, 63, 115–128. [Google Scholar] [CrossRef]
- He, S.; Yin, Q.; Xiong, Y.; Li, J.; Liu, D. Characterization of heat stress affecting the growth performance, blood biochemical profile, and redox status in male and female broilers at market age. Trop. Anim. Health Prod. 2020, 52, 3833–3841. [Google Scholar] [CrossRef] [PubMed]
- Onagbesan, O.M.; Uyanga, V.A.; Oso, O.; Tona, K.; Oke, O.E. Alleviating heat stress effects in poultry: Updates on methods and mechanisms of actions. Front. Vet. Sci. 2023, 10, 1255520. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, K.; Kumar, H.; Park, W.; Byun, M.; Lim, D.; Kemp, S.; Te Pas, M.F.W.; Kim, J.-M.; Park, J.-E. Cardiac and skeletal muscle transcriptome response to heat stress in kenyan chicken ecotypes adapted to low and high altitudes reveal differences in thermal tolerance and stress response. Front. Genet. 2019, 10, 993. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Hsieh, J.C.F.; Schmidt, C.J.; Zhu, Q.; Lamont, S.J. Liver transcriptome response to hyperthermic stress in three distinct chicken lines. BMC Genom. 2016, 17, 955. [Google Scholar] [CrossRef] [PubMed]
- Duangjinda, M.; Tunim, S.; Duangdaen, C.; Boonkum, W. Hsp70 genotypes and heat tolerance of commercial and native chickens reared in hot and humid conditions. Braz. J. Poult. Sci 2017, 19, 7–18. [Google Scholar] [CrossRef]
- Hako, T.B.A.; Yoniwo, S.N. The “Naked neck” gene and the adaptability of the native chicken to heat stress on station in Cameroon. Bio-Research 2023, 21, 1881–1895. [Google Scholar] [CrossRef]
- Barreto Sánchez, A.L.; Wang, Q.; Thiam, M.; Wang, Z.; Zhang, J.; Zhang, Q.; Zhang, N.; Li, Q.; Wen, J.; Zhao, G. Liver transcriptome response to heat stress in Beijing you chickens and Guang ming broilers. Genes 2022, 13, 416. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.A.; Iraqi, M.M.; Khalil, M.H.; El-Gendi, G.M.; EL Nagar, A.G. Genetic evaluation of additive and heterotic effects for growth traits in crossing four Egyptian strains of chickens. Egypt. J. Anim. Prod. 2022, 59, 57–64. [Google Scholar] [CrossRef]
- Johnsson, M.; Henriksen, R.; Höglund, A.; Fogelholm, J.; Jensen, P.; Wright, D. Genetical genomics of growth in a chicken model. BMC Genom. 2018, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Manjula, P.; Park, H.B.; Seo, D.; Choi, N.; Jin, S.; Ahn, S.J.; Heo, K.N.; Kang, B.S.; Lee, J.H. Estimation of heritability and genetic correlation of body weight gain and growth curve parameters in Korean native chicken. Asian-Australas. J. Anim. Sci. 2018, 31, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Peng, D.; Gu, X.; Gong, Y.; Sheng, Z.; Hu, X. Polygenic basis and variable genetic architectures contribute to the complex nature of body weight —A genome-wide study in four Chinese indigenous chicken breeds. Front. Genet. 2018, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Sinpru, P.; Bunnom, R.; Poompramun, C.; Kaewsatuan, P.; Sornsan, S.; Kubota, S.; Molee, W.; Molee, A. Association of growth hormone and insulin-like growth factor I genotype with body weight, dominance of body weight, and mRNA expression in Korat slow-growing chickens. Anim. Biosci. 2021, 34, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-R.; Lin, C.-Y.; Cheng, Y.-S.; Lin, D.-Y.; Huang, T.-P.; Hung, K.-H.; Liang, H.-M. Genetic parameters for body weight and egg production traits in Taiwan native chicken homozygous for the heat shock protein 70 gene. Asian J. Agric. Biol. 2018, 6, 396–402. [Google Scholar]
- Sesay, A.R. Impact of heat stress on chicken performance, welfare, and probable mitigation strategies. Int. J. Environ. Clim. 2022, 12, 3120–3133. [Google Scholar] [CrossRef]
- Bhawa, S.; Morêki, J.C.; Machete, J.B. Poultry management strategies to alleviate heat stress in hot climates: A review. J. Worlds Poult. Res. 2023, 13, 1–19. [Google Scholar] [CrossRef]
- Schmidt, C.J.; Kim, D.K.; Pendarvis, G.K.; Abasht, B.; McCarthy, F.M. Proteomic insight into human directed selection of the domesticated chicken Gallus gallus. PLoS ONE 2023, 18, e0289648. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Liang, Q.; Liu, C.; Li, S. Genomic analyses reveal adaptation to hot arid and harsh environments in native chickens of China. Front. Genet. 2020, 11, 582355. [Google Scholar] [CrossRef] [PubMed]
- Gheyas, A.; Vallejo-Trujillo, A.; Kebede, A.; Dessie, T.; Hanotte, O.; Smith, J. Whole genome sequences of 234 indigenous African chickens from Ethiopia. Sci. Data 2022, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.L.; Nestor, K.E.; Velleman, S.G. Continual selection for increased 16 wk body weight on turkey growth and meat quality: 50 generation update. J. Appl. Poult. Res. 2019, 28, 658–668. [Google Scholar] [CrossRef]
- Lillie, M.; Honaker, C.F.; Siegel, P.B.; Carlborg, Ö. Bidirectional selection for body weight on standing genetic variation in a chicken model. G3 Genes Genomes Genet. 2019, 9, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, L.A.; Porter, T.E.; Ellestad, L.E. The effect of commercial genetic selection on somatotropic gene expression in broilers: A potential role for insulin-like growth factor binding proteins in regulating broiler growth and body composition. Front. Physiol. 2022, 13, 935311. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.; Raymundo, A.; Martins, L.L.; Lordelo, M.; de Almeida, A.M. The naked neck gene in the domestic chicken: A genetic strategy to mitigate the impact of heat stress in poultry production—A review. Animals 2023, 13, 1007. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.L.J.; Zaytsoff, S.J.M.; Montina, T.; Inglis, G.D. Corticosterone-mediated physiological stress alters liver, kidney, and breast muscle metabolomic profiles in chickens. Animals 2021, 11, 3056. [Google Scholar] [CrossRef] [PubMed]
- Mignon-Grasteau, S.; Beaumont, C.; Ricard, F.H. Genetic analysis of a selection experiment on the growth curve of chickens. Poult. Sci. 2000, 80, 849–854. [Google Scholar] [CrossRef] [PubMed]

| Synthetic Chicken Lines | Kaimook e-san | Soi Pet | ||||
| Variances/Heritability | Va | Ve | h2 (SE) | Va | Ve | h2 (SE) |
| Traits | ||||||
| BW0 | 8.77 | 10.89 | 0.45 (0.05) | 7.22 | 11.02 | 0.40 (0.05) |
| BW4 | 1812 | 2835 | 0.39 (0.05) | 1715 | 2763 | 0.38 (0.05) |
| BW8 | 3782 | 7687 | 0.33 (0.05) | 3544 | 7511 | 0.32 (0.04) |
| BW12 | 12,602 | 28,364 | 0.31 (0.04) | 11,999 | 27,421 | 0.30 (0.04) |
| BW14 | 17,277 | 38,752 | 0.31 (0.04) | 15,600 | 35,552 | 0.30 (0.04) |
| ADG0–4 | 1.02 | 1.98 | 0.34 (0.03) | 0.89 | 2.00 | 0.31 (0.04) |
| ADG4–8 | 2.18 | 4.59 | 0.32 (0.03) | 1.77 | 4.22 | 0.30 (0.04) |
| ADG8–12 | 2.85 | 6.09 | 0.32 (0.03) | 2.55 | 6.52 | 0.28 (0.04) |
| ADG12–14 | 3.30 | 7.22 | 0.31 (0.03) | 3.01 | 7.99 | 0.27 (0.03) |
| BrC8 | 0.60 | 1.22 | 0.33 (0.03) | 0.54 | 1.32 | 0.29 (0.03) |
| BrC12 | 0.85 | 1.90 | 0.31 (0.03) | 0.75 | 2.01 | 0.27 (0.03) |
| BrC14 | 1.02 | 2.66 | 0.28 (0.03) | 0.84 | 2.45 | 0.26 (0.03) |
| Synthetic chicken lines Variances/Heritability | Soi Nin | Kaen Thong | ||||
| Va | Ve | h2 (SE) | Va | Ve | h2 (SE) | |
| Traits | ||||||
| BW0 | 7.01 | 11.25 | 0.38 (0.05) | 6.87 | 12.01 | 0.36 (0.05) |
| BW4 | 1599 | 2944 | 0.35 (0.05) | 1423 | 3012 | 0.32 (0.04) |
| BW8 | 3457 | 7422 | 0.32 (0.04) | 3002 | 7315 | 0.29 (0.04) |
| BW12 | 11,524 | 26,782 | 0.30 (0.04) | 9625 | 25,676 | 0.27 (0.03) |
| BW14 | 14,100 | 34,256 | 0.29 (0.04) | 11,562 | 33,253 | 0.26 (0.03) |
| ADG0–4 | 0.86 | 1.99 | 0.30 (0.04) | 0.75 | 1.89 | 0.28 (0.03) |
| ADG4–8 | 1.68 | 4.22 | 0.28 (0.03) | 1.42 | 4.01 | 0.26 (0.03) |
| ADG8–12 | 2.35 | 6.58 | 0.26 (0.03) | 1.86 | 5.89 | 0.24 (0.03) |
| ADG12–14 | 2.88 | 8.56 | 0.25 (0.03) | 2.12 | 7.53 | 0.22 (0.03) |
| BrC8 | 0.50 | 1.45 | 0.26 (0.03) | 0.45 | 1.40 | 0.24 (0.03) |
| BrC12 | 0.65 | 1.85 | 0.26 (0.03) | 0.52 | 1.77 | 0.23 (0.03) |
| BrC14 | 0.79 | 2.35 | 0.25 (0.03) | 0.64 | 2.22 | 0.22 (0.03) |
| Chicken Lines | Kaimook e-san | Soi Pet | Soi Nin | Kaen Thong | ||||
|---|---|---|---|---|---|---|---|---|
| BW traits (g/gen) | BW | Heat tolerance | BW | Heat tolerance | BW | Heat tolerance | BW | Heat tolerance |
| BW0 | 0.15 | −0.02 | 0.13 | −0.01 | 0.10 | −0.01 | 0.05 | −0.00 |
| BW4 | 6.23 | −0.62 | 5.99 | −0.58 | 5.65 | −0.40 | 5.02 | −0.35 |
| BW8 | 9.10 | −0.91 | 8.44 | −0.78 | 8.01 | −0.66 | 7.45 | −0.57 |
| BW12 | 12.12 | −1.21 | 10.89 | −1.07 | 9.42 | −0.76 | 7.85 | −0.69 |
| BW14 | 14.92 | −1.49 | 13.03 | −1.26 | 11.18 | −0.96 | 8.10 | −0.85 |
| ADG traits (g/gen) | ADG | Heat tolerance | ADG | Heat tolerance | ADG | Heat tolerance | ADG | Heat tolerance |
| ADG0–4 | 0.54 | −0.06 | 0.38 | −0.04 | 0.32 | −0.03 | 0.25 | −0.02 |
| ADG4–8 | 1.38 | −0.17 | 1.21 | −0.12 | 1.14 | −0.09 | 1.01 | −0.07 |
| ADG8–12 | 2.04 | −0.24 | 1.88 | −0.19 | 1.65 | −0.17 | 1.42 | −0.13 |
| ADG12–14 | 2.51 | −0.30 | 2.29 | −0.26 | 2.00 | −0.22 | 1.94 | −0.17 |
| BrC traits (cm/gen) | BrC | Heat tolerance | BrC | Heat tolerance | BrC | Heat tolerance | BrC | Heat tolerance |
| BrC8 | 0.25 | −0.05 | 0.18 | −0.05 | 0.14 | −0.04 | 0.12 | −0.03 |
| BrC12 | 0.55 | −0.10 | 0.46 | −0.09 | 0.40 | −0.07 | 0.36 | −0.05 |
| BrC14 | 0.92 | −0.13 | 0.77 | −0.12 | 0.68 | −0.10 | 0.60 | −0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonkum, W.; Wiangnak, S.; Chankitisakul, V. Long-Term Heat Stress and Genetic Responses in Growth Traits of Thai Native Synthetic Chicken Lines. Animals 2025, 15, 2130. https://doi.org/10.3390/ani15142130
Boonkum W, Wiangnak S, Chankitisakul V. Long-Term Heat Stress and Genetic Responses in Growth Traits of Thai Native Synthetic Chicken Lines. Animals. 2025; 15(14):2130. https://doi.org/10.3390/ani15142130
Chicago/Turabian StyleBoonkum, Wuttigrai, Supawan Wiangnak, and Vibuntita Chankitisakul. 2025. "Long-Term Heat Stress and Genetic Responses in Growth Traits of Thai Native Synthetic Chicken Lines" Animals 15, no. 14: 2130. https://doi.org/10.3390/ani15142130
APA StyleBoonkum, W., Wiangnak, S., & Chankitisakul, V. (2025). Long-Term Heat Stress and Genetic Responses in Growth Traits of Thai Native Synthetic Chicken Lines. Animals, 15(14), 2130. https://doi.org/10.3390/ani15142130







