Feeding a Bitter Mix of Gentian and Grape Seed Extracts with Caffeine Reduces Appetite and Body Fat Deposition and Improves Meat Colour in Pigs
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Animals, Housing, and Diets
2.2. Growth Performance and Carcass Traits
2.3. Meat Quality Assessment
2.4. Proteomics
2.4.1. Sample Preparation
2.4.2. Mass Spectrometry
2.4.3. Protein Identification
2.5. Statistical Analysis
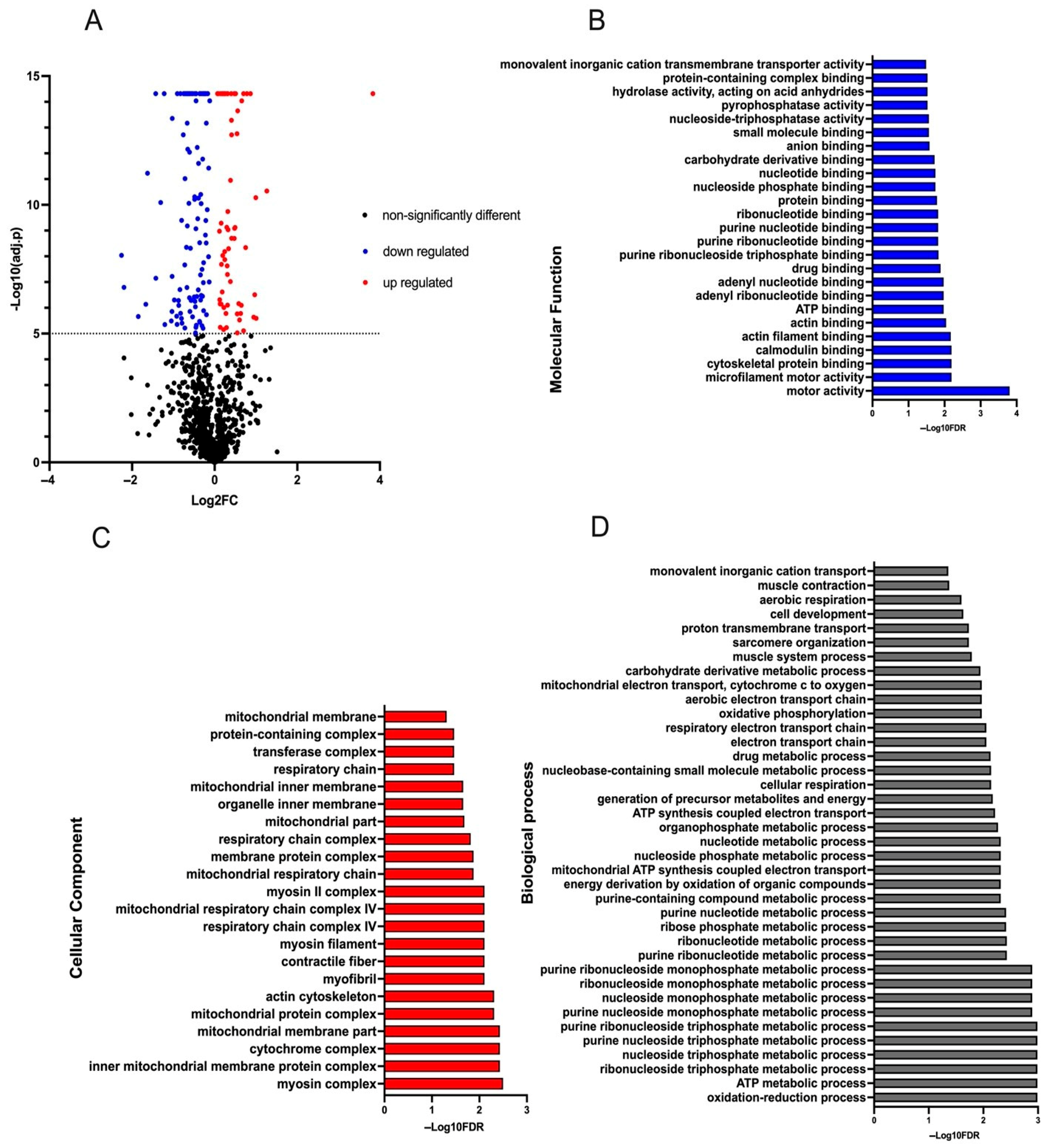
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| a* | Redness |
| ADFI | Average daily feed intake |
| ADG | Average daily gain |
| ANOVA | Analysis of Variance |
| ATP | Adenosine triphosphate |
| b* | Yellowness |
| BM | Bitter mix |
| DAP | Differentially abundant proteins |
| DIA-NN | data-independent acquisition neural network |
| FCR | Feed conversion ration |
| GG | Gentian and grape seed extract |
| GO | Gene Ontology |
| HSCW | Hot standard carcass weight |
| L* | Lightness |
| SWATH | Sequential Windowed Acquisition of all Theoretical fragment ions |
| ZenoTOF | Zeno time-of-flight |
References
- Ngapo, T.M.; Martin, J.F.; Dransfield, E. International preferences for pork appearance: II. Factors influencing consumer choice. Food Qual. Prefer. 2007, 18, 139–151. [Google Scholar] [CrossRef]
- Papanagiotou, P.; Tzimitra-Kalogianni, I.; Melfou, K. Consumers’ expected quality and intention to purchase high quality pork meat. Meat Sci. 2013, 93, 449–454. [Google Scholar] [CrossRef]
- Campbell, R.G.; Curic, D.M.; Taverner, M.R. Effects of sex and energy intake between 48 and 90 kg live weight on protein deposition in growing pigs. Anim. Sci. 1985, 40, 497–503. [Google Scholar] [CrossRef]
- Navarro, M.; Dunshea, F.; Lisle, A.; Roura, E. Feeding a oleic acid (C18:1) diet improves pleasing flavour attributes in pork. Food Chem. 2021, 357, 129770. [Google Scholar] [CrossRef] [PubMed]
- Boddicker, N.; Gabler, N.K.; Spurlock, M.E.; Nettleton, D.; Dekkers, J.C.M. Effects of ad libitum and restricted feeding on early production performance and body composition of Yorkshire pigs selected for reduced residual feed intake. Animal 2011, 5, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Dunshea, F.R.; Cox, M.L.; Borg, M.R.; Sillence, M.N.; Harris, D.R. Porcine somatotropin (pST) administered using a commercial delivery system improves growth performance of rapidly growing, group-housed finisher pigs. Aust. J. Agric. Res. 2002, 53, 287–293. [Google Scholar] [CrossRef]
- Apple, J.K.; Maxwell, C.V.; Sawyer, J.T.; Kutz, B.R.; Rakes, L.K.; Davis, M.E.; Johnson, Z.B.; Carr, S.N.; Armstrong, T.A. Interactive effect of ractopamine and dietary fat source on quality characteristics of fresh pork bellies. J. Anim. Sci. 2007, 85, 2682–2690. [Google Scholar] [CrossRef]
- Rikard-Bell, C.; Curtis, M.A.; van Barneveld, R.J.; Mullan, B.P.; Edwards, A.C.; Gannon, N.J.; Henman, D.J.; Hughes, P.E.; Dunshea, F.R. Ractopamine hydrochloride improves growth performance and carcass composition in immunocastrated boars, intact boars, and gilts. J. Anim. Sci. 2009, 87, 3536–3543. [Google Scholar] [CrossRef]
- Alemanno, A.; Capodieci, G. Testing the Limits of Global Food Governance: The Case of Ractopamine. Eur. J. Risk Regul. 2012, 3, 400–407. Available online: https://ssrn.com/abstract=2133908 (accessed on 27 March 2025). [CrossRef]
- Niño, A.M.M.; Granja, R.H.M.M.; Wanschel, A.C.B.A.; Salerno, A.G. The challenges of ractopamine use in meat production for export to European Union and Russia. Food Control 2017, 72, 289–292. [Google Scholar] [CrossRef]
- Marcoux, M.; Pomar, C.; Faucitano, L.; Brodeur, C. The relationship between different pork carcass lean yield definitions and the market carcass value. Meat Sci. 2007, 75, 94–102. [Google Scholar] [CrossRef]
- Bee, G.; Kragten, S.A.; Früh, B.; Girard, M. Impact of 100% organic diets on pig performance, carcass composition and carcass nutrient deposition efficiency. Org. Agric. 2021, 11, 421–433. [Google Scholar] [CrossRef]
- Liu, F.; Brewster, C.J.; Gilmour, S.L.; Henman, D.J.; Smits, R.J.; Luxford, B.G.; Dunshea, F.R.; Pluske, J.R.; Campbell, R.G. Relationship between energy intake and growth performance and body composition in pigs selected for low backfat thickness. J. Anim. Sci. 2021, 99, skab342. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.J.; Gupta, O.P.; Tariq, M.; Arora, R.B. Effect of caffeine and coffee on serum cholesterol, free fatty acids and triglycerides levels in pigs. Indian J. Med. Res. 1970, 58, 125–129. [Google Scholar] [PubMed]
- Cunningham, H.M. Effect of caffeine on nitrogen retention, carcass composition, fat mobilization and the oxidation of C14-labeled body fat in pigs. J. Anim. Sci. 1968, 27, 424–430. [Google Scholar] [CrossRef]
- Cunningham, H.M. Effect of caffeine on growth, feed efficiency and leanness of growing pigs and its interaction with calcium, zinc and corn oil. Can. J. Anim. Sci. 1971, 51, 95–102. [Google Scholar] [CrossRef]
- Rosenfeld, L.S.; Mihalov, J.J.; Carlson, S.J.; Mattia, A. Regulatory status of caffeine in the United States. Nutr. Rev. 2014, 72, 23–33. [Google Scholar] [CrossRef]
- Australia New Zealand Food Standards Code Standard 2.6.4 Formulated Caffeinated Beverages. Food Standards Australia New Zealand Act 1991. Available online: https://www.legislation.gov.au/F2015L00467/latest/text (accessed on 15 January 2025).
- Kyriazakis, I.; Emmans, G.C. The effect of protein source on the diets selected by pigs given a choice between a low and high protein food. Physiol. Behav. 1993, 53, 683–688. [Google Scholar] [CrossRef]
- Roura, E.; Humphrey, B.; Tedó, G.; Ipharraguerre, I. Unfolding the codes of short-term feed appetence in farm and companion animals. A comparative oronasal nutrient sensing biology review. Can. J. Anim. Sci. 2008, 88, 535–558. [Google Scholar] [CrossRef]
- Solà-Oriol, D.; Roura, E.; Torrallardona, D. Feed preference in pigs: Effect of selected protein, fat, and fiber sources at different inclusion rates. J. Anim. Sci. 2011, 89, 3219–3227. [Google Scholar] [CrossRef]
- Wang, J.; Fu, M.; Navarro, M.; Roura, E. A double-choice model to quantify negative preference to bitterness in pigs. Anim. Prod. Sci. 2017, 57, 2422. [Google Scholar] [CrossRef]
- Rojas, M.C.; Brewer, M.S. Effect of natural antioxidants on oxidative stability of cooked, refrigerated beef and pork. J. Food Sci. 2007, 72, S282–S288. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Pastorelli, G.; Cannata, S.; Tavaniello, S.; Maiorano, G.; Corino, C. Effect of long term dietary supplementation with plant extract on carcass characteristics meat quality and oxidative stability in pork. Meat Sci. 2013, 95, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Soladoye, O.P.; Juárez, M.L.; Aalhus, J.L.; Shand, P.; Estévez, M. Protein Oxidation in Processed Meat: Mechanisms and Potential Implications on Human Health. Compr. Rev. Food Sci. Food Saf. 2015, 14, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Kempster, A.J.; Chadwick, J.P.; Jones, D.W.; Cuthbertson, A. An evaluation of the Hennessy and Chong Fat Depth Indicator, and the Ulster Probe, for use in pig carcass classification and grading. Anim. Sci. 1981, 33, 319–324. [Google Scholar] [CrossRef]
- Akit, H.; Collins, C.L.; Fahri, F.T.; Hung, A.T.; D’Souza, D.N.; Leury, B.J.; Dunshea, F.R. Dietary lecithin improves dressing percentage and decreases chewiness in the longissimus muscle in finisher gilts. Meat Sci. 2014, 96, 1147–1151. [Google Scholar] [CrossRef]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Bouton, P.E.; Harris, P.V.; Shorthose, W.R. Effect of ultimate pH upon the water-holding capacity and tenderness of mutton. J. Food Sci. 1971, 36, 435–439. [Google Scholar] [CrossRef]
- Bouton, P.E.; Harris, P.V. A comparison of some objective methods used to assess meat tenderness. J. Food Sci. 1972, 37, 218–221. [Google Scholar] [CrossRef]
- Wiśniewski, J.R. Filter-Aided Sample Preparation for Proteome Analysis. In Microbial Proteomics: Methods and Protocols; Becher, D., Ed.; Springer: New York, NY, USA, 2018; pp. 3–10. [Google Scholar] [CrossRef]
- Gillet, L.C.; Navarro, P.; Tate, S.; Röst, H.; Selevsek, N.; Reiter, L.; Bonner, R.; Aebersold, R. Targeted Data Extraction of the MS/MS Spectra Generated by Data-independent Acquisition: A New Concept for Consistent and Accurate Proteome Analysis. Mol. Cell Proteom. 2012, 11, O111.016717. [Google Scholar] [CrossRef]
- Demichev, V.; Messner, C.B.; Vernardis, S.I.; Lilley, K.S.; Ralser, M. DIA-NN: Neural networks and interference correction enable deep proteome coverage in high throughput. Nat. Methods 2020, 17, 41–44. [Google Scholar] [CrossRef]
- Choi, M.; Chang, C.Y.; Clough, T.; Broudy, D.; Killeen, T.; MacLean, B.; Vitek, O. MSstats: An R package for statistical analysis of quantitative mass spectrometry-based proteomic experiments. Bioinformatics 2014, 30, 2524–2526. [Google Scholar] [CrossRef]
- Nelson, S.L.; Sanregret, J.D. Response of pigs to bitter-tasting compounds. Chem. Senses 1997, 22, 129–132. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roura, E.; Fu, M. Taste receptors and feed intake in pigs (130 years of research: Then, now and future). Anim. Feed Sci. Technol. 2017, 233, 3–12. [Google Scholar] [CrossRef]
- Orozco-Gregorio, H.; Mota-Rojas, D.; Bonilla-Jaime, H.; Trujillo-Ortega, M.E.; Becerril-Herrera, M.; Hernández-González, R.; Villanueva-García, D. Effects of administration of caffeine on metabolic variables in neonatal pigs with peripartum asphyxia. Am. J. Vet. Res. 2010, 71, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Nowland, T.L.; Kind, K.; Hebart, M.L.; van Wettere, W.H.E.J. Caffeine supplementation at birth, but not 8 to 12 h post-birth, increased 24 h pre-weaning mortality in piglets. Animal 2020, 14, 1529–1535. [Google Scholar] [CrossRef]
- Oksbjerg, N.; Sørensen, M.T. Separate and combined effects of ephedrine and caffeine on protein and lipid deposition in finishing pigs. Anim. Sci. 1995, 60, 299–305. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Barbosa, S.; Pinheiro, V.; Mourão, J.L.; Outor-Monteiro, D. The effect of olive leaves supplementation on the feed digestibility, growth performances of pigs and quality of pork meat. Meat Sci. 2009, 82, 438–443. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Ribeirinha, T.; Silva, A.; Gonçalves, R.; Pinheiro, V.; Mourão, J.L.; Outor-Monteiro, D. Effects of the dietary incorporation of olive leaves on growth performance, digestibility, blood parameters and meat quality of growing pigs. J. Sci. Food Agric. 2014, 94, 3023–3029. [Google Scholar] [CrossRef]
- Fu, M.; Collins, C.L.; Henman, D.J.; Roura, E. Some bitter compounds show potential for decreasing feed intake and fat deposition while others improve growth and feed conversion ratio in finishing pigs. Anim. Prod. Sci. 2015, 55, 1543. [Google Scholar] [CrossRef]
- Wei, S.; Zheng, Y.; Zhang, M.; Zheng, H.; Yan, P. Grape seed procyanidin extract inhibits adipogenesis and stimulates lipolysis of porcine adipocytes in vitro. J. Anim. Sci. 2018, 96, 2753–2762. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Lee, C.G.; Kim, J.; Yeo, S.; Kim, J.A.; Choi, C.W.; Jeong, S.Y. Antiobesity Effects of Gentiana lutea Extract on 3T3-L1 Preadipocytes and a High-Fat Diet-Induced Mouse Model. Molecules 2020, 25, 2453. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.S.; McKeith, F.K. Consumer-rated quality characteristics as related to purchase intent of fresh pork. J. Food Sci. 1999, 64, 171–174. [Google Scholar] [CrossRef]
- Swatland, H.J. Optical dispersion through muscle fibers isolated from pork. Food Res. Int. 2002, 35, 559–564. [Google Scholar] [CrossRef]
- Bidner, B.S.; Ellis, M.; Brewer, M.S.; Campion, D.; Wilson, E.R.; McKeith, F.K. Effect of ultimate ph on the quality characteristics of pork. J. Muscle Foods 2004, 15, 139–154. [Google Scholar] [CrossRef]
- Carpenter, R.; O’Grady, M.N.; O’Callaghan, Y.C.; O’Brien, N.M.; Kerry, J.P. Evaluation of the antioxidant potential of grape seed and bearberry extracts in raw and cooked pork. Meat Sci. 2007, 76, 604–610. [Google Scholar] [CrossRef]
- Xu, M.; Chen, X.; Huang, Z.; Chen, D.; Li, M.; He, J.; Chen, H.; Zheng, P.; Yu, J.; Luo, Y.; et al. Effects of dietary grape seed proanthocyanidin extract supplementation on meat quality, muscle fiber characteristics and antioxidant capacity of finishing pigs. Food Chem. 2022, 367, 130781. [Google Scholar] [CrossRef]
- Mei, H.; Li, Y.; Wu, S.; He, J. Natural plant polyphenols contribute to the ecological and healthy swine production. J. Anim. Sci. Biotechnol. 2024, 15, 146. [Google Scholar] [CrossRef]
- Nold, R.A.; Romans, J.R.; Costello, W.J.; Libal, G.W. Characterization of muscles from boars, barrows, and gilts slaughtered at 100 or 110 kilograms: Differences in fat, moisture, color, water-holding capacity, and collagen. J. Anim. Sci. 1999, 77, 1746–1754. [Google Scholar] [CrossRef]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How Muscle Structure and Composition Influence Meat and Flesh Quality. Sci. World J. 2016, 2016, 3182746. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Marrocco, C.; Zolla, V.; D’Andrea, M.; Zolla, L. Meat quality of the longissimus lumborum muscle of Casertana and Large White pigs: Metabolomics and proteomics intertwined. J. Proteom. 2011, 75, 610–627. [Google Scholar] [CrossRef]
- Bendall, J.R.; Swatland, H.J. A review of the relationships of pH with physical aspects of pork quality. Meat Sci. 1988, 24, 85–126. [Google Scholar] [CrossRef] [PubMed]
- Bee, G.; Biolley, C.; Guex, G.; Herzog, W.; Lonerga, S.M.; Huff-Lonergan, E. Effects of available dietary carbohydrate and preslaughter treatment on glycolytic potential, protein degradation, and quality traits of pig muscles. J. Anim. Sci. 2006, 84, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Monin, G.; Sellier, P. Pork of low technological quality with a normal rate of muscle pH fall in the immediate post-mortem period: The case of the Hampshire breed. Meat Sci. 1985, 13, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Mitacek, R.M.; Ke, Y.; Prenni, J.E.; Jadeja, R.; VanOverbeke, D.L.; Mafi, G.G.; Ramanathan, R. Mitochondrial Degeneration, Depletion of NADH, and Oxidative Stress Decrease Color Stability of Wet-Aged Beef Longissimus Steaks. J. Food Sci. 2019, 84, 38–50. [Google Scholar] [CrossRef]
- Spires, M.D.; Bodmer, J.S.; Beline, M.; Wicks, J.C.; Zumbaugh, M.D.; Shi, T.H.; Reichert, B.T.; Schinckel, A.P.; Grant, A.L.; Gerrard, D.E. Postmortem Metabolism and Pork Quality Development Are Affected by Electrical Stimulation across Three Genetic Lines. Animals 2023, 13, 2599. [Google Scholar] [CrossRef]
- King, D.A.; Hunt, M.C.; Barbut, S.; Claus, J.R.; Cornforth, D.P.; Joseph, P.; Kim, Y.H.B.; Lindahl, G.; Mancini, R.A.; Nair, M.N.; et al. American Meat Science Association Guidelines for Meat Color Measurement. Meat Muscle Biol. 2023, 6, 1–81. [Google Scholar] [CrossRef]
- Kwasiborski, A.; Sayd, T.; Chambon, C.; Santé-Lhoutellier, V.; Rocha, D.; Terlouw, C. Pig Longissimus lumborum proteome: Part II: Relationships between protein content and meat quality. Meat Sci. 2008, 80, 982–996. [Google Scholar] [CrossRef]
- Żelechowska, E.; Przybylski, W.; Jaworska, D.; Santé-Lhoutellier, V. Technological and sensory pork quality in relation to muscle and drip loss protein profiles. Eur. Food Res. Technol. 2012, 234, 883–894. [Google Scholar] [CrossRef]
- Kim, G.-D.; Jeong, J.-Y.; Yang, H.-S.; Hur, S.J. Differential abundance of proteome associated with intramuscular variation of meat quality in porcine longissimus thoracis et lumborum muscle. Meat Sci. 2019, 149, 85–95. [Google Scholar] [CrossRef]
- Suman, P.; Wang, Y.; Gagaoua, M.; Kiyimba, F.; Ramanathan, R. Proteomic approaches to characterize biochemistry of fresh beef color. J. Proteom. 2023, 281, 104893. [Google Scholar] [CrossRef]
- Lee, S.; Phillips, A.L.; Liebler, D.C.; Faustman, C. Porcine oxymyoglobin and lipid oxidation in vitro. Meat Sci. 2003, 63, 241–247. [Google Scholar] [CrossRef]
| Con | Caf | BM 0.5 1 | BM 1.0 | BM 1.5 | BM 2.0 | GG | |
|---|---|---|---|---|---|---|---|
| Ingredients, % | |||||||
| Wheat | 46.72 | 46.72 | 46.72 | 46.72 | 46.72 | 46.72 | 46.72 |
| Barley | 35.00 | 35.00 | 35.00 | 35.00 | 35.00 | 35.00 | 35.00 |
| Soybean Meal | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Canola Meal | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 |
| Water | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Tallow mixer | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 |
| Salt | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Limestone | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 |
| DL-Methionine | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Caffeine | - | 0.05 | 0.0125 | 0.025 | 0.0375 | 0.05 | - |
| Grape seed extract | - | - | 0.01875 | 0.0375 | 0.0562 | 0.075 | 0.075 |
| Gentian plant extract | - | - | 0.01875 | 0.0375 | 0.0562 | 0.075 | 0.075 |
| Lysine-HCL | 0.47 | 0.47 | 0.47 | 0.47 | 0.47 | 0.47 | 0.47 |
| Threonine | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Premix 2 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Calculated nutrients, % | |||||||
| Dry matter | 89.19 | 89.19 | 89.19 | 89.19 | 89.19 | 89.19 | 89.19 |
| Digestible energy, MJ/kg | 13.83 | 13.83 | 13.83 | 13.83 | 13.83 | 13.83 | 13.83 |
| Crude protein | 14.64 | 14.64 | 14.64 | 14.64 | 14.64 | 14.64 | 14.64 |
| Ether extract | 2.28 | 2.28 | 2.28 | 2.28 | 2.28 | 2.28 | 2.28 |
| Fibre | 3.82 | 3.82 | 3.82 | 3.82 | 3.82 | 3.82 | 3.82 |
| Ash | 4.06 | 4.06 | 4.06 | 4.06 | 4.06 | 4.06 | 4.06 |
| Available Phosphorus | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Available Calcium | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| Available Lysine | 0.86 | 0.86 | 0.86 | 0.86 | 0.86 | 0.86 | 0.86 |
| Dietary Treatments | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Control | Caffeine 1 | BM 2 | GG 3 | SEM | Treat | Sex | Sex x Treat | |
| Day 0 | Initial weight (kg) | 66.16 | 67.00 | 67.67 | 66.60 | 7.948 | 0.591 | 0.123 | 0.397 |
| Day 42 | Final weight (kg) | 104.81 | 101.33 | 102.45 | 105.80 | 1.320 | 0.052 | 0.001 | 0.826 |
| Day 0–21 | |||||||||
| ADFI (kg/d) | 2.21 a | 2.13 ab | 1.97 b | 2.20 a | 0.047 | 0.003 | 0.337 | 0.471 | |
| ADG (kg/d) | 0.82 | 0.78 | 0.73 | 0.84 | 0.031 | 0.069 | 0.135 | 0.867 | |
| FCR | 2.68 | 2.72 | 2.77 | 2.53 | 0.064 | 0.082 | 0.002 | 0.371 | |
| Day 21–42 | |||||||||
| ADFI (kg/d) | 2.53 | 2.39 | 2.47 | 2.55 | 0.102 | 0.618 | 0.007 | 0.721 | |
| ADG (kg/d) | 1.03 | 0.92 | 0.96 | 1.02 | 0.036 | 0.126 | 0.007 | 0.730 | |
| FCR | 2.60 | 2.47 | 2.50 | 2.55 | 0.116 | 0.868 | 0.583 | 0.792 | |
| Day 0–42 | |||||||||
| ADFI (kg/d) | 2.45 | 2.30 | 2.27 | 2.41 | 0.063 | 0.145 | 0.073 | 0.557 | |
| ADG (kg/d) | 0.96 a | 0.88 b | 0.88 b | 0.96 a | 0.019 | 0.003 | <0.001 | 0.365 | |
| FCR | 2.59 | 2.56 | 2.53 | 2.54 | 0.069 | 0.938 | 0.085 | 0.611 | |
| Carcass quality | |||||||||
| HSCW (kg) | 78.96 | 76.60 | 76.63 | 78.86 | 0.731 | 0.024 | 0.691 | 0.808 | |
| P2 backfat (mm) | 11.10 a | 10.38 b | 10.40 b | 11.05 ab | 0.173 | 0.002 | 0.356 | 0.158 | |
| Loin depth (mm) | 54.86 | 53.18 | 54.20 | 54.55 | 0.470 | 0.085 | <0.001 | 0.155 | |
| Dressing (%) | 75.61 | 75.79 | 74.36 | 74.62 | 0.489 | 0.082 | <0.001 | 0.499 | |
| BM 1 (g/kg) | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | 0 2 | 0.5 | 1.0 | 1.5 | 2.0 | SEM | Linear 3 | Quadratic 4 | |
| Day 0 | Initial weight (kg) | 66.16 | 66.63 | 66.03 | 66.23 | 67.67 | 4.901 | 0.239 | 0.284 |
| Day 42 | Final weight (kg) | 104.69 | 104.36 | 103.38 | 101.18 | 102.31 | 0.892 | 0.070 | 0.726 |
| Day 0–21 | |||||||||
| ADFI (kg/day) | 2.19 | 2.10 | 2.12 | 2.09 | 1.97 | 0.026 | <0.001 | 0.459 | |
| ADG (kg/day) | 0.82 | 0.80 | 0.85 | 0.81 | 0.72 | 0.016 | 0.016 | 0.009 | |
| FCR | 2.68 | 2.65 | 2.51 | 2.70 | 2.70 | 0.039 | 0.722 | 0.057 | |
| Day 21–42 | |||||||||
| ADFI (kg/day) | 2.59 | 2.59 | 2.48 | 2.37 | 2.39 | 0.064 | 0.063 | 0.838 | |
| ADG (kg/day) | 1.03 | 1.00 | 0.94 | 0.90 | 0.97 | 0.022 | 0.037 | 0.077 | |
| FCR | 2.57 | 2.69 | 2.64 | 2.66 | 2.48 | 0.086 | 0.669 | 0.258 | |
| Day 0–42 | |||||||||
| ADFI (kg/day) | 2.43 | 2.44 | 2.39 | 2.26 | 2.26 | 0.044 | 0.031 | 0.827 | |
| ADG (kg/day) | 0.96 | 0.93 | 0.92 | 0.88 | 0.88 | 0.013 | <0.001 | 0.564 | |
| FCR | 2.56 | 2.63 | 2.63 | 2.59 | 2.51 | 0.054 | 0.660 | 0.307 | |
| Carcass quality | |||||||||
| HSCW (kg) | 78.86 | 78.50 | 78.84 | 76.02 | 76.70 | 0.561 | 0.015 | 0.873 | |
| P2 backfat (mm) | 11.26 | 10.89 | 10.68 | 10.27 | 10.51 | 0.135 | 0.001 | 0.201 | |
| Loin depth (mm) | 54.79 | 54.27 | 53.67 | 52.90 | 54.06 | 0.397 | 0.269 | 0.110 | |
| Dressing (%) | 75.55 | 75.36 | 75.48 | 75.22 | 74.37 | 0.275 | 0.042 | 0.285 | |
| Dietary Treatments | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Control | Caffeine | BM 1 | GG 2 | SEM | Treat 3 | Sex | Sex x Treat |
| Drip loss (%) | 3.59 | 4.26 | 4.60 | 3.17 | 0.006 | 0.284 | 0.448 | 0.206 |
| pH 45 min | 6.14 | 6.35 | 6.30 | 6.24 | 0.064 | 0.161 | 0.697 | 0.725 |
| pH 24 h | 5.51 | 5.46 | 5.50 | 5.50 | 0.027 | 0.466 | 0.655 | 0.323 |
| Lightness (L*) | 47.46 | 48.08 | 46.80 | 47.57 | 1.047 | 0.597 | 0.372 | 0.511 |
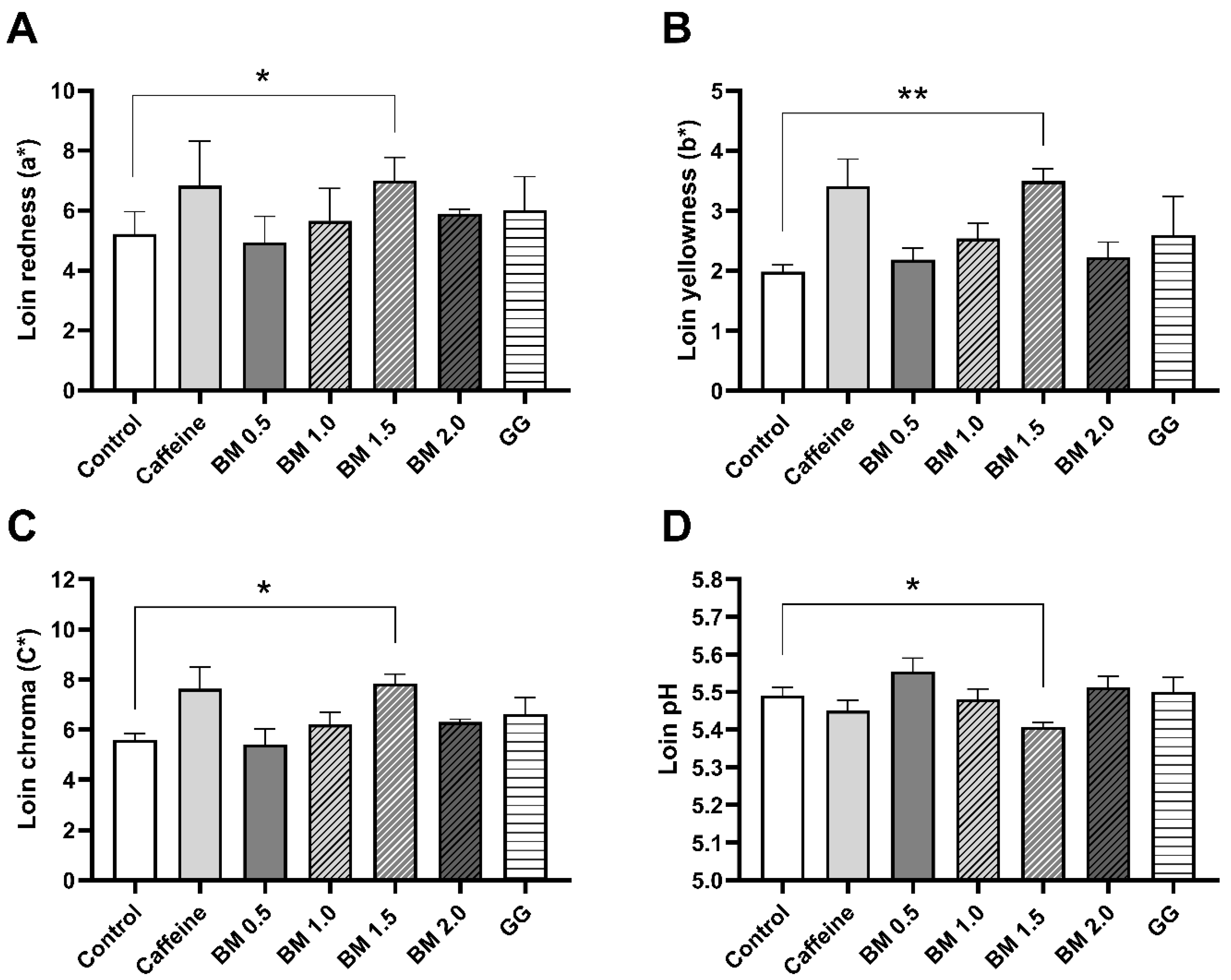
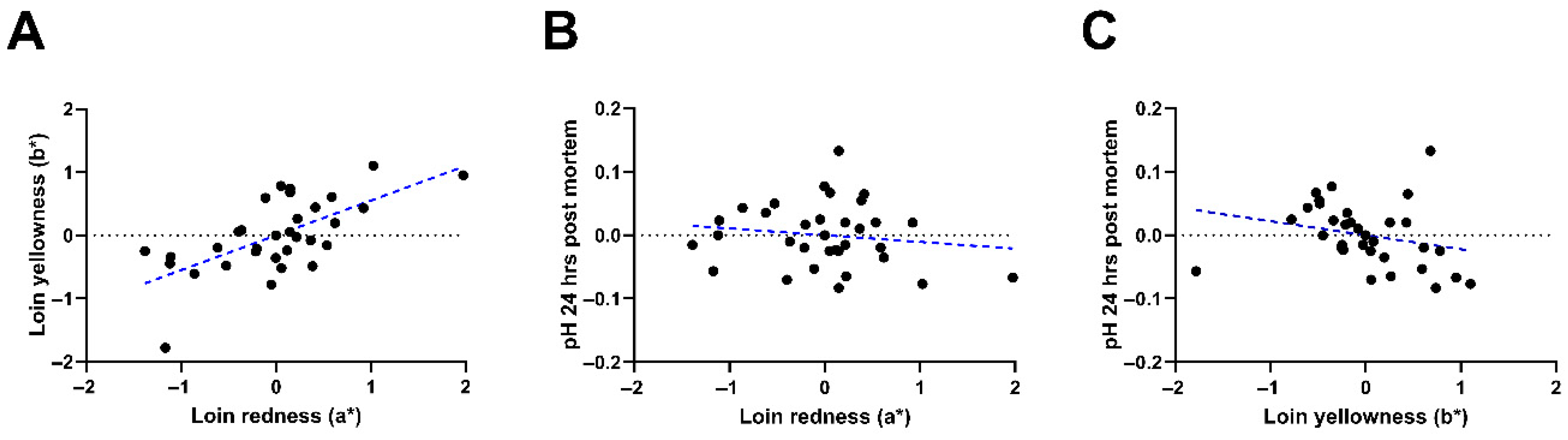
| Redness (a*) | 5.44 | 6.57 | 5.95 | 6.16 | 0.375 | 0.133 | 0.201 | 0.198 |
| Yellowness (b*) | 1.90 | 3.10 | 2.36 | 2.73 | 0.331 | 0.084 | 0.465 | 0.203 |
| Hue angle 3 (h°) | 19.59 | 25.03 | 21.55 | 22.35 | 2.545 | 0.421 | 0.797 | 0.406 |
| Chroma 4 (C*) | 5.78 | 7.27 | 6.41 | 6.80 | 0.432 | 0.082 | 0.193 | 0.174 |
| Cook loss (%) | 19.36 | 21.10 | 20.90 | 19.52 | 0.069 | 0.174 | 0.296 | 0.251 |
| Shear force (N) | 44.55 | 42.67 | 55.08 | 37.53 | 3.677 | 0.083 | 0.977 | 0.993 |
| BM 1 (g/kg) | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | 0 2 | 0.5 | 1.0 | 1.5 | 2.0 | SEM | Linear 3 | Quadratic 4 |
| Drip loss (%) | 3.60 | 3.10 | 3.55 | 5.11 | 4.60 | 0.005 | 0.328 | 0.694 |
| pH 45 min | 6.14 | 6.21 | 6.20 | 6.12 | 6.30 | 0.044 | 0.142 | 0.453 |
| pH 24 h | 5.52 | 5.56 | 5.48 | 5.41 | 5.50 | 0.023 | 0.351 | 0.267 |
| Lightness (L*) | 47.46 | 47.08 | 48.11 | 49.06 | 46.80 | 0.588 | 0.719 | 0.176 |
| Redness (a*) | 5.45 | 5.00 | 5.65 | 6.94 | 5.95 | 0.274 | 0.013 | 0.919 |
| Yellowness (b*) | 1.90 | 2.20 | 2.54 | 3.46 | 2.36 | 0.194 | 0.019 | 0.086 |
| Hue angle 5 (h°) | 19.59 | 24.00 | 24.18 | 26.47 | 21.55 | 1.194 | 0.315 | 0.020 |
| Chroma 6 (C*) | 5.78 | 5.50 | 6.20 | 7.77 | 6.41 | 0.309 | 0.010 | 0.740 |
| Cook loss (%) | 19.36 | 19.60 | 18.90 | 20.12 | 20.90 | 0.005 | 0.252 | 0.152 |
| Shear force (N) | 44.55 | 48.76 | 48.04 | 40.29 | 49.32 | 2.631 | 0.918 | 0.647 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, M.; Tan, X.; Liu, F.; Navarro, M.; Hoffman, L.C.; Roura, E. Feeding a Bitter Mix of Gentian and Grape Seed Extracts with Caffeine Reduces Appetite and Body Fat Deposition and Improves Meat Colour in Pigs. Animals 2025, 15, 2129. https://doi.org/10.3390/ani15142129
Müller M, Tan X, Liu F, Navarro M, Hoffman LC, Roura E. Feeding a Bitter Mix of Gentian and Grape Seed Extracts with Caffeine Reduces Appetite and Body Fat Deposition and Improves Meat Colour in Pigs. Animals. 2025; 15(14):2129. https://doi.org/10.3390/ani15142129
Chicago/Turabian StyleMüller, Maximiliano, Xinle Tan, Fan Liu, Marta Navarro, Louwrens C. Hoffman, and Eugeni Roura. 2025. "Feeding a Bitter Mix of Gentian and Grape Seed Extracts with Caffeine Reduces Appetite and Body Fat Deposition and Improves Meat Colour in Pigs" Animals 15, no. 14: 2129. https://doi.org/10.3390/ani15142129
APA StyleMüller, M., Tan, X., Liu, F., Navarro, M., Hoffman, L. C., & Roura, E. (2025). Feeding a Bitter Mix of Gentian and Grape Seed Extracts with Caffeine Reduces Appetite and Body Fat Deposition and Improves Meat Colour in Pigs. Animals, 15(14), 2129. https://doi.org/10.3390/ani15142129






