Characterizing the Dynamic Protein and Amino Acid Deposition in Tissues of Pregnant Gilts: Implications for Stage-Specific Nutritional Strategies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Step 1: Data Collection
2.2. Step 2: Curve Development
| Tissue/Studies | Number of Time Points | Gestation Period, Days | Selected | |
|---|---|---|---|---|
| Wet Weight, kg | Crude Protein, % of Wet Weight | |||
| Allantoic fluid | ||||
| Knight et al. (1977) [12] | 11 | 11 | 20–100 | ✔ |
| Tarraf and Knight (1995) [13] | 4 | NR | 40–100 | |
| Wu et al. (2017) [14] | 7 | NR | 20–114 | |
| Amniotic fluid | ||||
| Knight et al. (1977) [12] | 9 | 9 | 30–100 | ✔ |
| Wu et al. (2017) [14] | 7 | NR | 20–114 | |
| Placenta | ||||
| Guimarães et al. (2014) [15] | 3 | NR | 50–106 | |
| Jang et al. (2017) [3] | 6 | 6 | 43–108 | |
| Knight et al. (1977) [12] | 11 | NR | 20–100 | |
| McPherson et al. (2004) [16] | NR | 6 | 45–110 | |
| Montes et al. (2018) [17] | 4 | NR | 30–90 | |
| Tarraf and Knight (1995) [13] | 4 | NR | 40–100 | |
| Vallet and Freking (2006) [18] | 4 | NR | 45–105 | |
| Vallet et al. (2010) [19] | 5 | NR | 25–105 | |
| Wise et al. (1997) [20] | 3 | NR | 30–104 | |
| Wright et al. (2016) [21] | 5 | NR | 22–42 | |
| Wu et al. (2017) [14] | 7 | 7 | 20–114 | ✔ |
| Uterus | ||||
| Jang et al. (2017) [3] | 6 | 6 | 43–108 | ✔ |
| Knight et al. (1977) [12] | 11 | NR | 20–100 | |
| Fetus | ||||
| Finch et al. (2002) [22] | 3 | NR | 30–114 | |
| Guimarães et al. (2014) [15] | 3 | NR | 50–106 | |
| Jang et al. (2017) [3] | 6 | 6 | 43–108 | |
| Ji et al. (2005) [2] | 6 | NR | 45–112 | |
| Knight et al. (1977) [12] | 11 | NR | 20–100 | |
| McPherson et al. (2004) [16] | 6 | 6 | 45–110 | |
| Montes et al. (2018) [17] | 4 | NR | 30–90 | |
| Tarraf and Knight (1995) [13] | 4 | NR | 40–100 | |
| Vallet and Freking (2006) [18] | 4 | NR | 45–105 | |
| Vallet and Freking (2007) [23] | 4 | NR | 45–105 | |
| Vallet et al. (2010) [19] | 5 | NR | 25–105 | |
| Wise et al. (1997) [20] | 3 | NR | 30–104 | |
| Wright et al. (2016) [21] | 5 | NR | 22–42 | |
| Wu et al. (1999) [4] | 5 | 5 | 40–114 | ✔ |
| Wu et al. (2017) [14] | 7 | NR | 20–114 | |
| Mammary gland | ||||
| Ji et al. (2006) [24] | 6 | 6 | 45–112 | ✔ |
| Sørensen et al. (2002) [25] | 12 | NR | 10–114 | |
| Studies | Number of Time Points | Gestation Period, Days | Selected |
|---|---|---|---|
| Clowes et al. (2003) [26] | 3 | 33–105 | |
| Corley et al. (1983) [27] | 5 | 90–112 | |
| Dunn and Speer (1991) [28] | 6 | 45–99 | |
| Jones and Maxwell (1982) [29] | 3 | 30–90 | |
| King and Brown (1993) [30] | 3 | 30–93 | |
| Miller et al. (2016) [9] | 5 | 42–112 | ✔ |
| Miller et al. (2018) [31] | 4 | 49–108 | |
| Navales et al. (2019) [32] | 3 | 41–107 | |
| Noblet and Etienne (1987) [33] | 3 | 60–110 | |
| Ramirez-Camba et al. (2020) [34] | 3 | 41–107 | |
| Willis and Maxwell (1984) [35] | 3 | 30–90 | |
| Yang et al. (2021) [36] | 3 | 35–98 |
| Tissue/Studies | Number of Time Points | Wet Basis | Crude Protein Basis | Selected |
|---|---|---|---|---|
| Allantoic fluid | ||||
| Li et al. (2014) [37] | 1 | × | NR | |
| Li et al. (2023) [38] | 1 | × | NR | |
| Wu et al. (1998) [39] | 2 | × | NR | ✔ |
| Amniotic fluid | ||||
| Li et al. (2014) [37] | 1 | × | NR | |
| Wu et al. (1998) [39] | 2 | × | NR | ✔ |
| Li et al. (2023) [38] | 1 | × | NR | |
| Placenta | ||||
| Jang et al. (2017) [3] | 6 | × | × | |
| Wu et al. (2017) [14] | 7 | NR | × | ✔ |
| Self et al. (2004) [40] 1 | 9 | × | NR | |
| Li et al. (2014) [37] | 1 | × | NR | |
| Wu et al. (1998) [41] | 2 | × | NR | |
| Uterus | ||||
| Jang et al. (2017) [3] | 6 | × | × | ✔ |
| Fetus | ||||
| Jang et al. (2017) [3] | 6 | × | × | ✔ |
| Wu et al. (1999) [4] | 5 | × | × | |
| Wu et al. (2017) [14] | 7 | × | NR | |
| Everts and Dekker (1995) [42] | 1 | × | × |
2.3. Step 3: Model Development
2.3.1. Growth Model
2.3.2. Daily CP Deposition Model
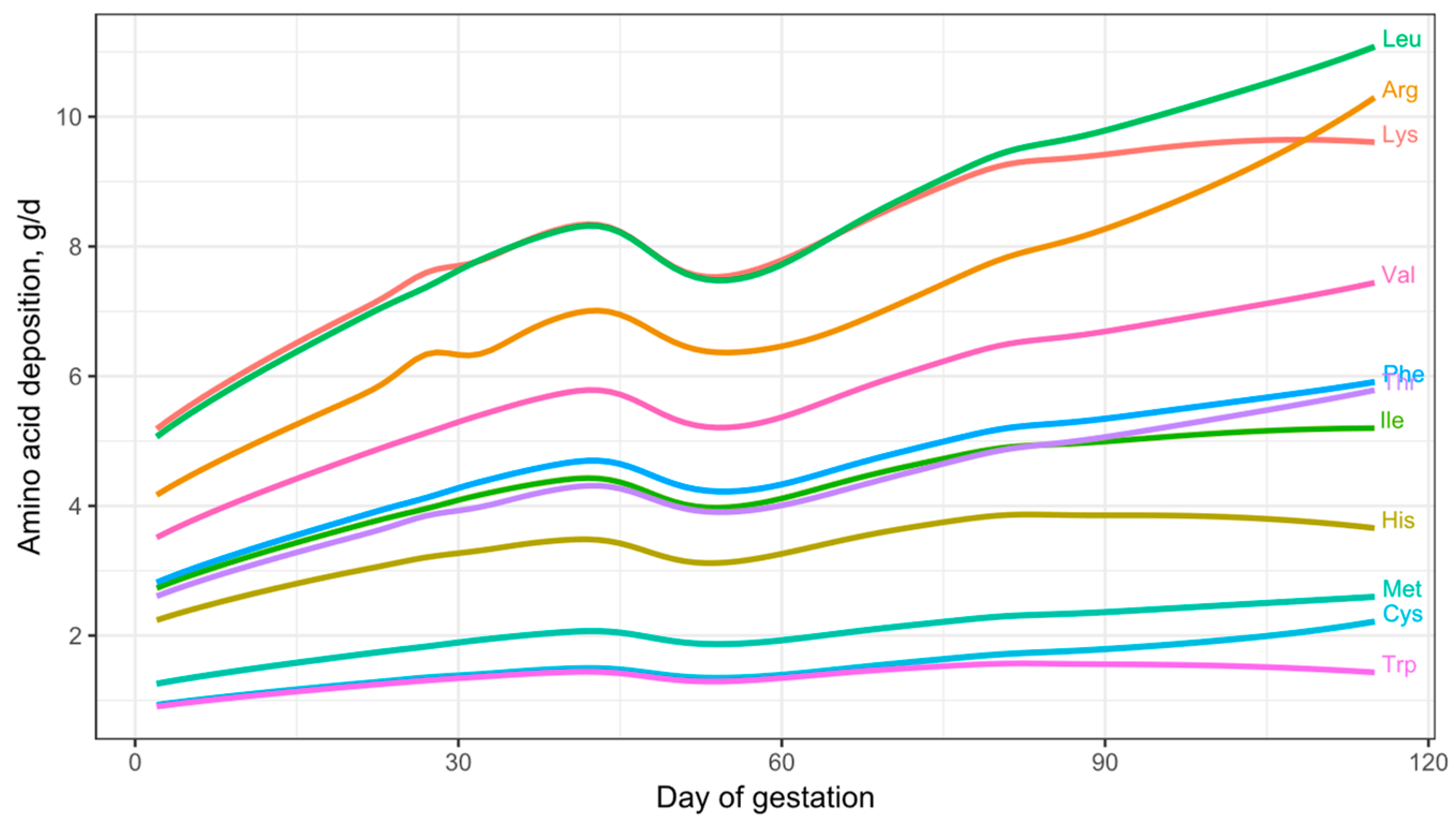
2.3.3. Daily Essential AA Deposition Model
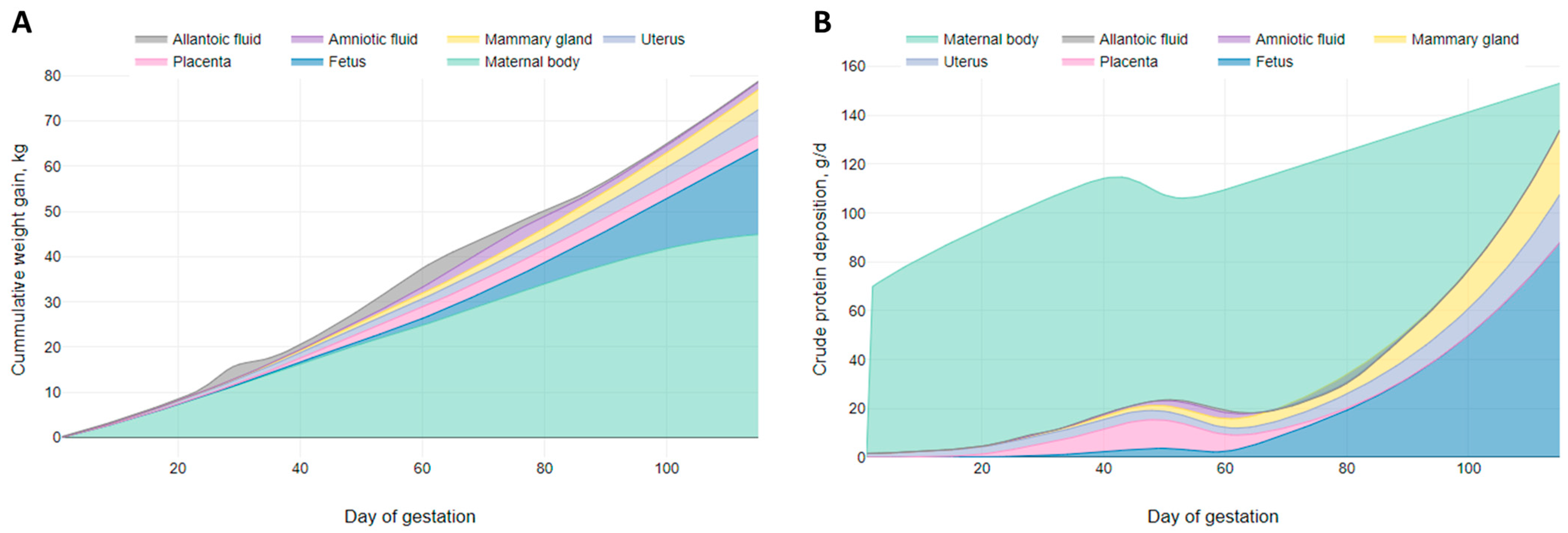
3. Results
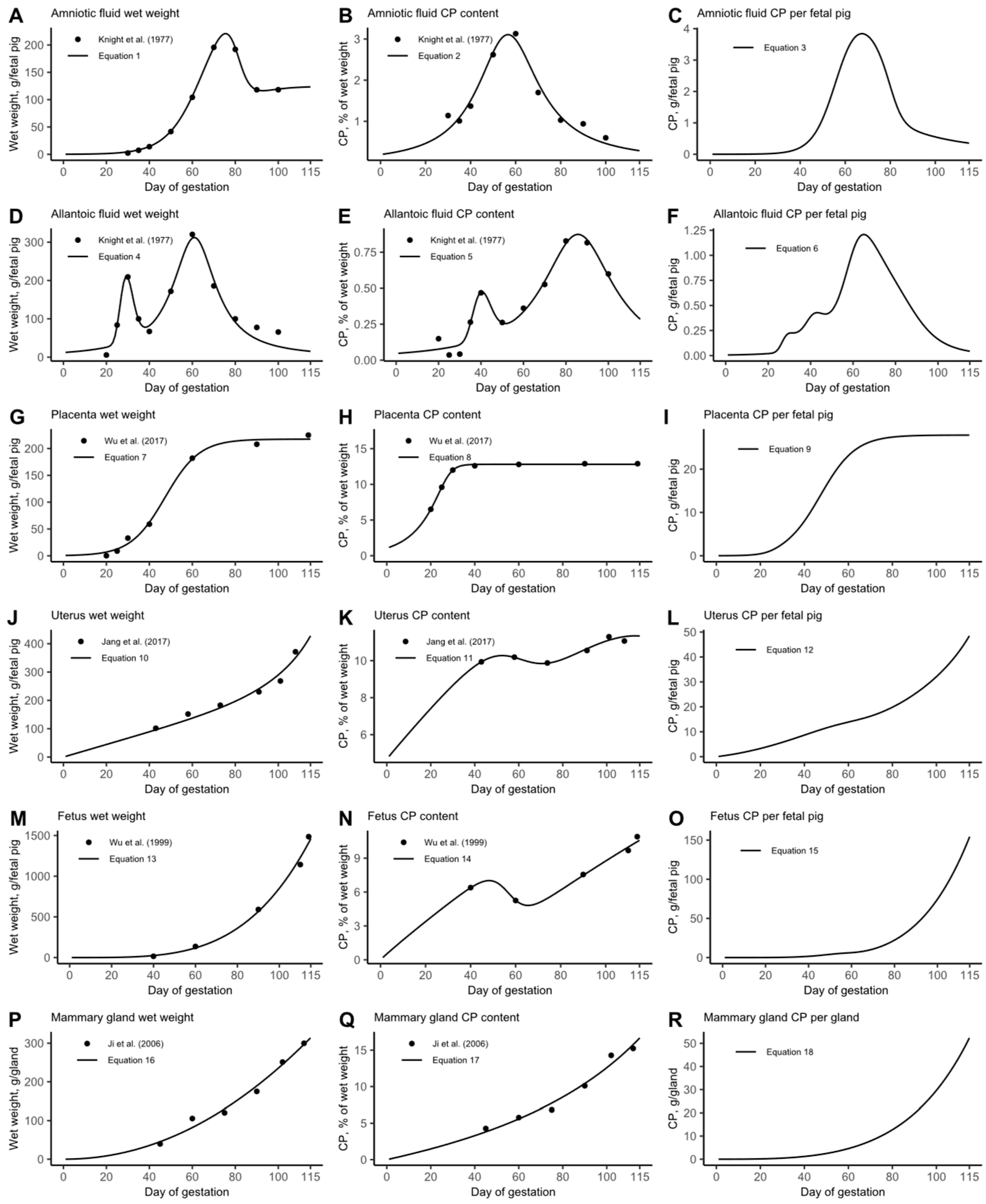
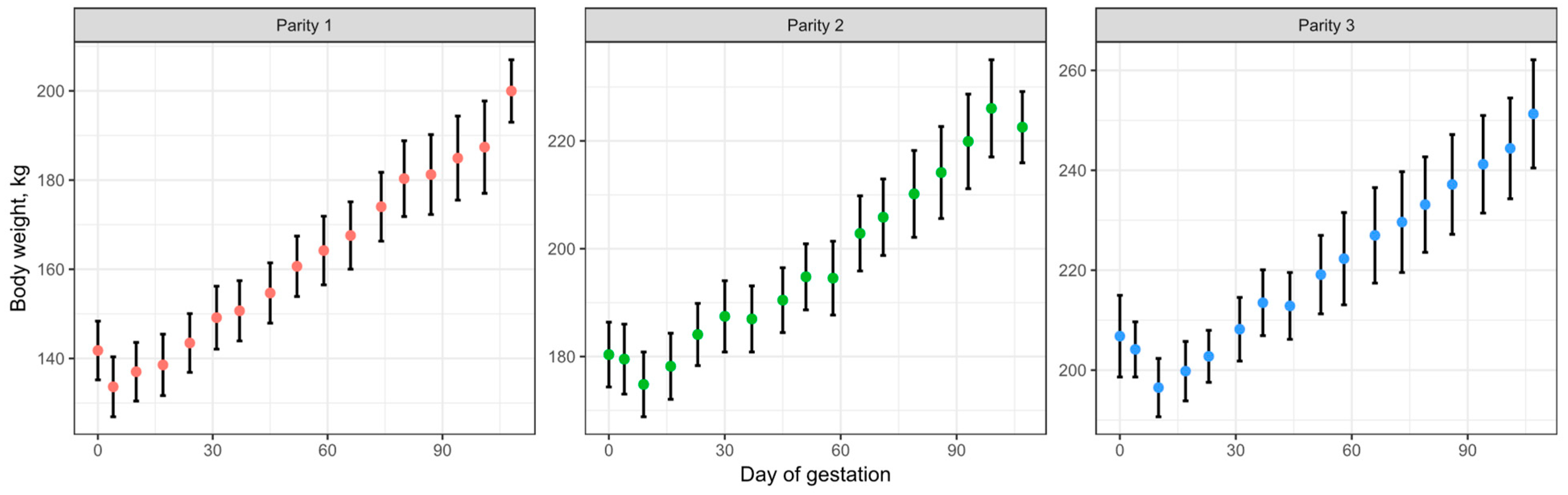
3.1. Growth Model
3.1.1. Growth Model Evaluation
3.1.2. Growth Model Adjustments to Fit Empirical Data (Model Calibration)
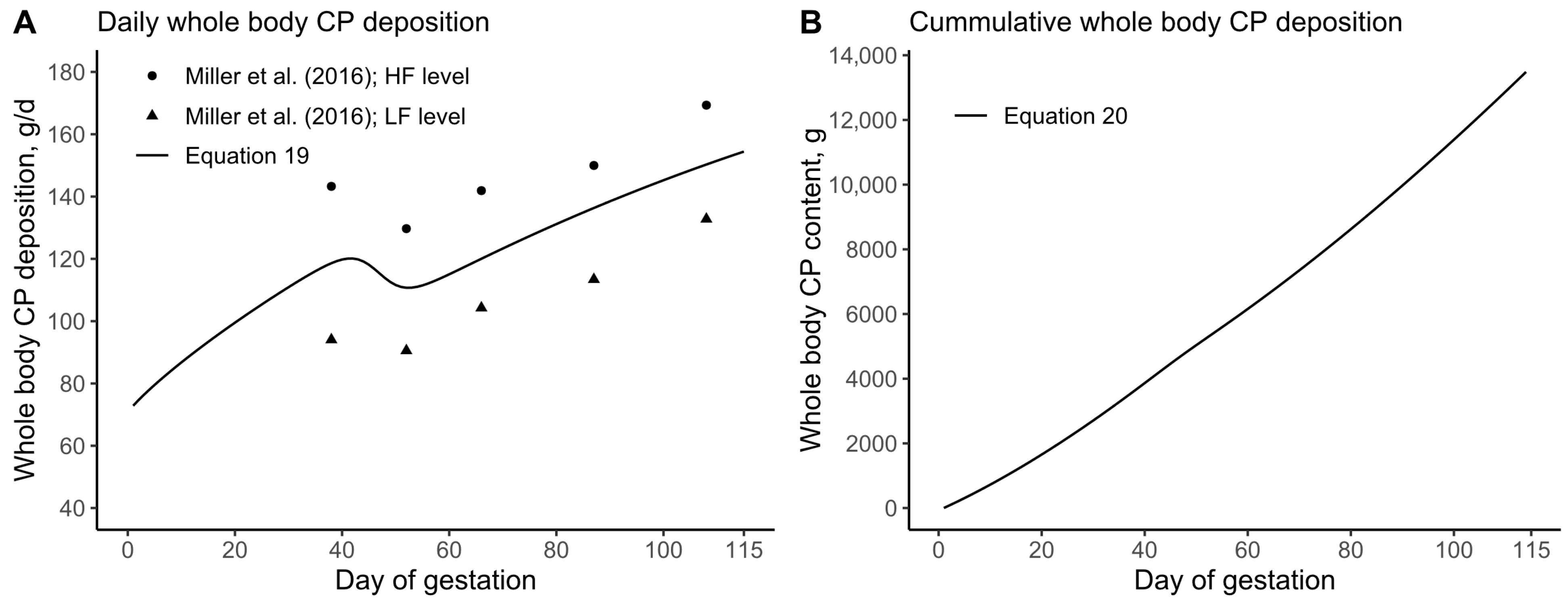
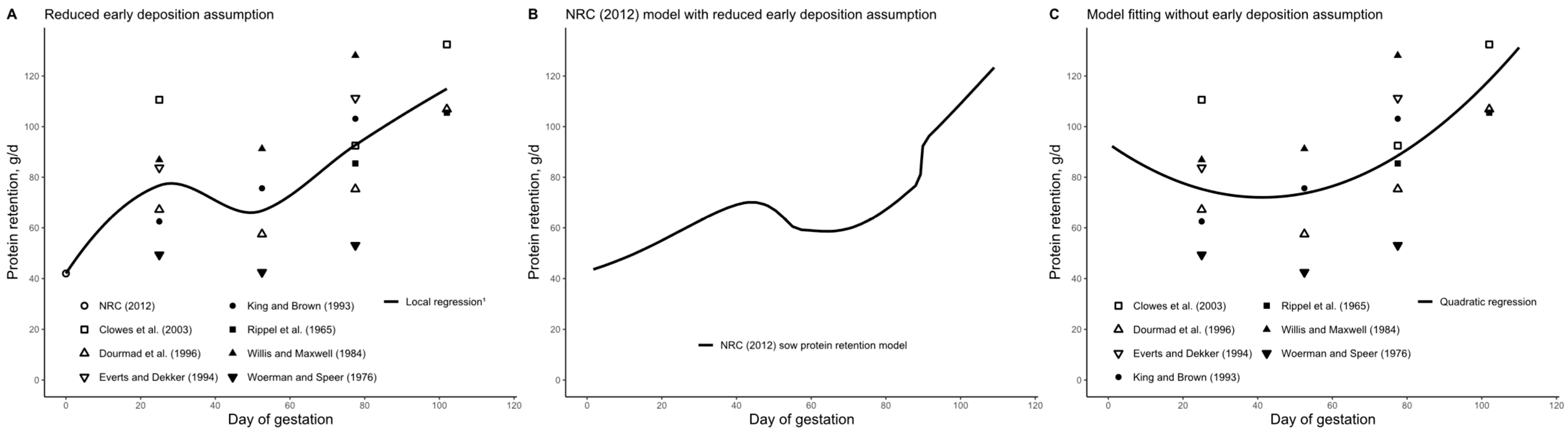
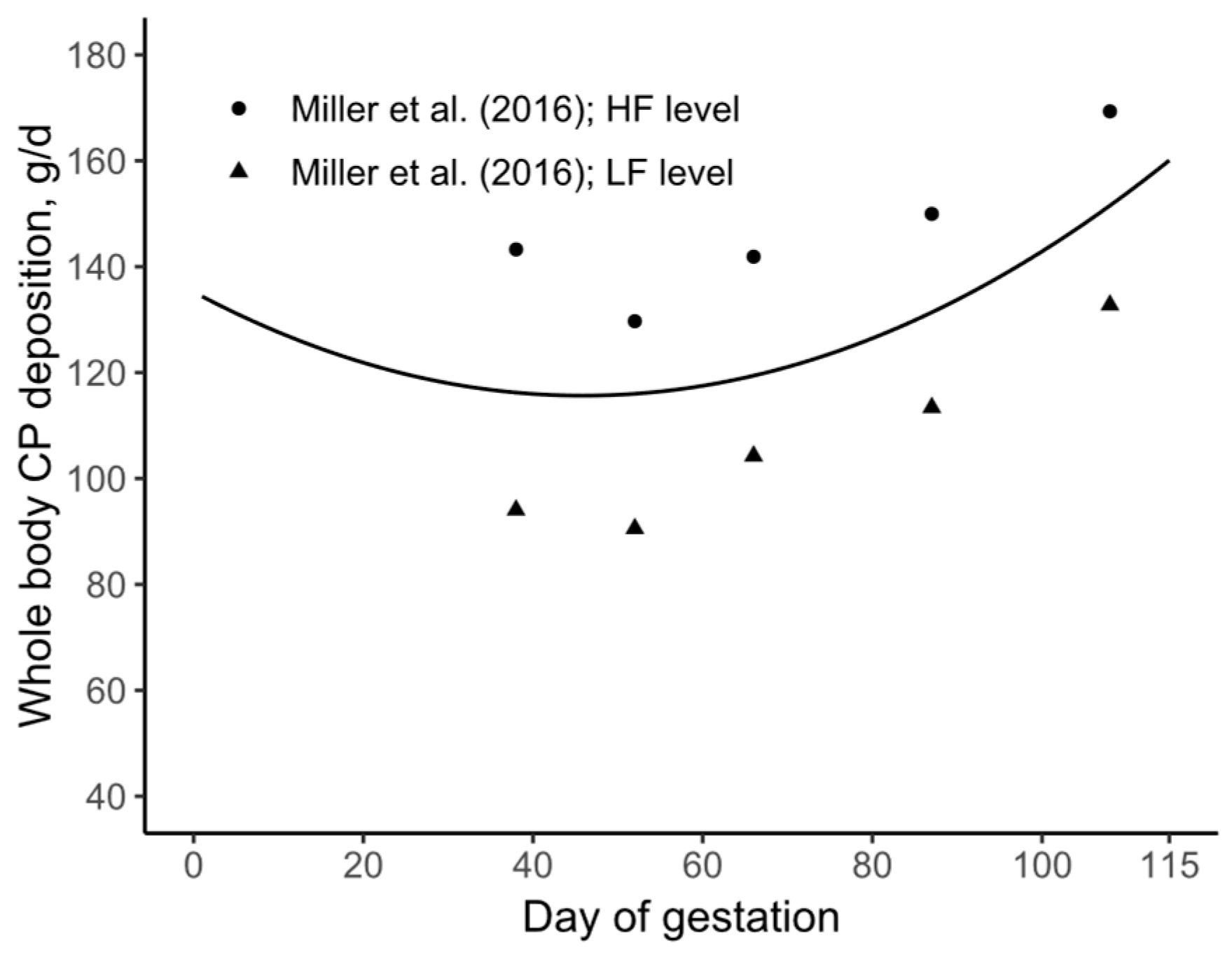
3.2. Daily CP Deposition Model
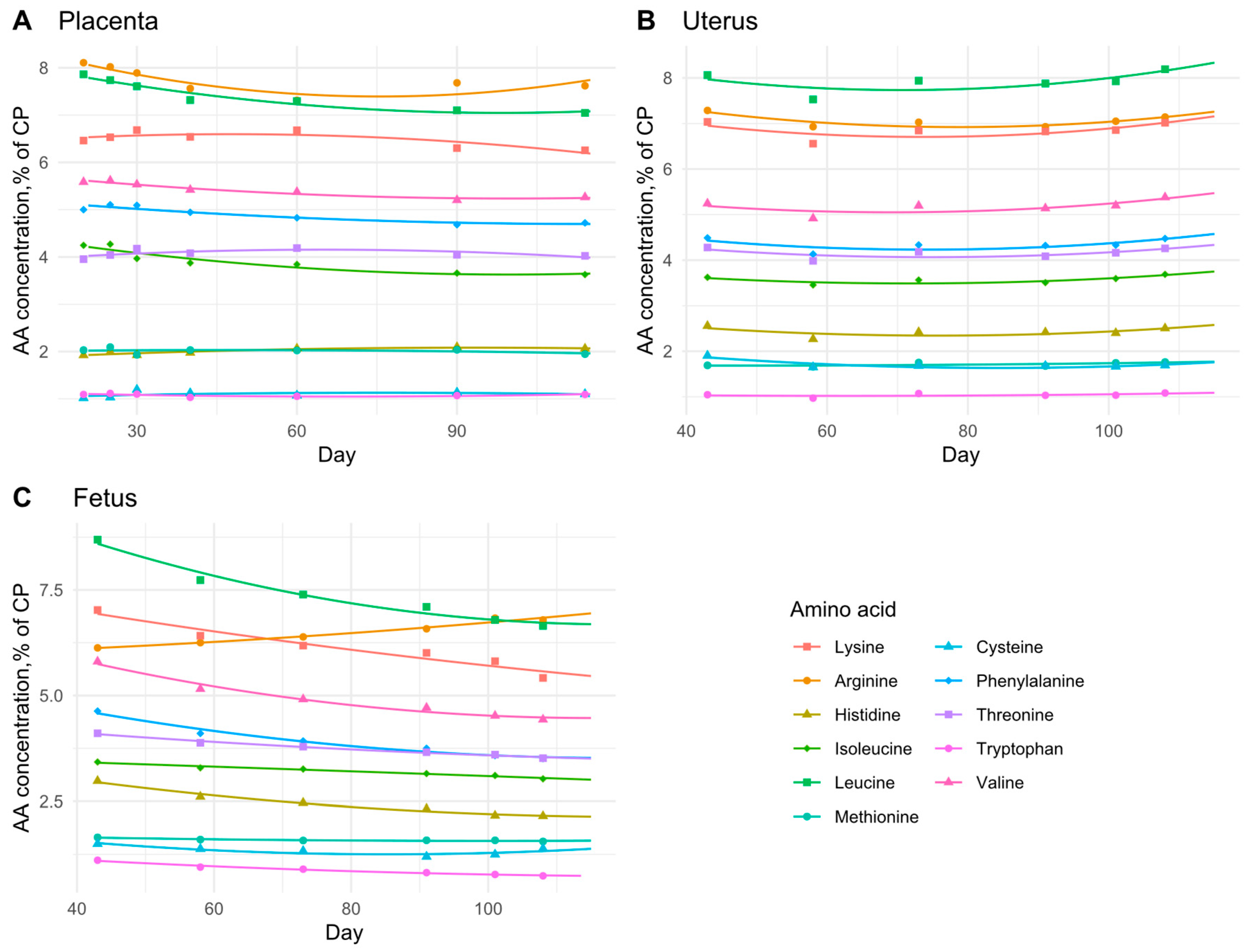
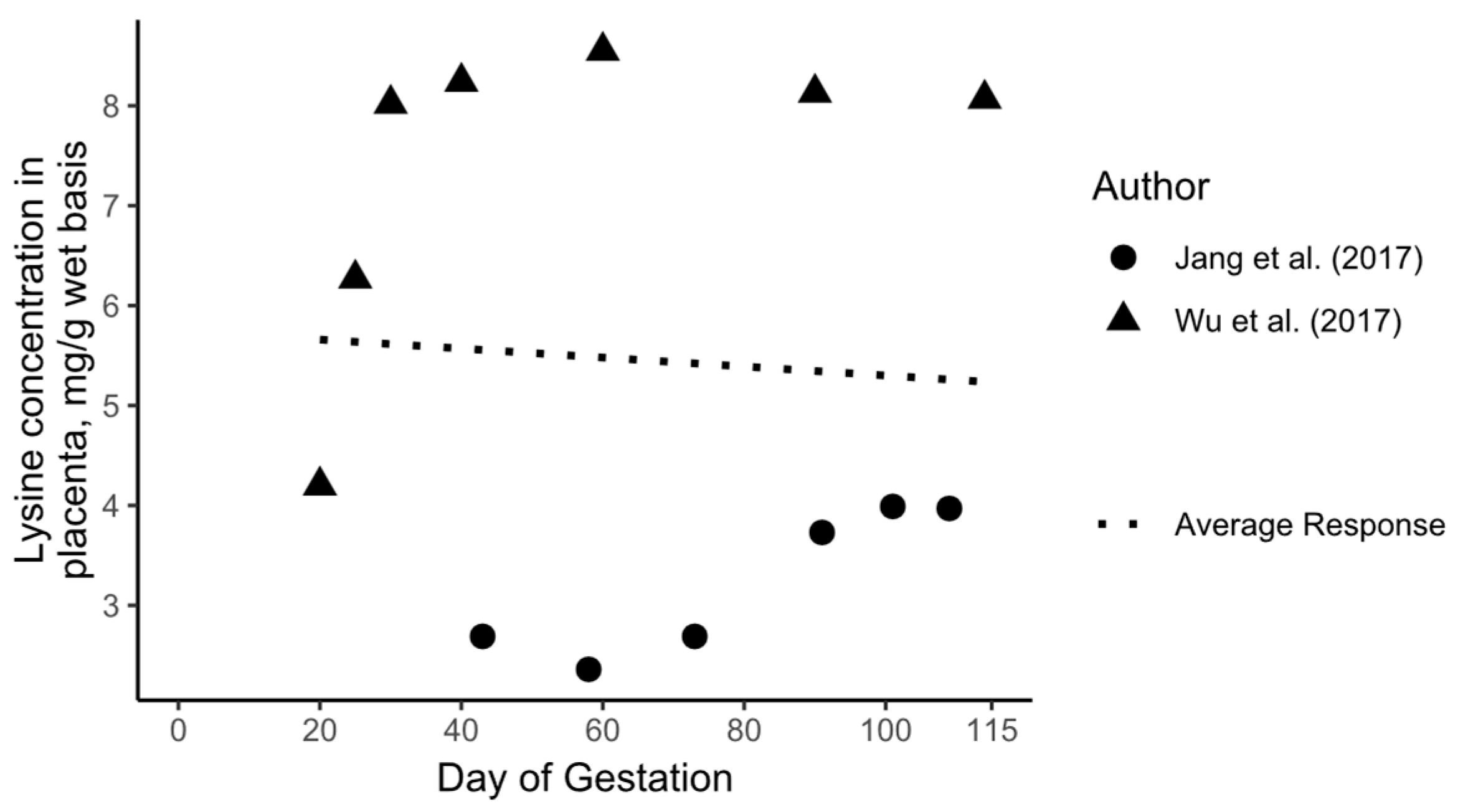
3.3. Daily AA Deposition Model
4. Discussion
4.1. Early Gestation
Time-Dependent Protein Deposition
4.2. Mid-Gestation
4.3. Late Gestation and the Entire Gestation Period
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Amino Acid |
| Arg | Arginine |
| BES | Best Evidence Synthesis |
| CP | Crude Protein |
| Cys Equation | Cysteine Equation |
| His | Histidine |
| Ile | Isoleucine |
| Leu | Leucine |
| Lys | Lysine |
| Met | Methionine |
| NRC | National Research Council |
| Phe | Phenylalanine |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| Thr | Threonine |
| Trp | Tryptophan |
| Val | Valine |
References
- NRC. Nutrient Requirements of Swine, 11th rev. ed.; The National Academies Press: Washington, DC, USA, 2012; p. 420.
- Ji, F.; Wu, G.; Blanton, J.R.; Kim, S.W. Changes in weight and composition in various tissues of pregnant gilts and their nutritional implications. J. Anim. Sci. 2005, 83, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Lauridsen, C.; Lærke, H.N.; Theil, P.K. Amino acid composition of fetus, placenta, and uterus in gilts throughout gestation. J. Anim. Sci. 2017, 95, 4448–4461. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ott, T.L.; Knabe, D.A.; Bazer, F.W. Amino acid composition of the fetal pig. J. Nutr. 1999, 129, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Slavin, R.E. Best-evidence synthesis: An alternative to meta-analytic and traditional reviews. Educ. Res. 1986, 15, 5–11. [Google Scholar] [CrossRef]
- Eysenck, H. Meta-analysis or best-evidence synthesis? J. Eval. Clin. Pract. 1995, 1, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Slavin, R.E. Best evidence synthesis: An intelligent alternative to meta-analysis. J. Clin. Epidemiol. 1995, 48, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Li, X.; Ji, F.; McPherson, R.L.; Blavi, L.; Beaulieu, A.D.; De Lange, C.F.M.; Kim, S.W. Dynamics of nitrogen retention in gestating gilts at two feeding levels. J. Anim. Sci. 2016, 94, 3353–3361. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hurley, W.L.; Wu, G. Changes in tissue composition associated with mammary gland growth during lactation in sows. J. Anim. Sci. 1999, 77, 2510–2516. [Google Scholar] [CrossRef] [PubMed]
- Hyams, D.G. CurveExpert Professional: Documentation. 2020. Available online: https://www.curveexpert.net/ (accessed on 15 March 2025).
- Knight, J.; Bazer, F.W.; Thatcher, W.W.; Franke, D.E.; Wallace, H.D. Conceptus development in intact and unilaterally hysterectomized-ovariectomized gilts: Interrelations among hormonal status, placental development, fetal fluids and fetal growth. J. Anim. Sci. 1977, 44, 620–637. [Google Scholar] [CrossRef] [PubMed]
- Tarraf, C.; Knight, J.W. Effect of intrauterine position on conceptus development, placental and endometrial release of progesterone and estrone in vitro, and concentration of steroid hormones in fetal fluids throughout gestation in swine. Domest. Anim. Endocrinol. 1995, 12, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Johnson, G.A.; Herring, C.M.; Seo, H.; Dai, Z.; Wang, X.; Wu, Z. Functional amino acids in the development of the pig placenta. Mol. Reprod. Dev. 2017, 84, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, G.C.; Menezes, M.P.; Borba, S.C.; Miranda-Moura, M.T.M.; Moura, C.E.B. Vascularization of broad ligament of uterus and its relationship with fetal and placental development in gilts. Theriogenology 2014, 82, 232–237. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R.L.; Ji, F.; Wu, G.; Blanton, J.R.; Kim, S.W. Growth and compositional changes of fetal tissues in pigs. J. Anim. Sci. 2004, 82, 2534–2540. [Google Scholar] [CrossRef] [PubMed]
- Montes, J.C.; Silva, H.V.R.; Chagas, L.M.; Bittar, R.E.; de Oliveira, M.E.F.; Pessoa, G.A.; Moreira, G.C.M. Aspects of sexual precocity and morphometry of uterus, placenta and embryos/fetuses in Piau breed and Commercial line gilts. Theriogenology 2018, 105, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Vallet, J.L.; Freking, B.A. Changes in fetal organ weights during gestation after selection for ovulation rate and uterine capacity in swine. J. Anim. Sci. 2006, 84, 2338–2345. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vallet, J.L.; Miles, J.R.; Freking, B.A. Effect of fetal size on fetal placental hyaluronan and hyaluronoglucosaminidases throughout gestation in the pig. Anim. Reprod. Sci. 2010, 118, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Wise, T.; Roberts, A.J.; Christenson, R.K. Relationships of light and heavy fetuses to uterine position, placental weight, gestational age, and fetal cholesterol concentrations. J. Anim. Sci. 1997, 75, 2197–2207. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.C.; Miles, J.R.; Brown-Brandl, T.M.; Lents, C.A.; Rempel, L.A. Uterine and placental characteristics during early vascular development in the pig from day 22 to 42 of gestation. Anim. Reprod. Sci. 2016, 164, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Finch, A.; Da Silva, P.; Ashworth, C.J. Patterns of fetal growth within Large White × Landrace and Chinese Meishan gilt litters at three stages of gestation. Reprod. Fertil. Dev. 2002, 14, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Vallet, J.L.; Freking, B.A. Differences in placental structure during gestation associated with large and small pig fetuses. J. Anim. Sci. 2007, 85, 3267–3275. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Hurley, W.; Kim, S. Characterization of mammary gland development in pregnant gilts. J. Anim. Sci. 2006, 84, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, M.T.; Sejrsen, K.; Purup, S. Mammary gland development in gilts. Livest. Prod. Sci. 2002, 75, 143–148. [Google Scholar] [CrossRef]
- Clowes, E.J.; Aherne, F.X.; Foxcroft, G.R.; Baracos, V.E. Phase-feeding protein to gestating sows over three parities reduced nitrogen excretion without affecting sow performance. Livest. Prod. Sci. 2003, 81, 235–246. [Google Scholar] [CrossRef]
- Corley, J.R.; Easter, R.A.; Norton, H.W.; Jensen, A.H. Amino acid supplementation of low-protein diets for swine: Effects of gestation treatment on reproductive performance of gilts and sows. J. Anim. Sci. 1983, 56, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.E.; Speer, V.C. Nitrogen requirement of pregnant gilts. J. Anim. Sci. 1991, 69, 2020–2025. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jones, R.D.; Maxwell, C.V. Growth, reproductive performance, and nitrogen balance of gilts as affected by protein intake and stage of gestation. J. Anim. Sci. 1982, 55, 848–856. [Google Scholar] [CrossRef] [PubMed]
- King, R.H.; Brown, W.G. Interrelationships between dietary protein level, energy intake, and nitrogen retention in pregnant gilts. J. Anim. Sci. 1993, 71, 2450–2456. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Li, X.; Ji, F.; Navales, R.A.; Beaulieu, A.D.; De Lange, C.F.M.; Kim, S.W. Variability in daily urinary nitrogen excretion of gestating gilts does not affect estimates of nitrogen retention. Can. J. Anim. Sci. 2018, 98, 603–605. [Google Scholar] [CrossRef]
- Navales, R.A.; Liu, H.; Ji, F.; Htoo, J.K.; Stein, H.H.; Kim, S.W. Efficiency of utilizing standardized ileal digestible lysine and threonine for whole-body protein retention in pregnant gilts during early, mid-, and late gestation. J. Anim. Sci. 2019, 97, 3016–3026. [Google Scholar] [CrossRef] [PubMed]
- Noblet, J.; Etienne, M. Metabolic utilization of energy and maintenance requirements in pregnant sows. Livest. Prod. Sci. 1987, 16, 243–257. [Google Scholar] [CrossRef]
- Ramirez-Camba, C.D.; Htoo, J.K.; Stein, H.H.; Kim, S.W. Efficiency of standardized ileal digestible lysine utilization for whole body protein deposition in pregnant gilts and sows during early-, mid-, and late-gestation. J. Anim. Sci. 2020, 98, skaa340. [Google Scholar] [CrossRef] [PubMed]
- Willis, G.M.; Maxwell, C.V. Influence of protein intake, energy intake, and stage of gestation on growth, reproductive performance, nitrogen balance, and carcass composition in gestating gilts. J. Anim. Sci. 1984, 58, 647–656. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, M.; Zhao, Y.; Liu, H.; Ji, F.; Kim, S.W. Dietary fiber in a low-protein diet during gestation affects nitrogen excretion in primiparous gilts, with possible influences from the gut microbiota. J. Anim. Sci. 2021, 99, skab121. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bazer, F.W.; Johnson, G.A.; Burghardt, R.C.; Wang, X.; Wu, G. Dietary supplementation with L-arginine between days 14 and 25 of gestation enhances embryonic development and survival in gilts. Amino Acids 2014, 46, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bazer, F.W.; Johnson, G.A.; Wang, X.; Wu, G. Dietary supplementation with L-citrulline improves placental angiogenesis and embryonic survival in gilts. Exp. Biol. Med. 2023, 248, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Cudd, T.A.; Meininger, C.J.; Spencer, T.E. Maternal dietary protein deficiency decreases amino acid concentrations in fetal plasma and allantoic fluid of pigs. J. Nutr. 1998, 128, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Self, J.T.; Spencer, T.E.; Johnson, G.A.; Hu, J.; Bazer, F.W.; Wu, G. Glutamine synthesis in the developing porcine placenta. Biol. Reprod. 2004, 70, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Cudd, T.A.; Meininger, C.J.; Spencer, T.E. Maternal dietary protein deficiency decreases nitric oxide synthase and ornithine decarboxylase activities in placenta and endometrium of pigs during early gestation. J. Nutr. 1998, 128, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Everts, H.; Dekker, R. Effect of protein supply during pregnancy on body composition of gilts and their products of conception. Livest. Prod. Sci. 1995, 43, 27–36. [Google Scholar] [CrossRef]
- Mahan, D.C.; Shields, R.G., Jr. Essential and nonessential amino acid composition of pigs from birth to 145 kilograms of body weight, and comparison to other studies. J. Anim. Sci. 1998, 76, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Liu, H.; Ji, F.; Kim, S.W. Effects of feeding growing-finishing pigs with low crude protein diets on growth performance, carcass characteristics, meat quality and nutrient digestibility in different areas of China. Anim. Feed Sci. Technol. 2019, 256, 114256. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, J.; Tor, M.; Pena, R.N.; Estany, J. Carcass lean-yield effects on the fatty acid and amino acid composition of Duroc pork and its technological quality after vacuum-aging. Anim. Prod. Sci. 2017, 58, 2335–2343. [Google Scholar] [CrossRef]
- Buis, R. Development and Application of a Precision Feeding Program Using Electronic Sow Feeders and Effect on Gestating Primiparous Sow Performance. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2016. Available online: https://bac-lac.on.worldcat.org/oclc/1252219753 (accessed on 15 March 2025).
- Thomas, L.L.; Stroshine, R.L.; Radcliffe, J.S.; Richert, B.T. Lessons learned from managing electronic sow feeders and collecting weights of gestating sows housed on a large commercial farm. J. Swine Health Prod. 2018, 26, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Stewart, V. Precision Feeding of Gestating Sows Across Three Parities Using Electronic Sow Feeders. Master’s Thesis, University of Guelph, Guelph, ON, Canada, 2020. [Google Scholar]
- Thomas, L.L. The Effects of Parity and Stage of Gestation on Whole Body and Maternal Growth and Feed Efficiency of Gestating Sows. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2017. Available online: http://hdl.handle.net/2097/35461 (accessed on 15 March 2025).
- Amoroso, E.C. Placentation. In Marshall’s Physiology of Reproduction, 3rd ed.; Parkes, A.S., Ed.; Longmans, Green and Co.: London, UK, 1952; pp. 127–311. [Google Scholar]
- Ripps, H.; Shen, W. Taurine: A “very essential” amino acid. Mol. Vis. 2012, 18, 2673. Available online: https://pubmed.ncbi.nlm.nih.gov/23170060/ (accessed on 15 March 2025). [PubMed]
- Li, R.; Sun, X.; Luo, Y.; Zhang, Y.; Deng, J. Concentration and composition of free amino acids and osmolalities of porcine oviductal and uterine fluid and their effects on development of porcine IVF embryos. Mol. Reprod. Dev. 2007, 74, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, H.; Albino, L.F.T.; Hannas, M.I.; Donzele, J.L.; Sakomura, N.K.; Perazzo, F.G. Brazilian Tables for Poultry and Swine: Composition of Feedstuffs and Nutrient Requirements, 5th ed.; Suprema: Visconde do Rio Branco, MG, Brazil, 2024; p. 529. [Google Scholar]
- Langendijk, P. Latest advances in sow nutrition during early gestation. Animals 2021, 11, 1720. [Google Scholar] [CrossRef] [PubMed]
- Ford, J., Jr.; Farmer, C.; Sorensen, M.T.; De Lange, C.F.M. Quantification of mammary gland tissue size and composition changes after weaning in sows. J. Anim. Sci. 2003, 81, 2583–2589. [Google Scholar] [CrossRef] [PubMed]
- Dourmad, J.-Y.; Etienne, M.; Noblet, J. Reconstitution of body reserves in multiparous sows during pregnancy: Effect of energy intake during pregnancy and mobilization during the previous lactation. J. Anim. Sci. 1996, 74, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Everts, H.; Dekker, R. Effect of nitrogen supply on the retention and excretion of nitrogen and on energy metabolism of pregnant sows. Anim. Sci. 1994, 59, 293–301. [Google Scholar] [CrossRef]
- Rippel, R.; Cromwell, G.L.; Hays, V.W.; Kratzer, D.D. Response of the gravid gilt to levels of protein as determined by nitrogen balance. J. Anim. Sci. 1965, 24, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Woerman, R.L.; Speer, V. Lysine requirement for reproduction in swine. J. Anim. Sci. 1976, 42, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Samuel, R.S. Maintenance Energy Metabolism in Non-Pregnant and Pregnant Sows. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2011. [Google Scholar] [CrossRef]
- Barash, V.; Shafrir, E. Mobilization of placental glycogen in diabetic rats. Placenta 1990, 11, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Coan, P.M.; Ferguson-Smith, A.C.; Burton, G.J. Origin and characteristics of glycogen cells in the developing murine placenta. Dev. Dyn. 2006, 235, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Tunster, S.J.; Creeth, H.D.J.; John, R.M. Placental glycogen stores and fetal growth: Insights from genetic mouse models. Reproduction 2020, 159, R213–R235. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; He, Y.; Chen, J.; Zhu, Y.; Du, H.; Shi, C.; Song, C. Isolation and characterization of porcine amniotic fluid-derived multipotent stem cells. PLoS ONE 2011, 6, e19964. [Google Scholar] [CrossRef] [PubMed]
- Loukogeorgakis, S.P.; De Coppi, P. Concise review: Amniotic fluid stem cells: The known, the unknown, and potential regenerative medicine applications. Stem Cells 2017, 35, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Fauza, D. Amniotic fluid and placental stem cells. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 877–891. [Google Scholar] [CrossRef] [PubMed]
- Ditadi, A.; Sturgeon, C.M.; Keller, G. Human and murine amniotic fluid c-Kit+ Lin− cells display hematopoietic activity. Blood 2009, 113, 3953–3960. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, A.A.; Anastasi, G.; Conforti, G.; Amato, A.; La Rocca, G.; Di Liegro, C.M. Endothelial properties of third-trimester amniotic fluid stem cells cultured in hypoxia. Stem Cell Res. Ther. 2015, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bagci, S.; Hamada, H.; Imanaka, M.; Suwa, M.; Morisawa, T.; Kondo, Y.; Koide, T. More than fetal urine: Enteral uptake of amniotic fluid as a major predictor for fetal growth during late gestation. Eur. J. Pediatr. 2016, 175, 825–831. [Google Scholar] [CrossRef] [PubMed]







| (1) | |
| (2) | |
| (3) | |
| (4) | |
| (5) | |
| (6) | |
| (7) | |
| (8) | |
| (9) | |
| (10) | |
| (11) | |
| (12) | |
| (13) | |
| (14) | |
| (15) | |
| (16) | |
| (17) | |
| (18) | |
| (19) | |
| (20) | |
| (21) | |
| (22) | |
| (23) | |
| (24) |
| Amino Acid | Placenta | Uterus | Fetus | ||||||
|---|---|---|---|---|---|---|---|---|---|
| a | b | c | a | b | c | a | b | c | |
| Lysine | 6.392 × 100 | 8.575 × 10−3 | −9.008 × 10−5 | 8.130 × 100 | −3.873 × 10−2 | 2.632 × 10−4 | 8.165 × 100 | −3.176 × 10−2 | 7.172 × 10−5 |
| Arginine | 8.575 × 100 | −3.387 × 10−2 | 2.242 × 10−4 | 8.519 × 100 | −4.057 × 10−2 | 2.575 × 10−4 | 5.900 × 100 | 2.906 × 10−3 | 5.373 × 10−5 |
| Histidine | 1.824 × 100 | 5.548 × 10−3 | −2.984 × 10−5 | 3.214 × 100 | −2.299 × 10−2 | 1.518 × 10−4 | 4.060 × 100 | −3.131 × 10−2 | 1.273 × 10−4 |
| Isoleucine | 4.561 × 100 | −1.869 × 10−2 | 9.358 × 10−5 | 4.213 × 100 | −2.015 × 10−2 | 1.402 × 10−4 | 3.634 × 100 | −5.129 × 10−3 | −2.704 × 10−6 |
| Leucine | 8.243 × 100 | −2.432 × 10−2 | 1.237 × 10−4 | 9.264 × 100 | −4.330 × 10−2 | 3.061 × 10−4 | 1.138 × 101 | −7.915 × 10−2 | 3.332 × 10−4 |
| Methionine | 1.996 × 100 | 1.483 × 10−3 | −1.556 × 10−5 | 1.721 × 100 | −1.554 × 10−3 | 1.718 × 10−5 | 1.801 × 100 | −4.641 × 10−3 | 2.314 × 10−5 |
| Cysteine | 1.003 × 100 | 3.290 × 10−3 | −2.163 × 10−5 | 2.598 × 100 | −2.283 × 10−2 | 1.352 × 10−4 | 2.331 × 100 | −2.528 × 10−2 | 1.480 × 10−4 |
| Phenylalanine | 5.282 × 100 | −1.015 × 10−2 | 4.402 × 10−5 | 5.359 × 100 | −3.044 × 10−2 | 2.054 × 10−4 | 6.099 × 100 | −4.309 × 10−2 | 1.799 × 10−4 |
| Threonine | 3.870 × 100 | 8.746 × 10−3 | −6.729 × 10−5 | 4.997 × 100 | −2.492 × 10−2 | 1.666 × 10−4 | 4.677 × 100 | −1.591 × 10−2 | 4.996 × 10−5 |
| Tryptophan | 1.162 × 100 | −3.337 × 10−3 | 2.441 × 10−5 | 1.119 × 100 | −3.121 × 10−3 | 2.485 × 10−5 | 1.543 × 100 | −1.244 × 10−2 | 4.742 × 10−5 |
| Valine | 5.832 × 100 | −1.189 × 10−2 | 5.929 × 10−5 | 6.013 × 100 | −2.781 × 10−2 | 2.009 × 10−4 | 7.709 × 100 | −5.614 × 10−2 | 2.431 × 10−4 |
| (25) | |
| (26) | |
| (27) | |
| (28) | |
| (29) | |
| (30) | |
| (31) | |
| (32) | |
| (33) | |
| (34) | |
| (35) | |
| (36) | |
| (37) | |
| (38) |
| (39) | |
| (40) | |
| (41) | |
| 00 | (42) |
| (43) | |
| (44) | |
| (45) | |
| (46) | |
| (47) | |
| (48) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez-Camba, C.D.; Urriola, P.E.; Levesque, C.L. Characterizing the Dynamic Protein and Amino Acid Deposition in Tissues of Pregnant Gilts: Implications for Stage-Specific Nutritional Strategies. Animals 2025, 15, 2126. https://doi.org/10.3390/ani15142126
Ramirez-Camba CD, Urriola PE, Levesque CL. Characterizing the Dynamic Protein and Amino Acid Deposition in Tissues of Pregnant Gilts: Implications for Stage-Specific Nutritional Strategies. Animals. 2025; 15(14):2126. https://doi.org/10.3390/ani15142126
Chicago/Turabian StyleRamirez-Camba, Christian D., Pedro E. Urriola, and Crystal L. Levesque. 2025. "Characterizing the Dynamic Protein and Amino Acid Deposition in Tissues of Pregnant Gilts: Implications for Stage-Specific Nutritional Strategies" Animals 15, no. 14: 2126. https://doi.org/10.3390/ani15142126
APA StyleRamirez-Camba, C. D., Urriola, P. E., & Levesque, C. L. (2025). Characterizing the Dynamic Protein and Amino Acid Deposition in Tissues of Pregnant Gilts: Implications for Stage-Specific Nutritional Strategies. Animals, 15(14), 2126. https://doi.org/10.3390/ani15142126









