1. Introduction
Aquatic organisms are continuously exposed to diverse microbial threats in their environment, requiring robust immune mechanisms to maintain homeostasis and prevent infection. In teleost fish, the immune system comprises both innate and adaptive components, with cytokines serving as key molecular messengers that coordinate immune responses [
1]. These small proteins mediate communication between immune cells, regulate inflammation, and orchestrate the activation, migration, and differentiation of leukocytes during pathogen exposure [
2].
Among the various cytokines,
tumor necrosis factor alpha (
TNFα) has emerged as a central pro-inflammatory mediator in both mammals and fish. It is known to induce programmed cell death, stimulate inflammatory cytokine cascades, and recruit innate immune cells to infection sites [
3]. In teleosts,
TNFα has been reported to enhance leukocyte activation, promote granulocyte infiltration, and modulate downstream signaling pathways essential for pathogen elimination [
4]. Studies in species such as zebrafish (
Danio rerio) [
5], rainbow trout (
Oncorhynchus mykiss) [
6], and olive flounder (
Paralichthys olivaceus) have demonstrated that
TNFα expression is induced upon bacterial and viral challenges in a tissue- and time-specific manner [
7,
8]. While
TNFα has been investigated in olive flounder, functional studies remain limited in other flatfish species, including the starry flounder, particularly regarding its biological role and potential biotechnological applications.
Beyond its role in immune regulation,
TNFα has recently gained attention as a potential molecular adjuvant—a biologically active agent capable of enhancing vaccine-induced immune responses when co-administered with antigens [
9]. Unlike traditional adjuvants such as mineral salts or emulsified oils, cytokine-based adjuvants provide immunological specificity by directly activating antigen-presenting cells and upregulating co-stimulatory molecules, which are essential for adaptive immunity [
10]. This immunostimulatory approach is of particular interest in aquaculture, where the development of effective vaccines is critical for reducing antibiotic use and controlling emerging infectious diseases.
To address these knowledge gaps, the present study focuses on the molecular and functional characterization of TNFα in the starry flounder, identified via transcriptomic analysis. We cloned the full-length cDNA of TNFα, produced recombinant protein using a cell-free expression system, and validated its biological activity through expression profiling, phagocytosis assays, and cytotoxicity evaluation. The aim of this study is to assess the immunological potential of recombinant TNFα (rTNFα) as a safe and effective molecular adjuvant in the starry flounder. The results are expected to support cytokine-based vaccine strategies and contribute to sustainable disease-management practices in aquaculture.
2. Materials and Methods
2.1. Ethics Statement, Experimental Animals, and Bacterial Strain
All experimental procedures involving animals were carried out in accordance with national ethical guidelines of South Korea and were approved by the Institutional Animal Care and Use Committee (IACUC) of the College of Marine Science, Gyeongsang National University (Approval No. GNU-241104-E0206; approved on 4 November 2024).
A total of 100 healthy starry flounders were obtained from a commercial aquaculture facility located in Pohang, Gyeongsangbuk-do, Republic of Korea. Fish were maintained in indoor recirculating seawater systems for a 2-week acclimation period and then held at a temperature of 25 ± 1 °C. Fish had an average total length of 21.5 ± 1.3 cm and an average body weight of 128.7 ± 18.2 g.
Prior to bacterial challenge, three individuals were randomly selected and examined for external and internal pathological signs to confirm their suitability for experimentation. The
Streptococcus parauberis strain PH0710 used in this study was obtained with official approval from the National Institute of Fisheries Science (Busan, Republic of Korea). Identification was performed by sequencing the 16S rRNA gene using the universal primer 27F (5′-AGAGTTTGATCMTGGCTCAG-3′), and sequence similarity was verified via BLAST (
http://www.ncbi.nlm.nih.gov/blast (accessed on 19 June 2025)) analysis in the NCBI database. A GenBank accession number is currently unavailable for this strain. Bacterial cultures were grown in Brain Heart Infusion (BHI) broth at 27 °C for 24 h, followed by centrifugation at 3000×
g for 10 min. The harvested cells were washed twice with sterile PBS, and the final concentration was adjusted to 1 × 10
7 CFU/mL, based on OD
600 measurements and confirmed through plate counting.
2.2. Sequence Validation and Gene Characterization of TNFα in the Starry Flounder
2.2.1. Cloning and Sequence Verification
The full-length open reading frame (ORF) of TNFα identified via NGS-based transcriptome analysis was validated by TA cloning and Sanger sequencing. Gene-specific primers were designed based on the terminal ORF sequences. PCR amplification was conducted using ExPrime Taq Premix (2×) (Genetbio, Daejeon, Republic of Korea) in a 20 µL reaction containing 10 µL of premix, 1 µL of each primer, 1 µL of cDNA template, and 7 µL of distilled water. Amplified products were electrophoresed on a 1.2% agarose gel containing 0.01% SafeView Classic (abm, New York, NY, USA), and target bands were extracted using the QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany).
Purified PCR products were ligated into the pGEM-T Easy Vector (Promega, Madison, WI, USA) and transformed into E. coli JM109 competent cells via heat shock. Following incubation in SOC medium at 37 °C for 4 h, the transformed cells were plated on LB agar supplemented with ampicillin, X-gal, and IPTG. Positive colonies were selected and cultured overnight in LB broth. Plasmids were extracted using the Hybrid-Q™ Plasmid Rapidprep Kit (GeneAll, Seoul, Republic of Korea), and plasmids were sequenced commercially (Bioneer, Daejeon, Republic of Korea).
2.2.2. Analysis and Phylogenetic Classification
The verified TNFα sequence was translated into an amino-acid sequence using GENETYX v8.0 (SDC Software Development, Tokyo, Japan). Homology searches were performed with BLAST against the NCBI protein database. Conserved domains and motifs were identified via the PROSITE tool and SMART database. Multiple sequence alignment was conducted using ClustalX2, and alignments were visualized in GeneDoc (version 2.7). A phylogenetic tree was constructed with MEGA v4.0 using the neighbor-joining method and 1000 bootstrap replicates to assess node reliability.
2.3. Expression Analysis of TNFα in the Starry Flounder
2.3.1. RNA Extraction and cDNA Synthesis
To evaluate tissue-specific and infection-induced expression of TNFα, total RNA was extracted from 12 tissues (brain, eye, gill, head kidney, heart, intestine, liver, muscle, skin, spleen, stomach, and trunk kidney) and blood-derived leukocytes from healthy starry flounder (n = 5). Peripheral blood was collected via caudal venipuncture and fractionated using a 53% Percoll gradient centrifugation to isolate leukocytes and erythrocytes.
For infection trials, fish were intraperitoneally injected with Streptococcus parauberis PH0710 (1 × 103 CFU/fish) suspended in PBS and maintained at 25 ± 1 °C. S. parauberis strain PH0710 was cultured in 15 mL of Brain Heart Infusion (BHI) broth (BD Difco™, Franklin Lakes, NJ, USA) at 27 °C for 24 h under constant agitation (200 rpm). Bacterial growth was monitored by measuring the optical density at 595 nm (OD595) and adjusted to 0.15, which corresponded to approximately 1 × 107 CFU/mL based on plate count validation. The culture was subsequently washed twice with sterile PBS and diluted to the target concentration of 1 × 103 CFU per 100 µL for injection. Tissues (brain, gill, heart, head kidney, liver, intestine, and spleen) were sampled at 0, 1, and 12 h, and at days 1, 3, 5, and 7 post-infection. All samples were stored at −80 °C until RNA isolation.
RNA extraction was carried out using RNAiso Plus (Takara, San Jose, CA, USA), followed by chloroform and PCI (phenol/chloroform/isoamyl alcohol, 25:24:1) phase separation. Genomic DNA was removed using recombinant DNase I (Takara, San Jose, CA, USA), and RNA was precipitated with isopropanol and sodium acetate. The final pellet was washed with 75% ethanol, dried, and resuspended in DEPC-treated water.
First-strand cDNA synthesis was performed using the PrimeScript™ 1st Strand cDNA Synthesis Kit (Takara, San Jose, CA, USA). Each 20 µL reaction included total RNA, random primers, dNTPs, reverse transcriptase, and RNase inhibitor, and followed the manufacturer’s protocol.
2.3.2. Real-Time PCR
Expression levels of TNFα were measured by SYBR Green-based real-time PCR using TB Green™ Premix Ex Taq™ (Tli RNaseH Plus) (Takara, San Jose, CA, USA). Each 25 µL reaction consisted of 12.5 µL of 2X premix, 1 µL each of forward and reverse primers (10 pmol), 1 µL of cDNA, and sterile distilled water. PCR was performed on a Thermal Cycler Dice® Real Time System III (Takara, San Jose, CA, USA) with the following conditions: 50 °C for 4 min, 95 °C for 10 min, followed by 45 cycles of 95 °C for 15 s and 60 °C for 30 s.
The elongation factor 1-alpha (EF-1α) gene was used as an internal control, and relative expression levels were calculated using the 2
−ΔΔCt method [
11]. Primer sequences are provided in
Table 1.
2.4. Production of Recombinant TNFα Protein
Recombinant TNFα protein was produced using a cell-free protein synthesis system (ExiProgen; Bioneer, Daejeon, Republic of Korea) in accordance with the manufacturer’s instructions. The TNFα gene was inserted into the pBIVT-2 expression vector containing a 6×His tag to facilitate purification. Protein synthesis was carried out at 30 °C in a reaction mixture comprising E. coli lysate supplemented with T7 RNA polymerase, ribosomes, tRNAs, amino acids, and an energy-regenerating system.
Following expression, the recombinant protein was purified using Ni-affinity chromatography and stored in a stabilization buffer (50 mM Tris-Cl, pH 7.6; 100 mM NaCl; 1 mM DTT; 0.1 mM EDTA; 0.05% NaN3; and 50% glycerol). Protein purity and molecular size were assessed via 12% SDS-PAGE, and concentration was quantified using the Bradford method with a commercial kit (Bio-Rad, Hercules, CA, USA).
2.5. Functional Evaluation of Recombinant TNFα as a Molecular Adjuvant
2.5.1. Phagocytic Activity Assay
Leukocytes were isolated from the kidney, spleen, and gill tissues of starry flounder using a 53% Percoll solution. The Percoll mixture was prepared by combining 26.5 mL of Percoll with 2.95 mL of 10× PBS and 20.55 mL of PBS. Tissues were passed through 100 µm strainers with 2 mL of RPMI medium (Sigma-Aldrich, Burlington, MA, USA), and cell suspensions were layered onto the 53% Percoll and centrifuged at 500× g for 15 min at 20 °C. The leukocyte layer was collected, washed twice with PBS, and erythrocytes were lysed with Red Blood Cell Lysing Buffer Hybri-Max (Sigma-Aldrich, Burlington, MA, USA). Final leukocyte suspensions were adjusted to 1 × 106 cells/mL.
Phagocytic activity was evaluated using S. parauberis PH0710 labeled with fluorescein isothiocyanate (FITC, 10 mg/mL in DMSO). Leukocytes were treated with FITC-S. parauberis in the presence of rTNFα at concentrations of 50, 100, 150, or 200 µg/mL. Control cells received only FITC-S. parauberis and PBS. All samples were incubated for 30 min at room temperature in the dark.
2.5.2. Hemolytic Activity Assay
Erythrocytes were isolated as described in
Section 2.5.1 and resuspended in PBS at a 4% concentration. rTNFα was diluted to six final concentrations (1, 10, 50, 100, 150, and 200 µg/mL). In a 96-well plate, 50 µL of erythrocyte suspension was mixed with 50 µL of each protein dilution. Positive controls were treated with 0.1% Triton X-100, and negative controls received PBS only. Following incubation at room temperature for 30 min, samples were centrifuged at 1000×
g for 10 min. Supernatants were transferred to a fresh plate, and absorbance was measured at 540 nm to quantify hemolytic activity.
2.5.3. Immune Induction by Molecular Adjuvants at Different Concentrations
To examine the time-dependent mRNA expression levels in the tissues of starry flounder stimulated with molecular adjuvants, rTNF protein was intraperitoneally injected into healthy fish at concentrations of 50, 100, and 150 μg/m. The fish were maintained at a water temperature of 15 ± 1 °C. At 0, 1, and 12 h and on days 1, 3, 5, and 7 post-injection, three fish were randomly selected, and their kidneys were excised. The tissues were stored at −80 °C until total RNA extraction. All protocols from RNA extraction to cDNA synthesis were performed as described in
Section 2.3.1.
mRNA expression levels of specific genes were measured by quantitative real-time PCR (RT-qPCR) with TB Green™ Premix Ex Taq™ (Takara, San Jose, CA, USA) using the SYBR Green method. The PCR reaction mixture consisted of 12.5 µL of TB Green Premix Ex Taq 2X (Tli RNaseH Plus, Takara Bio, San Jose, CA, USA), 1 µL each of the forward and reverse primers (10 pmol), 1 µL of cDNA template, and sterilized distilled water to a final volume of 25 µL. The specific primers used for the RT-qPCR analysis, including IL-1β, TNF, and EF-1α, along with their sequences, are provided below. PCR was performed using the Thermal Cycler Dice® Real-Time System III (Takara, San Jose, CA, USA) with the following conditions: 4 min at 50 °C, 10 min at 95 °C, followed by 45 cycles of 15 s at 95 °C and 30 s at 60 °C. The cycle threshold (Ct) values were normalized to those of elongation factor 1-alpha (EF-1α) using the delta delta Ct method. The specific primers used for the RT-qPCR analysis were as follows: IL-1β (forward: 5′-TGC AGT GGT CAA GAT GAT GGA-3′, reverse: 5′-AGT TGC GGT GAA GTC AAG CAG-3′), TNF (forward: 5′-ATC TGG AGA CCC ACG ACT GT-3′, reverse: 5′-TCC AGA AGA GGA TGT CGG TT-3′), and EF-1α (forward: 5′-TGA CGA GAT CGA GAA GTC CA-3′, reverse: 5′-GAC ATT GTC ACC AGG GAG GT-3′).
2.6. Statistical Analysis
All experiments were conducted in triplicate, and data are presented as mean ± standard deviation (SD). Statistical significance was determined by one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test using SPSS version 19 (IBM, Armonk, NY, USA). Differences were considered statistically significant at * p < 0.05 and highly significant at ** p < 0.01.
4. Discussion
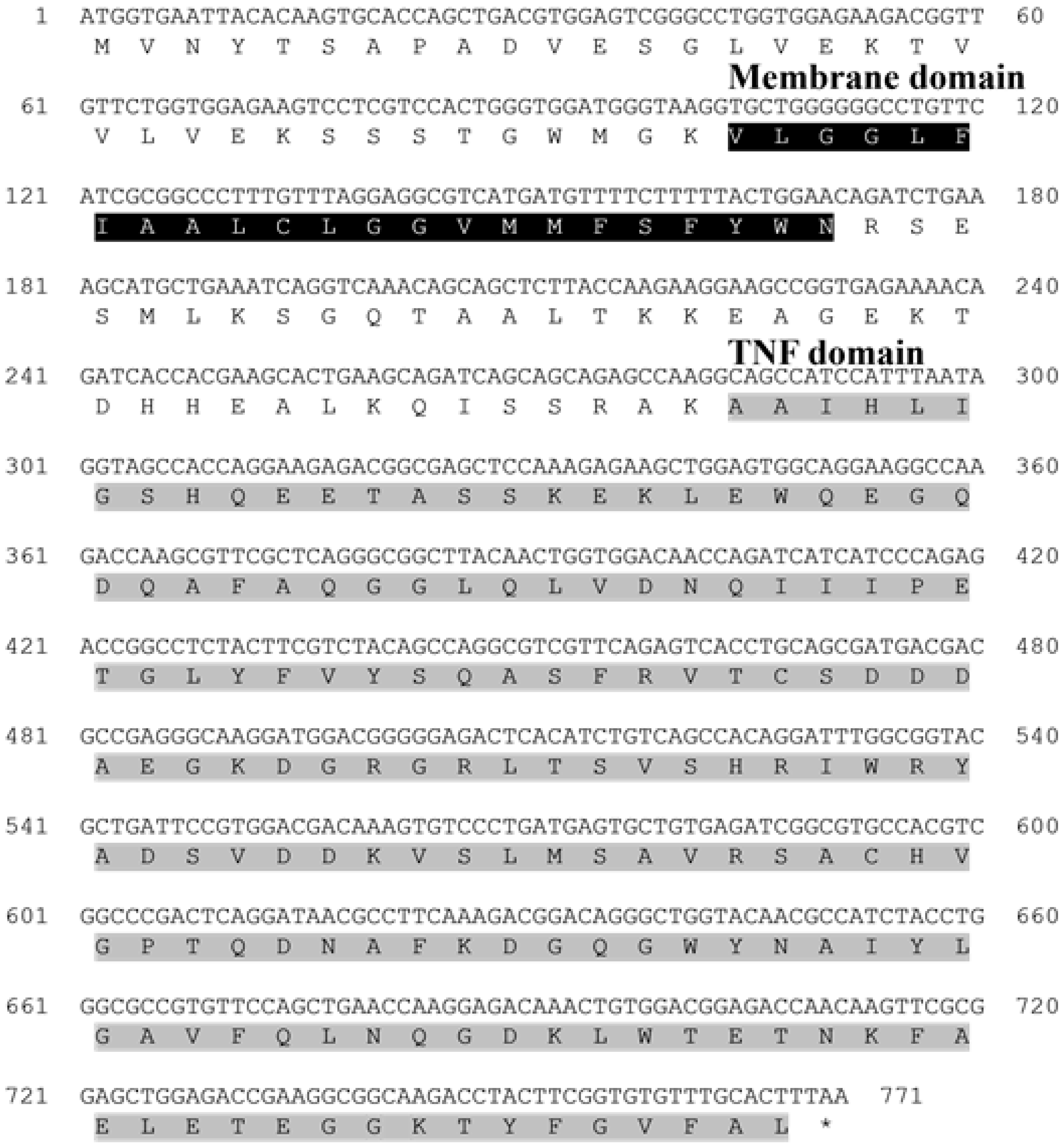
In this study, TNFα was identified in the starry flounder through next-generation sequencing (NGS) analysis, and its sequence characteristics were investigated. Conserved domains and functional motifs were annotated using the PROSITE profile database and the Simple Modular Architecture Research Tool (SMART). Multiple sequence alignment and phylogenetic analysis were performed to infer the potential immunological roles of the identified TNFα gene in the starry flounder.
TNFα is a key pro-inflammatory cytokine involved in the regulation of acute immune responses during infection in mammals. The PsTNFα gene identified in this study comprised a 771 bp open reading frame (ORF) encoding 257 amino acids. Structural analysis revealed the presence of a conserved TNF domain and a predicted transmembrane region, indicating that PsTNFα shares the typical features of the TNFα family. Sequence alignment showed 91.57% identity with European flounder TNFα, and phylogenetic analysis clustered PsTNFα with homologues from other marine teleosts, suggesting evolutionary conservation. These results indicate that PsTNFα likely plays a central role in the modulation of inflammatory responses and innate immunity in fish.
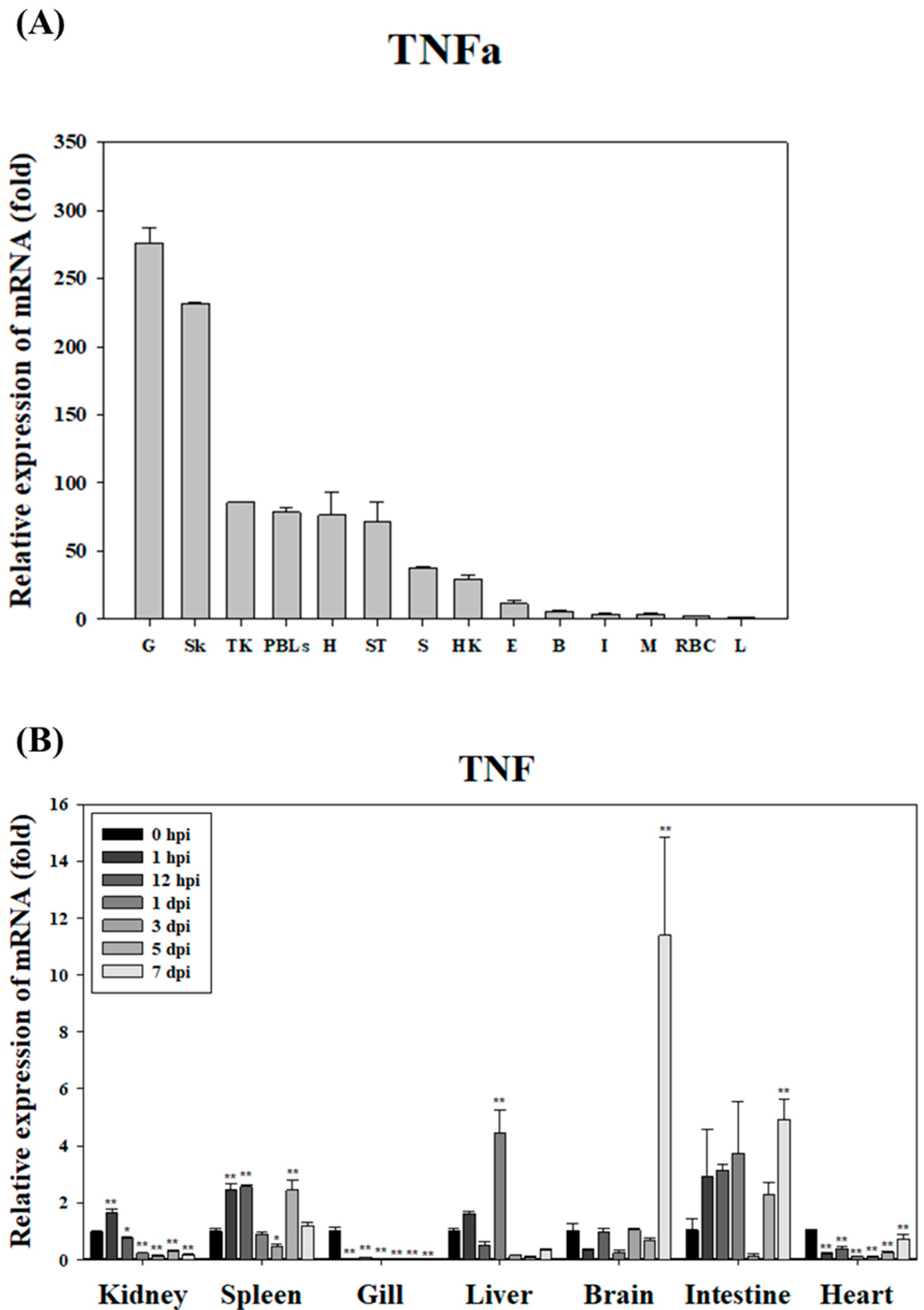
In this study,
TNFα expression in healthy starry flounder was found to be high in pathogen-contact tissues, such as the gills, skin, and head kidney. This suggests that
TNFα may promote inflammatory responses at the sites of pathogen invasion, thereby contributing to early defense mechanisms. Following infection, TNFα expression decreased in the gills and heart, but increased in certain tissues, such as the brain and intestine. This indicates that
TNFα may regulate immune responses in a tissue-specific manner. Notably, the significant increase in
TNFα expression observed in the brain on day 7 post-infection implies that the central nervous system may play a role in regulating inflammation and controlling the spread of infection. This finding suggests that the nervous system could be an integral part of the immune system. Tissue-specific expression of
TNFα has also been reported in white crucian carp, where recombinant TNFα1 played a crucial role in gut immune regulation following pathogen exposure, underscoring its diverse immunomodulatory functions across species [
12].
These results align with previous studies showing that
TNFα acts as a regulator of inflammation and immune responses during the early stages of infection in fish [
2]. Similarly, studies have reported that
TNFα expression in mollusks and fish is differentially regulated across tissues depending on bacterial or viral stimuli [
13]. This study suggests that
TNFα in the starry flounder may also possess mechanisms to regulate its expression in specific tissues to respond effectively to infections. Therefore,
TNFα appears to play an important role in inflammation and early defense against infections in the starry flounder, with its tissue-specific expression representing a strategic mechanism for localized immune responses.
In the present study, we successfully produced and characterized rTNFα derived from starry flounder and confirmed its immunological activity through a series of functional assays. Functionally, rTNFα demonstrated a concentration-dependent enhancement of phagocytic activity in both peripheral blood leukocytes (PBLs) and trunk kidney leukocytes. Maximal activation occurred at 150 μg/mL, supporting the notion that TNFα serves as a key regulator of innate immune responses in fish. Interestingly, PBLs and trunk kidney leukocytes showed slightly different response patterns, possibly due to differences in cell composition and activation thresholds, as trunk kidney leukocytes include a more heterogeneous population, including hematopoietic precursors. These findings are consistent with previous studies reporting that recombinant TNFα enhances phagocytic function in various teleost species [
14,
15,
16,
17,
18]. For example, in the rainbow trout (
Oncorhynchus mykiss),
TNFα stimulation increased macrophage respiratory burst and phagocytosis, while in the zebrafish (
Danio rerio), recombinant TNFα induced inflammatory gene expression and phagocytic activation in macrophage-like cells [
16]. These parallels suggest that the enhanced phagocytosis observed in our study reflects conserved biological activity of
TNFα across fish species. The immune-enhancing effect of rTNFα is likely mediated through
TNF receptor signaling pathways such as
TNFR1 and
TNFR2, which activate downstream effectors, including NF-κB and MAPKs, leading to increased immune-cell activation and antimicrobial activity. In our study, phagocytic activity slightly declined at the highest concentration (200 μg/mL), suggesting a possible feedback inhibition or functional exhaustion, as previously observed with other immunostimulants such as levamisole and β-glucans [
19,
20,
21]. This highlights the importance of optimizing cytokine dosage to maximize immunostimulatory effects while avoiding overstimulation. Overall, our results demonstrate that rTNFα can effectively activate innate immune cells in vitro without inducing cytotoxicity, as confirmed by hemolysis assays. These findings support the potential utility of rTNFα as an immunostimulant in aquaculture, particularly in vaccine adjuvant strategies aimed at enhancing early-phase immune responses and reducing reliance on antibiotics.
The observed upregulation of IL-1β and TNF mRNA following rTNFα stimulation confirms the immunostimulatory potential of the recombinant protein in the starry flounder. IL-1β is a key pro-inflammatory cytokine that plays a central role in initiating innate immune responses, while TNF is involved in amplifying inflammatory signaling and regulating apoptosis. The significant induction of both genes at early time points suggests that rTNFα effectively activates inflammatory pathways, thereby enhancing the host’s ability to respond to pathogenic threats. These findings support the hypothesis that rTNFα can serve as a functional molecular adjuvant by modulating key immune mediators in fish.
Collectively, these findings suggest that TNFα, beyond its canonical pro-inflammatory role, holds promise as a molecular adjuvant in fish immunology.
Its ability to boost phagocytic activity, paired with a favorable safety profile, supports its application in the design of next-generation vaccines. Notably, recombinant TNFα has also been shown to confer protective effects as an oral vaccine adjuvant in the European sea bass, where it enhanced mucosal immune responses against
Vibrio infection through modulation of the CCL25/CCR9 axis [
22]. Unlike traditional adjuvants, cytokines like
TNFα may offer precise immunological tuning with minimal off-target effects.
Future work should expand toward in vivo validation and field-based trials to assess its adjuvant efficacy under commercial aquaculture conditions. The foundational insights provided by this study may inform cytokine engineering strategies and contribute to sustainable fish health management by reducing antibiotic dependency.
To account for the possibility of nonspecific immune stimulation, the recombinant rTNFα protein used in this study was purified through Triton X-114 phase separation, a method widely used to remove endotoxin contamination. Although a direct quantification of endotoxin levels (e.g., via LAL assay) was not performed, several lines of evidence support the biological activity of rTNFα as the primary cause of the observed immune responses. The purified protein was confirmed by SDS-PAGE and Western blotting, and it induced a concentration-dependent increase in phagocytic activity, which aligns with the known functional role of TNFα in immune-cell activation. Moreover, the hemolysis assay confirmed the absence of cytotoxicity, indicating that the immune effects were not caused by nonspecific cell damage. Nevertheless, we recognize the importance of further validating these results through the inclusion of endotoxin-only controls in future studies.