Effects of Andrographolide-Loaded Nanostructured Lipid Carriers on Growth, Feed Efficiency, and Resistance to Streptococcus agalactiae in Nile Tilapia (Oreochromis niloticus)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Andrographolide-Loaded Nanostructured Lipid Carriers
2.3. Particle Characterization
2.4. Encapsulation Efficiency and In Vitro Release of AND-NLC
2.5. Fourier-Transform Infrared (FTIR) Spectroscopy Analysis
2.6. Evaluation of Antimicrobial Activity
2.7. Evaluation of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.8. Feed Supplementation
2.9. Experimental Design
2.10. Growth Performance and Feed Utilization
2.11. Length–Weight Relationship, Growth Pattern, and Relative Condition Factor
2.12. Disease Resistance of Nile Tilapia Against S. agalactiace ENC06
2.13. Statistical Analysis
3. Results
3.1. Physicochemical Properties
3.2. FTIR Analysis
3.3. Antibacterial Activity
3.4. Effect of Experimental Diets on Growth Performance and Feed Utilization of Nile Tilapia
3.5. Effect of Time on Growth Performance and Feed Utilization of Experimental Diets
3.6. Effects of Experimental Diets on Hepatosomatic Index
3.7. Effects of Experimental Diets on Length–Weight Relationship and Growth Pattern
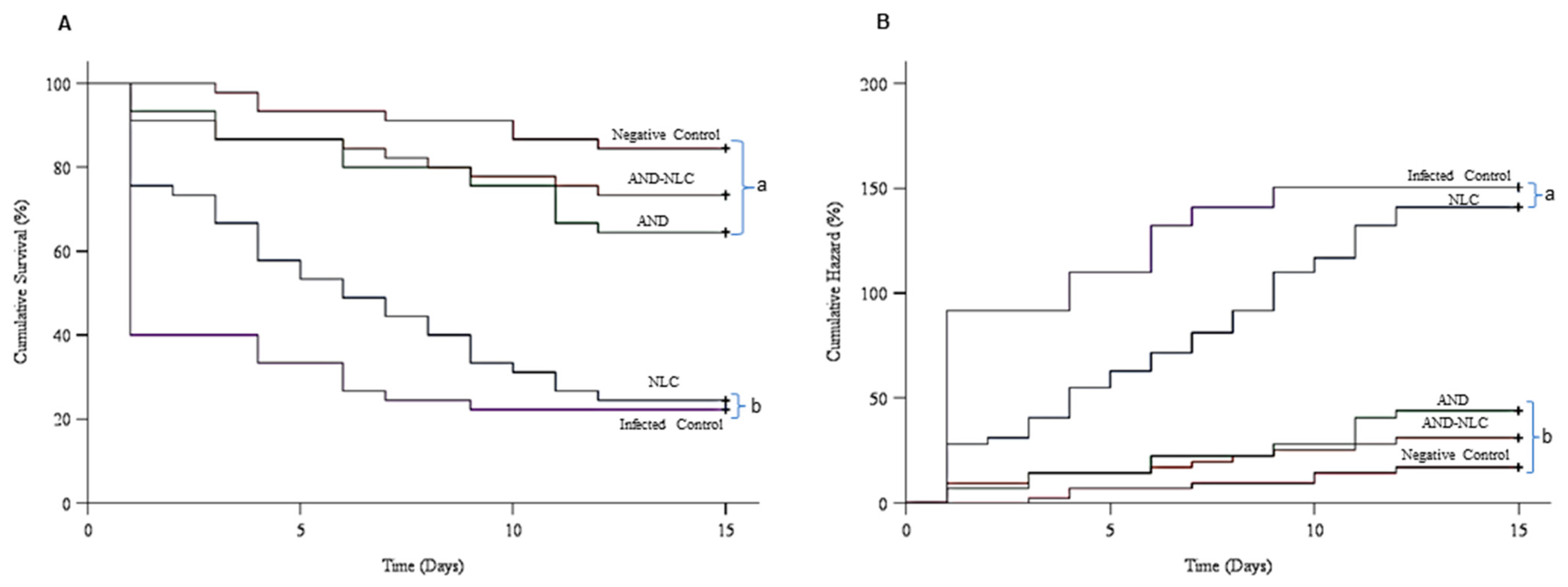
3.8. Effects of Experimental Diets on Survivorship of Nile Tilapia Following S. agalactiae Challenge (ENC06)
3.9. Cox Regression Analysis of Treatment Effects on Survival Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2023: Urbanization, Agrifood Systems Transformation and Healthy Diets Across the Rural–Urban Continuum; Food & Agriculture Organization: Rome, Italy, 2023; Volume 2023. [Google Scholar]
- Bjørndal, T.; Dey, M.; Tusvik, A. Economic analysis of the contributions of aquaculture to future food security. Aquaculture 2024, 578, 740071. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2024—Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar]
- El-Sayed, A.-F.M. Tilapia Culture; CABI Publishing: Wallingford, UK, 2006. [Google Scholar]
- Ibrahim, R.E.; Elshopakey, G.E.; Abd El-Rahman, G.I.; Ahmed, A.I.; Altohamy, D.E.; Zaglool, A.W.; Younis, E.M.; Abdelwarith, A.A.; Davies, S.J.; Al-Harthi, H.F. Palliative role of colloidal silver nanoparticles synthetized by moringa against Saprolegnia spp. infection in Nile Tilapia: Biochemical, immuno-antioxidant response, gene expression, and histopathological investigation. Aquac. Rep. 2022, 26, 101318. [Google Scholar] [CrossRef]
- Mahboub, H.H.; Nada, H.S.; Abdel-Ghany, H.M.; Ghanem, R.; Ahmed Ismail, T.; Abdel Rahman, A.N. Detection, diagnosis, Koch’s postulate, hepatorenal and antioxidant indicators for some systemic pathogenic fungi invading the liver and kidneys of African catfish (Clarias gariepinus) in Egypt with a histopathological approach. Aquac. Res. 2022, 53, 2670–2685. [Google Scholar] [CrossRef]
- Abdallah, E.S.H.; Metwally, W.G.M.; Abdel-Rahman, M.A.M.; Albano, M.; Mahmoud, M.M. Streptococcus agalactiae Infection in Nile Tilapia (Oreochromis niloticus): A Review. Biology 2024, 13, 914. [Google Scholar] [CrossRef] [PubMed]
- Kamble, M.T.; Gallardo, W.; Salin, K.R.; Pumpuang, S.; Chavan, B.R.; Bhujel, R.C.; Medhe, S.V.; Kettawan, A.; Thompson, K.D.; Pirarat, N. Effect of Moringa oleifera leaf extract on the growth performance, hematology, innate immunity, and disease resistance of Nile tilapia (Oreochromis niloticus) against Streptococcus agalactiae Biotype 2. Animals 2024, 14, 953. [Google Scholar] [CrossRef] [PubMed]
- Kayansamruaj, P.; Pirarat, N.; Kondo, H.; Hirono, I.; Rodkhum, C. Genomic comparison between pathogenic Streptococcus agalactiae isolated from Nile tilapia in Thailand and fish-derived ST7 strains. Infect. Genet. Evol. 2015, 36, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Kayansamruaj, P.; Pirarat, N.; Katagiri, T.; Hirono, I.; Rodkhum, C. Molecular characterization and virulence gene profiling of pathogenic Streptococcus agalactiae populations from tilapia (Oreochromis sp.) farms in Thailand. J. Vet. Diagn. Investig. 2014, 26, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Suanyuk, N.; Kong, F.; Ko, D.; Gilbert, G.L.; Supamattaya, K. Occurrence of rare genotypes of Streptococcus agalactiae in cultured red tilapia Oreochromis sp. and Nile tilapia O. niloticus in Thailand—Relationship to human isolates? Aquaculture 2008, 284, 35–40. [Google Scholar] [CrossRef]
- Evans, J.J.; Bohnsack, J.F.; Klesius, P.H.; Whiting, A.A.; Garcia, J.C.; Shoemaker, C.A.; Takahashi, S. Phylogenetic relationships among Streptococcus agalactiae isolated from piscine, dolphin, bovine and human sources: A dolphin and piscine lineage associated with a fish epidemic in Kuwait is also associated with human neonatal infections in Japan. J. Med. Microbiol. 2008, 57, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Bohnsack, J.F.; Takahashi, S.; Oliver, K.A.; Chan, M.-S.; Kunst, F.; Glaser, P.; Rusniok, C.; Crook, D.W.; Harding, R.M. Multilocus sequence typing system for group B streptococcus. J. Clin. Microbiol. 2003, 41, 2530–2536. [Google Scholar] [CrossRef] [PubMed]
- Delannoy, C.M.; Zadoks, R.N.; Crumlish, M.; Rodgers, D.; Lainson, F.A.; Ferguson, H.; Turnbull, J.; Fontaine, M.C. Genomic comparison of virulent and non-virulent Streptococcus agalactiae in fish. J. Fish Dis. 2016, 39, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Delannoy, C.M.; Crumlish, M.; Fontaine, M.C.; Pollock, J.; Foster, G.; Dagleish, M.P.; Turnbull, J.F.; Zadoks, R.N. Human Streptococcus agalactiae strains in aquatic mammals and fish. BMC Microbiol. 2013, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Kayansamruaj, P.; Soontara, C.; Unajak, S.; Dong, H.T.; Rodkhum, C.; Kondo, H.; Hirono, I.; Areechon, N. Comparative genomics inferred two distinct populations of piscine pathogenic Streptococcus agalactiae, serotype Ia ST7 and serotype III ST283, in Thailand and Vietnam. Genomics 2019, 111, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Kannika, K.; Pisuttharachai, D.; Srisapoome, P.; Wongtavatchai, J.; Kondo, H.; Hirono, I.; Unajak, S.; Areechon, N. Molecular serotyping, virulence gene profiling and pathogenicity of Streptococcus agalactiae isolated from tilapia farms in Thailand by multiplex PCR. J. Appl. Microbiol. 2017, 122, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, N.N.; Abasubong, K.P.; Erasmus, V.N.; Kamble, M.T. Sustainable Feed Ingredients and Additives for Aquaculture Farming; Springer: Singapore, 2024. [Google Scholar]
- Pepi, M.; Focardi, S. Antibiotic-resistant bacteria in aquaculture and climate change: A challenge for health in the Mediterranean area. Int. J. Environ. Res. Public Health 2021, 18, 5723. [Google Scholar] [CrossRef] [PubMed]
- Kamble, M.T.; Chavan, B.R.; Ataguba, G.; Azpeitia, T.; Medhe, S.; Jain, S.; Jadhav, R. Application of Moringa oleifera for development of sustainable and biosecure aquaculture. Aquac. Indones. 2015, 15, 64–73. [Google Scholar] [CrossRef]
- Kamble, M.T.; Soowannayan, C.; Chaicherd, S.; Medhe, S.V.; Rudtanatip, T.; Pissuwan, D.; Wongprasert, K. Bimetallic nanoparticles with sulfated galactan eliminate Vibrio parahaemolyticus in shrimp Penaeus vannamei. Fish Shellfish Immunol. 2024, 151, 109753. [Google Scholar] [CrossRef] [PubMed]
- Kamble, M.T.; Salin, K.R.; Chavan, B.R.; Medhe, S.V.; Thompson, K.D.; Pirarat, N. Length-weight relationship and condition factor of Nile tilapia (Oreochromis niloticus) fed diets supplemented with guava and star gooseberry leaf extract. F1000Research 2024, 13, 540. [Google Scholar] [CrossRef] [PubMed]
- Trullàs, C.; Sewaka, M.; Rodkhum, C.; Chansue, N.; Boonanuntanasarn, S.; Kamble, M.T.; Pirarat, N. Effects of jerusalem artichoke (Helianthus tuberosus) as a prebiotic supplement in the diet of red tilapia (Oreochromis spp.). Animals 2022, 12, 2882. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, R.; Zaini Asmawi, M.; Sadikun, A. A bitter plant with a sweet future? A comprehensive review of an oriental medicinal plant: Andrographis paniculata. Phytochem. Rev. 2012, 11, 39–75. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204. [Google Scholar] [CrossRef] [PubMed]
- Subash, K. In silico pharmacokinetic and toxicological properties prediction of bioactive compounds from Andrographis paniculata. Natl. J. Physiol. Pharm. Pharmacol. 2020, 10, 537–542. [Google Scholar]
- Lapmanee, S.; Rimsueb, N.; Bunwatcharaphansakun, P.; Namdee, K.; Wongchitrat, P.; Bhubhanil, S.; Supkamonseni, N.; Charoenphon, N.; Inchan, A.; Saenmuangchin, R. Oral andrographolide loaded lipid nanocarriers alleviate stress behaviors and hippocampal damage in TNF alpha induced neuroinflammatory mice. Sci. Rep. 2025, 15, 11939. [Google Scholar] [CrossRef] [PubMed]
- Dafur, G.S.; Harun, A.; Kub, T.N.T.; Bakar, R.A.; Harun, A. A Systematic Review on the Antimicrobial Activity of Andrographolide. J. Microbiol. Biotechnol. 2024, 35, e2408028. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Li, H.; Gao, S.; Quan, Y.; Wang, Y.; Yi, L.; Wang, Y. Andrographolide reverses the susceptibility of Streptococcus suis to aminoglycoside antibiotics by proton motive force. BMC Vet. Res. 2025, 21, 63. [Google Scholar] [CrossRef] [PubMed]
- Basha, K.A.; Raman, R.P.; Prasad, K.P.; Kumar, K.; Nilavan, E.; Kumar, S. Effect of dietary supplemented andrographolide on growth, non-specific immune parameters and resistance against Aeromonas hydrophila in Labeo rohita (Hamilton). Fish Shellfish Immunol. 2013, 35, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Fang, L.; Du, G. Andrographolide. In Natural Small Molecule Drugs from Plants; Springer: Singapore, 2018; Volume 1, pp. 357–362. [Google Scholar]
- Songvut, P.; Boonyarattanasoonthorn, T.; Nuengchamnong, N.; Junsai, T.; Kongratanapasert, T.; Supannapan, K.; Khemawoot, P. Enhancing oral bioavailability of andrographolide using solubilizing agents and bioenhancer: Comparative pharmacokinetics of Andrographis paniculata formulations in beagle dogs. Pharm. Biol. 2024, 62, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Yostawonkul, J.; Kitiyodom, S.; Kamble, M.T.; Supchukun, K.; Saengkrit, N.; Sukkarun, P.; Medhe, S.V.; Thompson, K.D.; Boonrungsiman, S.; Temisak, S. Immersion of nanostructured lipid carriers loaded with 17-alpha methyltestosterone for masculinization of red tilapia (Oreochromis sp.). Aquaculture 2024, 586, 740780. [Google Scholar] [CrossRef]
- Yostawonkul, J.; Kitiyodom, S.; Supchukun, K.; Thumrongsiri, N.; Saengkrit, N.; Pinpimai, K.; Hajitou, A.; Thompson, K.D.; Rattanapinyopituk, K.; Maita, M.; et al. Masculinization of red tilapia (Oreochromis spp.) using 17α-methyltestosterone-loaded alkyl polyglucosides integrated into nanostructured lipid carriers. Animals 2023, 13, 1364. [Google Scholar] [CrossRef] [PubMed]
- Kaewmalun, S.; Yata, T.; Kitiyodom, S.; Yostawonkul, J.; Namdee, K.; Kamble, M.T.; Pirarat, N. Clove oil-nanostructured lipid carriers: A platform of herbal anesthetics in whiteleg shrimp (Penaeus vannamei). Foods 2022, 11, 3162. [Google Scholar] [CrossRef] [PubMed]
- Yostawonkul, J.; Kamble, M.T.; Sakuna, K.; Madyod, S.; Sukkarun, P.; Medhe, S.V.; Rodkhum, C.; Pirarat, N.; Sewaka, M. Effects of Mangosteen (Garcinia mangostana) peel extract loaded in nanoemulsion on growth performance, immune response, and disease resistance of Nile tilapia (Oreochromis niloticus) against Aeromonas veronii infection. Animals 2023, 13, 1798. [Google Scholar] [CrossRef] [PubMed]
- Kamble, M.T.; Rudtanatip, T.; Soowannayan, C.; Nambunruang, B.; Medhe, S.V.; Wongprasert, K. Depolymerized fractions of sulfated galactans extracted from Gracilaria fisheri and their antibacterial activity against Vibrio parahaemolyticus and Vibrio harveyi. Mar. Drugs 2022, 20, 469. [Google Scholar] [CrossRef] [PubMed]
- Pirarat, N.; Pinpimai, K.; Chankow, K.; Malila, K.; Chansue, N.; Niyomtham, W.; Rodkhum, C. In vitro efficacy of human-derived probiotic, Lactobacillus rhamnosus against pathogenic bacteria in fish and frogs. Thai J. Vet. Med. 2015, 39, 305–310. [Google Scholar] [CrossRef]
- Pauly, D. Some Simple Methods for the Assessment of Tropical Fish Stocks; Food & Agriculture Organization: Rome, Italy, 1983. [Google Scholar]
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Le Cren, E.D. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201–219. [Google Scholar] [CrossRef]
- Amend, D.F. Potency testing of fish vaccines. Fish Biol. Serodiagn. Vaccines 1981, 49, 447–454. [Google Scholar]
- Luque-Alcaraz, A.G.; Maldonado-Arriola, J.A.; Hernández-Abril, P.A.; Álvarez-Ramos, M.E.; Hernández-Téllez, C.N. Zein nanoparticles loaded with Vitis vinifera L. grape pomace extract: Synthesis and characterization. Nanomaterials 2025, 15, 539. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, Q.-j.; Miao, Y.-L.; Luo, S.-Q.; Wang, H.-C.; Zhang, W.-P. Effect of solid lipid’s structure on nanostructured lipid carriers encapsulated with sun filter: Characterisation, photo-stability and in vitro release. J. Microencapsul. 2017, 34, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Lúcio, M.; Giannino, N.; Barreira, S.; Catita, J.; Gonçalves, H.; Ribeiro, A.; Fernandes, E.; Carvalho, I.; Pinho, H.; Cerqueira, F. Nanostructured lipid carriers enriched hydrogels for skin topical administration of quercetin and omega-3 fatty acid. Pharmaceutics 2023, 15, 2078. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.C.; Yañez, O.; Salas-Huenuleo, E.; Morales, J.O. Development of a nanostructured lipid carrier (NLC) by a low-energy method, comparison of release kinetics and molecular dynamics simulation. Pharmaceutics 2021, 13, 531. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Sobral, P.J.d.A. Plant protein-based delivery systems: An emerging approach for increasing the efficacy of lipophilic bioactive compounds. Molecules 2021, 27, 60. [Google Scholar] [CrossRef] [PubMed]
- Maeki, M.; Uno, S.; Niwa, A.; Okada, Y.; Tokeshi, M. Microfluidic technologies and devices for lipid nanoparticle-based RNA delivery. J. Control. Release 2022, 344, 80–96. [Google Scholar] [CrossRef] [PubMed]
- De, A.K.; Bera, T. Analytical method development, validation and stability studies by RP-HPLC method for simultaneous estimation of andrographolide and curcumin in co-encapsulated nanostructured lipid carrier drug delivery system. Int. J. Appl. Pharm. 2021, 13, 73–86. [Google Scholar] [CrossRef]
- Indrati, O.; Martien, R.; Rohman, A.; Nugroho, A.K. Development of nanoemulsion-based hydrogel containing andrographolide: Physical properties and stability evaluation. J. Pharm. Bioallied Sci. 2020, 12, S816–S820. [Google Scholar] [CrossRef] [PubMed]
- Surini, S.; Nastiti, P.D.; Putri, A.R.; Putri, K.S. Formulation of andrographolide transfersomes gel for transdermal delivery: A preliminary study. Int. J. Appl. Pharm. 2020, 12, 187–191. [Google Scholar] [CrossRef]
- Vairo, C.; Basas, J.; Pastor, M.; Palau, M.; Gomis, X.; Almirante, B.; Gainza, E.; Hernandez, R.; Igartua, M.; Gavaldà, J. In vitro and in vivo antimicrobial activity of sodium colistimethate and amikacin-loaded nanostructured lipid carriers (NLC). Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102259. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Wang, G.; Li, N.; Wang, X.; Kang, X.; Mao, Y.; Wang, G. Insights into the effects and mechanism of andrographolide-mediated recovery of susceptibility of methicillin-resistant Staphylococcus aureus to β-Lactam Antibiotics. Microbiol. Spectr. 2023, 11, e0297822. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Jiang, X.; Xu, X.; Jiang, C.; Kang, R.; Jiang, X. Andrographolide inhibits biofilm and virulence in listeria monocytogenes as a quorum-sensing inhibitor. Molecules 2022, 27, 3234. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef] [PubMed]
- Cé, R.; Pacheco, B.Z.; Ciocheta, T.M.; Barbosa, F.S.; de CS Alves, A.; Dallemole, D.R.; Lavayen, V.; Guterres, S.S.; Steppe, M.; Pohlmann, A.R. Antibacterial activity against Gram-positive bacteria using fusidic acid-loaded lipid-core nanocapsules. React. Funct. Polym. 2021, 162, 104876. [Google Scholar] [CrossRef]
- Alalaiwe, A.; Wang, P.-W.; Lu, P.-L.; Chen, Y.-P.; Fang, J.-Y.; Yang, S.-C. Synergistic anti-MRSA activity of cationic nanostructured lipid carriers in combination with oxacillin for cutaneous application. Front. Microbiol. 2018, 9, 1493. [Google Scholar] [CrossRef] [PubMed]
- Manan, A.; Aqib, A.I.; Shahbaz, A.; Khan, S.R.; Akram, K.; Majeed, H.; Muneer, A.; Murtaza, M.; Afrasiab, M.; Merola, C. Modification of the drug resistance of emerging milk-borne pathogens through sodium alginate-based antibiotics and nanoparticles. Front. Vet. Sci. 2023, 10, 1130130. [Google Scholar] [CrossRef] [PubMed]
- Talib, A.; Manzoor, K.; Ijaz, A.; Adnan, F.; Javed, F.; Khan, A. Encapsulated virgin coconut oil as a nanoscale in vitro solution against multiple drug resistant Staphylococcus aureus. Micro Nano Lett. 2021, 16, 9–15. [Google Scholar] [CrossRef]
- Syukri, Y.; Martien, R.; Lukitaningsih, E.; Nugroho, A.E. Novel Self-Nano Emulsifying Drug Delivery System (SNEDDS) of andrographolide isolated from Andrographis paniculata Nees: Characterization, in-vitro and in-vivo assessment. J. Drug Deliv. Sci. Technol. 2018, 47, 514–520. [Google Scholar] [CrossRef]
- Sridharan, B.; Lee, M.-J. Current developments in nano/micro-formulations for enhanced delivery and bioactivity of andrographolide. Mater. Today: Proc. 2021, 45, 4746–4752. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Eissa, E.-S.H.; Tawfik, W.A.; Abd Elnabi, H.E.; Saadony, S.; Bazina, W.K.; Ahmed, R.A. Dietary curcumin nanoparticles promoted the performance, antioxidant activity, and humoral immunity, and modulated the hepatic and intestinal histology of Nile tilapia fingerlings. Fish Physiol. Biochem. 2022, 48, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M.; Razek, N.A.; Abdel-Rahman, A.M. Immunostimulatory effect of dietary chitosan nanoparticles on the performance of Nile tilapia, Oreochromis niloticus (L.). Fish Shellfish Immunol. 2019, 88, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Ashry, A.M.; Hassan, A.M.; Habiba, M.M.; El-Zayat, A.; El-Sharnouby, M.E.; Sewilam, H.; Dawood, M.A. The impact of dietary curcumin on the growth performance, intestinal antibacterial capacity, and haemato-biochemical parameters of gilthead seabream (Sparus aurata). Animals 2021, 11, 1779. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to enhance the bioavailability and physiological functions of polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef] [PubMed]
- Chari, F.H.; Akrami, R.; Ghelichi, A.; Ebrahimi, P. The effect of Lavandula officinalis nanoemulsion on growth performance, body composition, haematology and immunity parameters of Oncorhynchus mykiss. J. Appl. Anim. Res. 2020, 48, 340–347. [Google Scholar] [CrossRef]
- Abd El-Hamid, M.I.; Ibrahim, S.M.; Eldemery, F.; El-Mandrawy, S.A.; Metwally, A.S.; Khalifa, E.; Elnahriry, S.S.; Ibrahim, D. Dietary cinnamaldehyde nanoemulsion boosts growth and transcriptomes of antioxidant and immune related genes to fight Streptococcus agalactiae infection in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2021, 113, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Min, T. Curcumin, curcumin nanoparticles and curcumin nanospheres: A review on their pharmacodynamics based on monogastric farm animal, poultry and fish nutrition. Pharmaceutics 2020, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Eissa, E.-S.H.; Alaidaroos, B.A.; Jastaniah, S.D.; Munir, M.B.; Shafi, M.E.; Abd El-Aziz, Y.M.; Bazina, W.K.; Ibrahim, S.b.; Eissa, M.E.; Paolucci, M. Dietary effects of nano curcumin on growth performances, body composition, blood parameters and histopathological alternation in red tilapia (Oreochromis sp.) challenged with Aspergillus flavus. Fishes 2023, 8, 208. [Google Scholar] [CrossRef]
- Ibrahim, D.; Arisha, A.H.; Khater, S.I.; Gad, W.M.; Hassan, Z.; Abou-Khadra, S.H.; Mohamed, D.I.; Ahmed Ismail, T.; Gad, S.A.; Eid, S.A. Impact of omega-3 fatty acids nano-formulation on growth, antioxidant potential, fillet quality, immunity, autophagy-related genes and Aeromonas hydrophila resistance in Nile tilapia (Oreochromis niloticus). Antioxidants 2022, 11, 1523. [Google Scholar] [CrossRef] [PubMed]
- Dube, E. Nanoparticle-enhanced fish feed: Benefits and challenges. Fishes 2024, 9, 322. [Google Scholar] [CrossRef]
- Yousefi, M.; Adineh, H.; Hoseini, S.M.; Hashemianfar, S.A.M.; Kulikov, E.V.; Petukhov, N.V.; Ryzhova, T.A. Effects of Laurus nobilis essential oil nano-particles on growth performance, antioxidant and immune responses to bacterial infection in Nile tilapia, Oreochromis niloticus. Aquaculture 2025, 596, 741821. [Google Scholar] [CrossRef]
- Goodrich, H.R.; Clark, T.D. Why do some fish grow faster than others? Fish Fish. 2023, 24, 796–811. [Google Scholar] [CrossRef]
- Shi, Y.; Zhong, L.; Liu, Y.; Zhang, J.; Lv, Z.; Li, Y.; Hu, Y. Effects of dietary andrographolide levels on growth performance, antioxidant capacity, intestinal immune function and microbioma of rice field eel (Monopterus albus). Animals 2020, 10, 1744. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, M.; Al-Sanea, M.M. Nanostructured lipid carriers (NLCs) as drug delivery platform: Advances in formulation and delivery strategies. Saudi Pharm. J. 2021, 29, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Noimoon, P.; Chitmanat, C. Efficacy of Dietary Herbal Extract and Nano-Quercetin on Growth, Innate Immune Responses, Antioxidant and Resistance of Nile Tilapia (Oreochromis niloticus) Against Streptococcus agalactiae Infection; Maejo University: Chiang Mai, Thailand, 2021. [Google Scholar]
- Elabd, H.; Mahboub, H.H.; Salem, S.M.; Abdelwahab, A.M.; Alwutayd, K.M.; Shaalan, M.; Ismail, S.H.; Abdelfattah, A.M.; Khalid, A.; Mansour, A.T. Nano-Curcumin/Chitosan modulates growth, biochemical, immune, and antioxidative profiles, and the expression of related genes in Nile tilapia, Oreochromis niloticus. Fishes 2023, 8, 333. [Google Scholar] [CrossRef]
- Abdelghany, M.F.; El-Sawy, H.B.; Abd El-hameed, S.A.; Khames, M.K.; Abdel-Latif, H.M.; Naiel, M.A. Effects of dietary Nannochloropsis oculata on growth performance, serum biochemical parameters, immune responses, and resistance against Aeromonas veronii challenge in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 107, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Sherif, A.; Alsokary, E.; Esam, H. Assessment of titanium dioxide nanoparticle as treatment of Aeromonas hydrophila infection in Oreochromis niloticus. J. Hell. Vet. Med. Soc. 2019, 70, 1697–1706. [Google Scholar] [CrossRef]
- Sherif, A.H.; Kassab, A.S. Multidrug-resistant Aeromonas bacteria prevalence in Nile tilapia broodstock. BMC Microbiol. 2023, 23, 80. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Hossain, M.; Khan, M.; Ali, M.; Aktar, S.; Moniruzzaman, M.; Khan, M. Influence of nanoparticle-based nano-nutrients on the growth performance and physiological parameters in tilapia (Oreochromis niloticus). RSC Adv. 2020, 10, 29918–29922. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.E.; Amer, S.A.; Farroh, K.Y.; Al-Gabri, N.A.; Ahmed, A.I.; El-Araby, D.A.; Ahmed, S.A. The effects of chitosan-vitamin C nanocomposite supplementation on the growth performance, antioxidant status, immune response, and disease resistance of Nile tilapia (Oreochromis niloticus) fingerlings. Aquaculture 2021, 534, 736269. [Google Scholar] [CrossRef]
- Roy, P.; Das, S.; Auddy, R.G.; Mukherjee, A. Engineered andrographolide nanosystems for smart recovery in hepatotoxic conditions. Int. J. Nanomed. 2014, 9, 4723–4735. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kamble, M.T.; Chaiyapechara, S.; Salin, K.R.; Bunphimpapha, P.; Chavan, B.R.; Bhujel, R.C.; Medhe, S.V.; Kettawan, A.; Thiyajai, P.; Thompson, K.D.; et al. Guava and Star gooseberry leaf extracts improve growth performance, innate immunity, intestinal microbial community, and disease resistance in Nile tilapia (Oreochromis niloticus) against Aeromonas hydrophila. Aquac. Rep. 2024, 35, 101947. [Google Scholar] [CrossRef]
- Das, S.; Pradhan, G.K.; Das, S.; Nath, D.; Saha, K.D. Enhanced protective activity of nano formulated andrographolide against arsenic induced liver damage. Chem. -Biol. Interact. 2015, 242, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Neamatallah, T.; Malebari, A.M.; Alamoudi, A.J.; Nazreen, S.; Alam, M.M.; Bin-Melaih, H.H.; Abuzinadah, O.A.; Badr-Eldin, S.M.; Alhassani, G.; Makki, L. Andrographolide nanophytosomes exhibit enhanced cellular delivery and pro-apoptotic activities in HepG2 liver cancer cells. Drug Deliv. 2023, 30, 2174209. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.D.; Mandal, T.; Hazra, M. Strategic approach in hepatic delivery of andrographolide: Key challenges and new insights. J. Herb. Med. 2020, 24, 100411. [Google Scholar] [CrossRef]
- Abdel-Ghany, H.M.; El-Sisy, D.M.; Salem, M.E.-S. A comparative study of effects of curcumin and its nanoparticles on the growth, immunity and heat stress resistance of Nile tilapia (Oreochromis niloticus). Sci. Rep. 2023, 13, 2523. [Google Scholar] [CrossRef] [PubMed]
- Chimsook, T. Formulation of Nanostructured Lipid Carriers Loaded with Algae Extract: A Detailed Study of Preparation and Evaluation of Antioxidant Potential for Stabilization of Fish Oil. Appl. Mech. Mater. 2015, 799, 42–46. [Google Scholar] [CrossRef]
- Rodrigues, A.T.; Mansano, C.F.; Khan, K.U.; Nascimento, T.M.; Boaratti, A.Z.; Sakomura, N.K.; Fernandes, J.B. Ideal profile of essential amino acids for Nile tilapia (Oreochromis niloticus) in the finishing growth phase. Aquac. Res. 2020, 51, 4724–4735. [Google Scholar] [CrossRef]
- Neu, D.; Boscolo, W.; Zaminhan, M.; Almeida, F.; Sary, C.; Furuya, W. Growth performance, biochemical responses, and skeletal muscle development of juvenile Nile tilapia, Oreochromis niloticus, fed with increasing levels of arginine. J. World Aquac. Soc. 2016, 47, 248–259. [Google Scholar] [CrossRef]
- Farhad, F.B.; Hashem, S.; Rana, K.S.; Salam, M. Growth performance and hematological responses of silver barb (Barbonymus gonionotus bleeker, 1850) fingerlings to dietary blanched moringa (Moringa oleifera lam.) leaf meal as a substitute of soybean meal. Heliyon 2023, 9, e13552. [Google Scholar] [CrossRef] [PubMed]
- Aiyelari, T.A.; Chaudhry, A.S. Plant protein-based diets can replace a fish meal-based diet for sustainable growth and body composition of zebrafish. bioRxiv 2020. [Google Scholar] [CrossRef]
- Pratiwy, F.M.; Kohbara, J.; AB, S. Effectiveness of Sargassum meal as feed additive on growth performance of nile tilapia, Oreochromis niloticus. Aquac. Sci. 2018, 66, 25–31. [Google Scholar]
- Ighwela, K.A.; Ahmed, A.B.; Abol-Munafi, A. Condition factor as an indicator of growth and feeding intensity of Nile tilapia fingerlings (Oreochromis niloticus) feed on different levels of maltose. Am.-Eurasian J. Agric. Environ. Sci. 2011, 11, 559–563. [Google Scholar]
- Nasr-Eldahan, S.; Nabil-Adam, A.; Shreadah, M.A.; Maher, A.M.; El-Sayed Ali, T. A review article on nanotechnology in aquaculture sustainability as a novel tool in fish disease control. Aquac. Int. 2021, 29, 1459–1480. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.I.; Campos, E.V.; De Oliveira, J.L.; Guilger-Casagrande, M.; De Lima, R.; Castanha, R.F.; De Castro, V.L.; Fraceto, L.F. Zein nanoparticles impregnated with eugenol and garlic essential oils for treating fish pathogens. ACS Omega 2020, 5, 15557–15566. [Google Scholar] [CrossRef] [PubMed]
- Salam, H.S.H.; Mohamed, W.M.; Aziz, S.A.A.A.; Mohammed, A.N.; Korni, F.M. Prevention of motile Aeromonas septicemia in Nile tilapia, Oreochromis niloticus, using thyme essential oil and its nano-emlusion. Aquac. Int. 2021, 29, 2065–2084. [Google Scholar] [CrossRef]
- El-Ekiaby, W.T. Basil oil nanoemulsion formulation and its antimicrobial activity against fish pathogen and enhance disease resistance against Aeromonas hydrophila in cultured Nile tilapia. Egypt. J. Aquac. 2019, 9, 13–33. [Google Scholar] [CrossRef]
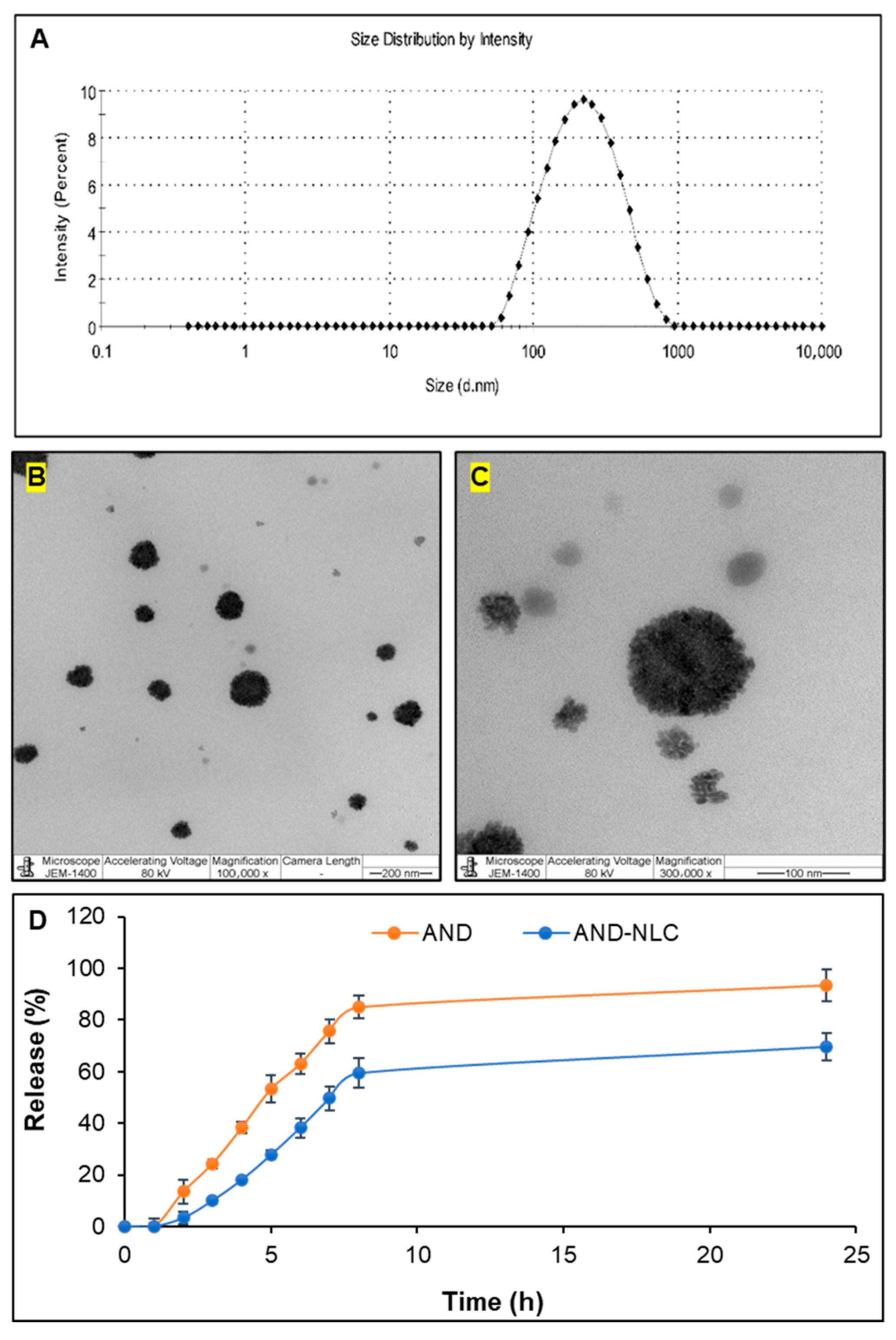





| Formulation | Size (nm) | Polydispersity Index | Zeta Potential (mV) | Encapsulation Efficiency (%) |
|---|---|---|---|---|
| NLC | 168.0 ± 2.2 | 0.262 ± 0.028 | −21.33 ± 1.0 | ND |
| AND-NLC | 189.6 ± 3.2 | 0.159 ± 0.038 | −20.0 ± 0.5 | 90.58 ± 0.13 |
| Formulation | IZD (mm) | MIC (ppm) | MBC (ppm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FPrA02 | FNA07 | ENC06 | FPrA02 | FNA07 | ENC06 | FPrA02 | FNA07 | ENC06 | |
| AND | 6 ± 0 a | 6 ± 0 a | 6 ± 0 a | 1000 | 1000 | 1000 | 2000 | 2000 | 2000 |
| AND-NLC | 13 ± 2 b | 13 ± 2 b | 14 ± 1 b | >2000 | >2000 | >2000 | >2000 | >2000 | >2000 |
| Parameters | Control | AND | AND-NLC | NLC |
|---|---|---|---|---|
| N | 90 | 90 | 90 | 90 |
| LMin-Max (cm) | 10.0–15 | 11.0–14.8 | 11.0–15.8 | 11.2–14.5 |
| Wmin-max (g) | 19.8–66.4 | 26.1–65.0 | 25.0–81.2 | 27.3–57.4 |
| a | −1.378 | −1.747 | −2.081 | −1.287 |
| b | 2.697 | 3.036 | 3.350 | 2.619 |
| SE (b) | 0.103 | 0.117 | 0.090 | 0.095 |
| CI (b) | 2.493–2.901 | 2.803–3.269 | 3.170–3.530 | 2.424–2.813 |
| r | 0.942 | 0.940 | 0.969 | 0.944 |
| R2 | 0.887 | 0.884 | 0.940 | 0.890 |
| p | 0.001 | 0.001 | 0.001 | 0.001 |
| t-test sig | 0.001 | 0.001 | 0.001 | 0.001 |
| Growth behavior | Negative allometry | Positive allometry | Positive allometry | Negative allometry |
| Kn | 1.195 a | 1.229 a | 1.052 b | 0.971 c |
| Min-Max | 0.962–1.452 | 1.016–1.463 | 0.918–1.236 | 0.876–1.188 |
| SE | 0.010 | 0.011 | 0.008 | 0.013 |
| Treatments | Mortality (%) | Survival (%) | RPS (%) |
|---|---|---|---|
| Infected control | 77.8 ± 2.2 a | 22.2 ± 2.2 a | 0.0 |
| Negative control | 15.5 ± 2.2 c | 84.5 ± 2.2 c | 80.0 ± 2.6 c |
| AND | 35.6 ± 4.4 b | 64.4 ± 4.4 b | 54.1 ± 6.4 b |
| AND-NLC | 26.7 ± 3.8 bc | 73.3 ± 3.8 bc | 65.6 ± 4.9 bc |
| NLC | 75.53 ± 2.2 a | 24.5 ± 2.2 a | 2.5 ± 0.8 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kengkittipat, W.; Kamble, M.T.; Kitiyodom, S.; Yostawonkul, J.; Sawatphakdee, G.; Thompson, K.D.; Medhe, S.V.; Pirarat, N. Effects of Andrographolide-Loaded Nanostructured Lipid Carriers on Growth, Feed Efficiency, and Resistance to Streptococcus agalactiae in Nile Tilapia (Oreochromis niloticus). Animals 2025, 15, 2117. https://doi.org/10.3390/ani15142117
Kengkittipat W, Kamble MT, Kitiyodom S, Yostawonkul J, Sawatphakdee G, Thompson KD, Medhe SV, Pirarat N. Effects of Andrographolide-Loaded Nanostructured Lipid Carriers on Growth, Feed Efficiency, and Resistance to Streptococcus agalactiae in Nile Tilapia (Oreochromis niloticus). Animals. 2025; 15(14):2117. https://doi.org/10.3390/ani15142117
Chicago/Turabian StyleKengkittipat, Warut, Manoj Tukaram Kamble, Sirikorn Kitiyodom, Jakarwan Yostawonkul, Gotchagorn Sawatphakdee, Kim D. Thompson, Seema Vijay Medhe, and Nopadon Pirarat. 2025. "Effects of Andrographolide-Loaded Nanostructured Lipid Carriers on Growth, Feed Efficiency, and Resistance to Streptococcus agalactiae in Nile Tilapia (Oreochromis niloticus)" Animals 15, no. 14: 2117. https://doi.org/10.3390/ani15142117
APA StyleKengkittipat, W., Kamble, M. T., Kitiyodom, S., Yostawonkul, J., Sawatphakdee, G., Thompson, K. D., Medhe, S. V., & Pirarat, N. (2025). Effects of Andrographolide-Loaded Nanostructured Lipid Carriers on Growth, Feed Efficiency, and Resistance to Streptococcus agalactiae in Nile Tilapia (Oreochromis niloticus). Animals, 15(14), 2117. https://doi.org/10.3390/ani15142117










