The Role of Gonadotropins and Growth Factor in Regulating Ras During Maturation in Cumulus–Oocyte Complexes of Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Porcine COCs
2.2. Treatment with FSH, LH, and EGF
2.3. Quantitative Reverse Transcription PCR
2.4. Statistical Analysis
3. Results
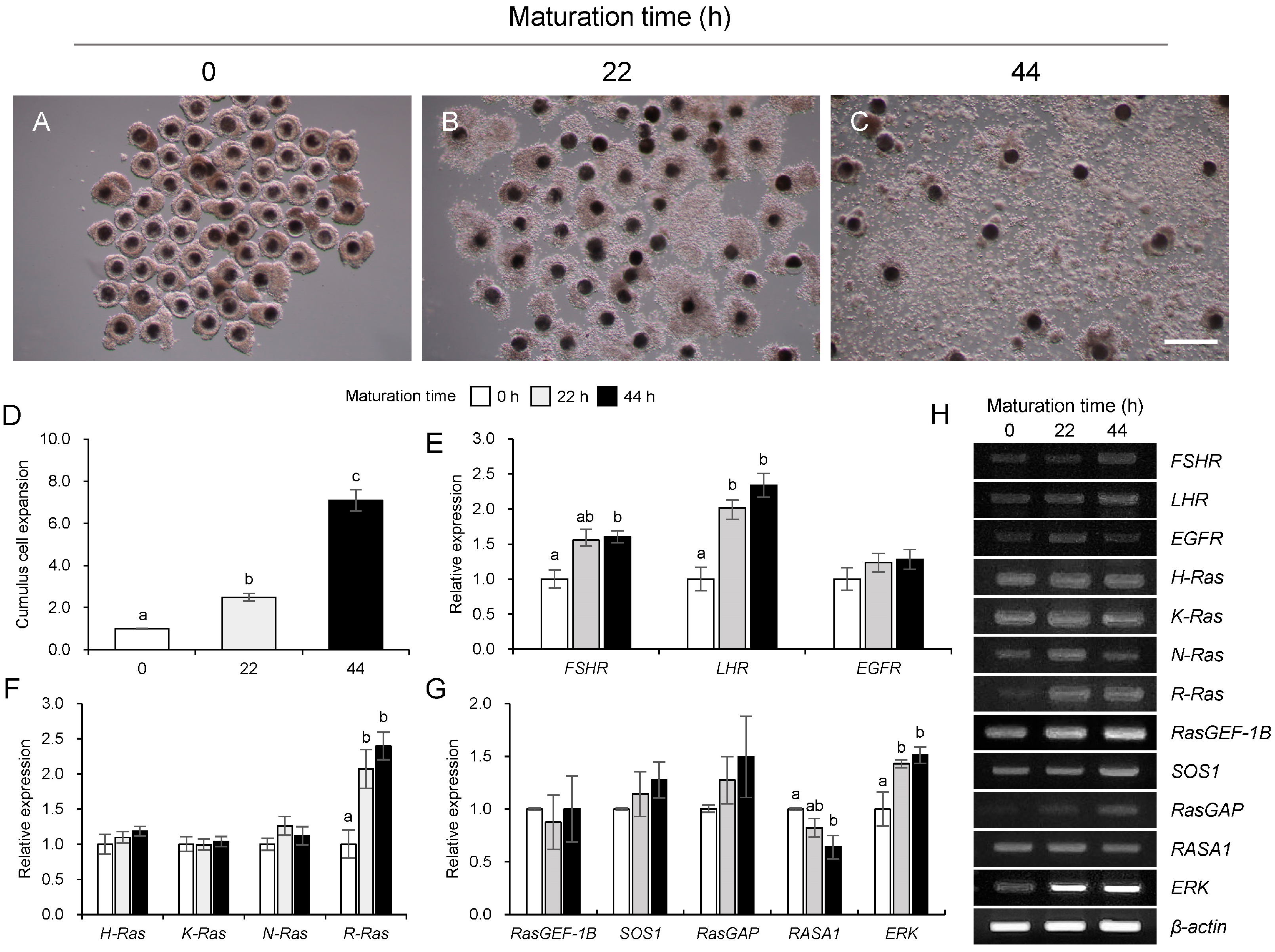
3.1. Changes in Ras and Its Regulators During Maturation in Porcine COCs
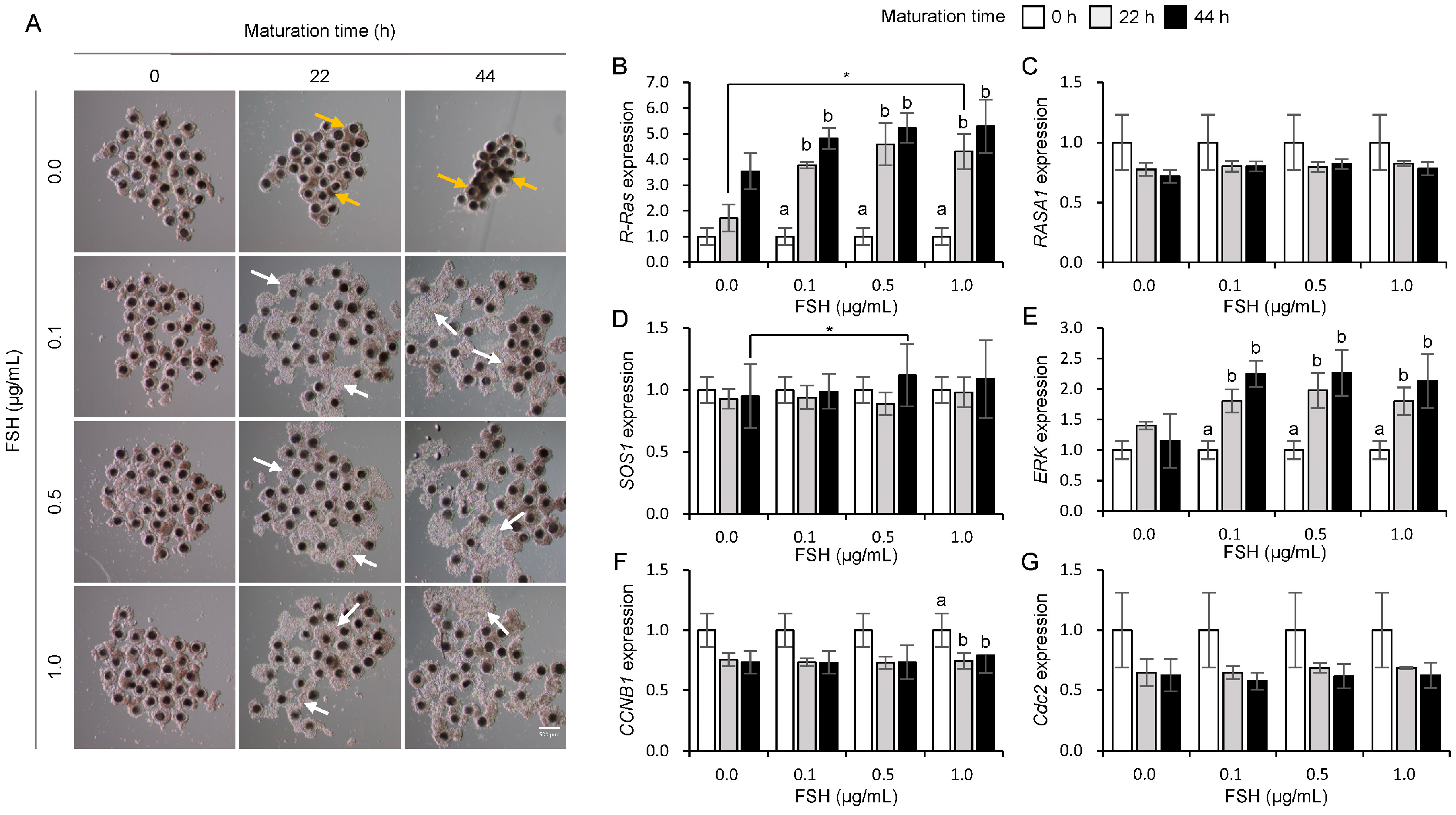
3.2. Influences of FSH on the Expression of Ras and Its Regulator Genes in Porcine COCs
3.3. Influences of hCG on the Expression of Ras and Its Regulator Genes in Porcine COCs
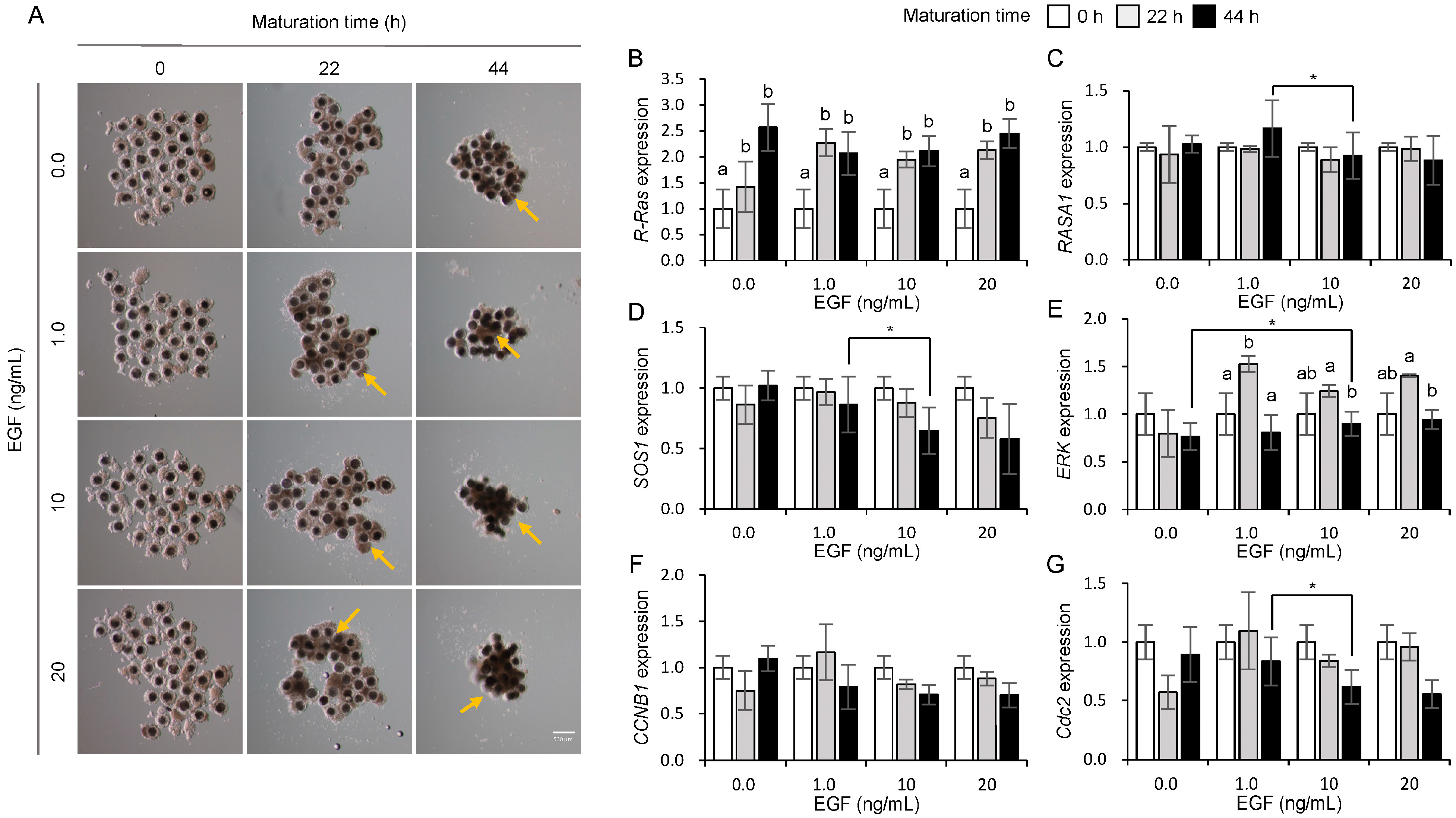
3.4. Influences of EGF on the Expression of Ras and Its Regulator Genes in Porcine COCs
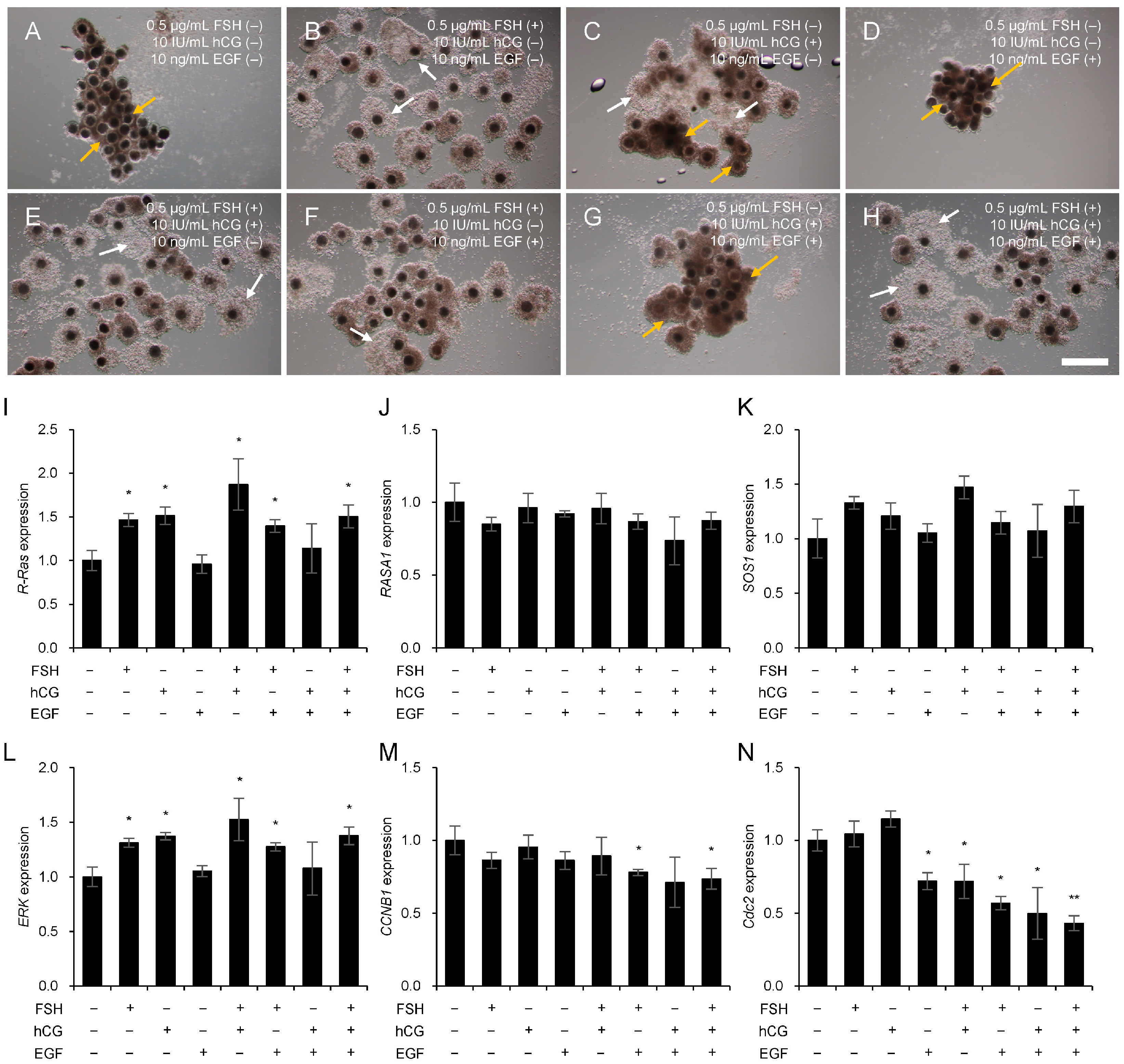
3.5. Mechanism of Ras Regulation by Gonadotropins and EGF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lonergan, P.; Fair, T. Maturation of oocytes in vitro. Annu. Rev. Anim. Biosci. 2016, 4, 255–268. [Google Scholar] [CrossRef]
- Robker, R.L.; Hennebold, J.D.; Russell, D.L. Coordination of ovulation and oocyte maturation: A good egg at the right time. Endocrinology 2018, 159, 3209–3218. [Google Scholar] [CrossRef]
- Richani, D.; Dunning, K.R.; Thompson, J.G.; Gilchrist, R.B. Metabolic co-dependence of the oocyte and cumulus cells: Essential role in determining oocyte developmental competence. Hum. Reprod. Update 2021, 27, 27–47. [Google Scholar] [CrossRef]
- Macaulay, A.D.; Gilbert, I.; Scantland, S.; Fournier, E.; Ashkar, F.; Bastien, A.; Saadi, H.A.S.; Gagné, D.; Sirard, M.-A.; Khandjian, É.W.; et al. Cumulus cell transcripts transit to the bovine oocyte in preparation for maturation. Biol. Reprod. 2016, 94, 16. [Google Scholar] [CrossRef] [PubMed]
- Turathum, B.; Gao, E.-M.; Chian, R.-C. The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells 2021, 10, 2292. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, A.; Kim, B.; Yeh, J. Luteinizing hormone action in human oocyte maturation and quality: Signaling pathways, regulation, and clinical impact. Reprod. Sci. 2020, 27, 1223–1252. [Google Scholar] [CrossRef] [PubMed]
- Field, S.L.; Dasgupta, T.; Cummings, M.; Orsi, N.M. Cytokines in ovarian folliculogenesis, oocyte maturation and luteinisation. Mol. Reprod. Dev. 2014, 81, 284–314. [Google Scholar] [CrossRef]
- Shimada, M.; Hernandez-Gonzalez, I.; Gonzalez-Robayna, I.; Richards, J.S. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: Key roles for prostaglandin synthase 2 and progesterone receptor. Mol. Endocrinol. 2006, 20, 1352–1365. [Google Scholar] [CrossRef]
- Su, Y.-Q.; Sugiura, K.; Li, Q.; Wigglesworth, K.; Matzuk, M.M.; Eppig, J.J. Mouse oocytes enable LH-induced maturation of the cumulus-oocyte complex via promoting EGF receptor-dependent signaling. Mol. Endocrinol. 2010, 24, 1230–1239. [Google Scholar] [CrossRef]
- Yong, H.; Oh, H.-I.; Lee, S.-H.; Cheong, H.-T.; Yalng, B.-K.; Park, C.-K. Treatment of epidermal growth factor (EGF) enhances nuclear maturation of porcine oocytes and stimulates expression of ER/Golgi transport proteins. Dev. Reprod. 2017, 21, 131–138. [Google Scholar] [CrossRef]
- Uhm, S.; Gupta, M.; Yang, J.; Chung, H.-J.; Min, T.; Lee, H. Epidermal growth factor can be used in lieu of follicle-stimulating hormone for nuclear maturation of porcine oocytes in vitro. Theriogenology 2010, 73, 1024–1036. [Google Scholar] [CrossRef]
- Richani, D.; Gilchrist, R.B. The epidermal growth factor network: Role in oocyte growth, maturation and developmental competence. Hum. Reprod. Update 2018, 24, 1–14. [Google Scholar] [CrossRef]
- Baszary, C.D.; Moniharapon, M. Epidermal Growth Factor as Trigger Mitotic Cleavage in Goat Cumulus Cell. J. Phys. Conf. Ser. 2020, 1463, 012019. [Google Scholar] [CrossRef]
- Hunter, M. Oocyte maturation and ovum quality in pigs. Rev. Reprod. 2000, 5, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Rebollo, A.; Martı, C. Ras proteins: Recent advances and new functions. Blood. J. Am. Soc. Hematol. 1999, 94, 2971–2980. [Google Scholar] [CrossRef]
- Simanshu, D.K.; Nissley, D.V.; McCormick, F. RAS proteins and their regulators in human disease. Cell 2017, 170, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Maldonado, C.; Zimmer, Y.; Medová, M. A comparative analysis of individual RAS mutations in cancer biology. Front. Oncol. 2019, 9, 1088. [Google Scholar] [CrossRef]
- Prior, I.A.; Hood, F.E.; Hartley, J.L. The frequency of Ras mutations in cancer. Cancer Res. 2020, 80, 2969–2974. [Google Scholar] [CrossRef]
- Pylayeva-Gupta, Y.; Grabocka, E.; Bar-Sagi, D. RAS oncogenes: Weaving a tumorigenic web. Nat. Rev. Cancer 2011, 11, 761–774. [Google Scholar] [CrossRef]
- Hobbs, G.A.; Der, C.J.; Rossman, K.L. RAS isoforms and mutations in cancer at a glance. J. Cell Sci. 2016, 129, 1287–1292. [Google Scholar] [CrossRef]
- McCormick, F. K-Ras protein as a drug target. J. Mol. Med. 2016, 94, 253–258. [Google Scholar] [CrossRef]
- Papke, B.; Der, C.J. Drugging RAS: Know the enemy. Science 2017, 355, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Vigil, D.; Cherfils, J.; Rossman, K.L.; Der, C.J. Ras superfamily GEFs and GAPs: Validated and tractable targets for cancer therapy? Nat. Rev. Cancer 2010, 10, 842–857. [Google Scholar] [CrossRef] [PubMed]
- Takashima, A.; Faller, D.V. Targeting the RAS oncogene. Expert Opin. Ther. Targets 2013, 17, 507–531. [Google Scholar] [CrossRef] [PubMed]
- Samatar, A.A.; Poulikakos, P.I. Targeting RAS–ERK signalling in cancer: Promises and challenges. Nat. Rev. Drug Discov. 2014, 13, 928–942. [Google Scholar] [CrossRef]
- Fan, H.-Y.; Liu, Z.; Mullany, L.K.; Richards, J.S. Consequences of RAS and MAPK activation in the ovary: The good, the bad and the ugly. Mol. Cell. Endocrinol. 2012, 356, 74–79. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, X.; Cao, R.; Liu, H.-L.; Cui, X.-S.; Kim, N.-H.; Rui, R.; Sun, S.-C. Small GTPase RhoA regulates cytoskeleton dynamics during porcine oocyte maturation and early embryo development. Cell Cycle 2014, 13, 3390–3403. [Google Scholar] [CrossRef]
- Gorczyca, G.; Wartalski, K.; Romek, M.; Samiec, M.; Duda, M. The Molecular Quality and Mitochondrial Activity of Porcine Cumulus–Oocyte Complexes Are Affected by Their Exposure to Three Endocrine-Active Compounds under 3D In Vitro Maturation Conditions. Int. J. Mol. Sci. 2022, 23, 4572. [Google Scholar] [CrossRef]
- Lee, S.-H.; Park, C.-K. Effect of magnetized extender on sperm membrane integrity and development of oocytes in vitro fertilized with liquid storage boar semen. Anim. Reprod. Sci. 2015, 154, 86–94. [Google Scholar] [CrossRef]
- Shimada, M.; Nishibori, M.; Isobe, N.; Kawano, N.; Terada, T. Luteinizing hormone receptor formation in cumulus cells surrounding porcine oocytes and its role during meiotic maturation of porcine oocytes. Biol. Reprod. 2003, 68, 1142–1149. [Google Scholar] [CrossRef]
- Abeydeera, L.R.; Wang, W.-H.; Cantley, T.C.; Rieke, A.; Prather, R.S.; Prather, R.S. Presence of epidermal growth factor during in vitro maturation of pig oocytes and embryo culture can modulate blastocyst development after in vitro fertilization. Mol. Reprod. Dev. Inc. Gamete Res. 1998, 51, 395–401. [Google Scholar] [CrossRef]
- Goitre, L.; Trapani, E.; Trabalzini, L.; Retta, S.F. The Ras superfamily of small GTPases: The unlocked secrets. Ras Signal. Methods Protoc. 2014, 1120, 1–18. [Google Scholar] [CrossRef]
- Toma-Fukai, S.; Shimizu, T. Structural insights into the regulation mechanism of small GTPases by GEFs. Molecules 2019, 24, 3308. [Google Scholar] [CrossRef]
- Li, M.; Liang, C.-G.; Xiong, B.; Xu, B.-Z.; Lin, S.-L.; Hou, Y.; Chen, D.-Y.; Schatten, H.; Sun, Q.-Y. PI3-kinase and mitogen-activated protein kinase in cumulus cells mediate EGF-induced meiotic resumption of porcine oocyte. Domest. Anim. Endocrinol. 2008, 34, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Okamoto, M.; Ikeda, M.; Okamoto, A.; Salkai, M.; Gunji, Y.; Nishimura, R.; Hishinuma, M.; Shimada, M. Protein kinase C (PKC) increases TACE/ADAM17 enzyme activity in porcine ovarian somatic cells, which is essential for granulosa cell luteinization and oocyte maturation. Endocrinology 2014, 155, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Dekel, N.; Lawrence, T.S.; Gilula, N.B.; Beers, W.H. Modulation of cell-to-cell communication in the cumulus-oocyte complex and the regulation of oocyte maturation by LH. Dev. Biol. 1981, 86, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Byskov, A.G.; Andersen, C.Y.; Hossaini, A.; Guoliang, X. Cumulus cells of oocyte-cumulus complexes secrete a meiosis-activating substance when stimulated with FSH. Mol. Reprod. Dev. Inc. Gamete Res. 1997, 46, 296–305. [Google Scholar] [CrossRef]
- Yokoo, M.; Sato, E. Cumulus-oocyte complex interactions during oocyte maturation. Int. Rev. Cytol. 2004, 235, 251–282. [Google Scholar] [CrossRef]
- Shimada, M.; Terada, T. FSH and LH induce progesterone production and progesterone receptor synthesis in cumulus cells: A requirement for meiotic resumption in porcine oocytes. Mol. Hum. Reprod. 2002, 8, 612–618. [Google Scholar] [CrossRef]
- Blaha, M.; Nemcova, L.; Kepkova, K.V.; Vodicka, P.; Prochazka, R. Gene expression analysis of pig cumulus-oocyte complexes stimulated in vitro with follicle stimulating hormone or epidermal growth factor-like peptides. Reprod. Biol. Endocrinol. 2015, 13, 113. [Google Scholar] [CrossRef][Green Version]
- Kalous, J.; Tetkova, A.; Kubelka, M.; Susor, A. Importance of ERK1/2 in regulation of protein translation during oocyte meiosis. Int. J. Mol. Sci. 2018, 19, 698. [Google Scholar] [CrossRef]
- Sugimura, S.; Ritter, L.J.; Rose, R.D.; Thompson, J.G.; Smitz, J.; Mottershead, D.G.; Gilchrist, R.B. Promotion of EGF receptor signaling improves the quality of low developmental competence oocytes. Dev. Biol. 2015, 403, 139–149. [Google Scholar] [CrossRef]
- Kim, J.-W.; Park, H.-J.; Jung, J.-M.; Yang, S.-G.; Kim, M.-J.; Kim, I.-S.; Jega, H.-G.; Koo, D.-B. Reversible effects of exogenous GM3 on meiotic maturation and cumulus cells expansion of porcine cumulus-oocyte complexes. J. Embryo Transf. 2018, 33, 287–296. [Google Scholar] [CrossRef]
- Reizel, Y.; Elbaz, J.; Dekel, N. Sustained activity of the EGF receptor is an absolute requisite for LH-induced oocyte maturation and cumulus expansion. Mol. Endocrinol. 2010, 24, 402–411. [Google Scholar] [CrossRef]
- Fan, H.-Y.; Richards, J.S. Minireview: Physiological and pathological actions of RAS in the ovary. Mol. Endocrinol. 2010, 24, 286–298. [Google Scholar] [CrossRef]
- Wei, S.; Shen, X.; Lai, L.; Liang, H.; Deng, Y.; Gong, Z.; Che, T. FSH receptor binding inhibitor impacts K-Ras and c-Myc of ovarian cancer and signal pathway. Oncotarget 2018, 9, 22498. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fan, H.-Y.; Shimada, M.; Liu, Z.; Cahill, N.; Noma, N.; Wu, Y.; Gossen, J.; Richards, J.S. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development 2008, 135, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Morgillo, F.; Troiani, T.; Ciardiello, F. Cancer resistance to therapies against the EGFR-RAS-RAF pathway: The role of MEK. Cancer Treat. Rev. 2017, 53, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Garrington, T.P.; Johnson, G.L. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 1999, 11, 211–218. [Google Scholar] [CrossRef]
- Cook, S.J.; McCormick, F. Inhibition by cAMP of Ras-dependent activation of Raf. Science 1993, 262, 1069–1072. [Google Scholar] [CrossRef]
- Hennig, A.; Markwart, R.; Esparza-Franco, M.A.; Ladds, G.; Rubio, I. Ras activation revisited: Role of GEF and GAP systems. Biol. Chem. 2015, 396, 831–848. [Google Scholar] [CrossRef]
- Hall, B.E.; Bar-Sagi, D.; Nassar, N. The structural basis for the transition from Ras-GTP to Ras-GDP. Proc. Natl. Acad. Sci. USA 2002, 99, 12138–12142. [Google Scholar] [CrossRef]

| Genes | Sequence (5′–3′) | Annealing Temp. (°C) | Cycle | Product Size (bp) |
|---|---|---|---|---|
| β-actin | F: GGACTTCGAGCAGGAGATGG | 60 | 30 | 233 |
| R: GCACCGTGTTGGCGTAGAGG | ||||
| FSHR | F: GATCCTGATCACCAGCCAATAC | 60 | 32 | 217 |
| R: GACTGAGAGCTCACTAGCAAAG | ||||
| LHR | F: CTGTCCTCTTTGTTCTCCTGAC | 60 | 32 | 191 |
| R: AGCAACACTACACCCATTCC | ||||
| EGFR | F: CCTTGGGAACTTGGAGATCACCTAC | 60 | 32 | 344 |
| R: TGTTGCTTAGAAAGTCGCTGTTGAC | ||||
| H-Ras | F: CGCCATCAACAACACCAAATC | 60 | 32 | 194 |
| R: TGGCTGAGGTCTCGATGTAA | ||||
| K-Ras | F: CAATGAGGGACCAGTACATGAG | 60 | 32 | 205 |
| R: GCTAAGTCCTGAGCCTGTTT | ||||
| N-Ras | F: TACAAACTGGTGGTGGTTGG | 60 | 32 | 219 |
| R: GCTAAGTCCTGAGCCTGTTT | ||||
| R-Ras | F: CCCACTATTGAAGACTCCTACAC | 60 | 32 | 205 |
| R: GAAGTCATCTCGGTCCTTGAC | ||||
| RasGEF-1B | F: GGCAGCACAAAGGTCTTTAAC | 60 | 35 | 201 |
| R: GGACACTCCACTTGTTTCCA | ||||
| SOS1 | F: CTGCTCACCTTACACCCAATAG | 60 | 35 | 231 |
| R: CACCACAGCTACCCTTTCTT | ||||
| RasGAP | F: CAAACGCACGAAGTCACAAC | 60 | 35 | 203 |
| R: CTGGCTTGATGATGGAGTCTT | ||||
| RASA1 | F: TACTTCCACCGACACTGAGATA | 60 | 35 | 221 |
| R: CTGCACAGACTTAGCCACTAAT | ||||
| ERK | F: GACCAGCTCAACCACATTCT | 60 | 32 | 213 |
| R: CCGACAGAAGCCAAGATAACA | ||||
| CCNB1 | F: GTGTCAGGCTTTCTCTGATGT | 60 | 32 | 199 |
| R: CCAGTCAATTAGGATGGCTCTC | ||||
| Cdc2 | F: GGTGTTCCTAGTACTGCCATTC | 60 | 32 | 179 |
| R: GAATCCATGAACTGACCAGGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seok, E.; Son, M.; Lee, S.; Cheong, H.-T.; Lee, S.-H. The Role of Gonadotropins and Growth Factor in Regulating Ras During Maturation in Cumulus–Oocyte Complexes of Pigs. Animals 2025, 15, 2100. https://doi.org/10.3390/ani15142100
Seok E, Son M, Lee S, Cheong H-T, Lee S-H. The Role of Gonadotropins and Growth Factor in Regulating Ras During Maturation in Cumulus–Oocyte Complexes of Pigs. Animals. 2025; 15(14):2100. https://doi.org/10.3390/ani15142100
Chicago/Turabian StyleSeok, Eunju, Minyoung Son, Seunghyung Lee, Hee-Tae Cheong, and Sang-Hee Lee. 2025. "The Role of Gonadotropins and Growth Factor in Regulating Ras During Maturation in Cumulus–Oocyte Complexes of Pigs" Animals 15, no. 14: 2100. https://doi.org/10.3390/ani15142100
APA StyleSeok, E., Son, M., Lee, S., Cheong, H.-T., & Lee, S.-H. (2025). The Role of Gonadotropins and Growth Factor in Regulating Ras During Maturation in Cumulus–Oocyte Complexes of Pigs. Animals, 15(14), 2100. https://doi.org/10.3390/ani15142100






