Selection for Growth Performance in Oreochromis niloticus Across Different Aquatic Environments Using Growth Hormone Gene Polymorphisms
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Broadstock Collection, Adaptation, and Tagging
2.3. Parent Crosses and Fry Collection
2.4. Genetic Analysis of the Broadstocks
2.4.1. Broadstock’s DNA Extraction, PCR Primer Design, and Partial GH Gene Amplification and Sequencing
2.4.2. Sequence Analysis and SNP Discovery
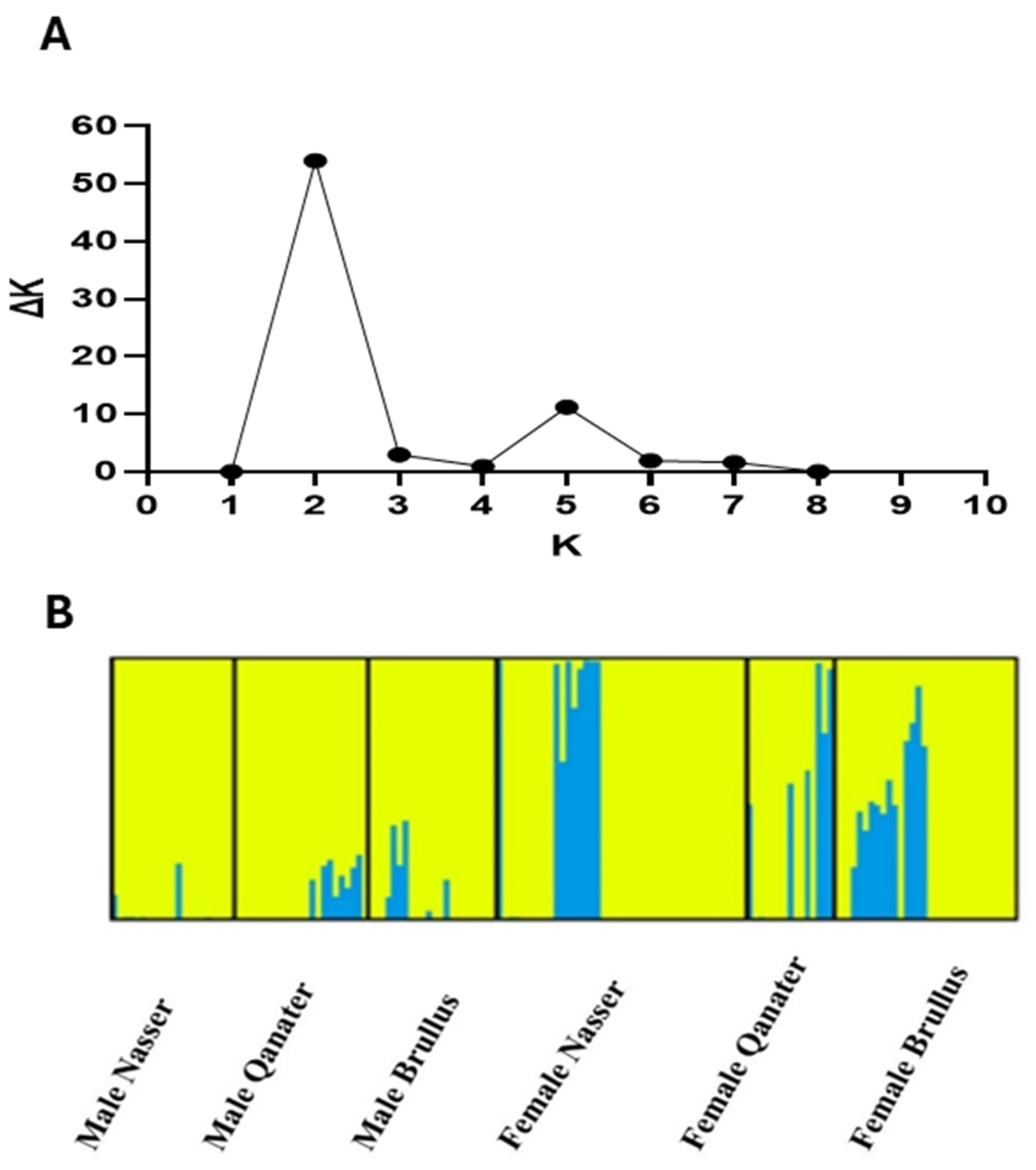
2.4.3. Population Structure and Kinship Analysis Using SNP Data
2.4.4. Association Analysis Between SNPs and Body Characteristics
2.4.5. Linkage Disequilibrium Analysis
2.5. Isolation of RNA and cDNA Synthesis from 72-Hour Larvae
2.5.1. RNA Extraction
2.5.2. Quantitative Real-Time PCR
2.6. Statistical Analysis
3. Results
3.1. Variation in Body Measurements Across Locations
3.2. Sequence Analysis and SNPs Discovery of the Growth Hormone (GH) Gene
3.3. Population Structure Assessment
3.4. Association Analysis Using SNPs
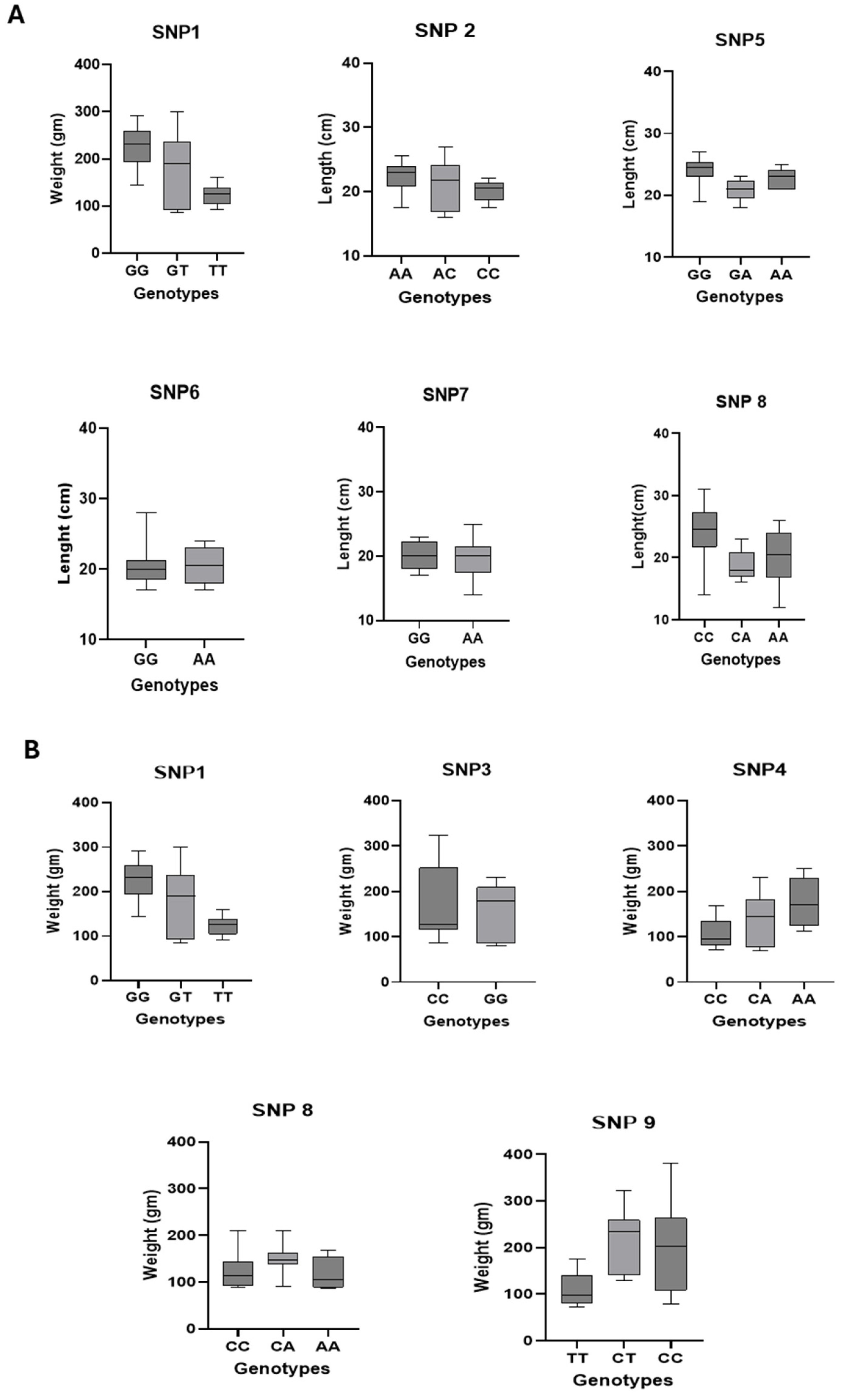
3.4.1. Associations Between SNPs and Morphometric Traits
3.4.2. Linkage Disequilibrium Analysis
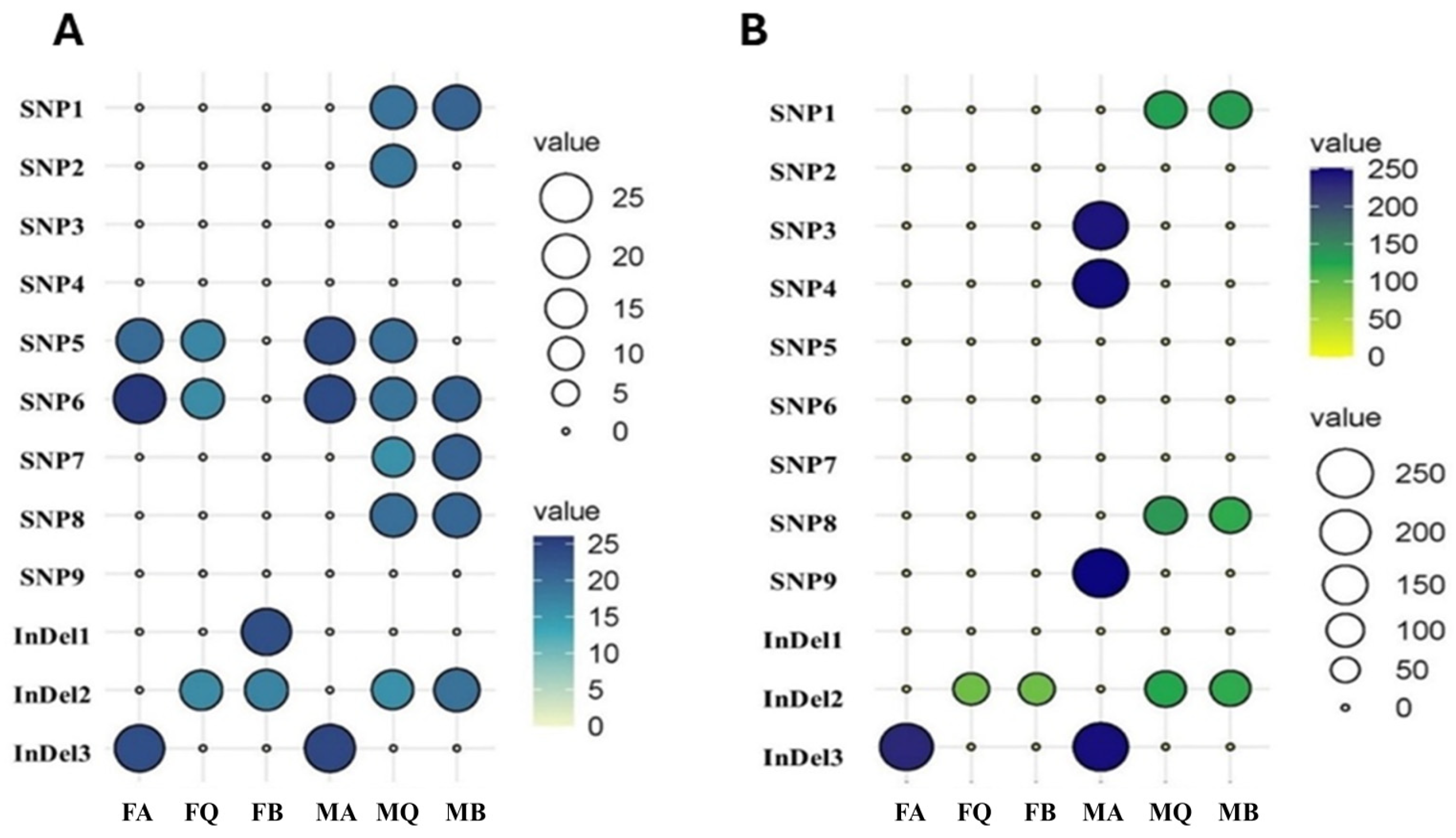
3.4.3. Population-Specific Effects of SNPs
3.4.4. Genotypic Distribution of SNP-Associated Traits
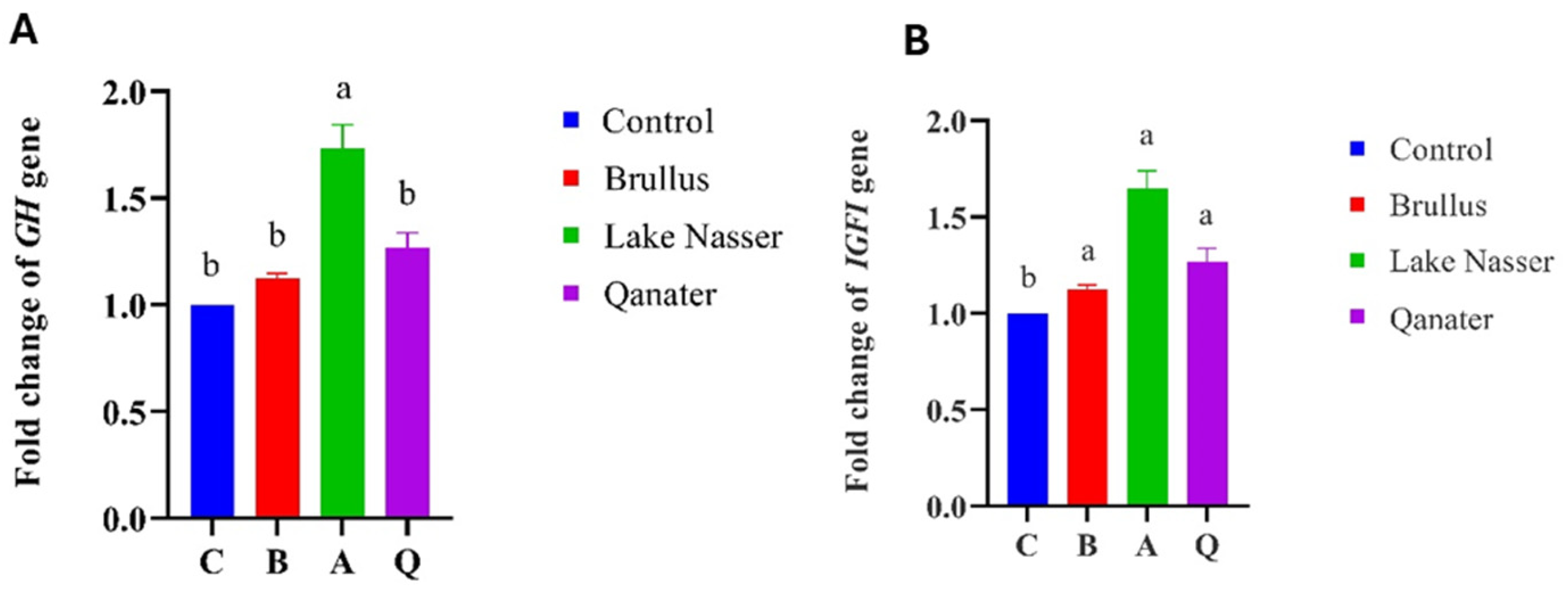
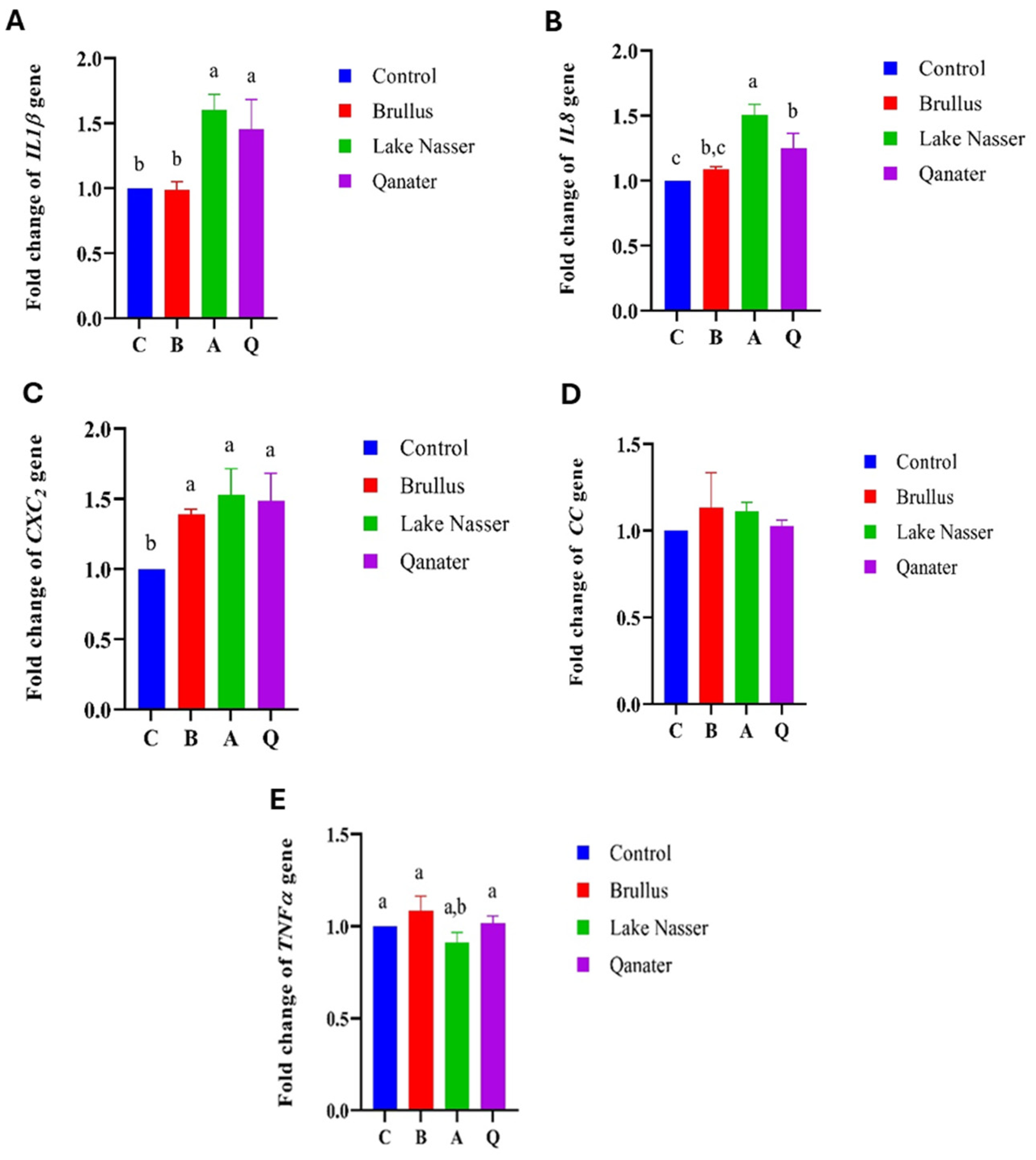
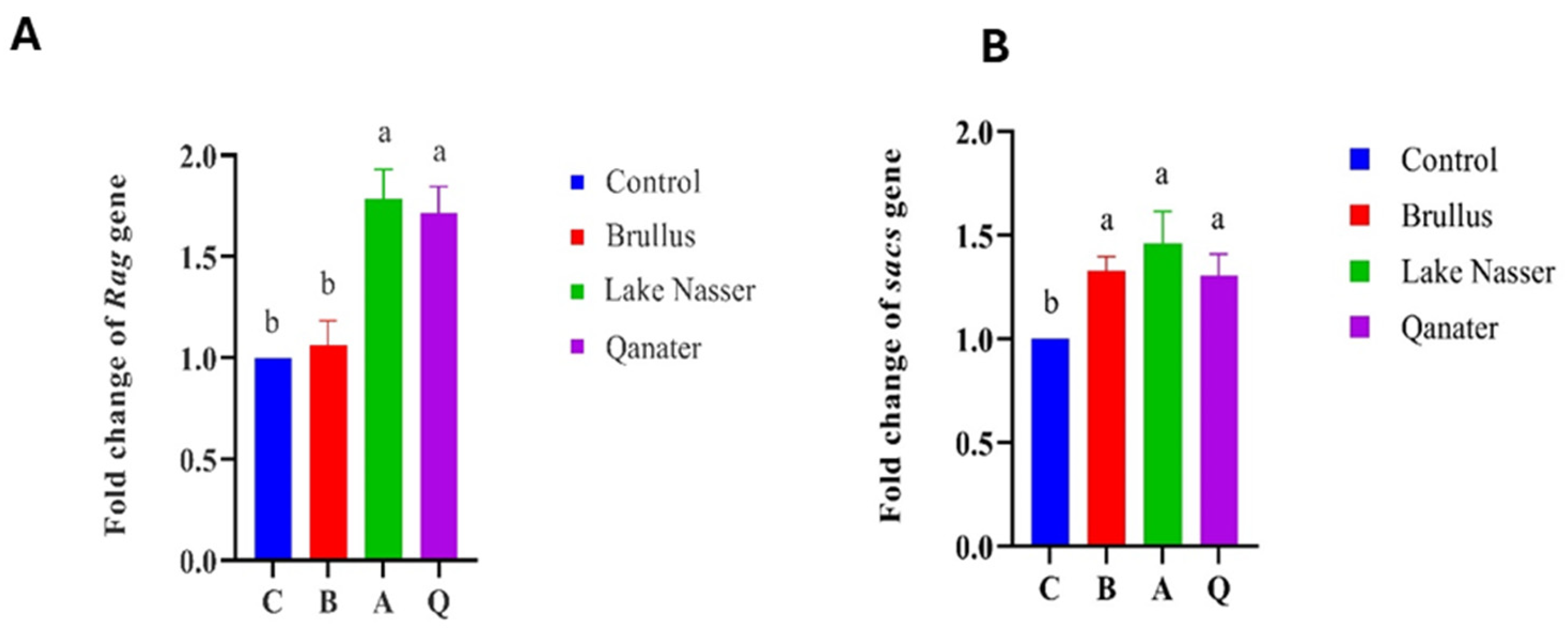
3.5. Gene Expression Profiles in F1 Progeny
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| SNP | Single Nucleotide Polymorphism |
| GH | Growth Hormone |
| IGF1 | Insulin-like Growth Factor I |
| IL1β | Interleukin 1 Beta |
| IL8 | Interleukin 8 |
| CC | CC Chemokine (β-chemokine) |
| CXC2 | CXC2 Chemokine (α-chemokine) |
| TNFα | Tumor Necrosis Factor Alpha |
| Rag | Recombination Activating Gene |
| Sacs | Sacsin Molecular Chaperone |
| MAS | Marker-Assisted Selection |
| SSR | Simple Sequence Repeat |
| HPS | Hypothalamic-Pituitary-Somatotropic |
| PCR | Polymerase Chain Reaction |
| FDR | False Discovery Rate |
| LD | Linkage Disequilibrium |
| qRT-PCR | Quantitative Real-Time Polymerase Chain Reaction |
| NIOF | National Institute of Oceanography and Fisheries |
| IACUC | Institutional Animal Care and Use Committee |
| bp | Base Pairs |
| BLAST | Basic Local Alignment Search Tool |
| MAF | Minor Allele Frequency |
| K | Number of Clusters (in STRUCTURE analysis) |
| ERK | Extracellular Signal-Regulated Kinase |
| PI3K | Phosphatidylinositol 3-Kinase |
| AKT | Protein Kinase B |
| JAK2 | Janus Kinase 2 |
| STAT | Signal Transducers and Activators of Transcription |
| GIFT | Genetically Improved Farmed Tilapia |
| GST | Genomar Supreme Tilapia |
| USA | United States of America |
| RNA | Ribonucleic Acid |
| DNA | Deoxyribonucleic Acid |
| cDNA | Complementary DNA |
| EL-Qanater | El-Qanater El-Khairia |
References
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.J.; Teletchea, F.; Tomasso, J.R.; et al. Achieving Sustainable Aquaculture: Historical and Current Perspectives and Future Needs and Challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Gjedrem, T.; Robinson, N.; Rye, M. The Importance of Selective Breeding in Aquaculture to Meet Future Demands for Animal Protein: A Review. Aquaculture 2012, 350–353, 117–129. [Google Scholar] [CrossRef]
- Siddique, M.A.B.; Mahalder, B.; Haque, M.M.; Bashar, A.; Hasan, M.M.; Shohan, M.H.; Talukdar, M.M.N.; Biswas, J.C.; Ahammad, A.K.S. Assessment of Embryonic and Larval Development of Nile Tilapia under the Traditional and Re-Circulatory Thermostatic System in Relation to Climatic and Water Quality Variations. Aquac. J. 2023, 3, 70–89. [Google Scholar] [CrossRef]
- Hamed, S.; El-Kassas, S.; Abo-Al-Ela, H.G.; Abdo, S.E.; Abou-Ismail, U.A.; Mohamed, R.A. Temperature and Feeding Frequency: Interactions with Growth, Immune Response, and Water Quality in Juvenile Nile Tilapia. BMC Vet. Res. 2024, 20, 520. [Google Scholar] [CrossRef]
- Soliman, N.F.; Yacout, D.M.M. Aquaculture in Egypt: Status, constraints and potentials. Aquac. Int. 2016, 24, 1201–1227. [Google Scholar] [CrossRef]
- Barría, A.; Trinh, T.Q.; Mahmuddin, M.; Benzie, J.A.H.; Chadag, V.M.; Houston, R.D. Genetic Parameters for Resistance to Tilapia Lake Virus (TiLV) in Nile Tilapia (Oreochromis niloticus). Aquaculture 2020, 522, 735126. [Google Scholar] [CrossRef]
- Fu, J.; Shen, Y.; Xu, X.; Li, J. Genetic Parameter Estimates for Growth of Grass Carp, Ctenopharyngodon idella, at 10 and 18months of Age. Aquaculture 2016, 450, 342–348. [Google Scholar] [CrossRef]
- Kjetså, M.H.; Ødegård, J.; Meuwissen, T.H.E. Accuracy of Genomic Prediction of Host Resistance to Salmon Lice in Atlantic Salmon (Salmo salar) Using Imputed High-Density Genotypes. Aquaculture 2020, 526, 735415. [Google Scholar] [CrossRef]
- Pasaribu, W.; Sukenda, S.; Nuryati, S. The Efficacy of Nile Tilapia (Oreochromis niloticus) Broodstock and Larval Immunization against Streptococcus agalactiae and Aeromonas hydrophila. Fishes 2018, 3, 16. [Google Scholar] [CrossRef]
- Das, S.; Sahoo, P.K. Markers for selection of disease resistance in fish: A review. Aquac. Int. 2014, 22, 1793–1812. [Google Scholar] [CrossRef]
- Song, H.; Dong, T.; Yan, X.; Wang, W.; Tian, Z.; Sun, A.; Dong, Y.; Zhu, H.; Hu, H. Genomic selection and its research progress in aquaculture breeding. Rev. Aquac. 2023, 15, 274–291. [Google Scholar] [CrossRef]
- Hakim, M.M.; Syed, M.A. Advances in molecular markers and their applications in aquaculture and fisheries. Genet. Aquat. Org. 2017, 1, 21–41. [Google Scholar] [CrossRef]
- Lind, C.E.; Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L. Selective Breeding in Fish and Conservation of Genetic Resources for Aquaculture. Reprod. Domest. Anim. 2012, 47, 255–263. [Google Scholar] [CrossRef]
- Alam, S.; Zafrin, M.S.; Alam, M.S. Microsatellite marker-based variation in the growth hormone genes of Nile tilapia (Oreochromis niloticus). Banglad. J. Fish. 2020, 32, 1–10. [Google Scholar] [CrossRef]
- Sandra, S.A.; Nasrullah, H.; Arfah, H.; Zairin, M., Jr.; Alimuddin, A. Growth and expression pattern of growth-related genes in the fast-growing giant gourami (Osphronemus goramy). Indones. Aquac. J. 2021, 16, 79–89. [Google Scholar] [CrossRef]
- Dias, M.A.D.; Reis Neto, R.V.; Bueno Filho, J.S.d.S.; Jaser, S.K.K.; Lago, A.d.A.; Hilsdorf, A.W.S. Growth Hormone Gene Polymorphism Associated with Grow-out Performance of Oreochromis niloticus Strains. Aquaculture 2019, 503, 105–110. [Google Scholar] [CrossRef]
- Hamzah, A.; Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L.; Yee, H.Y.; Mohd Nor, S.A. Performance of the Genetically Improved Farmed Tilapia (GIFT) Strain over Ten Generations of Selection in Malaysia. Pertanika J. Trop. Agric. Sci. 2014, 37, 411–429. [Google Scholar]
- Yáñez, J.M.; Joshi, R.; Yoshida, G.M. Genomics to Accelerate Genetic Improvement in Tilapia. Anim. Genet. 2020, 51, 658–674. [Google Scholar] [CrossRef]
- Triantaphyllopoulos, K.A.; Cartas, D.; Miliou, H. Factors Influencing GH and IGF-I Gene Expression on Growth in Teleost Fish: How Can Aquaculture Industry Benefit? Rev. Aquac. 2020, 12, 1637–1662. [Google Scholar] [CrossRef]
- Eze, F. Marker-assisted selection in fish: A review. Asian J. Fish. Aquat. Res. 2019, 3, 1–11. [Google Scholar] [CrossRef]
- Duan, B.; Kang, T.; Wan, H.; Liu, W.; Zhang, F.; Mu, S.; Guan, Y.; Li, Z.; Tian, Y.; Kang, X. Microsatellite Markers Reveal Genetic Diversity and Population Structure of Portunus trituberculatus in the Bohai Sea, China. Sci. Rep. 2023, 13, 8668. [Google Scholar] [CrossRef]
- Thomas, B.L.; Gomelsky, B.; Delomas, T.A.; Novelo, N.D. Genetic variability of Oreochromis niloticus strains as determined by microsatellite DNA markers. N. Am. J. Aquac. 2021, 83, 177–183. [Google Scholar] [CrossRef]
- Wenne, R. Single Nucleotide Polymorphism Markers with Applications in Conservation and Exploitation of Aquatic Natural Populations. Animals 2023, 13, 1089. [Google Scholar] [CrossRef]
- Chishty, S.M.S.; Chotipuntu, P.; Direkbusarakom, S.; Wuthisuthimethavee, S. Identification of growth-related EST-derived microsatellite marker in Oreochromis niloticus. Walailak J. Sci. Technol. 2020, 17, 867–879. [Google Scholar] [CrossRef]
- Van Oppen, M.J.H.; Coleman, M.A. Advancing the protection of marine life through genomics. PLoS Biol. 2022, 20, e3001801. [Google Scholar] [CrossRef]
- Ndandala, C.B.; Dai, M.; Mustapha, U.F.; Li, X.; Liu, J.; Huang, H.; Li, G.; Chen, H. Current research and future perspectives of GH and IGFs family genes in somatic growth and reproduction of teleost fish. Aquac. Rep. 2022, 26, 101289. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M.; Fitzsimmons, K. From Africa to the world—The journey of Oreochromis niloticus. Rev. Aquac. 2023, 15, 6–21. [Google Scholar] [CrossRef]
- Barría, A.; Trịnh, T.Q.; Mahmuddin, M.; Peñaloza, C.; Papadopoulou, A.; Gervais, O.; Chadag, V.M.; Benzie, J.A.H.; Houston, R.D. A major quantitative trait locus affecting resistance to tilapia lake virus in farmed Oreochromis niloticus. Heredity 2021, 127, 334–343. [Google Scholar] [CrossRef]
- Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L.; Hamzah, A.; Bakar, K.R.A.; Yee, H.Y. Genetic improvement of Oreochromis niloticus with special reference to the work conducted by the WorldFish Center with the GIFT strain. Rev. Aquac. 2011, 3, 27–41. [Google Scholar] [CrossRef]
- Neira, R.; García, X.; Lhorente, J.P.; Filp, M.; Yáñez, J.M.; Cascante, A.M. Evaluation of the Growth and Carcass Quality of Diallel Crosses of Four Strains of Nile Tilapia (Oerochromis niloticus). Aquaculture 2016, 451, 213–222. [Google Scholar] [CrossRef]
- Qiao, T.F.; Jiao, J.P.; Huang, D.D.; Liu, Z.M.; Wen, J.P.; Wang, Z.B.; Xia, J.H. Genome-wide identification of DNA methylation marks associated with resistance to Streptococcus agalactiae in the GIFT strain of Oreochromis niloticus. Aquaculture 2025, 595, 741530. [Google Scholar] [CrossRef]
- Awad, S.T.; Hemeda, S.A.; El Nahas, A.F.; Abbas, E.M.; Abdel-Razek, M.A.S.; Ismail, M.; Mamoon, A.; Ali, F.S. Gender-specific responses in gene expression of Oreochromis niloticus to heavy metal pollution in different aquatic habitats. Sci. Rep. 2024, 14, 14671. [Google Scholar] [CrossRef]
- Zouakh, D.E.; Chebel, F.; Bouaziz, A.; Kara, M.H. Reproduction, age and growth of Tilapia zillii (Cichlidae) in Oued Righ wetland (southeast Algeria). Cybium 2016, 40, 235–243. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Liu, C.; Wang, L.; Dong, Y.; Sun, D.; Wen, H.; Zhang, K.; Qi, X.; Li, Y. Genome-wide association study and genomic prediction for growth traits in spotted sea bass (Lateolabrax maculatus) using insertion and deletion markers. Anim. Res. One Health 2024, 2, 400–416. [Google Scholar] [CrossRef]
- Johnston, S.E.; Orell, P.; Pritchard, V.L.; Kent, M.P.; Lien, S.; Niemelä, E.; Erkinaro, J.; Primmer, C.R. Genome-wide SNP analysis reveals a genetic basis for sea-age variation in a wild population of Atlantic salmon (Salmo salar). Mol. Ecol. 2014, 23, 3452–3468. [Google Scholar] [CrossRef]
- Zhang, C.; Wen, H.; Zhang, Y.; Zhang, K.; Qi, X.; Li, Y. First Genome-Wide Association Study and Genomic Prediction for Growth Traits in Spotted Sea Bass (Lateolabrax maculatus) Using Whole-Genome Resequencing. Aquaculture 2023, 566, 739194. [Google Scholar] [CrossRef]
- Ali, F.S.; Nazmi, H.M.; Abdelaty, B.S.; El-Far, A.M.; Goda, A.M. Genetic improvement of farmed Nile tilapia (Oreochromis niloticus) through selective breeding in Egypt. Int. J. Fish. Aquat. Stud. 2017, 5, 395–401. [Google Scholar]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Nader, M.M.; Salem, H.M.; El-Tahan, A.M.; Soliman, S.M.; Khafaga, A.F. Effect of environmental factors on growth performance of Oreochromis niloticus. Int. J. Biometeorol. 2022, 66, 2183–2194. [Google Scholar] [CrossRef]
- El-Greisy, Z.A.; El-Gamal, A.E. Monosex production of tilapia, Oreochromis niloticus, using different doses of 17α-methyltestosterone with respect to the degree of sex stability after one year of treatment. Egypt. J. Aquat. Res. 2012, 38, 59–66. [Google Scholar] [CrossRef]
- Gunasekera, R.M.; Shim, K.F.; Lam, T.J. Influence of Protein Content of Broodstock Diets on Larval Quality and Performance in Nile Tilapia, Oreochromis niloticus (L.). Aquaculture 1996, 146, 245–259. [Google Scholar] [CrossRef]
- Fujimura, K.; Okada, N. Development of the embryo, larva and early juvenile of Nile tilapia Oreochromis niloticus (Pisces: Cichlidae): Developmental staging system. Devel. Growth Different. 2007, 49, 301–324. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M. Reproduction and seed production. In Tilapia Culture; CABI Publishing: Wallingford, UK, 2006; pp. 112–138. [Google Scholar] [CrossRef]
- Asahida, T.; Kobayashi, T.; Saitoh, K.; Nakayama, I. Tissue preservation and total DNA extraction from fish stored at ambient temperature using buffers containing high concentration of urea. Fish. Sci. 1996, 62, 727–730. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucl. Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Megahed, E.T.; Abbas, E.M.; El Nahas, A.F.; Hemeda, S.A. Genetic variation of Diplodus sargus and Diplodus vulgaris in four Mediterranean coastal regions of Egypt based on microsatellites. Egypt. J. Aquat. Biol. Fish. 2020, 24, 203–215. [Google Scholar] [CrossRef]
- Ewing, B.; Green, P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998, 8, 186–194. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Dereeper, A.; Homa, F.; Andres, G.; Sempere, G.; Sarah, G.; Hueber, Y.; Dufayard, J.-F.; Ruiz, M. SNiPlay3: A web-based application for exploration and large-scale analyses of genomic variations. Nucleic Acids Res. 2015, 43, W295–W300. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Rosenberg, G. A Place for Consciousness: Probing the Deep Structure of the Natural World; Oxford University Press: Oxford, UK, 2004; ISBN 0190290587. [Google Scholar]
- Ritland, K. Estimators for pairwise relatedness and individual inbreeding coefficients. Genet. Res. 1996, 67, 175–185. [Google Scholar] [CrossRef]
- Hardy, O.J.; Vekemans, X. SPAGeDi: A versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol. Ecol. Notes 2002, 2, 618–620. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for Association Mapping of Complex Traits in Diverse Samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Cappa, E.P.; Martínez, M.C.; Garcia, M.N.; Villalba, P.V.; Marcucci Poltri, S.N. Effect of population structure and kinship relationships on the results of association mapping tests of growth and wood quality traits in four Eucalyptus populations. BMC Proc. 2011, 5, P23. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef]
- Gabriel, S.B.; Schaffner, S.F.; Nguyen, H.; Moore, J.M.; Roy, J.; Blumenstiel, B.; Higgins, J.; DeFelice, M.; Lochner, A.; Faggart, M. The structure of haplotype blocks in the human genome. Science 2002, 296, 2225–2229. [Google Scholar] [CrossRef]
- Abo-Al-Ela, H.G.; El-Nahas, A.F.; Mahmoud, S.; Ibrahim, E.M. The extent to which immunity, apoptosis and detoxification gene expression interact with 17 alpha-methyltestosterone. Fish Shellfish Immunol. 2017, 60, 289–298. [Google Scholar] [CrossRef]
- El-Leithy, A.A.A.; Hemeda, S.A.; El Naby, W.S.H.A.; El Nahas, A.F.; Hassan, S.A.H.; Awad, S.T.; El-Deeb, S.I.; Helmy, Z.A. Optimum salinity for Nile tilapia (Oreochromis niloticus) growth and mRNA transcripts of ion-regulation, inflammatory, stress- and immune-related genes. Fish Physiol. Biochem. 2019, 45, 1217–1232. [Google Scholar] [CrossRef]
- Ming, C.; Rui, W.; Liping, L.; Huang, T.; Weiyi, H.; Jian, L.; Chao, L.; Aiying, L.; Honglin, L.; Wanwen, L. Sequence and evolution differences of Oreochromis niloticus CXC contribute to the diversification of cellular immune responses in tilapias with treatment of Streptococcus iniae. J. Anim. Vet. Adv. 2013, 12, 303–313. Available online: http://docsdrive.com/pdfs/medwelljournals/javaa/2013/303-311.pdf (accessed on 1 March 2024).
- Elkatatny, N.M.; El Nahas, A.F.; Helal, M.A.; Fahmy, H.A.; Tanekhy, M. The impacts of seasonal variation on the immune status of Nile tilapia larvae and their response to different immunostimulants feed additives. Fish Shellfish Immunol. 2020, 96, 270–278. [Google Scholar] [CrossRef]
- Ghazy, H.A.; Abdel-Razek, M.A.S.; El Nahas, A.F.; Mahmoud, S. Assessment of complex water pollution with heavy metals and pyrethroid pesticides on transcript levels of metallothionein and immune related genes. Fish Shellfish Immunol. 2017, 68, 318–326. [Google Scholar] [CrossRef]
- Puerto, M.; Gutierrez-Praena, D.; Prieto, A.; Pichardo, S.; Jos, A.; Miguel-Carrasco, J.L.; Vazquez, C.M.; Cameán, A.M. Subchronic Effects of Cyanobacterial Cells on the Transcription of Antioxidant Enzyme Genes in Tilapia Nilotica (Oreochromis niloticus). Ecotoxicology 2011, 20, 479–490. [Google Scholar] [CrossRef]
- Phumyu, N.; Boonanuntanasarn, S.; Jangprai, A.; Yoshizaki, G.; Na-Nakorn, U. Pubertal effects of 17 alpha-methyltestosterone on GH-IGF-related genes of the hypothalamic–pituitary–liver–gonadal axis and other biological parameters in male, female and sex-reversed Nile tilapia. Gen. Comp. Endocrinol. 2012, 177, 278–292. [Google Scholar] [CrossRef]
- Pholchamat, S.; Vialle, R.; Luang-In, V.; Phadee, P.; Wang, B.; Wang, T.; Secombes, C.J.; Wangkahart, E. Evaluation of the efficacy of MONTANIDETM GR01, a new adjuvant for feed-based vaccines, on the immune response and protection against Streptococcus agalactiae in oral vaccinated Nile tilapia (Oreochromis niloticus) under laboratory and on-farm conditions. Fish Shellfish Immunol. 2024, 149, 109567. [Google Scholar] [CrossRef]
- Ruenkoed, S.; Pholoeng, A.; Nontasan, S.; Panprommin, D.; Mongkolwit, K.; Wangkahart, E. Assessing the impact of acidifiers on growth performance, innate immune capacity, response to ammonia nitrogen stress, digestive enzyme activity, intestinal histology, and gene expression of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2025, 162, 110315. [Google Scholar] [CrossRef]
- Tawfik, W.; Nassef, E.; Bakr, A.; Hegazi, E.; Ismail, T.A.; Abdelazim, A.M.; El-Nagar, S.H.; Sabike, I.; Fadl, S.E.; Sharoba, A.M. Orange pulp in Nile tilapia (Oreochromis niloticus) diets: Growth performance, biochemical parameters and gene expression for growth and fat metabolism. Aqua. Rep. 2022, 22, 100970. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2(−ΔΔCT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinformat. Biomathemat. 2013, 3, 71–85. [Google Scholar]
- Musa, A.M.; Mohammed, E.B.; Alla, A.A.A.; Alfatih, W.M.; Manofal, A.M. Evaluation study on the growth performance of four local strains of Nile tilapia (Oreochromis niloticus) in Sudan. Aquat. Sci. Technol. 2021, 9, 1. [Google Scholar] [CrossRef]
- Teichert, N.; Lizé, A.; Cabral, H.; Acou, A.; Trancart, T.; Virag, L.S.; Feunteun, E.; Carpentier, A. Decoupling carry-over effects from environment in fish nursery grounds. Sci. Total Environ. 2023, 857, 159487. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Cooke, S.J. Ecological carryover effects complicate conservation. Ambio 2015, 44, 582–591. [Google Scholar] [CrossRef]
- Jaser, S.K.K.; Dias, M.A.D.; Lago, A.d.A.; Reis Neto, R.V.; Hilsdorf, A.W.S. Single Nucleotide Polymorphisms in the Growth Hormone Gene of Oreochromis niloticus and Their Association with Growth Performance. Aquac. Res. 2017, 48, 5835–5845. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M. Comparative growth performance and feed utilization of four local strains of Nile tilapia (Oreochromis niloticus). In Proceedings of the Sixth International Symposium on Tilapia in Aquaculture, Manila, Philippines, 12–16 September 2004; Volume 11, pp. 510–517. Available online: http://hdl.handle.net/10576/9845 (accessed on 1 March 2004).
- Chen, B.; Xiao, W.; Zou, Z.; Zhu, J.; Li, D.; Yu, J.; Yang, H. Ghrelin gene single nucleotide polymorphisms and their effects on Nile tilapia (Oreochromis niloticus) growth. Aquac. Rep. 2020, 18, 100469. [Google Scholar] [CrossRef]
- El-Far, A.; Aly, W.; El-Haweet, A.E.D.; Nasr-Allah, A.; Karisa, H. Fisheries management based on gear selectivity of a tropical reservoir, Lake Nasser, Egypt. Egypt. J. Aquat. Res. 2020, 46, 71–77. [Google Scholar] [CrossRef]
- Faggion, S.; Vandeputte, M.; Vergnet, A.; Clota, F.; Blanc, M.O.; Sanchez, P.; Ruelle, F.; Allal, F. Sex dimorphism in European sea bass (Dicentrarchus labrax L.): New insights into sex-related growth patterns during very early life stages. PLoS ONE 2021, 16, e0239791. [Google Scholar] [CrossRef]
- Wan, Z.Y.; Lin, G.; Yue, G. Genes for sexual body size dimorphism in hybrid tilapia (Oreochromis sp. × Oreochromis mossambicus). Aquac. Fish. 2019, 4, 231–238. [Google Scholar] [CrossRef]
- Cleveland, B.M.; Weber, G.M. Effects of sex steroids on expression of genes regulating growth-related mechanisms in rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 2015, 216, 103–115. [Google Scholar] [CrossRef]
- Wang, C.; Liu, B.; Chen, M.; Ning, J.; Lu, X.; Wang, C. Mutations in growth-related genes induced by EMS treatment in scallops. Front. Genet. 2022, 13, 879844. [Google Scholar] [CrossRef]
- Hu, Z.; Holzschuh, J.; Driever, W. Loss of DDB1 leads to transcriptional P53 pathway activation in proliferating cells, cell cycle deregulation, and apoptosis in zebrafish embryos. PLoS ONE 2015, 10, e0134299. [Google Scholar] [CrossRef]
- Li, Z.; Pearlman, A.H.; Hsieh, P. DNA mismatch repair and the DNA damage response. DNA Rep. 2016, 38, 94–101. [Google Scholar] [CrossRef]
- Mohamed, E.; Hussein, M.; El-Geziry, T.M.; Beltagy, A.; Sheta, M. Water exchange characteristics between Lake Burullus and the Mediterranean Sea. Egypt. J. Aquat. Biol. Fish. 2022, 26, 427–442. [Google Scholar] [CrossRef]
- Abdelazim, N.M.; Mohamed, B.A.; Abdel-Fattah, S. Egyptian Coastal Lakes and Wetlands: Part II Climate Change and Biodiversity; Springer: Berlin/Heidelberg, Germany, 2019; Volume 49, ISBN 9783319936109. [Google Scholar]
- Salih, S.Q.; Allawi, M.F.; Yousif, A.A.; Armanuos, A.M.; Saggi, M.K.; Ali, M.; Shahid, S.; Al-Ansari, N.; Yaseen, Z.M.; Chau, K.W. Viability of the advanced adaptive neuro-fuzzy inference system model on reservoir evaporation process simulation: Case study of Nasser Lake in Egypt. Eng. Appl. Comput. Fluid Mech. 2019, 13, 878–891. [Google Scholar] [CrossRef]
- Farhat, H.I.; Aly, W. Effect of site on sedimentological characteristics and metal pollution in two semi-enclosed embayments of great freshwater reservoir: Lake Nasser, Egypt. J. Afr. Earth Sci. 2018, 141, 194–206. [Google Scholar] [CrossRef]
- Kadry, S.M.; Tayel, S.I.; Afify, M.F.H.; El-Sayed, R.A. Eco-histopathological studies Oreochromis niloticus fish living in Damietta Branch in Egypt. World J. Pharm. Sci. 2015, 3, 815–825. [Google Scholar]
- Osman, A.G.M.; Abuel-Fadl, K.Y.; Kloas, W. In situ evaluation of the genotoxic potential of the River Nile: II. Detection of DNA strand-breakage and apoptosis in Oreochromis niloticus niloticus (Linnaeus, 1758) and Clarias gariepinus (Burchell, 1822). Mut. Res. Genet. Toxicol. Environ. Mut. 2012, 747, 14–21. [Google Scholar] [CrossRef]
- Abdel-Aal, E.I.; Haroon, A.M.; Ibrahim, S.M.; Abd El-Aziz, G.S.; Sabae, S.A.; Gaber, K.M.; Goher, M.E. Ecological status of Lake Nasser khors, Egypt, before operating the Grand Ethiopian Renaissance Dam. Stoch. Environ. Res. Risk Assess. 2023, 37, 1229–1245. [Google Scholar] [CrossRef]
- Shaltout, K.H.; Khalil, M.T.; Al-Sodany, Y.M. Lake Burullus: Burullus Protected Area; Publication of National Biodiversity Unit No. 13; Egyptian Environmental Affairs Agency: Cairo, Egypt, 2005. [Google Scholar]
- Marsh, J.N.; Vega-Trejo, R.; Jennions, M.D.; Head, M.L. Why does inbreeding reduce male paternity? Effects on sexually selected traits. Evolution 2017, 71, 2728–2737. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Rolshausen, G.; Uren Webster, T.M.; Tyler, C.R. Adaptive capabilities and fitness consequences associated with pollution exposure in fish. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160042. [Google Scholar] [CrossRef]
- Houston, R.D.; Bean, T.P.; Macqueen, D.J.; Gundappa, M.K.; Jin, Y.H.; Jenkins, T.L.; Selly, S.L.C.; Martin, S.A.M.; Stevens, J.R.; Santos, E.M. Harnessing genomics to fast-track genetic improvement in aquaculture. Nat. Rev. Genet. 2020, 21, 389–409. [Google Scholar] [CrossRef]
- Dunham, R. Aquaculture and Fisheries Biotechnology: Genetic Approaches; CABI: Wallingford, UK, 2023; ISBN 1789243467. [Google Scholar] [CrossRef]
- Robledo, D.; Ogwang, J.; Byakora, E.; Nascimento-Schulze, J.C.; Benda, K.K.; Fraslin, C.; Salisbury, S.; Solimo, M.; Mayega, J.F.; Peter, B. Genetic Diversity and Population Structure of Farmed and Wild Nile Tilapia (Oreochromis niloticus) in Uganda: The Potential for Aquaculture Selection and Breeding Programs. Genomics 2024, 116, 110781. [Google Scholar] [CrossRef]
- Kajungiro, R.A.; Palaiokostas, C.; Pinto, F.A.L.; Mmochi, A.J.; Mtolera, M.; Houston, R.D.; de Koning, D.J. Population Structure and Genetic Diversity of Nile Tilapia (Oreochromis niloticus) Strains Cultured in Tanzania. Front. Genet. 2019, 10, 1269. [Google Scholar] [CrossRef]
- Fagbémi, M.N.A.; Pigneur, L.-M.; André, A.; Smitz, N.; Gennotte, V.; Michaux, J.R.; Mélard, C.; Lalèyè, P.A.; Rougeot, C. Genetic Structure of Wild and Farmed Nile Tilapia (Oreochromis niloticus) Populations in Benin Based on Genome Wide SNP Technology. Aquaculture 2021, 535, 736432. [Google Scholar] [CrossRef]
- García-Fernández, C.; Sánchez, J.A.; Blanco, G. SNP-Haplotypes: An Accurate Approach for Parentage and Relatedness Inference in Gilthead Sea Bream (Sparus aurata). Aquaculture 2018, 495, 582–591. [Google Scholar] [CrossRef]
- Cuevas-Rodríguez, B.L.; Sifuentes-Rincón, A.M.; Ambriz-Morales, P.; García-Ulloa, M.; Valdez-González, F.J.; Rodríguez-González, H. Novel Single Nucleotide Polymorphisms in Candidate Genes for Growth in Tilapia (Oreochromis niloticus). Rev. Bras. Zootec. 2016, 45, 345–348. [Google Scholar] [CrossRef]
- Li, M.J.; Liu, W.S.; Luo, W.; Zhang, X.Q.; Zhu, W.L.; Wang, J.; Liao, L.Y.; Li, G.H. Polymorphisms and Their Association with Growth Traits in the Growth Hormone Gene of Yellow Catfish, Pelteobagrus fulvidraco. Aquaculture 2017, 469, 117–123. [Google Scholar] [CrossRef]
- Naby, H.A.E.; Aboukila, R.S.; Hemeda, S.A.E.; El Nahas, A.F.; El Naby, W.S. Molecular Characterization of GHR1 Gene and Expression Analysis of Some Growth-Related Genes in Oreochromis niloticus. Adv. Anim. Vet. Sci. 2021, 9, 1025–1033. [Google Scholar] [CrossRef]
- Parker, G.A. The Evolution of Sexual Size Dimorphism in Fish. J. Fish Biol. 1992, 41, 1–20. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Early Environment Influences Later Performance in Fishes. J. Fish Biol. 2014, 85, 151–188. [Google Scholar] [CrossRef]
- Syed, R.; Masood, Z.; Ul Hassan, H.; Khan, W.; Mushtaq, S.; Ali, A.; Gul, Y.; Jafari, H.; Habib, A.; Ishaq Ali Shah, M.; et al. Growth Performance, Haematological Assessment and Chemical Composition of Nile Tilapia, Oreochromis niloticus (Linnaeus, 1758) Fed Different Levels of Aloe vera Extract as Feed Additives in a Closed Aquaculture System. Saudi J. Biol. Sci. 2022, 29, 296–303. [Google Scholar] [CrossRef]
- Sem, A.; Ap, M.; Mg, O. Broodstock Fecundity and Fry Survivability in Nile Tilapia (Oreochromis niloticus): Effects of Dietary Nucleotide Supplementation in an on-Farm Feeding Trial. In Proceedings of the International Conference on Fisheries and Aquaculture, Barcelona, Spain, 9–10 September 2024; Volume 11, pp. 1–15. [Google Scholar] [CrossRef]
- Christie, M.R.; Marine, M.L.; Fox, S.E.; French, R.A.; Blouin, M.S. A Single Generation of Domestication Heritably Alters the Expression of Hundreds of Genes. Nat. Commun. 2016, 7, 10676. [Google Scholar] [CrossRef]
- Mahboub, H.H.; Eltanahy, A.; Omran, A.; Mansour, A.T.; Safhi, F.A.; Alwutayd, K.M.; Khamis, T.; Husseiny, W.A.; Ismail, S.H.; Yousefi, M.; et al. Chitosan Nanogel Aqueous Treatment Improved Blood Biochemicals, Antioxidant Capacity, Immune Response, Immune-Related Gene Expression and Infection Resistance of Nile Tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2024, 269, 110876. [Google Scholar] [CrossRef]
- Mahboub, H.H.; Elsheshtawy, H.M.; Sheraiba, N.I.; Fahmy, E.M.; Masoud, S.R.; Mohamed, E.A.A.; Abdelnaeim, N.S.; Mohamed, D.I.; Ismail, T.A.; Ahmed, S.A.A. Dietary Black Cumin (Nigella sativa) Improved Hemato-Biochemical, Oxidative Stress, Gene Expression, and Immunological Response of Nile Tilapia (Oreochromis niloticus) Infected by Burkholderia cepacia. Aquac. Rep. 2022, 22, 100943. [Google Scholar] [CrossRef]
- Fuentes, E.N.; Valdés, J.A.; Molina, A.; Björnsson, B.T. Regulation of skeletal muscle growth in fish by the growth hormone–insulin-like growth factor system. Gen. Comp. Endocrinol. 2013, 192, 136–148. [Google Scholar] [CrossRef]
- Opazo, R.; Valladares, L.; Romero, J. Comparison of gene expression patterns of key growth genes between different rate growths in zebrafish (Danio rerio) siblings. Lat. Am. J. Aquat. Res 2017, 45, 766–775. [Google Scholar] [CrossRef]
- Devlin, R.H.; Sakhrani, D.; Tymchuk, W.E.; Rise, M.L.; Goh, B. Domestication and growth hormone transgenesis cause similar changes in gene expression in coho salmon (Oncorhynchus kisutch). Proc. Natl. Acad. Sci. USA 2009, 106, 3047–3052. [Google Scholar] [CrossRef]
- Jeay, S.; Sonenshein, G.E.; Postel-Vinay, M.-C.; Kelly, P.A.; Baixeras, E. Growth hormone can act as a cytokine controlling survival and proliferation of immune cells: New insights into signaling pathways. Mol. Cell. Endocrinol. 2002, 188, 1–7. [Google Scholar] [CrossRef]
- Yada, T. Growth hormone and fish immune system. Gen. Comp. Endocrinol. 2007, 152, 353–358. [Google Scholar] [CrossRef]
- Franz, A.C.; Faass, O.; Kollner, B.; Shved, N.; Link, K.; Casanova, A.; Wenger, M.; D’cotta, H.; Baroiller, J.F.; Ullrich, O. Endocrine and local IGF-I in the bony fish immune system. Biology 2016, 5, 9. [Google Scholar] [CrossRef]
- Zhu, W.; Su, J. Immune functions of phagocytic blood cells in teleost. Rev. Aquac. 2022, 14, 630–646. [Google Scholar] [CrossRef]
- Pontigo, J.P.; Vargas-Chacoff, L. Growth hormone (GH) and growth hormone release factor (GRF) modulate the immune response in the SHK-1 cell line and leukocyte cultures of head kidney in Atlantic salmon. Gen. Comp. Endocrinol. 2021, 300, 113631. [Google Scholar] [CrossRef]
- Shved, N.; Berishvili, G.; Mazel, P.; Baroiller, J.F.; Eppler, E. Growth hormone (GH) treatment acts on the endocrine and autocrine/paracrine GH/IGF-axis and on TNF-α expression in bony fish pituitary and immune organs. Fish Shellfish Immunol. 2011, 31, 944–952. [Google Scholar] [CrossRef]
- Nakharuthai, C.; Srisapoome, P. Molecular Identification and Dual Functions of Two Different CXC Chemokines in Nile Tilapia (Oreochromis niloticus) against Streptococcus agalactiae and Flavobacterium columnare. Microorganisms 2020, 8, 1058. [Google Scholar] [CrossRef]
- Hanna, M.I.; El Sayed, A.T.; El Megeed, O.H.A.; Ibrahim, M.A.; Korany, R.M.S.; Attia, M.M. Assessment of a New Protocol Strategy to Control the Ectoparasitic Infestation in Nile Tilapia (Oreochromis niloticus) Using Efficient Natural Products. BMC Vet. Res. 2025, 21, 15. [Google Scholar] [CrossRef]
- Chovatia, R.M.; Acharya, A.; Rasal, K.D.; Bedekar, M.K.; Jeena, K.; Rathinam, R.B.; Dinakaran, C.; Tripathi, G. Ontogeny and Tissue Specific Expression Profiles of Recombination Activating Genes (RAGs) during Development in Nile Tilapia (Oreochromis niloticus). Gene Expr. Patterns 2024, 52, 119358. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, J.E.; Goo, I.B.; Hwang, J.A.; Im, J.H.; Choi, H.S.; Lee, J.H. Expression of Immune-Related Genes during Loach (Misgurnus anguillicaudatus) Embryonic and Early Larval Development. Devel. Reprod. 2015, 19, 181–187. [Google Scholar] [CrossRef]
- Lee, J.-W.; Yang, H.; Noh, J.K.; Kim, H.C.; Park, C.-J.; Park, J.-W.; Hwang, I.J.; Kim, S.Y.; Lee, J.-H. RAG-1 and IgM genes, markers for early development of the immune system in Olive Flounder (Paralichthys olivaceus). Devel. Reproduct. 2014, 18, 99–106. [Google Scholar] [CrossRef]




| Gene Name and Code | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Target Size (bp) | Linkage Group | Accession Number | Reference |
|---|---|---|---|---|---|---|
| CXC2 chemokine | CTATCCATGGAGCCTCAGGT | CTTCTTGAGCGTGGCAATAA | 146 | LG6 | XM_003452201.5 | [59] |
| Interleukin-1 Beta (IL1β) | AGAGCAGCAATTCAGAGC | GTGCTGATGTACCAGT | 514 | LG12 | XM_019365842.2 | [60] |
| Interleukin-8 (IL8) | GCACTGCCGCTGCATTAAG | GCAGTGGGAGTTGGGAAGAA | 85 | LG6 | XM_019359413.2 | [61] |
| CC-chemokine | ACAGAGCCGATCTTGGGTTACTTG | TGAAGGAGAGGCGGTGGATGTTAT | 228 | LG9 | XM_019362411.2 | [60] |
| “Recombination Activating Gene1 (rag1)” | AAGACAGTGCCTGCACATCA | CTCAGGAACAACTGGTCCCC | 104 | LG7 | XM_019361952.2 | [62] |
| Sacsin gene (sacs) | ACATCACTGATAGCACCCGC | TCATGGTGGGATCTGGACCT | 82 | LG14 | XM_019367592.2 | [62] |
| Tumer necrosis factorα (TNFα) | GGTTAGTTGAGAAGAAATCACCTGCA | GTCGTCGCTATTCCCGCAGATCA | 407 | LG11 | NM_001279533.1 | [63] |
| Insulin like growth factor1 (IGF1) | TTGTCTGTGGAGAG CGAGGCT | CAGCTTTGGAAGCA GCACTCGT | 202 | LG17 | XM_019346352.2 | [60] |
| Growth hormone (GH) | TAATGGGAGAGGGA AGATGG | CTCTGCGATGTAAT TCAGGA | 90 | LG4 | XM_003442542 | [64] |
| βeta-actin | TGG CAA TGA GAG GTT CCG | TGCTGTTGTAGGTGGTTTCG | 136 | LG4 | XM_003443127.5 | [65] |
| Attribute | SNP/InDel | Position in Consensus Sequence | p-Value | % Phenotypic Variance (R2) |
|---|---|---|---|---|
| Body length | SNP1 * | 36 | 0.006 | 4.9 |
| SNP2 | 116 | 0.02 | 3.5 | |
| SNP5 | 211 | 0.016 | 3.8 | |
| SNP6 | 240 | 0.015 | 3.8 | |
| SNP7 | 330 | 0.008 | 4.5 | |
| SNP8 * | 492 | 0.007 | 4.7 | |
| InDel1 | 572 | 0.005 | 4.9 | |
| InDel2 * | 581 | 0 | 14.8 | |
| InDel3 * | 582 | 0 | 23.4 | |
| Body weight | SNP1 * | 36 | 0.028 | 3.1 |
| SNP3 | 134 | 0.042 | 2.6 | |
| SNP4 | 174 | 0.045 | 2.6 | |
| SNP8 * | 492 | 0.029 | 3.1 | |
| SNP9 | 539 | 0.028 | 3.1 | |
| InDel2 * | 581 | 0 | 18.2 | |
| InDel3 * | 582 | 0 | 36.0 |
| SNP/InDel | FA | FQ | FB | MA | MQ | MB |
|---|---|---|---|---|---|---|
| SNP1 | 8 | 1 | ||||
| SNP2 | 4 | |||||
| SNP3 | 6 | |||||
| SNP4 | 5 | |||||
| SNP5 | 1 | 1 | 9 | 5 | ||
| SNP6 | 1 | 2 | 7 | 5 | 6 | |
| SNP7 | 2 | 3 | ||||
| SNP8 | 6 | 1 | ||||
| SNP9 | 6 | |||||
| InDel1 | 8 | 1 | ||||
| InDel2 | 8 | 1 | 8 | 1 | ||
| InDel3 | 10 | 6 | ||||
| Total | 12 | 11 | 9 | 39 | 38 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, F.S.; Awad, S.T.; Ismail, M.; Hemeda, S.A.; El Nahas, A.F.; Abbas, E.M.; Mamoon, A.; Nazmi, H.; El-Haroun, E. Selection for Growth Performance in Oreochromis niloticus Across Different Aquatic Environments Using Growth Hormone Gene Polymorphisms. Animals 2025, 15, 2097. https://doi.org/10.3390/ani15142097
Ali FS, Awad ST, Ismail M, Hemeda SA, El Nahas AF, Abbas EM, Mamoon A, Nazmi H, El-Haroun E. Selection for Growth Performance in Oreochromis niloticus Across Different Aquatic Environments Using Growth Hormone Gene Polymorphisms. Animals. 2025; 15(14):2097. https://doi.org/10.3390/ani15142097
Chicago/Turabian StyleAli, Fawzia S., Simone T. Awad, Mohamed Ismail, Shabaan A. Hemeda, Abeer F. El Nahas, Eman M. Abbas, Ahmed Mamoon, Hani Nazmi, and Ehab El-Haroun. 2025. "Selection for Growth Performance in Oreochromis niloticus Across Different Aquatic Environments Using Growth Hormone Gene Polymorphisms" Animals 15, no. 14: 2097. https://doi.org/10.3390/ani15142097
APA StyleAli, F. S., Awad, S. T., Ismail, M., Hemeda, S. A., El Nahas, A. F., Abbas, E. M., Mamoon, A., Nazmi, H., & El-Haroun, E. (2025). Selection for Growth Performance in Oreochromis niloticus Across Different Aquatic Environments Using Growth Hormone Gene Polymorphisms. Animals, 15(14), 2097. https://doi.org/10.3390/ani15142097






