The Role of Lipid Metabolic Reprogramming in the Hibernation of Chipmunks
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
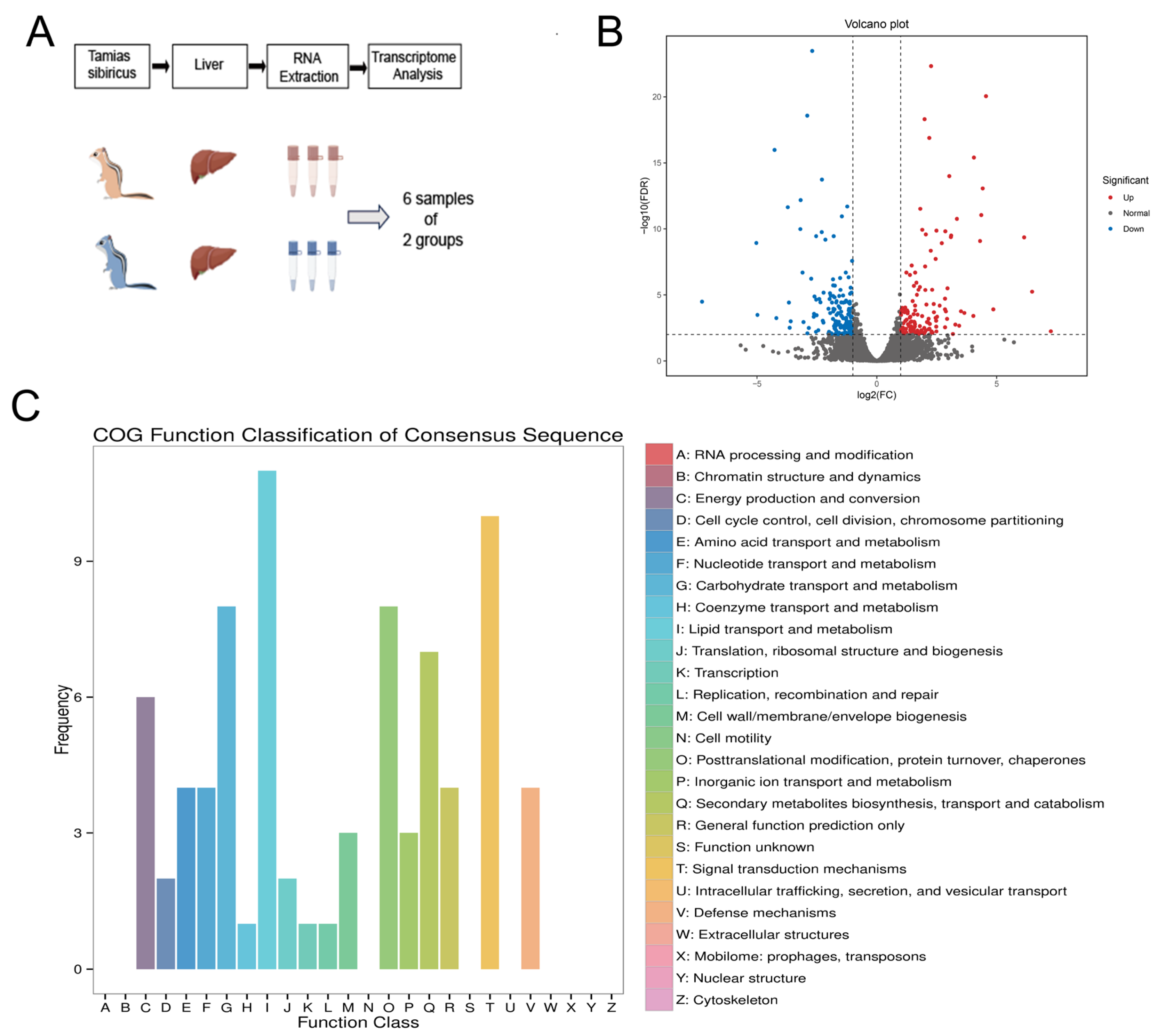
2.1. Animals and Study Design
2.2. Histological Analysis of the Liver Tissues
2.3. Glycogen Content Detection
2.4. Blood Glucose Measurement
2.5. ATP Assay
2.6. Fatty Acid Analysis
2.7. Transcriptome Sequencing
2.8. Real-Time PCR
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Effects of Hibernation on Liver Morphology of Chipmunk
3.2. The Energy Source of Chipmunk Livers During Hibernation
3.3. Proportion of Fatty Acids in the Liver During Hibernation
3.4. Transcriptome Analysis of the Liver of Hibernating Chipmunks
3.5. The Effect of Hibernation on Lipid Metabolic Genes Expression in the Liver of Chipmunks
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Staples, J.F. Metabolic Flexibility: Hibernation, Torpor, and Estivation. Compr. Physiol. 2016, 6, 737–771. [Google Scholar] [CrossRef]
- Wältermann, M.; Steinbüchel, A. Neutral lipid bodies in prokaryotes: Recent insights into structure, formation, and relationship to eukaryotic lipid depots. J. Bacteriol. 2005, 187, 3607–3619. [Google Scholar] [CrossRef] [PubMed]
- Olsen, L.; Thum, E.; Rohner, N. Lipid metabolism in adaptation to extreme nutritional challenges. Dev. Cell 2021, 56, 1417–1429. [Google Scholar] [CrossRef]
- Mukerjee, S.; Saeedan, A.S.; Ansari, M.N.; Singh, M. Polyunsaturated fatty acids mediated regulation of membrane biochemistry and tumor cell membrane integrity. Membranes 2021, 11, 479. [Google Scholar] [CrossRef]
- Price, E.R. The physiology of lipid storage and use in reptiles. Biol. Rev. 2017, 92, 1406–1426. [Google Scholar] [CrossRef]
- Liu, J.G.; Jiang, H.; Zhang, H.; Zhang, X.; Jia, Z.G.; Yu, H. Effects of hibernation on colonic epithelial tissue and gut microbiota in wild chipmunks (Tamias sibiricus). Animals 2024, 14, 1498. [Google Scholar] [CrossRef]
- Lyman, C.P.; Chatfield, P.O. Physiology of hibernation in mammals. Physiol. Rev. 1955, 35, 403–425. [Google Scholar] [CrossRef]
- Hindle, A.G.; Martin, S.L. Intrinsic circannual regulation of brown adipose tissue form and function in tune with hibernation. Am. J. Physiol. Endocrinol. Metab. 2014, 306, 284–299. [Google Scholar] [CrossRef]
- Ruiz-Núñez, B.; Dijck-Brouwer, D.J.; Muskiet, F.A. The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J. Nutr. Biochem. 2016, 36, 1–20. [Google Scholar] [CrossRef]
- Storey, K.B. Out cold: Biochemical regulation of mammalian hibernation-a mini-review. Gerontology 2010, 56, 220–230. [Google Scholar] [CrossRef]
- Lyman, C.P. Hibernation and Torpor in Mammals and Birds; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Nedergaard, J.; Cannon, B. Mammalian hibernation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1990, 326, 669–686. [Google Scholar]
- Boyer, B.B.; Barnes, B.M. Molecular and metabolic aspects of mammalian hibernation: Expression of the hibernation phenotype results from the coordinated regulation of multiple physiological and molecular events during preparation for and entry into torpor. Bioscience 1999, 49, 713–724. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem.-Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef]
- Carey, H.V.; Andrews, M.T.; Martin, S.L. Mammalian hibernation: Cellular and molecular responses to depressed metabolism and low temperature. Physiol. Rev. 2003, 83, 1153–1181. [Google Scholar] [CrossRef]
- MacLean, A.; Legendre, F.; Appanna, V.D. The tricarboxylic acid (TCA) cycle: A malleable metabolic network to counter cellular stress. Crit. Rev. Biochem. Mol. Biol. 2023, 58, 81–97. [Google Scholar] [CrossRef]
- Saxton, M.W.; Perry, B.W.; Hutzenbiler, B.D.E.; Trojahn, S.; Gee, A.; Brown, A.P.; Merrihew, G.E.; Park, J.; Cornejo, O.E.; MacCoss, M.J. Serum plays an important role in reprogramming the seasonal transcriptional profile of brown bear adipocytes. Iscience 2022, 25, 105084. [Google Scholar] [CrossRef]
- Wu, G.; Baumeister, R.; Heimbucher, T. Molecular mechanisms of lipid-based metabolic adaptation strategies in response to cold. Cells 2023, 12, 1353. [Google Scholar] [CrossRef] [PubMed]
- Hiroshima, Y.; Yamamoto, T.; Watanabe, M.; Baba, Y.; Shinohara, Y. Effects of cold exposure on metabolites in brown adipose tissue of rats. Mol. Genet. Metab. Rep. 2018, 15, 36–42. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.C.; Caramujo, M.J. The various roles of fatty acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [PubMed]
- Zierfuss, B.; Buda, A.; Villoria-González, A.; Logist, M.; Fabjan, J.; Parzer, P.; Battin, C.; Vandersteene, S.; Dijkstra, I.M.; Waidhofer-Söllner, P. Saturated very long-chain fatty acids regulate macrophage plasticity and invasiveness. J. NeuroInflamm. 2022, 19, 305. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Liu, X.; Zhao, Z.; Tao, S.; Xu, Q.; Zhao, J.; Dai, Z.; Zhang, G.; Han, D. Galactooligosaccharides and Limosilactobacillus reuteri synergistically alleviate gut inflammation and barrier dysfunction by enriching Bacteroides acidifaciens for pentadecanoic acid biosynthesis. Nat. Commun. 2024, 15, 9291. [Google Scholar] [CrossRef] [PubMed]
- Masquio, D.C.L.; de Piano-Ganen, A.; Oyama, L.M.; da Silveira Campos, R.M.; Santamarina, A.B.; Gomes, A.D.O.; Moreira, R.G.; Corgosinho, F.C.; do Nascimento, C.M.O.; Tock, L. The role of free fatty acids in the inflammatory and cardiometabolic profile in adolescents with metabolic syndrome engaged in interdisciplinary therapy. J. Nutr. Biochem. 2016, 33, 136–144. [Google Scholar] [CrossRef]
- D’Ambrosio, D.N.; Clugston, R.D.; Blaner, W.S. Vitamin A metabolism: An update. Nutrients 2011, 3, 63–103. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cai, S.Y.; Boyer, J.L. The role of the retinoid receptor, RAR/RXR heterodimer, in liver physiology. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2021, 1867, 166085. [Google Scholar] [CrossRef] [PubMed]
- Tattoli, I.; Mathew, A.R.; Verrienti, A.; Pallotta, L.; Severi, C.; Andreola, F.; Cavallucci, V.; Giorgi, M.; Massimi, M.; Bencini, L. The Interplay between Liver and Adipose Tissue in the Onset of Liver Diseases: Exploring the Role of Vitamin Deficiency. Cells 2024, 13, 1631. [Google Scholar] [CrossRef]
- Saeed, A.; Dullaart, R.P.; Schreuder, T.C.; Blokzijl, H.; Faber, K.N. Disturbed vitamin A metabolism in non-alcoholic fatty liver disease (NAFLD). Nutrients 2017, 10, 29. [Google Scholar] [CrossRef]
- Saeed, A.; Bartuzi, P.; Heegsma, J.; Dekker, D.; Kloosterhuis, N.; de Bruin, A.; Jonker, J.W.; van de Sluis, B.; Faber, K.N. Impaired hepatic vitamin A metabolism in NAFLD mice leading to vitamin A accumulation in hepatocytes. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 309–325.e303. [Google Scholar] [CrossRef]
- Lai, X.; Wu, A.; Bing, Y.; Liu, Y.; Luo, J.; Yan, H.; Zheng, P.; Yu, J.; Chen, D. Retinoic acid protects against lipopolysaccharide-induced ferroptotic liver injury and iron disorders by regulating Nrf2/HO-1 and RARβ signaling. Free Radic. Biol. Med. 2023, 205, 202–213. [Google Scholar] [CrossRef]
- Steinhoff, J.S.; Wagner, C.; Taschler, U.; Wulff, S.; Kiefer, M.F.; Petricek, K.M.; Wowro, S.J.; Oster, M.; Flores, R.E.; Yang, N. Acute retinol mobilization by retinol-binding protein 4 in mouse liver induces fibroblast growth factor 21 expression. J. Lipid Res. 2022, 63, 100268. [Google Scholar] [CrossRef]
- Harder, D.; Roman, R.; Gebremedhin, D. Molecular mechanisms controlling nutritive blood flow: Role of cytochrome P450 enzymes. Acta Physiol. Scand. 2000, 168, 543–549. [Google Scholar] [CrossRef]
- Tahri-Joutey, M.; Andreoletti, P.; Surapureddi, S.; Nasser, B.; Cherkaoui-Malki, M.; Latruffe, N. Mechanisms mediating the regulation of peroxisomal fatty acid beta-oxidation by PPARα. Int. J. Mol. Sci. 2021, 22, 8969. [Google Scholar] [CrossRef]
- Cheng, C.F.; Ku, H.C.; Lin, H. PGC-1α as a pivotal factor in lipid and metabolic regulation. Int. J. Mol. Sci. 2018, 19, 3447. [Google Scholar] [CrossRef]





| Primer Name | Sequence (5′–3′) |
|---|---|
| P450 2C55 (LOC124096538) | CAACTCTCCCTCTTTCTGC |
| ACACACCACACTCATCCTG | |
| P450 2C18 (LOC124096544) | CTTATCCCAACCAATCTGC |
| TGAGTCCATCAAGGTGCT | |
| P450 2C55 (LOC101972885) | TGTCTGCTTCTTGTCTCACTC |
| ATCATCCAGGGCTTCCTT | |
| P450 2C5-like (LOC101972028) | TTTCCTCAACTCCTCCACC |
| ACAAGACGCTTCTCCCTCA | |
| P450 26A1 (LOC107154487) | GATGACCCGCAATCTCTT |
| ACGAGTGCTCAATCAACAG | |
| P450 2C18-like (LOC101976849) | TGTCCAAAGAAGAGCAGTG |
| GGTTGATGATAAGGAGAGCA | |
| Acyl-CoA Desaturase1 (LOC124984655) | GGTTATTTGGGTAGTTGGC |
| TCACTGACCTGGGATTGT | |
| Apoc3 | CCCTGACTTCACACATCTCC |
| TGCTACCCACTCTCCTTCAC | |
| P450 4B1 (LOC124963612) | GGTAAAGGAGAGGAGGCAA |
| AACGACACCATCTACGGTC | |
| Cpt1a | AAACCCACCAGGCTACAGTG |
| GCAGGTCCACATCATTTGC | |
| PCK1 | TCTCGGGTGATGATGACT |
| GAAATGTGACCAGGAGTGA | |
| EHHADH | CCATTGCCACTGTTATGAAC |
| GCTTGCTGCCTTCTTCTAA | |
| GAPDH | GTGATGCTGGTGCTGAATA |
| GCTGACAATCTTGAGGGA |
| Gene Name | Con | Hib | Ratio | Ratio (Up/Down) |
|---|---|---|---|---|
| Marmota monax cytochrome P450 2C55 (LOC124096538) | 444.8867 | 117.8467 | 3.775132 | −3.78 |
| Marmota monax cytochrome P450 2C18 (LOC124096544) | 439.3067 | 46.88 | 9.370876 | −9.37 |
| Ictidomys tridecemlineatus cytochrome P450 2C55 (LOC101972885) | 79.36333 | 16.56667 | 4.790543 | −4.79 |
| Ictidomys tridecemlineatus cytochrome P450 2C5-like (LOC101972028) | 239.2967 | 74.70667 | 3.20315 | −3.20 |
| Marmota monax retinol dehydrogenase 16 (Rdh16) | 107.9867 | 50.68667 | 2.130475 | −2.13 |
| Marmota cytochrome P450 26A1 (LOC107154487) | 1.173333 | 7.206667 | 6.142045 | 6.14 |
| Ictidomys tridecemlineatus cytochrome P450 2C18-like (LOC101976849) | 639.6767 | 116.9067 | 5.471687 | −5.47 |
| Ictidomys tridecemlineatus cytochrome P450 2C18-like (LOC101976849) | 809.18 | 443.1967 | 1.825781 | −1.83 |
| Marmota 17-beta-Hydroxysteroid dehydrogenase 13(LOC107153606) | 443.02 | 211.03 | 2.099322 | −2.10 |
| Marmota monax cytochrome P450 2C5 (LOC124096537) | 400.53 | 113.7567 | 3.520937 | −3.52 |
| Marmota monax cytochrome P450 2C18 (LOC124096535) | 112.2567 | 50.06667 | 2.242144 | −2.24 |
| Marmota cytochrome P450 2C18-like (LOC107149742) | 433.4967 | 44.40333 | 9.762706 | −9.76 |
| Gene Name | Con | Hib | Ratio | Ratio (Up/Down) |
|---|---|---|---|---|
| Sciurus carolinensis acyl-CoA Desaturase1 (LOC124984655) | 30.17 | 96.65 | 3.203513 | 3.20 |
| Ictidomys tridecemlineatus apolipoprotein C3 (Apoc3) | 2.723333 | 13.03 | 4.784578 | 4.78 |
| Ictidomys tridecemlineatus glycerol kinase (Gk) | 46.00667 | 23.46 | 1.961068 | −1.96 |
| Sciurus carolinensis cytochrome P450 4B1 (LOC124963612) | 444.8867 | 117.8467 | 3.775132 | −3.78 |
| Marmota monax enoyl-CoA hydratase and 3-hydroxyacyl CoA dehydrogenase (Ehhadh) | 526.05 | 91.17333 | 5.769779 | −5.77 |
| Urocitellus parryii angiopoietin-like 4 (Angptl4) | 100.91 | 27.60333 | 3.655718 | −3.66 |
| Marmota carnitine palmitoyltransferase 1A (Cpt1a) | 92.10333 | 33.17667 | 2.776148 | −2.78 |
| Marmota monax phosphoenolpyruvate carboxykinase1 (Pck1), transcript variant X1 | 758.3533 | 76.38333 | 9.928257 | −9.93 |
| Marmota flaviventris 3-hydroxy-3-methylglutaryl-CoA synthase 2 (Hmgcs2) | 1303.4 | 579.67 | 2.248521 | −2.25 |
| Marmota monax 7-alpha-hydroxycholest-4-en-3-one 12-alpha-hydroxylase (LOC124087568), transcript variant X1 | 106.2467 | 3.553333 | 29.90056 | −29.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Wang, C.; Liu, J.; Liu, Q.; Tian, Y.; Li, X.; Lu, W.; Zhang, D.; Yu, H. The Role of Lipid Metabolic Reprogramming in the Hibernation of Chipmunks. Animals 2025, 15, 2091. https://doi.org/10.3390/ani15142091
Huang M, Wang C, Liu J, Liu Q, Tian Y, Li X, Lu W, Zhang D, Yu H. The Role of Lipid Metabolic Reprogramming in the Hibernation of Chipmunks. Animals. 2025; 15(14):2091. https://doi.org/10.3390/ani15142091
Chicago/Turabian StyleHuang, Mingrui, Chong Wang, Juntao Liu, Qing Liu, Ye Tian, Xiaohui Li, Wei Lu, Dawei Zhang, and Huimei Yu. 2025. "The Role of Lipid Metabolic Reprogramming in the Hibernation of Chipmunks" Animals 15, no. 14: 2091. https://doi.org/10.3390/ani15142091
APA StyleHuang, M., Wang, C., Liu, J., Liu, Q., Tian, Y., Li, X., Lu, W., Zhang, D., & Yu, H. (2025). The Role of Lipid Metabolic Reprogramming in the Hibernation of Chipmunks. Animals, 15(14), 2091. https://doi.org/10.3390/ani15142091






