Impact of Dietary Enrichment with Omega-3 Polyunsaturated Fatty Acids from Extruded Linseed and Padina pavonica Algae Extract on Growth Performance and Metabolic Status in Fattening Rabbits
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Experimental Design
2.2. Productive Performance
2.3. Blood Sampling and Measurements of Hormones and Metabolites
2.4. Statistical Analysis
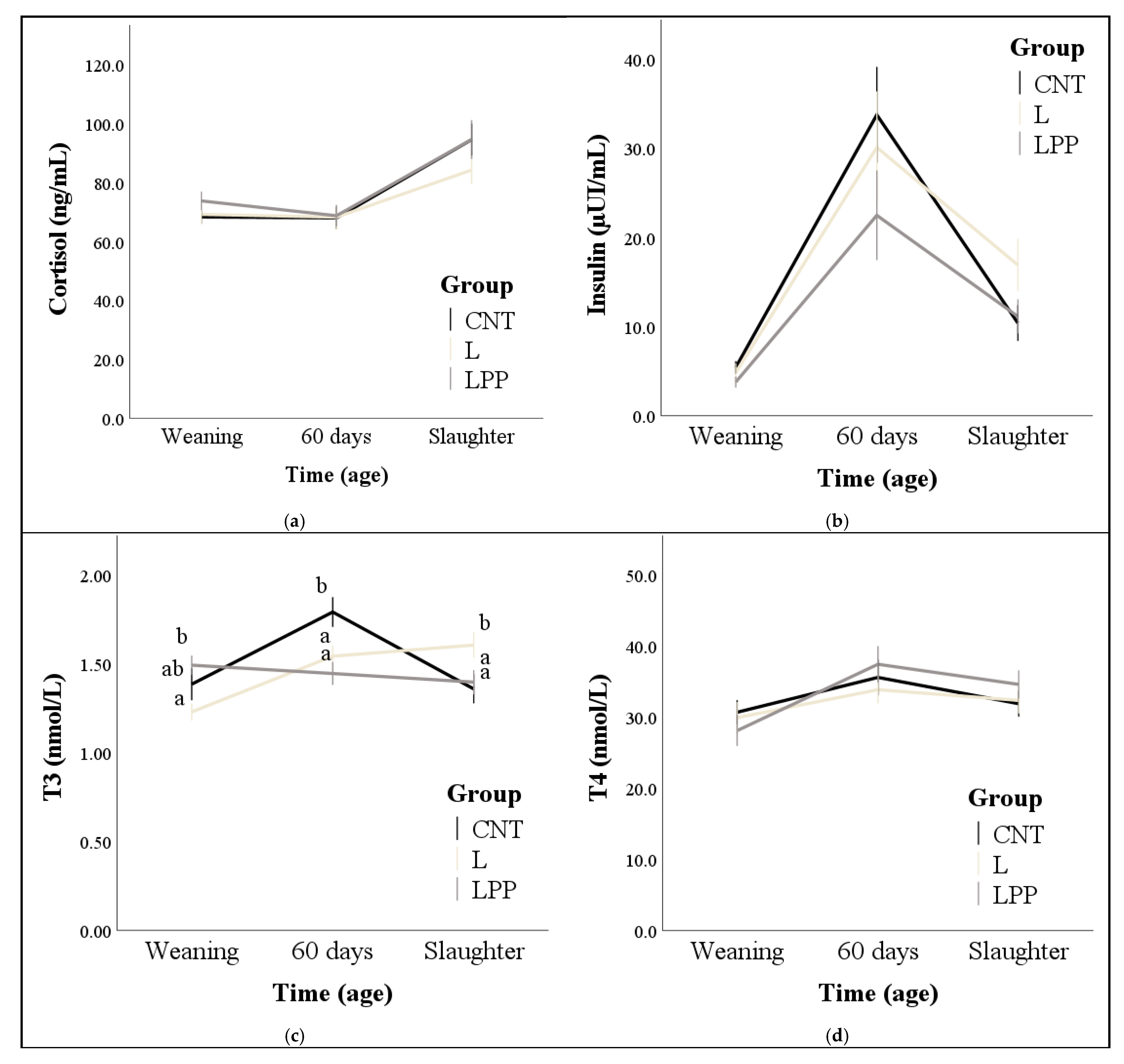
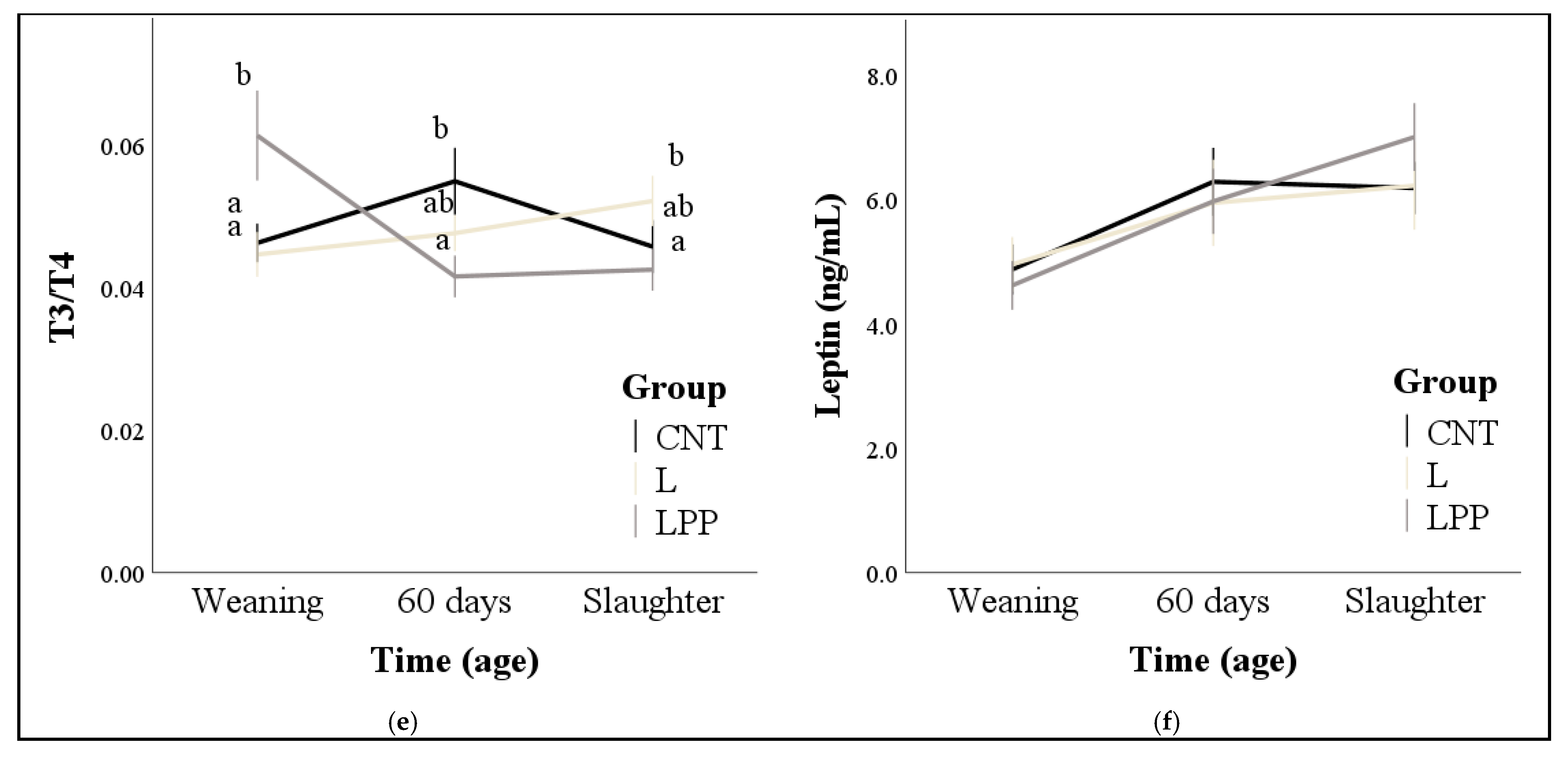
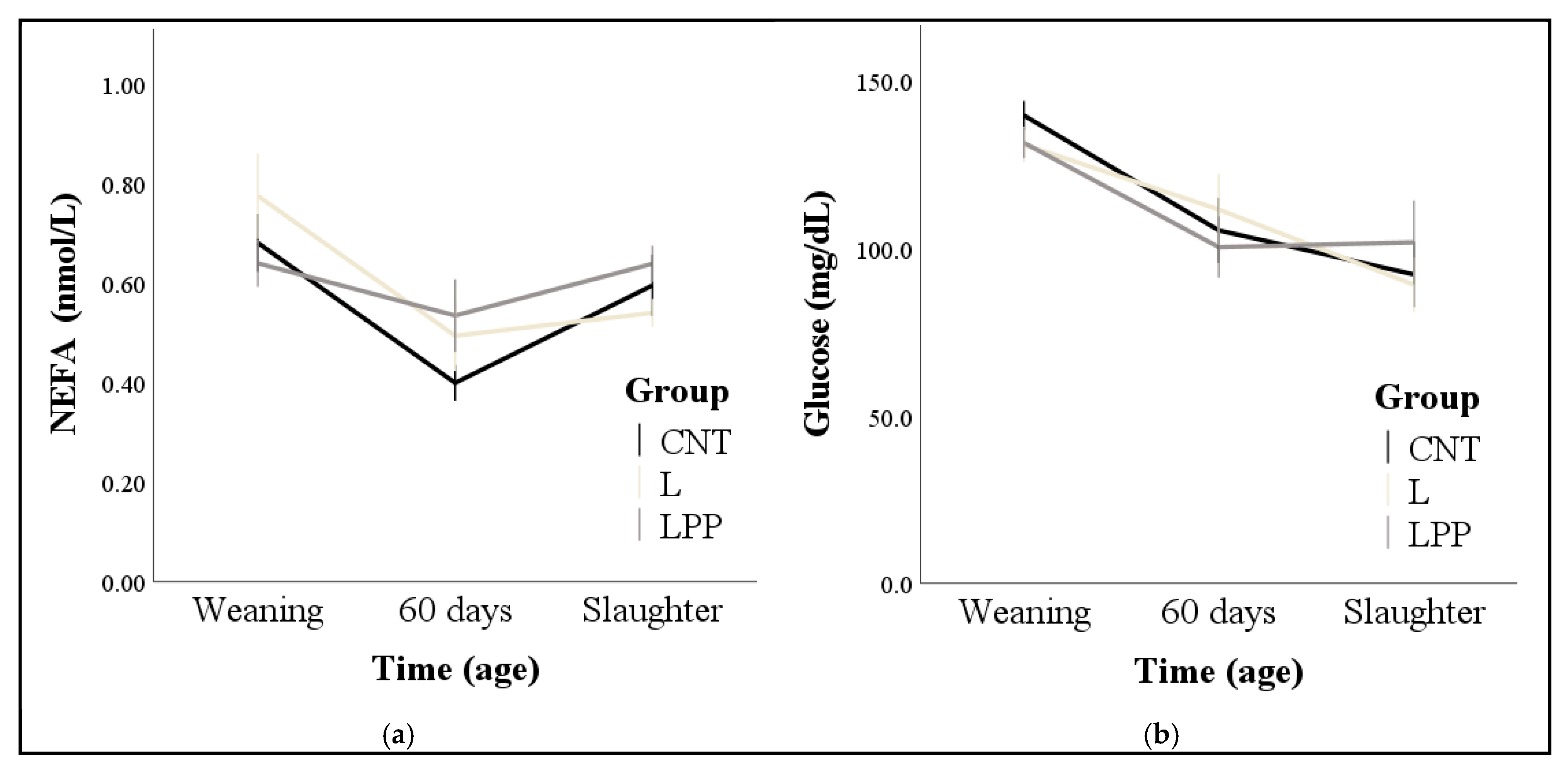
3. Results
3.1. Productive Parameters
3.2. Hormones and Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agradi, S.; Sulce, M.; Menchetti, L.; Vigo, D.; Castrica, M.; Barbato, O.; Andoni, E.; Quattrone, A.; Munga, A.; Marongiu, M.L.; et al. Dietary Supplementation with N-3 Polyunsaturated Fatty Acids: Effects on Reproductive and Productive Performance and Meat Quality in Rabbit Breeding. Anim. Nutr. 2023, 14, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Bivolarski, B.L.; Vachkova, E.G. Morphological and Functional Events Associated to Weaning in Rabbits. J. Anim. Physiol. Anim. Nutr. 2014, 98, 9–18. [Google Scholar] [CrossRef]
- Curone, G.; Biscarini, F.; Cotozzolo, E.; Menchetti, L.; Dal Bosco, A.; Riva, F.; Cremonesi, P.; Agradi, S.; Mattioli, S.; Castiglioni, B.; et al. Could Dietary Supplementation with Different Sources of N-3 Polyunsaturated Fatty Acids Modify the Rabbit Gut Microbiota? Antibiotics 2022, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Agradi, S.; Cremonesi, P.; Menchetti, L.; Balzaretti, C.; Severgnini, M.; Riva, F.; Castiglioni, B.; Draghi, S.; Di Giancamillo, A.; Castrica, M.; et al. Bovine Colostrum Supplementation Modulates the Intestinal Microbial Community in Rabbits. Animals 2023, 13, 976. [Google Scholar] [CrossRef] [PubMed]
- El-Sabrout, K.; Khalifah, A.; Ciani, F. Current Applications and Trends in Rabbit Nutraceuticals. Agriculture 2023, 13, 1424. [Google Scholar] [CrossRef]
- Quattrone, A.; Belabbas, R.; Fehri, N.E.; Agradi, S.; Mazzola, S.M.; Barbato, O.; Dal Bosco, A.; Mattioli, S.; Failla, S.; Abdel-Kafy, E.-S.M.; et al. The Effect of Dietary Plant-Derived Omega 3 Fatty Acids on the Reproductive Performance and Gastrointestinal Health of Female Rabbits. Vet. Sci. 2024, 11, 457. [Google Scholar] [CrossRef]
- Fehri, N.E.; Contò, M.; Castrica, M.; Quattrone, A.; Renzi, G.; Di Giovanni, S.; Agradi, S.; Vigo, D.; Brecchia, G.; Menchetti, L.; et al. Effects of Diets Containing Extruded Linseed and Padina Pavonica Algae on Meat Rabbit: Carcass Performance and Meat Quality. Foods 2025, 14, 274. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Castrica, M.; Contò, M.; Fehri, N.E.; Curone, G.; Balzaretti, C.M.; Andoni, E.; Quattrone, A.; Vigo, D.; Agradi, S.; Menchetti, L.; et al. Quality and Microbial Changes in Omega-3-Enriched Rabbit Meat Packaged with an Active Absorbent Pad in MAP. Foods 2025, 14, 404. [Google Scholar] [CrossRef]
- Matics, Z.; Cullere, M.; Szín, M.; Gerencsér, Z.; Szabó, A.; Fébel, H.; Odermatt, M.; Radnai, I.; Dalle Zotte, A.; Szendrő, Z. Effect of a Dietary Supplementation with Linseed Oil and Selenium to Growing Rabbits on Their Productive Performances, Carcass Traits and Fresh and Cooked Meat Quality. J. Anim. Physiol. Anim. Nutr. 2017, 101, 685–693. [Google Scholar] [CrossRef]
- Bianchi, M.; Petracci, M.; Cavani, C. The Influence of Linseed on Rabbit Meat Quality. World Rabbit Sci. 2010, 17, 97–107. [Google Scholar] [CrossRef]
- Menchetti, L.; Barbato, O.; Sforna, M.; Vigo, D.; Mattioli, S.; Curone, G.; Tecilla, M.; Riva, F.; Brecchia, G. Effects of Diets Enriched in Linseed and Fish Oil on the Expression Pattern of Toll-like Receptors 4 and Proinflammatory Cytokines on Gonadal Axis and Reproductive Organs in Rabbit Buck. Oxidative Med. Cell. Longev. 2020, 2020, 4327470. [Google Scholar] [CrossRef] [PubMed]
- El-Moghazy, M.; Zedan, N.S.; El-Atrsh, A.M.; El-Gogary, M.; Tousson, E. The Possible Effect of Diets Containing Fish Oil (Omega-3) on Hematological, Biochemical and Histopathogical Alterations of Rabbit Liver and Kidney. Biomed. Prev. Nutr. 2014, 4, 371–377. [Google Scholar] [CrossRef]
- Al-Soufi, S.; García, J.; Muíños, A.; López-Alonso, M. Marine Macroalgae in Rabbit Nutrition—A Valuable Feed in Sustainable Farming. Animals 2022, 12, 2346. [Google Scholar] [CrossRef] [PubMed]
- Abu Hafsa, S.H.; Khalel, M.S.; El-Gindy, Y.M.; Hassan, A.A. Nutritional Potential of Marine and Freshwater Algae as Dietary Supplements for Growing Rabbits. Ital. J. Anim. Sci. 2021, 20, 784–793. [Google Scholar] [CrossRef]
- Ander, B.P.; Edel, A.L.; McCullough, R.; Rodriguez-Leyva, D.; Rampersad, P.; Gilchrist, J.S.C.; Lukas, A.; Pierce, G.N. Distribution of Omega-3 Fatty Acids in Tissues of Rabbits Fed a Flaxseed-Supplemented Diet. Metabolism 2010, 59, 620–627. [Google Scholar] [CrossRef]
- Abedi, E.; Sahari, M.A. Long-Chain Polyunsaturated Fatty Acid Sources and Evaluation of Their Nutritional and Functional Properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef]
- Dalle Zotte, A.; Szendrő, Z. The Role of Rabbit Meat as Functional Food. Meat Sci. 2011, 88, 319–331. [Google Scholar] [CrossRef]
- Petracci, M.; Bianchi, M.; Cavani, C. Development of Rabbit Meat Products Fortified with N-3 Polyunsaturated Fatty Acids. Nutrients 2009, 1, 111–118. [Google Scholar] [CrossRef]
- Eiben, C.; Végi, B.; Virág, G.; Gódor-Surmann, K.; Maró, A.; Odermatt, M.; Zsédely, E.; Tóth, T.; Schmidt, J. Effect of Different Dietary Ratios of Sunflower and Linseed Oils on Growth and Carcass Traits of Rabbits. Livest. Sci. 2010, 131, 15–22. [Google Scholar] [CrossRef]
- Prim, C.R.; Baroncini, L.A.V.; Précoma, L.B.; Caron, P.H.L.; Winter, G.; Poletti, M.O.D.; Précoma, D.B. Effects of Linseed Consumption for a Short Period of Time on Lipid Profile and Atherosclerotic Lesions in Rabbits Fed a Hypercholesterolaemic Diet. Br. J. Nutr. 2012, 107, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Benatmane, F.; Kouba, M.; Youyou, A.; Mourot, J. Effect of a Linseed Diet on Lipogenesis, Fatty Acid Composition and Stearoyl-CoA-Desaturase in Rabbits. Animal 2011, 5, 1993–2000. [Google Scholar] [CrossRef]
- Grigorova, N.; Ivanova, Z.; Bjorndal, B.; Vachkova, E.; Penchev, G.; Berge, R.; Ribarski, S.; Georgieva, T.M.; Yonkova, P.; Georgiev, I.P. Effect of Fish Oil Supplementation and Restricted Feeding on Body Fat Distribution and Blood Lipid Profile in a Rabbit Model of Castration-Induced Obesity. Res. Vet. Sci. 2019, 124, 99–105. [Google Scholar] [CrossRef]
- Rizwan Tariq, M.; Issa Khan, M.; Ahmad, Z.; Ahmed, S.; Sameen, A.; Sameem Javed, M. Development of Healthier Rabbit Meat by Supplementation of Linseed in the Feed and Its Impact on Human Blood Lipid Profile. J. Food Process. Preserv. 2017, 41, e13194. [Google Scholar] [CrossRef]
- Pardilhó, S.; Cotas, J.; Pereira, L.; Oliveira, M.B.; Dias, J.M. Marine Macroalgae in a Circular Economy Context: A Comprehensive Analysis Focused on Residual Biomass. Biotechnol. Adv. 2022, 60, 107987. [Google Scholar] [CrossRef]
- Shafay, S.E.; El-Sheekh, M.; Bases, E.; El-Shenody, R. Antioxidant, Antidiabetic, Anti-Inflammatory and Anticancer Potential of Some Seaweed Extracts. Food Sci. Technol. 2022, 42, e20521. [Google Scholar] [CrossRef]
- Germoush, M.O.; Elgebaly, H.A.; Hassan, S.; Kamel, E.M.; Bin-Jumah, M.; Mahmoud, A.M. Consumption of Terpenoids-Rich Padina Pavonia Extract Attenuates Hyperglycemia, Insulin Resistance and Oxidative Stress, and Upregulates PPARγ in a Rat Model of Type 2 Diabetes. Antioxidants 2019, 9, 22. [Google Scholar] [CrossRef]
- Naveen, J.; Baskaran, R.; Baskaran, V. Profiling of Bioactives and in Vitro Evaluation of Antioxidant and Antidiabetic Property of Polyphenols of Marine Algae Padina tetrastromatica. Algal Res. 2021, 55, 102250. [Google Scholar] [CrossRef]
- Moheimanian, N.; Mirkhani, H.; Purkhosrow, A.; Sohrabipour, J.; Jassbi, A.R. In Vitro and In Vivo Antidiabetic, α-Glucosidase Inhibition and Antibacterial Activities of Three Brown Algae, Polycladia Myrica, Padina Antillarum, and Sargassum Boveanum, and a Red Alga, Palisada Perforata from the Persian Gulf. Iran. J. Pharm. Res. IJPR 2023, 22, e133731. [Google Scholar] [CrossRef]
- Čagalj, M.; Fras Zemljič, L.; Kraševac Glaser, T.; Mežnar, E.; Sterniša, M.; Smole Možina, S.; Razola-Díaz, M.D.C.; Šimat, V. Seasonal Changes in Chemical Profile and Antioxidant Activity of Padina Pavonica Extracts and Their Application in the Development of Bioactive Chitosan/PLA Bilayer Film. Foods 2022, 11, 3847. [Google Scholar] [CrossRef]
- Minetti, M.; Bernardini, G.; Biazzo, M.; Gutierrez, G.; Geminiani, M.; Petrucci, T.; Santucci, A. Padina Pavonica Extract Promotes In Vitro Differentiation and Functionality of Human Primary Osteoblasts. Mar. Drugs 2019, 17, 473. [Google Scholar] [CrossRef]
- de Blas, C.; Wiseman, J. (Eds.) Nutrition of the Rabbit, 2nd ed.; CABI: Wallingford, UK; Cambridge, MA, USA, 2010; ISBN 978-1-84593-669-3. [Google Scholar]
- Association of Official Analytical Chemist (AOAC). Official Methods of Analysis, 20th ed.; AOAC: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Soest, P.J.V.; Wine, R.H. Use of Detergents in the Analysis of Fibrous Feeds. IV. Determination of Plant Cell-Wall Constituents. J. AOAC Int. 1967, 50, 50–55. [Google Scholar] [CrossRef]
- Maertens, L.; Moermans, R.; Groote, G. Prediction of the Apparent Digestible Energy Content of Commercial Pelleted Feeds for Rabbits. J. Appl. Rabbit Res. 1988, 11, 60–67. [Google Scholar]
- Menchetti, L.; Andoni, E.; Barbato, O.; Canali, C.; Quattrone, A.; Vigo, D.; Codini, M.; Curone, G.; Brecchia, G. Energy Homeostasis in Rabbit Does during Pregnancy and Pseudopregnancy. Anim. Reprod. Sci. 2020, 218, 106505. [Google Scholar] [CrossRef]
- García-García, R.M.; Rebollar, P.G.; Arias-Álvarez, M.; Sakr, O.G.; Bermejo-Álvarez, P.; Brecchia, G.; Gutierrez-Adan, A.; Zerani, M.; Boiti, C.; Lorenzo, P.L. Acute Fasting before Conception Affects Metabolic and Endocrine Status without Impacting Follicle and Oocyte Development and Embryo Gene Expression in the Rabbit. Reprod. Fertil. Dev. 2011, 23, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Rommers, J.M.; Boiti, C.; De Jong, I.; Brecchia, G. Performance and Behaviour of Rabbit Does in a Group-Housing System with Natural Mating or Artificial Insemination. Reprod. Nutr. Dev. 2006, 46, 677–687. [Google Scholar] [CrossRef]
- Al-Janab, A.A.; Alsalami, M.S.; Mohammed, A.B.; Al-Douri, A.A.R. Omega-3 as a Dietary Supplement in Rabbits: Effect on the Growth Rate, Blood Parameters and Lipid Profiles. Adv. Anim. Vet. Sci. 2022, 10, 1887–2089. [Google Scholar] [CrossRef]
- Benita, M.; Dubinsky, Z.; Iluz, D. Padina Pavonica: Morphology and Calcification Functions and Mechanism. Am. J. Plant Sci. 2018, 9, 1156–1168. [Google Scholar] [CrossRef]
- Ansari, A.A.; Ghanem, S.M. Growth Attributes and Biochemical Composition of Padina pavonica (L.) from the Red Sea, in Response to Seasonal Alterations of Tabuk, Saudi Arabia. Egypt. J. Aquat. Res. 2019, 45, 139–144. [Google Scholar] [CrossRef]
- Gidenne, T.; Garreau, H.; Drouilhet, L.; Aubert, C.; Maertens, L. Improving Feed Efficiency in Rabbit Production, a Review on Nutritional, Technico-Economical, Genetic and Environmental Aspects. Anim. Feed Sci. Technol. 2017, 225, 109–122. [Google Scholar] [CrossRef]
- Øverland, M.; Mydland, L.T.; Skrede, A. Marine Macroalgae as Sources of Protein and Bioactive Compounds in Feed for Monogastric Animals. J. Sci. Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Okab, A.B.; Samara, E.M.; Abdoun, K.A.; Rafay, J.; Ondruska, L.; Parkanyi, V.; Pivko, J.; Ayoub, M.A.; Al-Haidary, A.A.; Aljumaah, R.S.; et al. Effects of Dietary Seaweed (Ulva lactuca) Supplementation on the Reproductive Performance of Buck and Doe Rabbits. J. Appl. Anim. Res. 2013, 41, 347–355. [Google Scholar] [CrossRef]
- Mordenti, A.L.; Sardi, L.; Bonaldo, A.; Pizzamiglio, V.; Brogna, N.; Cipollini, I.; Tassinari, M.; Zaghini, G. Influence of Marine Algae (Schizochytrium spp.) Dietary Supplementation on Doe Performance and Progeny Meat Quality. Livest. Sci. 2010, 128, 179–184. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Meineri, G. Effects of Diets with Increasing Levels of Spirulina Platensis on the Performance and Apparent Digestibility in Growing Rabbits. Livest. Sci. 2008, 118, 173–177. [Google Scholar] [CrossRef]
- Trocino, A.; Zomeño, C.; Birolo, M.; Di Martino, G.; Stefani, A.; Bonfanti, L.; Bertotto, D.; Gratta, F.; Xiccato, G. Impact of Pre-Slaughter Transport Conditions on Stress Response, Carcass Traits, and Meat Quality in Growing Rabbits. Meat Sci. 2018, 146, 68–74. [Google Scholar] [CrossRef]
- Bozzo, G.; Dimuccio, M.M.; Casalino, G.; Ceci, E.; D’Amico, F.; Petrontino, A.; Bonerba, E.; Camarda, A.; Circella, E. Preliminary Evidence Regarding the Detection of Cortisol and IL-6 to Assess Animal Welfare in Various Rabbit Housing Systems. Agriculture 2022, 12, 1622. [Google Scholar] [CrossRef]
- Feng, Y.; Fan, H.; Liang, X.; Wang, X.; Gao, G.; Gun, S. Environmental Enrichment Changes Rabbits’ Behavior, Serum Hormone Level and Further Affects Cecal Microbiota. PeerJ 2022, 10, e13068. [Google Scholar] [CrossRef]
- Weekers, F.; Giulietti, A.-P.; Michalaki, M.; Coopmans, W.; Van Herck, E.; Mathieu, C.; Van den Berghe, G. Metabolic, Endocrine, and Immune Effects of Stress Hyperglycemia in a Rabbit Model of Prolonged Critical Illness. Endocrinology 2003, 144, 5329–5338. [Google Scholar] [CrossRef]
- George, S.A.; Khan, S.; Briggs, H.; Abelson, J.L. CRH-Stimulated Cortisol Release and Food Intake in Healthy, Non-Obese Adults. Psychoneuroendocrinology 2010, 35, 607–612. [Google Scholar] [CrossRef]
- Georgiev, I. Relationships between Plasma Concentrations of Epidermal Growth Factor, Insulin and Iodated Thyroid Hormones in Early and Normal Weaned Rabbits. Rev. Médecine Vét. 2010, 161, 30–36. [Google Scholar]
- Afsar, B.; Ay, M. The Relationships between Cortisol Levels, Insulin Levels, and Thyroid Hormones with 24-h Urinary Sodium Excretion in Never Treated Essential Hypertensive Patients. ARYA Atheroscler. 2014, 10, 159–163. [Google Scholar] [PubMed]
- Chmurska-Gąsowska, M.; Sowińska, N.; Pałka, S.; Kmiecik, M.; Lenarczyk-Knapik, J.; Migdał, Ł. Non-Invasive Measurement of Thyroid Hormones in Domestic Rabbits. Animals 2021, 11, 1194. [Google Scholar] [CrossRef]
- Robson, H.; Siebler, T.; Shalet, S.M.; Williams, G.R. Interactions between GH, IGF-I, Glucocorticoids, and Thyroid Hormones during Skeletal Growth. Pediatr. Res. 2002, 52, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Palme, R. Monitoring Stress Hormone Metabolites as a Useful, Non-Invasive Tool for Welfare Assessment in Farm Animals. Anim. Welf. 2012, 21, 331–337. [Google Scholar] [CrossRef]
- Rommers, J.M.; Boiti, C.; Brecchia, G.; Meijerhof, R.; Noordhuizen, J.P.T.M.; Decuypere, E.; Kemp, B. Metabolic Adaptation and Hormonal Regulation in Young Rabbit Does during Long-Term Caloric Restriction and Subsequent Compensatory Growth. Anim. Sci. 2004, 79, 255–264. [Google Scholar] [CrossRef]
- Snoj, T.; Jenko, Z.; Cebulj-Kadunc, N. Fluctuations of Serum Cortisol, Insulin and Non-Esterified Fatty Acid Concentrations in Growing Ewes over the Year. Ir. Vet. J. 2014, 67, 22. [Google Scholar] [CrossRef]
- Gengatharan, A.; Mohamad, N.V.; Zahari, C.N.M.C.; Vijayakumar, R. Seaweeds as Emerging Functional Foods and Therapeutics for Colorectal Cancer Management. Discov. Food 2025, 5, 128. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Song, Y.J.; Sawamura, M.; Ikeda, K.; Igawa, S.; Yamori, Y. Soluble Dietary Fibre Improves Insulin Sensitivity by Increasing Muscle GLUT-4 Content in Stroke-Prone Spontaneously Hypertensive Rats. Clin. Exp. Pharmacol. Physiol. 2000, 27, 41–45. [Google Scholar] [CrossRef]
- Moon, M.K.; Kang, G.H.; Kim, H.H.; Han, S.K.; Koo, Y.D.; Cho, S.W.; Kim, Y.A.; Oh, B.-C.; Park, D.J.; Chung, S.S.; et al. Thyroid-Stimulating Hormone Improves Insulin Sensitivity in Skeletal Muscle Cells via cAMP/PKA/CREB Pathway-Dependent Upregulation of Insulin Receptor Substrate-1 Expression. Mol. Cell. Endocrinol. 2016, 436, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Smyth, P.P.A. Iodine, Seaweed, and the Thyroid. Eur. Thyroid. J. 2021, 10, 101–108. [Google Scholar] [CrossRef]
- Li, H.; Matheny, M.; Nicolson, M.; Tümer, N.; Scarpace, P.J. Leptin Gene Expression Increases with Age Independent of Increasing Adiposity in Rats. Diabetes 1997, 46, 2035–2039. [Google Scholar] [CrossRef] [PubMed]
- Barb, C.R.; Hausman, G.J.; Houseknecht, K.L. Biology of Leptin in the Pig. Domest. Anim. Endocrinol. 2001, 21, 297–317. [Google Scholar] [CrossRef]
- Martínez-Sánchez, N. There and Back Again: Leptin Actions in White Adipose Tissue. Int. J. Mol. Sci. 2020, 21, 6039. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.; Asakawa, A.; Amitani, H.; Inui, A. Stimulation of Leptin Secretion by Insulin. Indian J. Endocrinol. Metab. 2012, 16, S543–S548. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Burke, S.L.; Head, G.A. Obesity-Related Hypertension and the Role of Insulin and Leptin in High-Fat–Fed Rabbits. Hypertension 2013, 61, 628–634. [Google Scholar] [CrossRef]
- Fortun-Lamothe, L. Energy Balance and Reproductive Performance in Rabbit Does. Anim. Reprod. Sci. 2006, 93, 1–15. [Google Scholar] [CrossRef]
- Peiró, R.; Argente, M.-J.; García, M.-L. Changes in Body Reserves, Non-Esterified Fatty Acids, and Leptin during the Reproductive Lifespan of the Rabbit Female. Animals 2023, 13, 3213. [Google Scholar] [CrossRef] [PubMed]
- Pomares, O.; Vales-Villamarín, C.; Pérez-Nadador, I.; Mejorado-Molano, F.J.; Soriano-Guillén, L.; Garcés, C. Plasma Non-Esterified Fatty Acid Levels Throughout Childhood and Its Relationship with Leptin Levels in Children. J. Clin. Med. 2024, 13, 7286. [Google Scholar] [CrossRef]
- Guzzardi, M.A.; Hodson, L.; Guiducci, L.; La Rosa, F.; Salvadori, P.A.; Burchielli, S.; Iozzo, P. The Role of Glucose, Insulin and NEFA in Regulating Tissue Triglyceride Accumulation: Substrate Cooperation in Adipose Tissue versus Substrate Competition in Skeletal Muscle. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 956–963. [Google Scholar] [CrossRef]
- Davis, T.A.; Suryawan, A.; Bush, J.A.; O’Connor, P.M.J.; Thivierge, M.C. Interaction of Amino Acids and Insulin in the Regulation of Protein Metabolism in Growing Animals. Can. J. Anim. Sci. 2003, 83, 357–364. [Google Scholar] [CrossRef]
| Ingredients (%) | CNT | L | LPP |
|---|---|---|---|
| Extruded linseed | - | 5 | 3.5 |
| “Padina Pavonica” algae extract | - | - | 0.2 |
| Wheat bran | 23.16 | 23.09 | 23.08 |
| Beet pulp | 11.5 | 9.33 | 11 |
| Wheat straw | 11 | 11 | 11 |
| Alfalfa | 10 | 12.5 | 10.17 |
| Sunflower husks | 9.95 | 6 | 10.78 |
| Barley | 9.5 | 9 | 9.5 |
| Sunflower seed meal | 8.83 | 14 | 9.17 |
| Soybean hulls | 0.17 | - | - |
| Toasted soybean seed | 5 | - | 1.50 |
| Sugarcane molasses | 3 | 3 | 3 |
| Wheat | 2.5 | 2.5 | 2.5 |
| Grape seed meal | 2.17 | 1.83 | 1.87 |
| Soybean oil | 0.55 | - | - |
| Palm oil | 0.33 | 0.33 | 0.33 |
| Carboxymethylcellulose | 0.2 | 0.2 | 0.2 |
| Liquid acidifier 1 | 0.15 | 0.15 | 0.15 |
| Calcium carbonate | 0.8 | 0.8 | 0.8 |
| Sodium chloride | 0.4 | 0.4 | 0.4 |
| Magnesium oxide | 0.15 | 0.15 | 0.15 |
| Oligo-vitamin supplement 2 | 0.25 | 0.25 | 0.25 |
| Methionine hydroxy analog | 0.15 | 0.14 | 0.15 |
| Lysine | 0.14 | 0.21 | 0.19 |
| L Threonine | 0.07 | 0.08 | 0.08 |
| Vitamin E 50% | 0.03 | 0.03 | 0.03 |
| Chemical Composition (g/100 g) | CNT | L | LPP |
|---|---|---|---|
| Dry matter | 88.61 | 88.89 | 88.85 |
| Crude protein | 15.03 | 15.28 | 15.13 |
| Crude fat | 3.56 | 3.75 | 3.57 |
| Ash | 7.95 | 8.04 | 8.10 |
| Crude fiber | 18.64 | 18.63 | 18.71 |
| NDF 1 | 36.13 | 36.22 | 36.15 |
| ADF 2 | 22.57 | 22.62 | 22.57 |
| ADL 3 | 6.50 | 6.50 | 6.50 |
| Met + Cys 4 | 0.6 | 0.6 | 0.6 |
| Lys 5 | 0.7 | 0.7 | 0.7 |
| Thr 6 | 0.58 | 0.58 | 0.58 |
| Digestible energy 7 | 2189.1 | 2190.4 | 2188.7 |
| CNT | L | LPP | |
|---|---|---|---|
| 14:0 | 0.33 | 0.29 | 0.32 |
| 16:0 | 16.75 | 15.74 | 16.11 |
| 16:1 n-7 | 0.19 | 0.23 | 0.21 |
| 17:0 | 0.12 | 0.11 | 0.09 |
| 18:0 | 6.80 | 6.94 | 7.18 |
| 18:1 n-9 | 19.41 | 18.63 | 18.60 |
| 18:1 n-7 | 1.25 | 1.01 | 1.20 |
| 18:2 n-6, LA 1 | 47.25 | 33.32 | 33.54 |
| 20:0 | 0.32 | 0.29 | 0.26 |
| 18:3 n-6, γ-ALA 2 | 0.23 | 0.30 | 0.25 |
| 18:3 n-3, α-ALA | 5.35 | 21.71 | 20.42 |
| 22:1 n-11 | 0.17 | 0.17 | 0.17 |
| 20:4 n-6, AA 3 | 0.06 | 0.05 | 0.19 |
| 20:5 n-3, EPA 4 | - | - | 0.09 |
| 22:5 n-3 DPA 5 | - | - | 0.06 |
| SFA 6 | 25.26 | 24.21 | 24.85 |
| MUFA 7 | 21.79 | 20.36 | 20.54 |
| PUFA n-6 8 | 47.60 | 33.72 | 34.04 |
| PUFA n-3 9 | 5.35 | 21.71 | 20.58 |
| Parameter | Group | Week Post-Weaning | Significance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | BW at Weaning | Group | Time | Group × Time | ||
| BW | CNT | 840 ± 26 | 1088 a ± 31 | 1315 a ± 31 | 1551 a ± 40 | 1828 a ± 47 | 2128a ± 61 | 2445 a ± 64 | <0.001 | 0.397 | <0.001 | 0.588 |
| L | 863 ± 23 | 1122 a ± 22 | 1362 a ± 20 | 1633 a ± 20 | 1888 a ± 34 | 2223a ± 33 | 2529 a ± 29 | |||||
| LPP | 889 ± 32 | 1126 a ± 40 | 1344 a ± 38 | 1560 a ± 38 | 1816 a ± 47 | 2151a ± 47 | 2436 a ± 53 | |||||
| Feed intake (g) | CNT | 100 ± 0 | 110 ± 0 | 120 ± 0 | 135 ± 0 | 150 ± 0 | 160 ± 0 | 160 ± 0 | - * | - * | - * | - * |
| L | 100 ± 0 | 110 ± 0 | 120 ± 0 | 135 ± 0 | 150 ± 0 | 160 ± 0 | 160 ± 0 | |||||
| LPP | 100 ± 0 | 110 ± 0 | 120 ± 0 | 135 ± 0 | 150 ± 0 | 160 ± 0 | 160 ± 0 | |||||
| ADG (g/d) | CNT | - | 36.6 a ± 2.25 | 32.5 a ± 2.1 | 33.7 a ± 2.0 | 40.6 a ± 2.0 | 47.3 a ± 2.8 | 41.6 a ± 4.3 | - | 0.002 | <0.001 | 0.958 |
| L | - | 38.4 a ± 2.06 | 34.3 a ± 2.5 | 38.6 a ± 2.2 | 40.7 a ± 1.7 | 49.3 a ± 2.1 | 45.6 a ± 4.4 | |||||
| LPP | - | 33.8 a ± 2.32 | 31.3 a ± 2.2 | 30.8 a ± 1.9 | 42.8 a ± 2.6 | 43.4 a ± 3.1 | 41.3 a ± 3.2 | |||||
| FCR | CNT | - | 3.51 a ± 0.41 | 4.15 a ± 0.30 | 4.47 a ± 0.35 | 3.63 a ± 0.32 | 3.63 a ± 0.32 | 4.91 a ± 0.58 | - | 0.003 | <0.001 | 0.695 |
| L | - | 4.18 a ± 1.08 | 4.22 a ± 0.43 | 3.94 a ± 0.39 | 3.80 a ± 0.22 | 3.80 a ± 0.22 | 4.06 a ± 0.29 | |||||
| LPP | - | 3.68 a ± 0.23 | 4.63 a ± 0.43 | 5.00 a ± 0.38 | 4.09 a ± 0.36 | 4.09 a ± 0.36 | 4.50 a ± 0.39 | |||||
| Time (Age) | Cortisol | Insulin | T3 | T4 | Leptin | NEFA | |
|---|---|---|---|---|---|---|---|
| Weaning | Insulin | −0.028 | -- | ||||
| T3 | −0.037 | −0.101 | -- | ||||
| T4 | 0.105 | 0.216 | 0.168 | -- | |||
| Leptin | 0.139 | 0.208 | 0.063 | −0.160 | -- | ||
| NEFA | 0.231 | −0.062 | −0.214 | −0.256 * | 0.465 ** | -- | |
| Glucose | 0.332 ** | 0.113 | −0.101 | 0.017 | 0.024 | 0.377 ** | |
| 60 days of age | Insulin | −0.450 ** | -- | ||||
| T3 | −0.364 ** | 0.369 ** | -- | ||||
| T4 | −0.405 ** | 0.252 | 0.421 ** | -- | |||
| Leptin | 0.106 | 0.019 | −0.031 | −0.090 | -- | ||
| NEFA | 0.217 | −0.152 | −0.298 * | −0.427 ** | 0.512 ** | -- | |
| Glucose | −0.169 | 0.042 | 0.063 | 0.286 * | −0.484 ** | −0.574 ** | |
| Slaughter | Insulin | 0.233 | -- | ||||
| T3 | 0.136 | 0.330 * | -- | ||||
| T4 | −0.067 | 0.212 | 0.175 | -- | |||
| Leptin | −0.235 | −0.121 | −0.032 | 0.049 | -- | ||
| NEFA | −0.061 | −0.280 * | −0.292 * | 0.012 | 0.239 | -- | |
| Glucose | 0.389 ** | 0.259 * | 0.194 | −0.196 | −0.209 | −0.335 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quattrone, A.; Beqiraj, D.; Fehri, N.E.; Belabbas, R.; Vigo, D.; Menchetti, L.; Barbato, O.; Failla, S.; Faustini, M.; Ghoneim, S.S.; et al. Impact of Dietary Enrichment with Omega-3 Polyunsaturated Fatty Acids from Extruded Linseed and Padina pavonica Algae Extract on Growth Performance and Metabolic Status in Fattening Rabbits. Animals 2025, 15, 2085. https://doi.org/10.3390/ani15142085
Quattrone A, Beqiraj D, Fehri NE, Belabbas R, Vigo D, Menchetti L, Barbato O, Failla S, Faustini M, Ghoneim SS, et al. Impact of Dietary Enrichment with Omega-3 Polyunsaturated Fatty Acids from Extruded Linseed and Padina pavonica Algae Extract on Growth Performance and Metabolic Status in Fattening Rabbits. Animals. 2025; 15(14):2085. https://doi.org/10.3390/ani15142085
Chicago/Turabian StyleQuattrone, Alda, Doriana Beqiraj, Nour Elhouda Fehri, Rafik Belabbas, Daniele Vigo, Laura Menchetti, Olimpia Barbato, Sebastiana Failla, Massimo Faustini, Shereen Salama Ghoneim, and et al. 2025. "Impact of Dietary Enrichment with Omega-3 Polyunsaturated Fatty Acids from Extruded Linseed and Padina pavonica Algae Extract on Growth Performance and Metabolic Status in Fattening Rabbits" Animals 15, no. 14: 2085. https://doi.org/10.3390/ani15142085
APA StyleQuattrone, A., Beqiraj, D., Fehri, N. E., Belabbas, R., Vigo, D., Menchetti, L., Barbato, O., Failla, S., Faustini, M., Ghoneim, S. S., Jemmali, B., Mattioli, S., Contò, M., Munga, A., Dal Bosco, A., Ben Salem, I., Ozuni, E., Or, M. E., Andoni, E., ... Curone, G. (2025). Impact of Dietary Enrichment with Omega-3 Polyunsaturated Fatty Acids from Extruded Linseed and Padina pavonica Algae Extract on Growth Performance and Metabolic Status in Fattening Rabbits. Animals, 15(14), 2085. https://doi.org/10.3390/ani15142085















