Alpha-Amylase Activity in Feline Saliva: An Analytical Validation of an Automated Assay for Its Measurement and a Pilot Study on Its Changes Following Acute Stress and Due to Urinary Tract Pathologies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis
2.2. Analytical Validation
- -
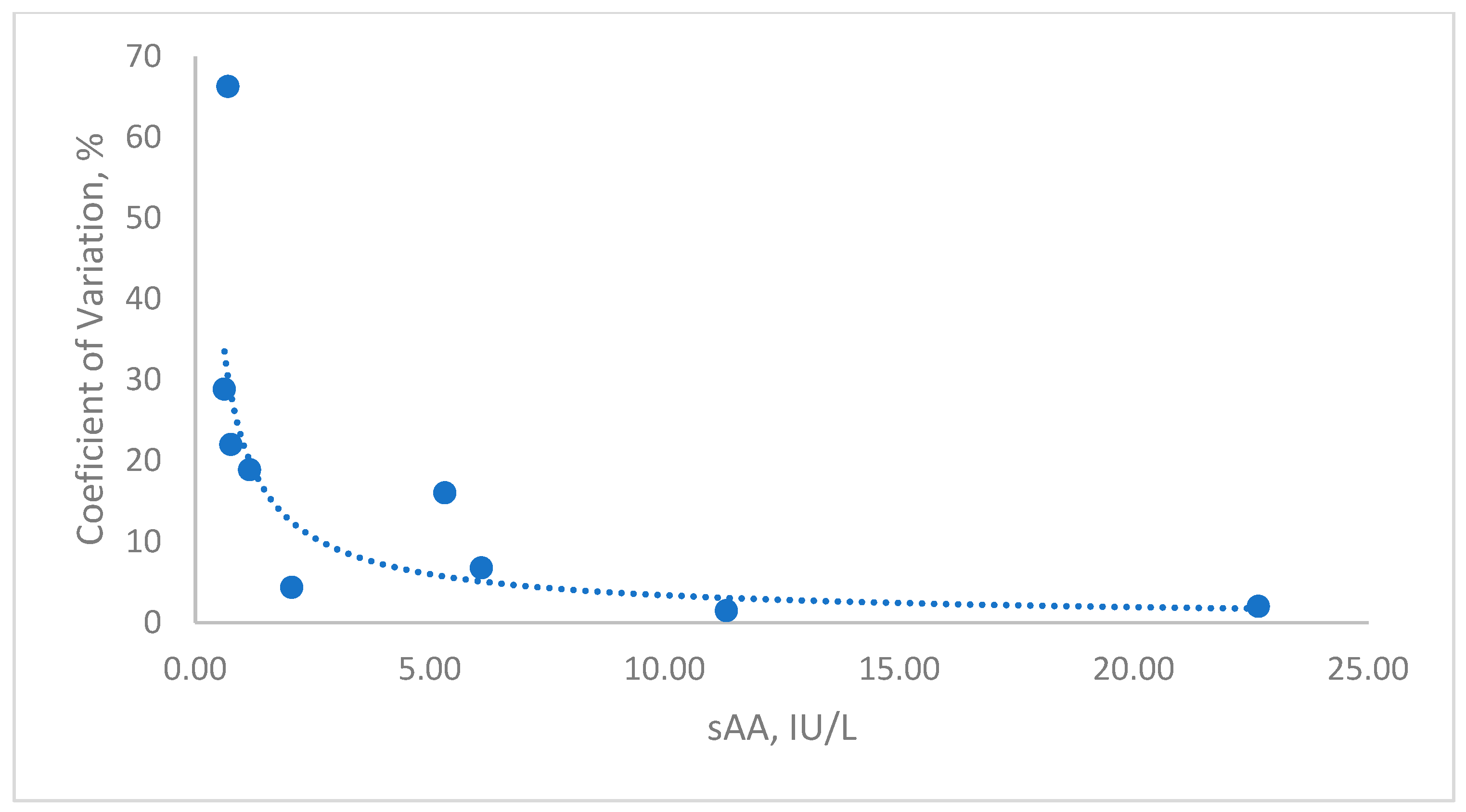
- Precision: Two feline saliva samples, one with low and one with high sAA activity, were analyzed. Intra-assay precision was determined by performing five consecutive measurements of each sample within a single analytical run. To evaluate inter-assay precision, each sample was measured once daily over a period of five days. To eliminate any impact from repeated freeze–thaw cycles, samples were stored in individual aliquots, using a fresh aliquot for each measurement. Results were reported as the coefficient of variation (CV), calculated by dividing the standard deviation by the mean of replicates and multiplying by 100%.
- -
- Accuracy: Since no reference method exists for determining sAA activity in cats, accuracy was assessed through dilution linearity. Two samples with distinct sAA activities were serially diluted to concentrations of 75%, 50%, 25%, and 12.5% using deionized water. Measured values were compared to expected concentrations by linear regression analysis.
- -
- Limit of Detection (LD): Defined as the smallest analyte concentration distinguishable from zero. This was calculated by analyzing the zero standard (deionized water) in 10 replicates and determining the mean plus three times the standard deviation.
- -
- Lower Limit of Quantification (LLOQ): Established as the lowest sAA activity that could be reliably quantified above the detection limit, maintaining a CV below 15%. A saliva sample was serially diluted in deionized water, and each dilution was tested in triplicate within the same run. The CV for each dilution was calculated as described above.
2.3. Changes in Stress and Disease
- -
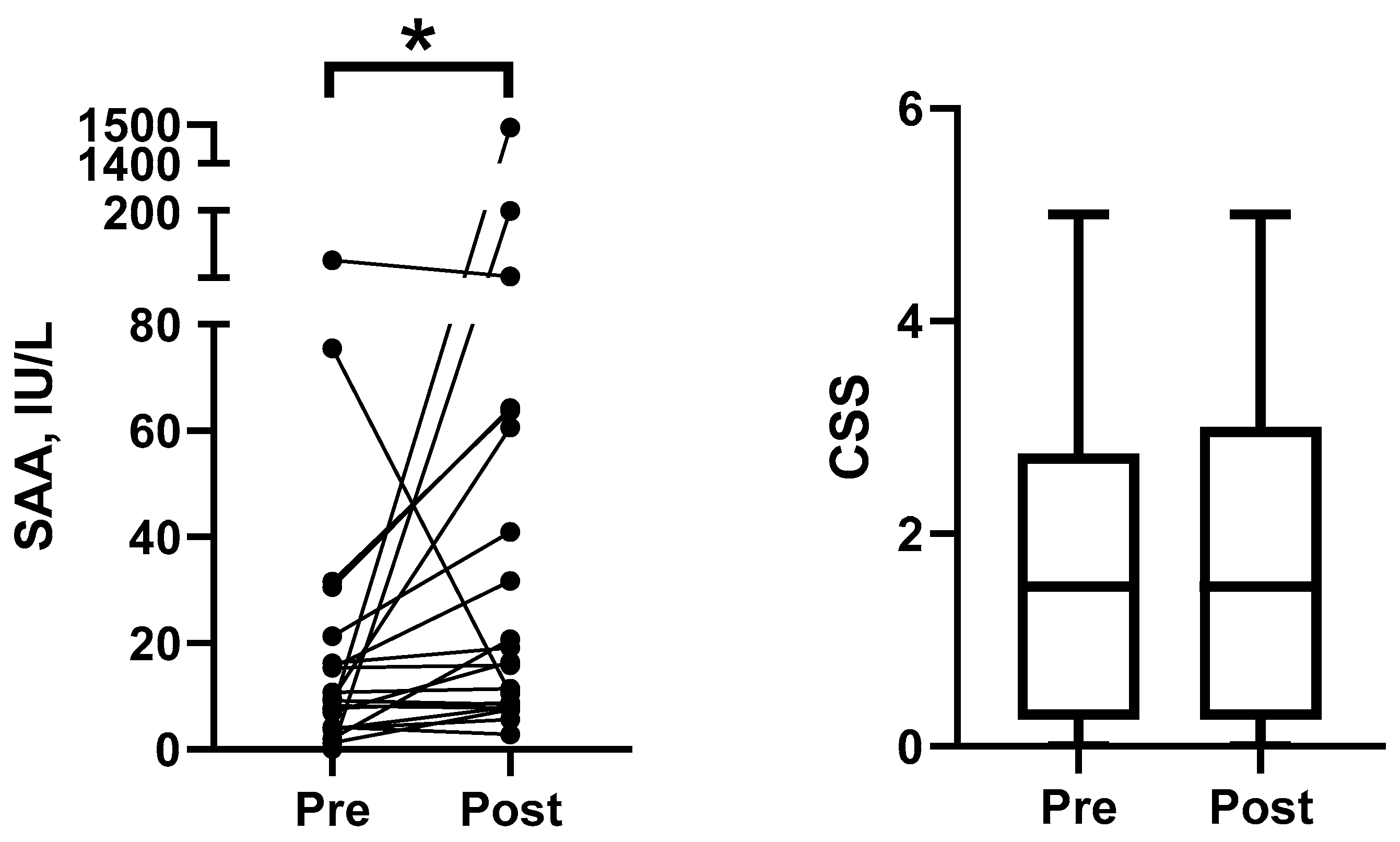
- Response to acute stress. To evaluate the dynamics of sAA in feline saliva in response to stress, saliva samples were collected from 21 healthy control animals before and immediately after the blood collection procedure. The included animals were adults (1–22 years), 12 females and 9 males with body weight (BW) between 2.8 and 6.6 kg and body condition score (BCS) of 4–7/9. All cats were healthy (except for overweight in 7 cases) on physical examination and blood analysis. The blood samples were collected for reasons other than the study, e.g., routine check-ups. Blood samples were obtained by the puncture of the jugular vein in all cats.
- -
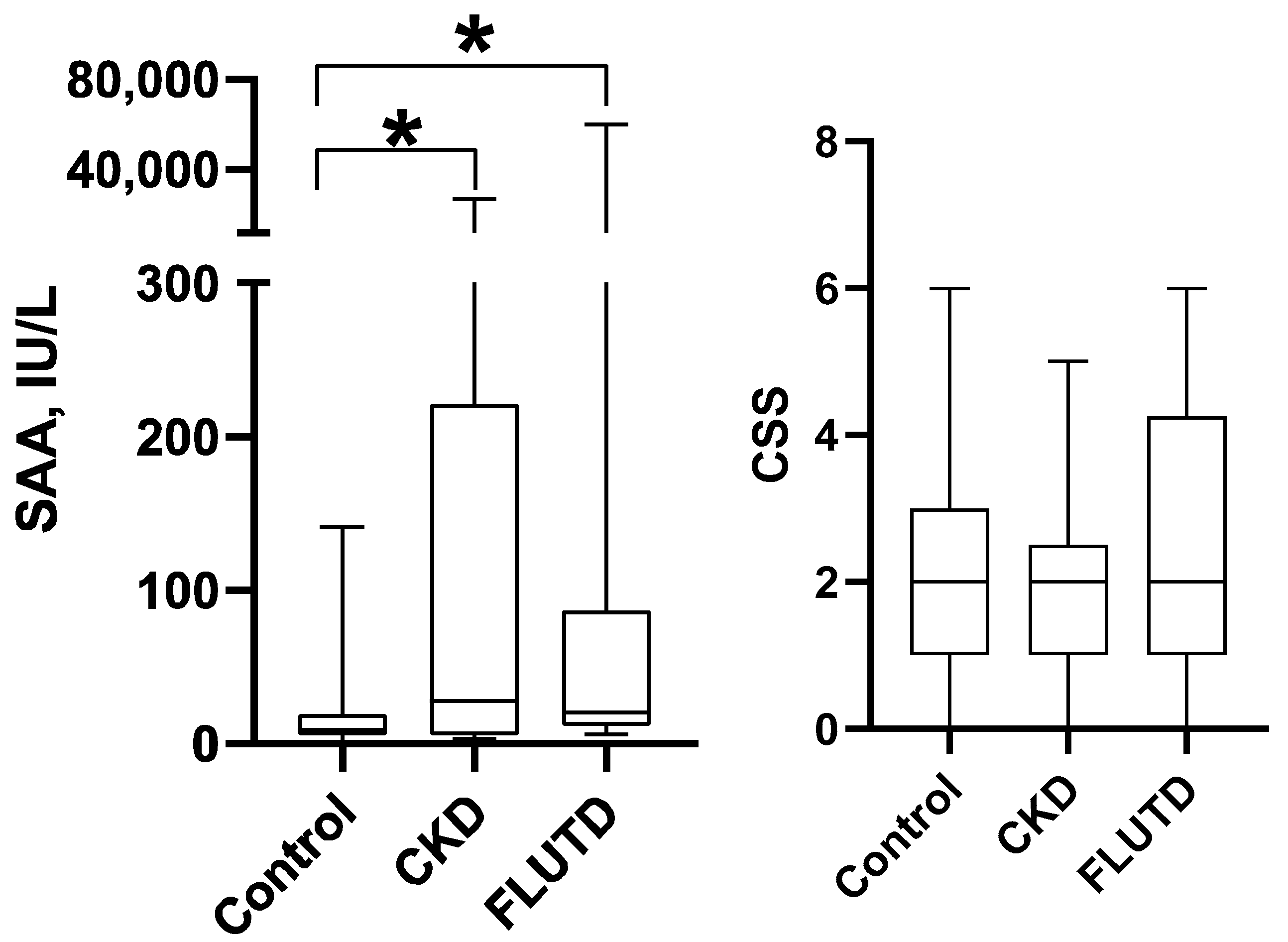
- Urinary tract diseases. To evaluate the possible changes in sAA in saliva of diseased cats, salivary samples were obtained from healthy and diseased animals with chronic kidney disease (CKD) and feline lower urinary tract disease (FLUTD). Healthy cats (Control Group) were the same as those included in study 1 (response to stress); the sample used was obtained before blood collection.
2.4. Saliva Sample Collection
2.5. Statistical Analysis
3. Results
3.1. Analytical Validation
3.2. Response to Acute Stress
3.3. Changes in Urinary Tract Diseases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, E.-H.; Park, S.-H.; Oh, Y.-I.; Seo, K.-W. Assessment of salivary alpha-amylase and cortisol as a pain related stress biomarker in dogs pre-and post-operation. BMC Vet. Res. 2022, 18, 31. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Beerda, B.; Schilder, M.B.H.; Van Hooff, J.; De Vries, H.W.; Mol, J.A. Behavioural, saliva cortisol and heart rate responses to different types of stimuli in dogs. Appl. Anim. Behav. Sci. 1998, 58, 365–381. [Google Scholar] [CrossRef]
- Bonne, N.J.; Wong, D.T. Salivary biomarker development using genomic, proteomic and metabolomic approaches. Genome Med. 2012, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Burbelo, P.D.; Bayat, A.; Lebovitz, E.E.; Iadarola, M.J. New technologies for studying the complexity of oral diseases. Oral Dis. 2012, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Tvarijonaviciute, A.; Martínez-Subiela, S.; López-Jornet, P.; Lamy, E. Saliva in Health and Disease–The Present and Future of a Unique Sample for Diagnosis; Springer: Cham, Switzerland, 2020; ISBN 978-3-030-37680-2. [Google Scholar]
- Schroers, M.; Meyer-Lindenberg, A. Performance and overview of clinically relevant areas of application of saliva testing in the cat. Front. Vet. Sci. 2024, 11, 1385345. [Google Scholar] [CrossRef] [PubMed]
- Nater, U.M.; Rohleder, N.; Gaab, J.; Berger, S.; Jud, A.; Kirschbaum, C.; Ehlert, U. Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. Int. J. Psychophysiol. 2005, 55, 333–342. [Google Scholar] [CrossRef]
- Rohleder, N.; Nater, U.M. Determinants of salivary alpha-amylase in humans and methodological considerations. Psychoneuroendocrinology 2009, 34, 469–485. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.D.; Henry, S.; Coste, C.; Tecles, F.; Escribano, D.; Cerón, J.J.; Hausberger, M. Changes in Saliva Analytes Correlate with Horses’ Behavioural Reactions to An Acute Stressor: A Pilot Study. Animals 2019, 9, 993. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.D.; Escribano, D.; Martín-Cuervo, M.; Tecles, F.; Cerón, J.J. Salivary alpha-amylase activity and cortisol in horses with acute abdominal disease: A pilot study. BMC Vet. Res. 2018, 14, 156. [Google Scholar] [CrossRef]
- Fuentes-Rubio, M.; Fuentes, F.; Otal, J.; Quiles, A.; Hevia, M.L. Validation of an assay for quantification of alpha-amylase in saliva of sheep. Can. J. Vet. Res. 2016, 80, 197–202. [Google Scholar] [PubMed]
- Fuentes, M.; Tecles, F.; Gutiérrez, A.; Otal, J.; Martínez-Subiela, S.; Cerón, J.J. Validation of an automated method for salivary alpha-amylase measurements in pigs (Sus scrofa domesticus) and its application as a stress biomarker. J. Vet. Diagn. Investig. 2011, 23, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Aguilar, M.D.; Tecles, F.; Martínez-Subiela, S.; Escribano, D.; Bernal, L.J.; Cerón, J.J. Detection and measurement of alpha-amylase in canine saliva and changes after an experimentally induced sympathetic activation. BMC Vet. Res. 2017, 13, 1–6. [Google Scholar] [CrossRef]
- Hong, H.R.; Oh, Y.I.; Kim, Y.J.; Seo, K.W. Salivary alpha-amylase as a stress biomarker in diseased dogs. J. Vet. Sci. 2019, 20, e46. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-H.; Lee, K.; Choi, H.-S.; Han, J.S.; Kim, S.-A. Hair Cortisol and Fe-BARQ: Evaluating Chronic Stress and Behavior in Cats with Chronic Kidney Disease. Animals 2025, 15, 889. [Google Scholar] [CrossRef]
- Kessler, M.R.; Turner, D.C. Stress and Adaptation of Cats (Felis Silvestris Catus) Housed Singly, in Pairs and in Groups in Boarding Catteries. Anim. Welf. 1997, 6, 243–254. [Google Scholar] [CrossRef]
- Laflamme, D. Development and validation of a body condition score system for dogs. Canine. Pract. 1997, 22, 10–15. [Google Scholar]
- Ettinger, S.J.; Feldman, E.C. Veterinary Internal Medicine; Elsevier: St. Louis, MO, USA, 2017; Volume 2, p. 2182. [Google Scholar]
- McCune, S. Temperament and the Welfare of Caged Cats. Doctoral Dissertation, University of Cambridge, Cambridge, UK, 1992. [Google Scholar]
- Parra, M.D.; Tecles, F.; Martínez-Subiela, S.; Cerón, J.J. C-reactive protein measurement in canine saliva. J. Vet. Diagn. Investig. 2005, 17, 139–144. [Google Scholar] [CrossRef]
- Amat, M.; Camps, T.; Manteca, X. Stress in owned cats: Behavioural changes and welfare implications. J. Feline Med. Surg. 2016, 18, 577–586. [Google Scholar] [CrossRef]
- Lloyd, J.K.F. Minimising Stress for Patients in the Veterinary Hospital: Why It Is Important and What Can Be Done about It. Vet. Sci. 2017, 4, 22. [Google Scholar] [CrossRef]
- Contreras, E.T.; Vanderstichel, R.; Hovenga, C.; Lappin, M.R. Evaluation of hair and nail cortisol concentrations and associations with behavioral, physical, and environmental indicators of chronic stress in cats. J. Vet. Intern. Med. 2021, 35, 2662–2672. [Google Scholar] [CrossRef] [PubMed]
- Minh, P.Q.A.; Nampimoon, T.; Sirirut, S.; Kalandakanond-Thongsong, S.; Benjanirut, C. Potential of nail cortisol for welfare assessment in shelter and owned cats. Appl. Anim. Behav. Sci. 2024, 280, 106422. [Google Scholar] [CrossRef]
- ISFM Guide to Feline Stress and Health; International Cat Care: Wiltshire, UK, 2016; ISBN 978-1-5262-0186-7.
- Crisi, P.E.; De Santis, F.; Giordano, M.V.; Cerasoli, I.; Colucci, F.; Di Tommaso, M.; Luciani, A. Evaluation of eutectic lidocaine/prilocaine cream for jugular blood sampling in cats. J. Feline Med. Surg. 2021, 23, 185–189. [Google Scholar] [CrossRef]
- Carney, H.C.; Little, S.; Brownlee-Tomasso, D.; Harvey, A.M.; Mattox, E.; Robertson, S.; Rucinsky, R.; Manley, D.S. American Association of Feline Practitioners, & International Society of Feline Medicine. AAFP and ISFM feline-friendly nursing care guidelines. J. Feline Med. Surg. 2012, 14, 337–349. [Google Scholar]
- Akcali, A.; Huck, O.; Tenenbaum, H.; Davideau, J.L.; Buduneli, N. Periodontal diseases and stress: A brief review. J. Oral Rehabil. 2013, 40, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Vermes, I.; Beishuizen, A. The hypothalamic-pituitary-adrenal response to critical illness. Best Pract. Res. Clin. Endocrinol. Metab. 2001, 15, 495–511. [Google Scholar] [CrossRef]
- Gulersoy, E.; Maden, M.; Parlak, T.M.; Sayin, Z. Diagnostic effectiveness of stress biomarkers in cats with feline interstitial and bacterial cystitis. Vet. Clin. Pathol. 2023, 52, 88–96. [Google Scholar] [CrossRef]
- Horwitz, D.F.; Rodan, I. Behavioral awareness in the feline consultation: Understanding physical and emotional health. J. Feline Med. Surg. 2018, 20, 423–436. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.D.; Escribano, D.; Martínez-Subiela, S.; Martínez-Miró, S.; Rubio, M.; Tvarijonaviciute, A.; Tecles, F.; Cerón, J.J. Influence of the way of reporting alpha-Amylase values in saliva in different naturalistic situations: A pilot study. PLoS ONE 2017, 12, e0180100. [Google Scholar] [CrossRef]

| Sample | Mean | Intra-CV% | Inter-CV |
|---|---|---|---|
| High | 91.0 | 3.2 | 5.3 |
| Low | 8.1 | 4.0 | 7.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cañadas-Vidal, E.; Muñoz-Prieto, A.; García-Martínez, J.D.; Ceron, J.J.; Pardo-Marín, L.; Tvarijonaviciute, A. Alpha-Amylase Activity in Feline Saliva: An Analytical Validation of an Automated Assay for Its Measurement and a Pilot Study on Its Changes Following Acute Stress and Due to Urinary Tract Pathologies. Animals 2025, 15, 2074. https://doi.org/10.3390/ani15142074
Cañadas-Vidal E, Muñoz-Prieto A, García-Martínez JD, Ceron JJ, Pardo-Marín L, Tvarijonaviciute A. Alpha-Amylase Activity in Feline Saliva: An Analytical Validation of an Automated Assay for Its Measurement and a Pilot Study on Its Changes Following Acute Stress and Due to Urinary Tract Pathologies. Animals. 2025; 15(14):2074. https://doi.org/10.3390/ani15142074
Chicago/Turabian StyleCañadas-Vidal, Esmeralda, Alberto Muñoz-Prieto, Juan D. García-Martínez, Jose J. Ceron, Luis Pardo-Marín, and Asta Tvarijonaviciute. 2025. "Alpha-Amylase Activity in Feline Saliva: An Analytical Validation of an Automated Assay for Its Measurement and a Pilot Study on Its Changes Following Acute Stress and Due to Urinary Tract Pathologies" Animals 15, no. 14: 2074. https://doi.org/10.3390/ani15142074
APA StyleCañadas-Vidal, E., Muñoz-Prieto, A., García-Martínez, J. D., Ceron, J. J., Pardo-Marín, L., & Tvarijonaviciute, A. (2025). Alpha-Amylase Activity in Feline Saliva: An Analytical Validation of an Automated Assay for Its Measurement and a Pilot Study on Its Changes Following Acute Stress and Due to Urinary Tract Pathologies. Animals, 15(14), 2074. https://doi.org/10.3390/ani15142074







