Impact of Light Spectrum on Tadpole Physiology and Gut Microbiota in the Dybowski’s Frog (Rana dybowskii)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Basic Metrics of R. dybowskii
2.3. Sex Identification Method Based on Gene Expression
2.4. Hormone Quantification via HPLC
2.5. DNA Extraction and 16S rRNA Gene Sequencing
2.6. Bioinformatics
2.7. Data Analysis
3. Results
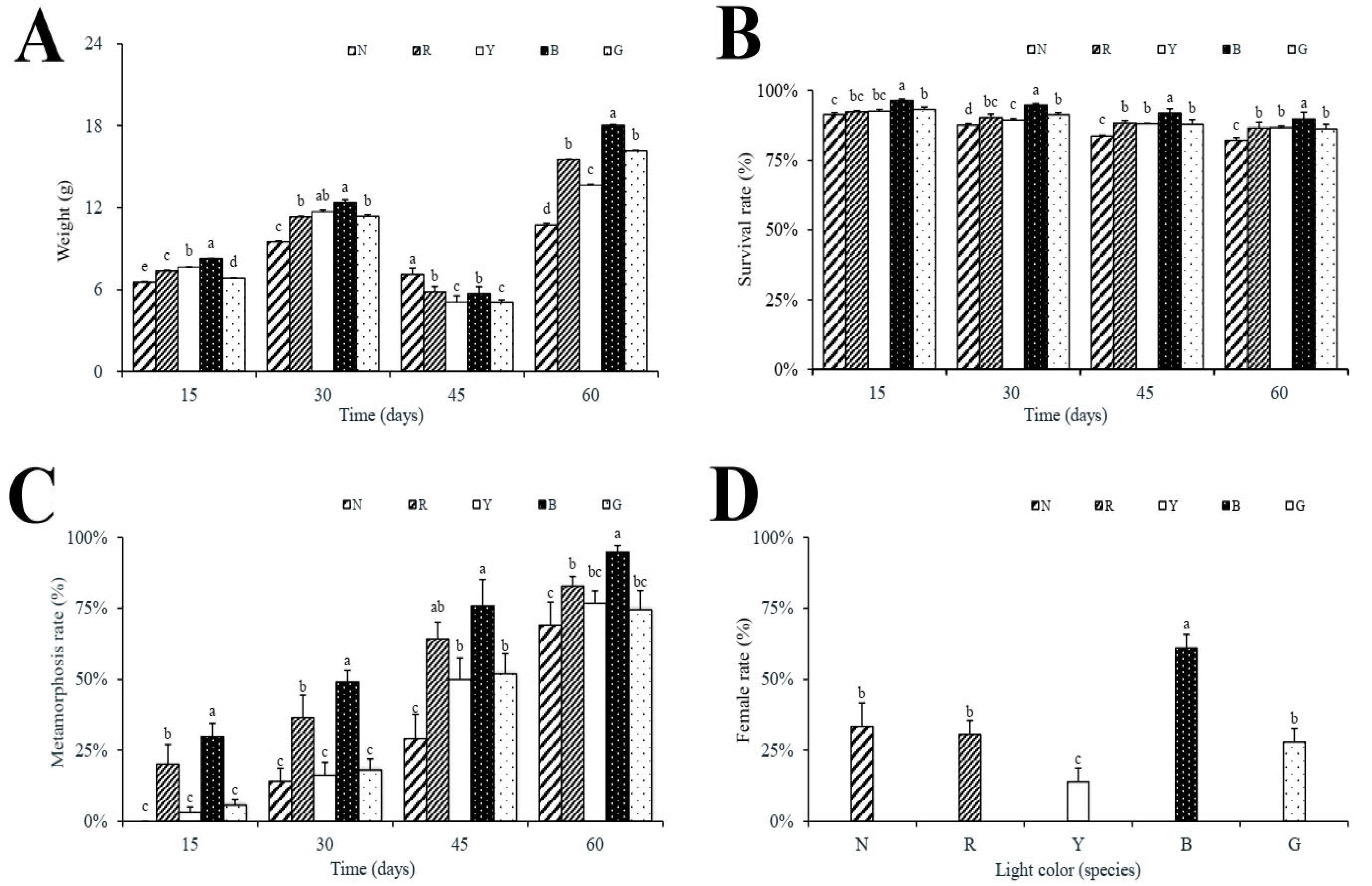
3.1. Growth, Survival, Metamorphosis, and Sex Ratio
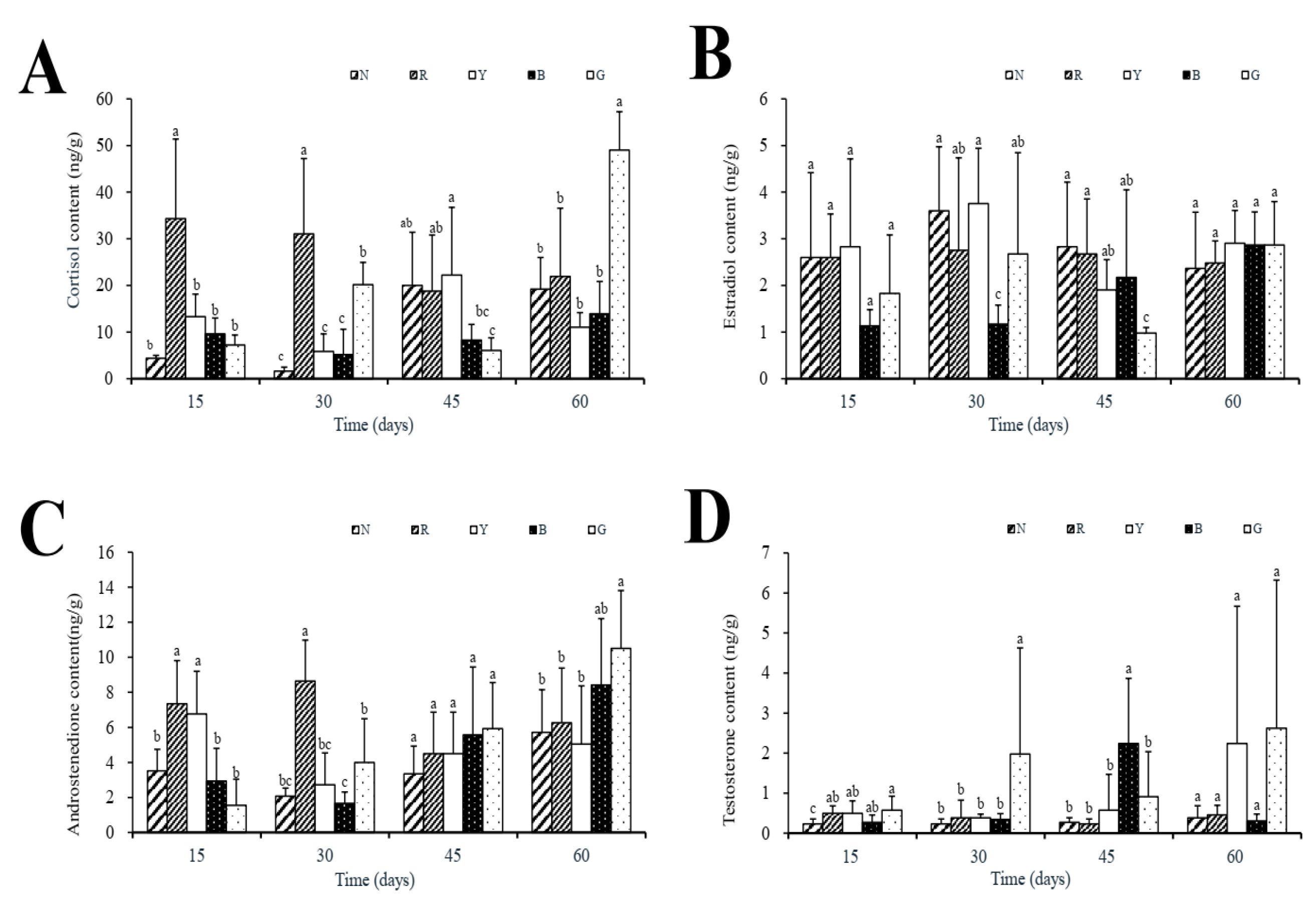
3.2. Steroid Hormone Levels
3.3. Pyrosequencing of the Gut Bacterial Community
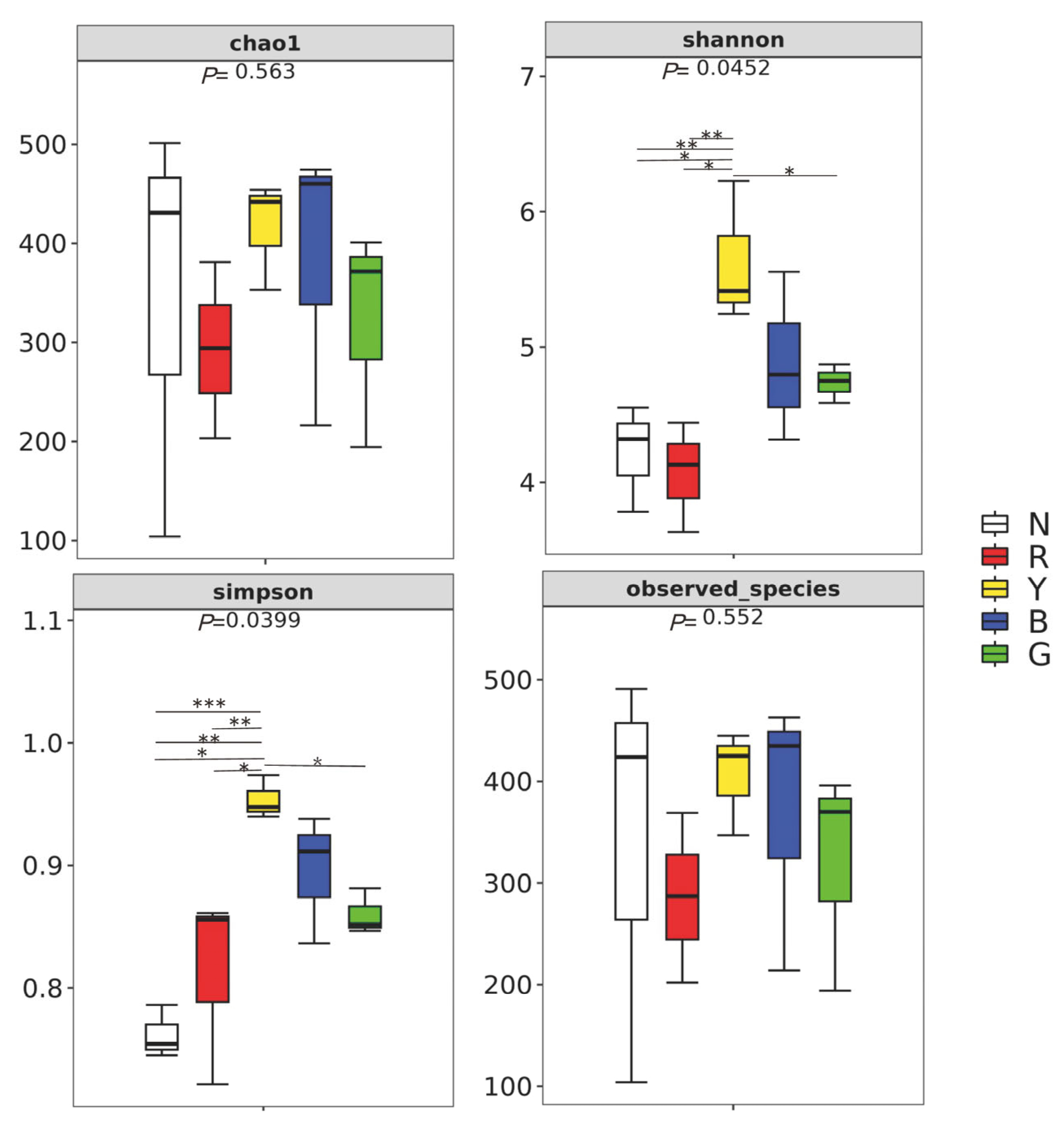
3.4. Microbial Alpha Diversity
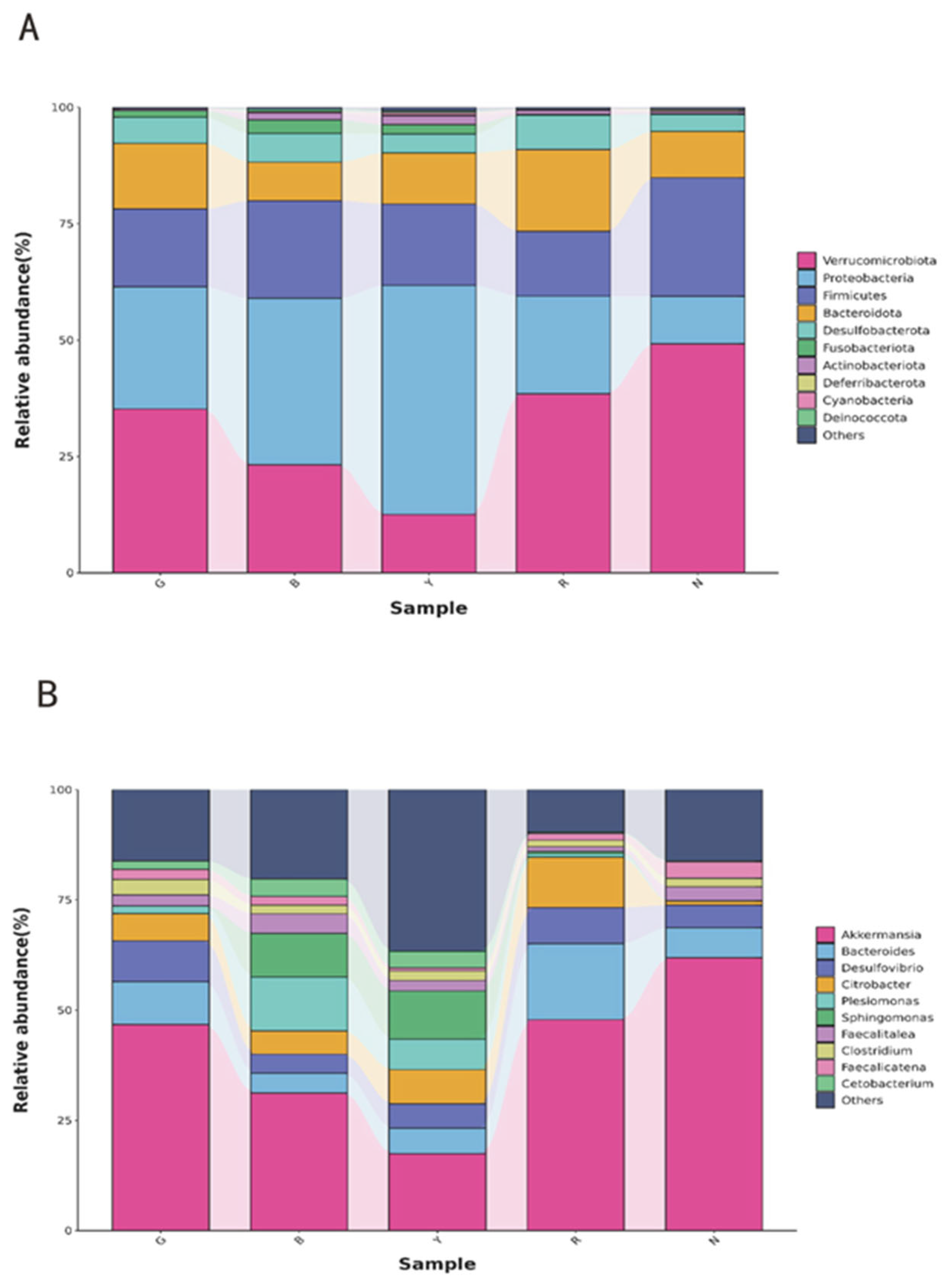
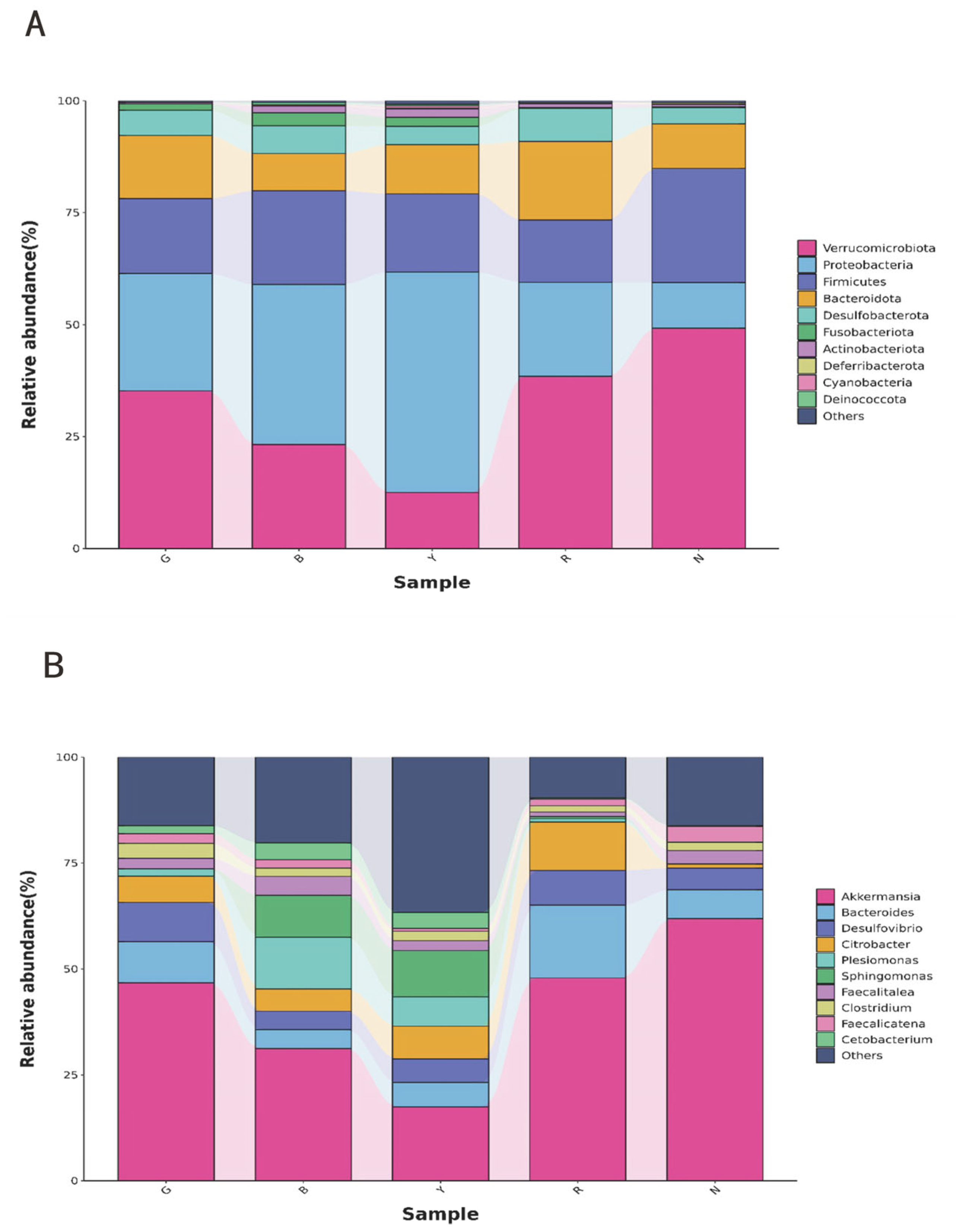
3.5. Gut Bacterial Taxonomic Abundance
3.6. Beta Diversity
3.7. Taxonomic Differences and Marker Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Wang, Z.; Luo, Q.; Li, H.; Wang, P.; Wang, J.; Li, D.; Wang, W.; Yuan, K.; Zhou, Y.; et al. Circadian Rhythm of Body Color Change in the Juvenile Chinese Giant Salamander (Andrias davidianus) Under Different Photoperiods. Animals 2025, 15, 1526. [Google Scholar] [CrossRef] [PubMed]
- Schott, R.K.; Fujita, M.K.; Streicher, J.W.; Gower, D.J.; Thomas, K.N.; Loew, E.R.; Bamba Kaya, A.G.; Bittencourt-Silva, G.B.; Guillherme Becker, C.; Cisneros-Heredia, D.; et al. Diversity and evolution of frog visual opsins: Spectral tuning and adaptation to distinct light environments. Mol. Biol. Evol. 2024, 41, msae049. [Google Scholar] [CrossRef]
- Ruchin, A.B. The effect of illumination and light spectrum on growth and larvae development of Pelophylax ridibundus (Amphibia: Anura). Biol. Rhythm Res. 2021, 52, 307–318. [Google Scholar] [CrossRef]
- Joshi, B.N.; Mohinuddin, K. Red light accelerates and melatonin retards metamorphosis of frog tadpoles. BMC Physiol. 2003, 3, 1–6. [Google Scholar] [CrossRef][Green Version]
- Buchanan, B.W. Observed and potential effects of artificial night lighting on anuran amphibians. In Ecological Consequences of Artificial Night Lighting; Rich, C., Longcore, T., Eds.; Island Press: Washington, DC, USA, 2006; pp. 192–220. [Google Scholar]
- Moore, F.L.; Boyd, S.K.; Kelley, D.B. Historical perspective: Hormonal regulation of behaviors in amphibians. Horm. Behav. 2005, 48, 373–383. [Google Scholar] [CrossRef]
- Leary, C.J. Hormones and acoustic communication in anuran amphibians. Integr. Comp. Biol. 2009, 49, 452–470. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.A.; DuRant, S.E.; Beck, M.L.; Ray, W.K.; Helm, R.F.; Romero, L.M. Cortisol is the predominant glucocorticoid in the giant paedomorphic hellbender salamander (Cryptobranchus alleganiensis). Gen. Comp. Endocrinol. 2020, 285, 113267. [Google Scholar] [CrossRef]
- Denver, R.J. Stress hormones mediate environment-genotype interactions during amphibian development. Gen. Comp. Endocrinol. 2009, 164, 20–31. [Google Scholar] [CrossRef]
- Sever, D.M.; Staub, N.L. Chapter 5 - Hormones, Sex Accessory Structures, and Secondary Sexual Characteristics in Amphibians. In Hormones and Reproduction of Vertebrates; Norris, D.O., Lopez, K.H., Eds.; Academic Press: London, UK, 2011; pp. 83–98. [Google Scholar]
- Ruiz-García, A.; Roco, Á.S.; Bullejos, M. Sex Differentiation in Amphibians: Effect of Temperature and Its Influence on Sex Reversal. Sex. Dev. 2021, 15, 157–167. [Google Scholar] [CrossRef]
- Hayes, T.B.; Collins, A.; Lee, M.; Mendoza, M.; Noriega, N.; Stuart, A.A.; Vonk, A. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc. Natl. Acad. Sci. USA 2002, 99, 5476–5480. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Q.; Liu, H.; Jin, J.; Yang, Y.; Zhu, X.; Han, D.; Zhou, Z.; Xie, S. Potential Functions of the Gut Microbiome and Modulation Strategies for Improving Aquatic Animal Growth. Rev. Aquac. 2025, 17, e12959. [Google Scholar] [CrossRef]
- Cao, H.; Shi, Y.; Wang, J.; Niu, Z.; Wei, L.; Tian, H.; Yu, F.; Gao, L. The intestinal microbiota and metabolic profiles of Strauchbufo raddei underwent adaptive changes during hibernation. Integr. Zool. 2024, 19, 612–630. [Google Scholar] [CrossRef]
- Song, X.; Zhang, J.; Song, J.; Zhai, Y. Decisive Effects of Life Stage on the Gut Microbiota Discrepancy Between Two Wild Populations of Hibernating Asiatic Toads (Bufo gargarizans). Front. Microbiol. 2021, 12, 665849. [Google Scholar] [CrossRef]
- Emerson, K.J.; Fontaine, S.S.; Kohl, K.D.; Woodley, S.K. Temperature and the microbial environment alter brain morphology in a larval amphibian. J. Exp. Biol. 2023, 226, jeb245333. [Google Scholar] [CrossRef] [PubMed]
- Kohl, K.D.; Cary, T.L.; Karasov, W.H.; Dearing, M.D. Restructuring of the amphibian gut microbiota through metamorphosis. Environ. Microbiol. Rep. 2013, 5, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Colombo, B.M.; Scalvenzi, T.; Benlamara, S.; Pollet, N. Microbiota and mucosal immunity in amphibians. Front. Immunol. 2015, 6, e111. [Google Scholar] [CrossRef]
- Bögi, C.; Levy, G.; Lutz, I.; Kloas, W. Functional genomics and sexual differentiation in amphibians. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 133, 559–570. [Google Scholar] [CrossRef]
- Gabor, C.R.; Villatoro-Castañeda, M.; Carlos-Shanley, C.; Ujhegyi, N.; Bókony, V. Gut Bacterial Communities Vary across Habitats and Their Diversity Increases with Increasing Glucocorticoids in Toad Tadpoles. Diversity 2023, 15, 23. [Google Scholar] [CrossRef]
- Moore, F.L.; Zoeller, R.T. Stress-induced inhibition of reproduction: Evidence of suppressed secretion of LH-RH in an amphibian. Gen. Comp. Endocrinol. 1985, 60, 252–258. [Google Scholar] [CrossRef]
- Lee, C.C.; Liang, F.; Lee, I.C.; Lu, T.H.; Shan, Y.Y.; Jeng, C.F.; Zou, Y.F.; Yu, H.T.; Chen, S.K. External light-dark cycle shapes gut microbiota through intrinsically photosensitive retinal ganglion cells. EMBO Rep. 2022, 23, e52316. [Google Scholar] [CrossRef]
- Nan, H.; Changyue, Y.; Jiaxin, J.; Xinmiao, Z.; Yingying, Z.; Hua, W.; Yingdong, L. Impact of photoperiods on the specific activities of immune and antioxidant enzymes in different tissues of Dybowski’s frog (Rana dybowskii). Bio. Rhythm Res. 2022, 53, 1790–1799. [Google Scholar] [CrossRef]
- Sui, X.; Li, X.-h.; Duan, M.-h.; Jia, A.-l.; Wang, Y.; Liu, D.; Li, Y.-P.; Qiu, Z.-D. Investigation of the anti-glioma activity of Oviductus ranae protein hydrolysate. Biomed. Pharmacother. 2016, 81, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Suryanti, S.; Santoso, H.; Lisminingsih, R.D. dengan Alizarin Red The Ossification Study and Tadpoles Morphokinetic of Rana catesbeiana Shaw. with Alizarin. J. Ilm. Biosaintropis Biosci.-Trop. 2020, 5, 52–58. [Google Scholar]
- Gomez-Mestre, I.; Kulkarni, S.; Buchholz, D.R. Mechanisms and consequences of developmental acceleration in tadpoles responding to pond drying. PLoS ONE 2013, 8, e84266. [Google Scholar] [CrossRef]
- Xiao, Y.; Liao, G.; Luo, W.; Xia, Y.; Zeng, X. Homology in Sex Determination in Two Distant Spiny Frogs, Nanorana quadranus and Quasipaa yei. Animals 2024, 14, 1849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shan, B.; Li, Y.J.G. Transcriptomic analysis of the differences in gene expression between testis and ovary of Dybowski’s frog (Rana dybowskii) in reproduction period. Gene 2025, 962, e149579. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet J. 2011, 17, 1138–1143. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Ramette, A. Multivariate analyses in microbial ecology. FEMS Microbiol. Ecol. 2007, 62, 142–160. [Google Scholar] [CrossRef]
- Zaura, E.; Keijser, B.J.; Huse, S.M.; Crielaard, W. Defining the healthy "core microbiome" of oral microbial communities. BMC Microbiol. 2009, 9, 1–12. [Google Scholar] [CrossRef]
- Ruchin, A. Light Spectrum Impacts on Early Development of Amphibians (Amphibia: Anura and Caudata). Pertanika J. Trop. Agric. Sci. 2018, 41, 1889–1897. [Google Scholar]
- Borah, B.K.; Renthlei, Z.; Tripathi, A.; Trivedi, A. Role of photoperiod, temperature and food on development of Polypedates teraiensis (Dubois, 1987) tadpoles. J. Environ. Biol. 2022, 43, 448–459. [Google Scholar] [CrossRef]
- Dananay, K.L.; Benard, M.F. Artificial light at night decreases metamorphic duration and juvenile growth in a widespread amphibian. Proc. R. Soc. B Biol. Sci. 2018, 285, e20180367. [Google Scholar] [CrossRef]
- Norris, D.O.; Lopez, K.H. Hormones and Reproduction in Amphibians and Reptiles. In Encyclopedia of Reproduction, 2nd ed.; Skinner, M.K., Ed.; Academic Press: Oxford, UK, 2018; pp. 374–384. [Google Scholar]
- Bliss, S.P.; Navratil, A.M.; Xie, J.; Roberson, M.S. GnRH signaling, the gonadotrope and endocrine control of fertility. Front. Neuroendocrinol. 2010, 31, 322–340. [Google Scholar] [CrossRef]
- Nakamura, M. Is a sex-determining gene(s) necessary for sex-determination in amphibians? Steroid hormones may be the key factor. Sex. Dev. 2013, 7, 104–114. [Google Scholar] [CrossRef]
- Choi, Y.J.; Park, S.G.N.R.; Jo, A.-H.; Kim, J.-H. Physiological Effect of Extended Photoperiod and Green Wavelength on the Pituitary Hormone, Sex Hormone and Stress Response in Chub Mackerel, Scomber japonicus. Fishes 2023, 8, 77. [Google Scholar] [CrossRef]
- Kılınç Uğurlu, A.; Bideci, A.; Demirel, M.A.; Take Kaplanoğlu, G.; Dayanır, D.; Gülbahar, Ö.; Deveci Bulut, T.S.; Döğer, E.; Çamurdan, M.O. Effects of Blue Light on Puberty and Ovary in Female Rats. J. Clin. Res. Pediatr. Endocrinol. 2023, 15, 365–374. [Google Scholar] [CrossRef]
- Iwama, B.G.K. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- Forsburg, Z.R.; Guzman, A.; Gabor, C.R. Artificial light at night (ALAN) affects the stress physiology but not the behavior or growth of Rana berlandieri and Bufo valliceps. Environ. Pollut. 2021, 277, e116775. [Google Scholar] [CrossRef]
- Paul, B.; Sterner, Z.R.; Buchholz, D.R.; Shi, Y.B.; Sachs, L.M. Thyroid and Corticosteroid Signaling in Amphibian Metamorphosis. Cells 2022, 11, 1595. [Google Scholar] [CrossRef]
- Cyr, N.E.; Romero, L.M. Identifying hormonal habituation in field studies of stress. Gen. Comp. Endocrinol. 2009, 161, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.S.; Gramapurohit, N.P. Effect of corticosterone on larval growth, antipredator behaviour and metamorphosis of Hylarana indica. Gen. Comp. Endocrinol. 2017, 251, 21–29. [Google Scholar] [CrossRef]
- Navarrete, P.; Espejo, R.T.; Romero, J. Molecular analysis of microbiota along the digestive tract of juvenile Atlantic salmon (Salmo salar L.). Microb. Ecol. 2009, 57, 550–561. [Google Scholar] [CrossRef]
- Li, P.; Zhang, J.; Liu, X.; Gan, L.; Xie, Y.; Zhang, H.; Si, J. The Function and the Affecting Factors of the Zebrafish Gut Microbiota. Front. Microbiol. 2022, 13, 903471. [Google Scholar] [CrossRef]
- Nikouli, E.; Meziti, A.; Smeti, E.; Antonopoulou, E.; Mente, E.; Kormas, K.A. Gut Microbiota of Five Sympatrically Farmed Marine Fish Species in the Aegean Sea. Microb. Ecol. 2021, 81, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Kohl, K.D.; Yahn, J. Effects of environmental temperature on the gut microbial communities of tadpoles. Environ. Microbiol. 2016, 18, 1561–1565. [Google Scholar] [CrossRef]
- Huang, B.H.; Chang, C.W.; Huang, C.W.; Gao, J.; Liao, P.C. Composition and functional specialists of the gut microbiota of frogs reflect habitat differences and agricultural activity. Front. Microbiol. 2017, 8, e2670. [Google Scholar] [CrossRef] [PubMed]
- Weng, F.C.; Yang, Y.J.; Wang, D. Functional analysis for gut microbes of the brown tree frog (Polypedates megacephalus) in artificial hibernation. BMC Genom. 2016, 17, e1024. [Google Scholar] [CrossRef]
- Shu, Y.; Hong, P.; Tang, D.; Qing, H.; Omondi Donde, O.; Wang, H.; Xiao, B.; Wu, H. Comparison of intestinal microbes in female and male Chinese concave-eared frogs (Odorrana tormota) and effect of nematode infection on gut bacterial communities. MicrobiologyOpen 2019, 8, e00749. [Google Scholar] [CrossRef]
- Bletz, M.C.; Goedbloed, D.J.; Sanchez, E.; Reinhardt, T.; Tebbe, C.C.; Bhuju, S.; Geffers, R.; Jarek, M.; Vences, M.; Steinfartz, S. Amphibian gut microbiota shifts differentially in community structure but converges on habitat-specific predicted functions. Nat. Commun. 2016, 7, e13699. [Google Scholar] [CrossRef]
- Anhê, F.F.; Nachbar, R.T.; Varin, T.V.; Trottier, J.; Dudonné, S.; Le Barz, M.; Feutry, P.; Pilon, G.; Barbier, O.; Desjardins, Y.; et al. Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. Gut 2019, 68, 453–464. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, H.; Yuan, F.; Li, N.; Huang, Q.; He, L.; Wang, L.; Liu, Z. Perilla oil has similar protective effects of fish oil on high-fat diet-induced nonalcoholic fatty liver disease and gut dysbiosis. BioMed Res. Int. 2016, 2016, e9462571. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.I.; Lee, M.K. Physicochemical Properties of Mealworm (Tenebrio molitor Larva) Oil and its Hypolipidemic Effect as a Replacement for Dietary Saturated Fat in Mice. Eur. J. Lipid Sci. Technol. 2022, 124, e2100213. [Google Scholar] [CrossRef]
- Passos, L.F.; Garcia, G.; Young, R.J. Comparing the bacterial communities of wild and captive golden mantella frogs: Implications for amphibian conservation. PLoS ONE 2018, 13, e0205652. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Liu, X.N.; Hu, Z.F.; Ding, J.F.; Bie, J.; Wang, H.B.; Zhang, J.T. Effects of Captivity and Season on the Gut Microbiota of the Brown Frog (Rana dybowskii). Front. Microbiol. 2019, 10, 1912. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Yang, L.; Cui, J.; Li, Y.; Jiang, X.; Meca, G.; Wang, S.; Feng, Y.; Zhao, Y.; Qin, J.; et al. Combined Analysis of the Effects of Exposure to Blue Light in Ducks Reveals a Reduction in Cholesterol Accumulation Through Changes in Methionine Metabolism and the Intestinal Microbiota. Front. Nutr. 2021, 8, e737059. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Blue Light Alters the Composition of the Jejunal Microbiota and Promotes the Development of the Small Intestine by Reducing Oxidative Stress. Antioxidants 2022, 11, 274. [Google Scholar] [CrossRef]
- Li, Q.M.; Zhou, Y.L.; Wei, Z.F.; Wang, Y. Phylogenomic Insights into Distribution and Adaptation of Bdellovibrionota in Marine Waters. Microorganisms 2021, 9, 757. [Google Scholar] [CrossRef]
- Yuan, Y.; Sepúlveda, M.S.; Bi, B.; Huang, Y.; Kong, L.; Yan, H.; Gao, Y. Acute polyethylene microplastic (PE-MPs) exposure activates the intestinal mucosal immune network pathway in adult zebrafish (Danio rerio). Chemosphere 2023, 311, 137048. [Google Scholar] [CrossRef]
- Danese, S.; Colombel, J.F.; Rieder, F.; Peyrin-Biroulet, L.; Siegmund, B.; Vermeire, S.; Dubinsky, M.; Schreiber, S.; Yarur, A.; Panaccione, R.; et al. P144 Modulation of Immunometabolism via NLRX1 or PLXDC2: Novel Bimodal Mechanisms for the Treatment of Inflammatory Bowel Diseases. J. Crohn’s Colitis 2024, 18, i443–i445. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, H.; Shan, B.; Hu, N.; Zhang, M.; Li, Y. Impact of Light Spectrum on Tadpole Physiology and Gut Microbiota in the Dybowski’s Frog (Rana dybowskii). Animals 2025, 15, 2066. https://doi.org/10.3390/ani15142066
Ji H, Shan B, Hu N, Zhang M, Li Y. Impact of Light Spectrum on Tadpole Physiology and Gut Microbiota in the Dybowski’s Frog (Rana dybowskii). Animals. 2025; 15(14):2066. https://doi.org/10.3390/ani15142066
Chicago/Turabian StyleJi, Haoyu, Baolong Shan, Nan Hu, Mingchao Zhang, and Yingdong Li. 2025. "Impact of Light Spectrum on Tadpole Physiology and Gut Microbiota in the Dybowski’s Frog (Rana dybowskii)" Animals 15, no. 14: 2066. https://doi.org/10.3390/ani15142066
APA StyleJi, H., Shan, B., Hu, N., Zhang, M., & Li, Y. (2025). Impact of Light Spectrum on Tadpole Physiology and Gut Microbiota in the Dybowski’s Frog (Rana dybowskii). Animals, 15(14), 2066. https://doi.org/10.3390/ani15142066





