Polymorphism of Keratin Gene KRT71 and Its Relationship with Wool Properties in Gansu Alpine Fine-Wool Sheep
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Animals and Sample Collection
2.3. Determination of Wool Production Traits
2.4. Protein Immunofluorescence Localization Analysis
2.5. Blood Genomic DNA Extraction
2.6. Detection of SNPs in the KRT71 Gene
2.7. Genotypic Detection of SNP Sites
2.8. Statistical Analysis
3. Results
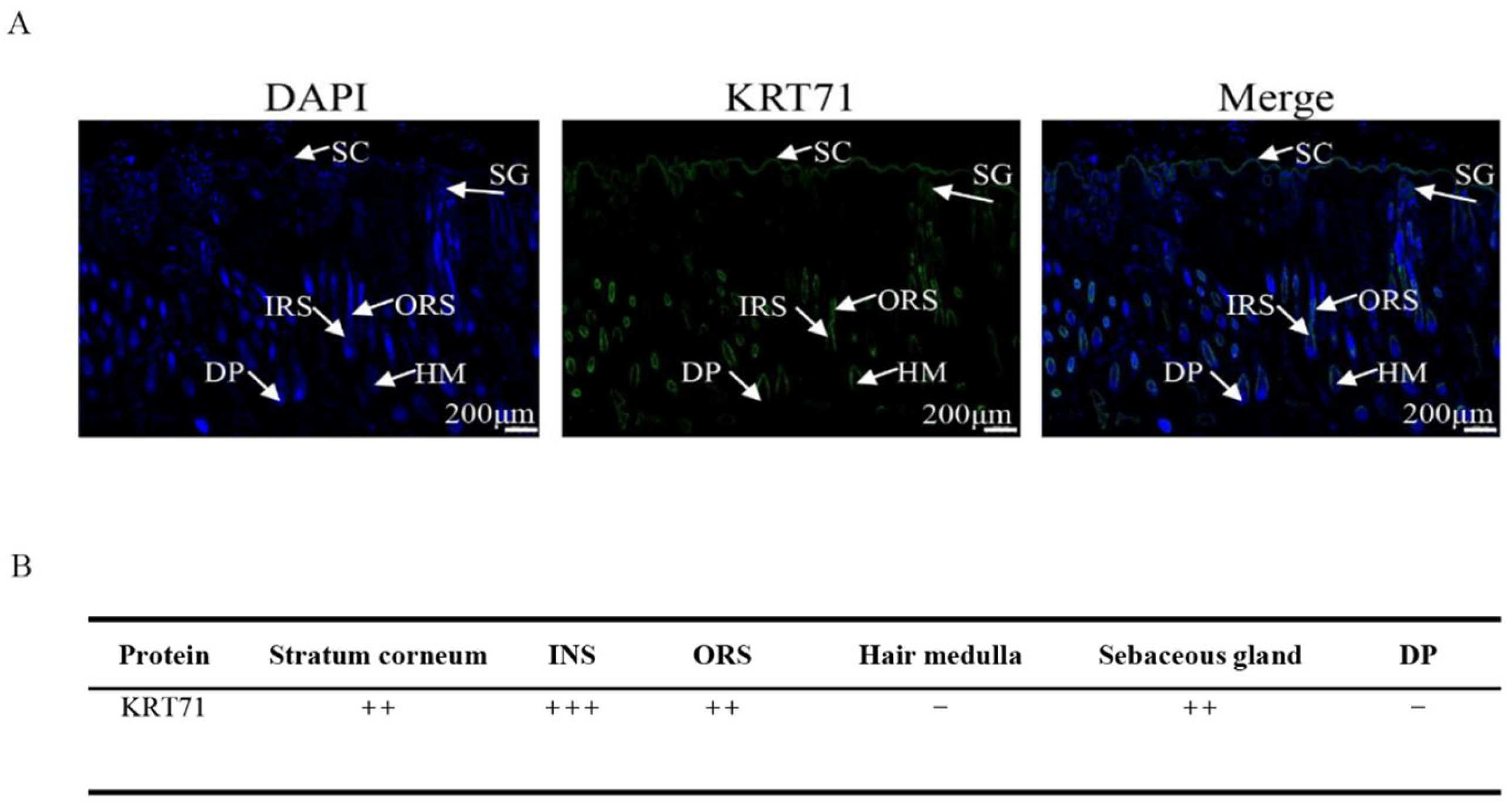
3.1. Localization of KRT71 Protein in Sheep Skin Tissue
3.2. Results of SNP Site Detection of KRT71 Gene in Gansu Alpine Fine-Wool Sheep
3.3. Genotyping Results of SNPs in KRT71 Gene
3.4. Population Genetic Polymorphism Analysis of the KRT71 Gene
3.5. Association Analysis of KRT71 Genotype with Wool Production Traits
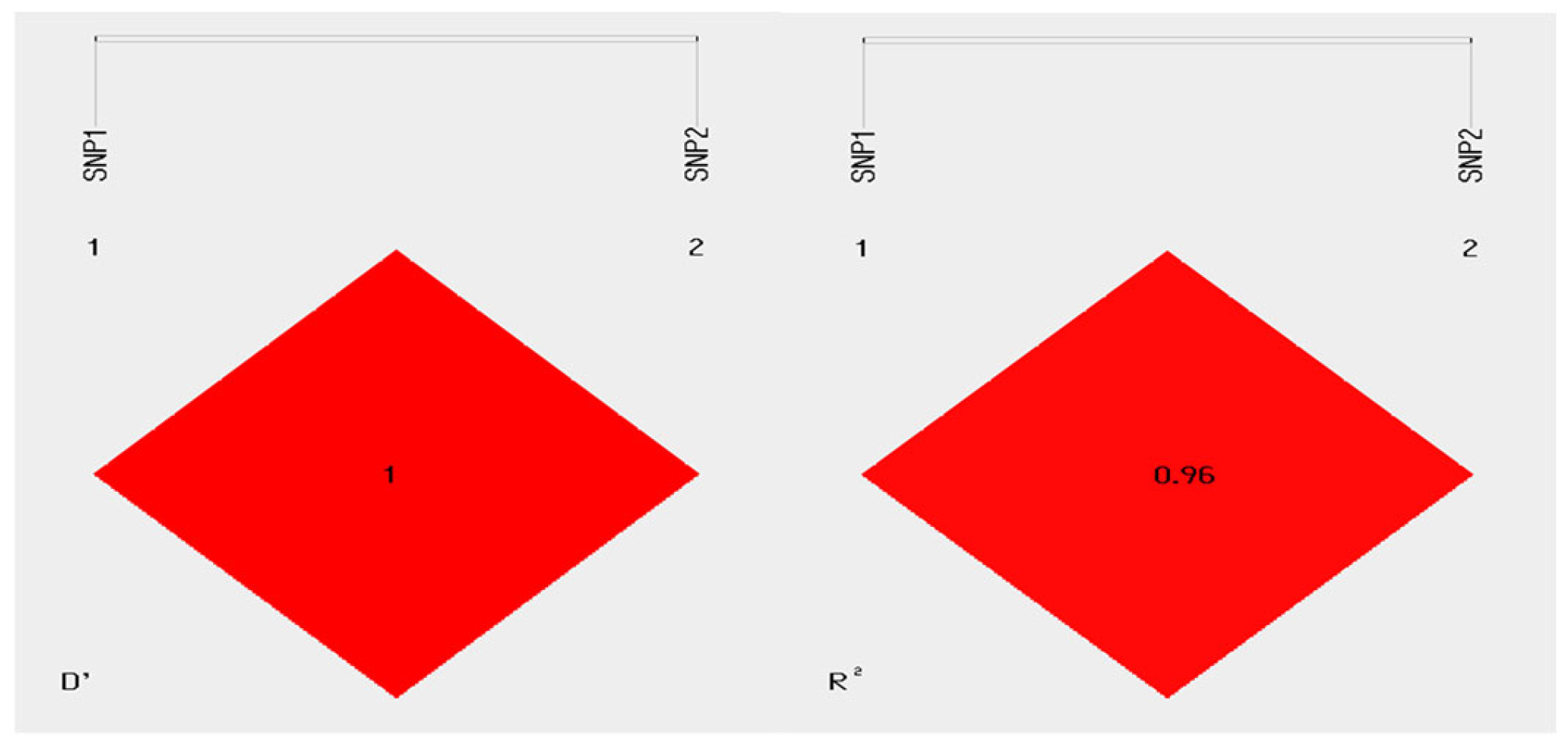
3.6. Linkage Disequilibrium Analysis of SNP Loci in the KRT71 Gene
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, Z.; Wells, C.M.; Carrington, C.G.; Hewitt, N.J. Thermal conductivity of wool and wool–hemp insulation. Int. J. Energy Res. 2006, 30, 37–49. [Google Scholar] [CrossRef]
- Patnaik, A.; Mvubu, M.; Muniyasamy, S.; Botha, A.; Anandjiwala, R.D. Thermal and sound insulation materials from waste wool and recycled polyester fibers and their biodegradation studies. Energy Build. 2015, 92, 161–169. [Google Scholar] [CrossRef]
- Pei, H.; Ma, X.; Pan, Y.; Han, T.; Lu, Z.; Wu, R.; Cao, X.; Zheng, J. Separation and purification of lanosterol, dihydrolanosterol, and cholesterol from lanolin by high-performance counter-current chromatography dual-mode elution method. J. Sep. Sci. 2019, 42, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Kicinska-Jakubowska, A.; Morales Villavicencio, A.; Zimniewska, M.; Przybylska, P.; Kwiatkowska, E. Evaluation of Wool Quality Parameters of Polish Sheep Breeds. J. Nat. Fibers 2022, 19, 5880–5887. [Google Scholar] [CrossRef]
- Zenda, M.; Malan, P.J.; Geyer, A.C. An analysis of the contribution of wool characteristics on price determination of Merino Wool and White Wool all combined in South Africa. Small Rumin. Res. 2023, 219, 106890. [Google Scholar] [CrossRef]
- Bromley, C.M.; Snowder, G.D.; Van Vleck, L.D. Genetic parameters among weight, prolificacy, and wool traits of Columbia, Polypay, Rambouillet, and Targhee sheep. J. Anim. Sci. 2000, 78, 846–858. [Google Scholar] [CrossRef]
- Burton, D.J.; Ludden, P.A.; Stobart, R.H.; Alexander, B.M. 50 years of the Wyoming ram test: How sheep have changed. J. Anim. Sci. 2015, 93, 1327–1331. [Google Scholar] [CrossRef]
- Cipriano, A.; Ballarino, M. The Ever-Evolving Concept of the Gene: The Use of RNA/Protein Experimental Techniques to Understand Genome Functions. Front. Mol. Biosci. 2018, 5, 20. [Google Scholar] [CrossRef]
- Gong, H.; Zhou, H.; Forrest, R.H.J.; Li, S.; Wang, J.; Dyer, J.M.; Luo, Y.; Hickford, J.G.H. Wool Keratin-Associated Protein Genes in Sheep—A Review. Genes 2016, 7, 24. [Google Scholar] [CrossRef]
- Zernov, N.V.; Skoblov, M.Y.; Marakhonov, A.V.; Shimomura, Y.; Vasilyeva, T.A.; Konovalov, F.A.; Abrukova, A.V.; Zinchenko, R.A. Autosomal Recessive Hypotrichosis with Woolly Hair Caused by a Mutation in the Keratin 25 Gene Expressed in Hair Follicles. J. Investig. Dermatol. 2016, 136, 1097–1105. [Google Scholar] [CrossRef]
- Coulombe, P.A.; Omary, M.B. ‘Hard’ and ‘soft’ principles defining the structure, function and regulation of keratin intermediate filaments. Curr. Opin. Cell Biol. 2002, 14, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Hobbs, R.P.; Coulombe, P.A. The expanding significance of keratin intermediate filaments in normal and diseased epithelia. Curr. Opin. Cell Biol. 2013, 25, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, A.; Farooq, M.; Fujikawa, H.; Inoue, A.; Ohyama, M.; Ehama, R.; Nakanishi, J.; Hagihara, M.; Iwabuchi, T.; Aoki, J.; et al. A Missense Mutation within the Helix Initiation Motif of the Keratin K71 Gene Underlies Autosomal Dominant Woolly Hair/Hypotrichosis. J. Investig. Dermatol. 2012, 132, 2342–2349. [Google Scholar] [CrossRef] [PubMed]
- Kuramoto, T.; Hirano, R.; Kuwamura, M.; Serikawa, T. Identification of the Rat Rex Mutation as a 7-bp Deletion at Splicing Acceptor Site of the Krt71 Gene. J. Vet. Med. Sci. 2010, 72, 909–912. [Google Scholar] [CrossRef]
- Duan, C.; Zhang, L.; Gao, K.; Guo, Y.; Liu, Y.; Zhang, Y. Cashmere production, skin characteristics, and mutated genes in crimped cashmere fibre goats. Animal 2022, 16, 100565. [Google Scholar] [CrossRef]
- Li, W.; Guo, J.; Li, F.; Niu, C. Evaluation of Crossbreeding of Australian Superfine Merinos with Gansu Alpine Finewool Sheep to Improve Wool Characteristics. PLoS ONE 2016, 11, e0166374. [Google Scholar] [CrossRef]
- Bolormaa, S.; Swan, A.A.; Brown, D.J.; Hatcher, S.; Moghaddar, N.; van der Werf, J.H.; Goddard, M.E.; Daetwyler, H.D. Multiple-trait QTL mapping and genomic prediction for wool traits in sheep. Genet. Sel. Evol. 2017, 49, 62. [Google Scholar] [CrossRef]
- He, Z.; Sun, H.; Zhao, F.; Ma, L.; Wang, J.; Liu, X.; Li, M.; Hao, Z.; Li, S. MicroRNA expression profiles reveal wool development and fineness regulation in Gansu alpine fine-wool sheep. Genomics 2024, 116, 110922. [Google Scholar] [CrossRef]
- GB1523-2013; Sheep Wool. Standardization Administration of the People’s Republic of China (SAC): Beijing, China, 2013.
- Diao, X.; Yao, L.; Wang, X.; Li, S.; Qin, J.; Yang, L.; He, L.; Zhang, W. Hair Follicle Development and Cashmere Traits in Albas Goat Kids. Animals 2023, 13, 617. [Google Scholar] [CrossRef]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar]
- Chai, W.; Zhou, H.; Gong, H.; Hickford, J.G.H. Variation in the ovine KRT34 promoter region affects wool traits. Small Rumin. Res. 2022, 206, 106586. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, R.; Li, F.; Yue, X. Five SNPs Within the FGF5 Gene Significantly Affect Both Wool Traits and Growth Performance in Fine-Wool Sheep (Ovis aries). Front. Genet. 2021, 12, 732097. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Zhou, H.; Gong, H.; Wang, C.; Hickford, J.G.H. Variation in the Exon 3–4 Region of Ovine KRT85 and Its Effect on Wool Traits. Animals 2024, 14, 2272. [Google Scholar] [CrossRef] [PubMed]
- Almaamory, Y.A.; Al-Anbari, N.N. Relationship of FASN Gene Polymorphism in Growth Performance and Wool Traits of Awassi Sheep. IOP Conf. Ser. Earth Environ. Sci. 2023, 1214, 012032. [Google Scholar] [CrossRef]
- Yue, L.; Lu, Z.; Guo, T.; Liu, J.; Yuan, C.; Yang, B. Association of SLIT3 and ZNF280B Gene Polymorphisms with Wool Fiber Diameter. Animals 2023, 13, 3552. [Google Scholar] [CrossRef]
- Xue, Q.; Huang, Y.; Cheng, C.; Wang, Y.; Liao, F.; Duan, Q.; Wang, X.; Miao, C. Progress in epigenetic regulation of milk synthesis, with particular emphasis on mRNA regulation and DNA methylation. Cell Cycle 2023, 22, 1675–1693. [Google Scholar] [CrossRef]
- He, Z.; Zhao, F.; Sun, H.; Hu, J.; Wang, J.; Liu, X.; Li, M.; Hao, Z.; Zhao, Z.; Shi, B.; et al. Screened of long non-coding RNA related to wool development and fineness in Gansu alpine fine-wool sheep. BMC Genom. 2025, 26, 8. [Google Scholar] [CrossRef]
- Kryuchkova-Mostacci, N.; Robinson-Rechavi, M. A benchmark of gene expression tissue-specificity metrics. Brief. Bioinform. 2016, 18, 205–214. [Google Scholar] [CrossRef]
- Grover, M.; Gang, S.S.; Troemel, E.R.; Barkoulas, M. Proteasome inhibition triggers tissue-specific immune responses against different pathogens in C. elegans. PLoS Biol. 2024, 22, e3002543. [Google Scholar] [CrossRef]
- Chen, R.; Zhou, H.; Li, A.; Cheng, X.; Liu, X.; Huang, F.; Wang, Y.; Liu, Y.; Gong, H.; Liu, X.; et al. Chemical Sectioning for Immunofluorescence Imaging. Anal. Chem. 2021, 93, 8698–8703. [Google Scholar] [CrossRef]
- Cohen, R.; Lee-Pullen, T.; Miller, T.J.; Meehan, K.; Fuller, K.; McCoy, M.J. Optimising multiplex immunofluorescence staining for characterising the tumour immune micro-environment. Methods 2023, 219, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Cotsarelis, G. The Biology of Hair Follicles. N. Engl. J. Med. 1999, 341, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Teama, S. DNA polymorphisms: DNA-based molecular markers and their application in medicine. In Genetic Diversity and Disease Susceptibility; Intechopen: London, UK, 2018; Volume 25. [Google Scholar]
- Gebert, M.; Jaśkiewicz, M.; Moszyńska, A.; Collawn, J.F.; Bartoszewski, R. The Effects of Single Nucleotide Polymorphisms in Cancer RNAi Therapies. Cancers 2020, 12, 3119. [Google Scholar] [CrossRef] [PubMed]
- Shatoff, E.; Bundschuh, R. Single nucleotide polymorphisms affect RNA-protein interactions at a distance through modulation of RNA secondary structures. PLoS Comput. Biol. 2020, 16, e1007852. [Google Scholar] [CrossRef]
- Van Kouwenhove, M.; Kedde, M.; Agami, R. MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nat. Rev. Cancer 2011, 11, 644–656. [Google Scholar] [CrossRef]
- Burd, C.G.; Dreyfuss, G. Conserved structures and diversity of functions of RNA-binding proteins. Science 1994, 265, 615–621. [Google Scholar] [CrossRef]
- Hurst, L.D. Molecular genetics: The sound of silence. Nature 2011, 471, 582–583. [Google Scholar] [CrossRef]
- Zhao, J.; Sauvage, C.; Bitton, F.; Causse, M. Multiple haplotype-based analyses provide genetic and evolutionary insights into tomato fruit weight and composition. Hortic. Res. 2022, 9, uhab009. [Google Scholar] [CrossRef]
- Holman, B.; Malau-Aduli, A. A review of sheep wool quality traits. Annu. Rev. Res. Biol. 2012, 2, 1–14. [Google Scholar]
- Runkel, F.; Klaften, M.; Koch, K.; Böhnert, V.; Büssow, H.; Fuchs, H.; Franz, T.; de Angelis, M.H. Morphologic and molecular characterization of two novel Krt71 (Krt2-6g) mutations: Krt71rco12 and Krt71rco13. Mamm. Genome 2006, 17, 1172–1182. [Google Scholar] [CrossRef]
- Shimomura, Y.; Wajid, M.; Petukhova, L.; Kurban, M.; Christiano, A.M. Autosomal-dominant woolly hair resulting from disruption of keratin 74 (KRT74), a potential determinant of human hair texture. Am. J. Hum. Genet. 2010, 86, 632–638. [Google Scholar] [CrossRef]


| Gene | Position | Forward Primer (5′ → 3′) | Reverse Primer (5′ → 3′) | Amplification Product Length (bp) |
|---|---|---|---|---|
| KRT71 | 5′ UTR | TTCATGTTGTTTCAGAGGTGGGGAG | CTCAGGGAAGACTATACGGGAAACCTT | 835 |
| E9 | TCTCTGCCATATTGTCCCCAGTGG | TCAGGAAGACACAGTCTGGGAGAAGTG | 535 |
| Gene | SNP | Position | Physical Position/bp | Variation Type | Amino Acid Change |
|---|---|---|---|---|---|
| KRT71 | SNP1 | 5′ UTR | g.133805316 | G → C | No change |
| SNP2 | Exon 9 | g.133812850 | G → A | No change |
| Genotype | Genotype Frequency(n) | Allele Frequency | χ2 | p-Value | |||
|---|---|---|---|---|---|---|---|
| SNP1 | GG | GC | CC | G | C | 0.617 | 0.432 |
| 0.924 (278) | 0.073 (22) | 0.003 (1) | 0.960 | 0.040 | |||
| SNP2 | GG | GA | AA | G | A | 0.470 | 0.493 |
| 0.920 (274) | 0.077 (23) | 0.003 (1) | 0.958 | 0.042 | |||
| SNP | Ho | He | PIC | Ne |
|---|---|---|---|---|
| SNP1 | 0.923 | 0.076 | 0.074 | 1.083 |
| SNP2 | 0.919 | 0.080 | 0.077 | 1.087 |
| SNP | Genotype | MFD (μm) | FDSD | CVFD (%) | MSL (mm) | MFC (Number/mm) | CF (%) | MSS (cN/dt) |
|---|---|---|---|---|---|---|---|---|
| SNP1 | GG | 21.508 ± 0.156 | 5.624 ± 0.073 | 25.142 ± 0.213 | 80.047 ± 0.768 a | 104.998 ± 0.815 | 87.482 ± 0.805 | 13.982 ± 0.303 |
| GC | 21.726 ± 0.628 | 5.395 ± 0.204 | 24.766 ± 0.551 | 75.454 ± 1.841 b | 106.138 ± 2.989 | 89.285 ± 3.034 | 15.848 ± 1.051 | |
| SNP2 | GG | 21.516 ± 0.158 | 5.619 ± 0.074 | 25.139 ± 0.214 | 79.963 ± 0.768 a | 104.913 ± 0.818 | 87.566 ± 0.805 | 13.986 ± 0.305 |
| GA | 21.733 ± 0.601 | 5.368 ± 0.196 | 24.645 ± 0.539 | 75.304 ± 1.766 b | 106.386 ± 2.861 | 89.500 ± 2.900 | 15.806 ± 1.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; He, Z.; Sun, H.; Wang, J.; Liu, X.; Hao, Z.; Li, M.; Li, S. Polymorphism of Keratin Gene KRT71 and Its Relationship with Wool Properties in Gansu Alpine Fine-Wool Sheep. Animals 2025, 15, 2028. https://doi.org/10.3390/ani15142028
Zhao F, He Z, Sun H, Wang J, Liu X, Hao Z, Li M, Li S. Polymorphism of Keratin Gene KRT71 and Its Relationship with Wool Properties in Gansu Alpine Fine-Wool Sheep. Animals. 2025; 15(14):2028. https://doi.org/10.3390/ani15142028
Chicago/Turabian StyleZhao, Fangfang, Zhaohua He, Hongxian Sun, Jiqing Wang, Xiu Liu, Zhiyun Hao, Mingna Li, and Shaobin Li. 2025. "Polymorphism of Keratin Gene KRT71 and Its Relationship with Wool Properties in Gansu Alpine Fine-Wool Sheep" Animals 15, no. 14: 2028. https://doi.org/10.3390/ani15142028
APA StyleZhao, F., He, Z., Sun, H., Wang, J., Liu, X., Hao, Z., Li, M., & Li, S. (2025). Polymorphism of Keratin Gene KRT71 and Its Relationship with Wool Properties in Gansu Alpine Fine-Wool Sheep. Animals, 15(14), 2028. https://doi.org/10.3390/ani15142028





