Behavioral, Endocrine, and Neuronal Responses to Odors in Lampreys
Simple Summary
Abstract
1. Introduction
2. Olfactory-Induced Behavior in Lampreys
2.1. Larval Stage
2.2. Metamorphosis and Juvenile Stage
2.3. Male Upstream Migration
2.3.1. Attractive Larval Migratory Pheromones
2.3.2. Repulsive Anti-Predator Cues
2.4. Female Upstream Migration
2.4.1. Identifying a Suitable River
2.4.2. Locating Spawning Grounds
2.5. Olfactory Spawning Behavior
2.5.1. 3-Keto Petromyzonol Sulfate
2.5.2. 3,12-Diketo-4,6-petromyzonene-24-sulfate
2.5.3. Other Bile Products
2.5.4. Spermine
2.6. Section Summary
3. Endocrine Signaling Induced by Pheromone Detection
3.1. The Hypothalamic-Pituitary-Gonadal Axis in Lampreys
3.1.1. Hypothalamic Gonadotropin-Releasing Hormones in Lampreys
3.1.2. Gonadal Hormones in Lampreys
3.2. Exposure to Pheromones Induces Gametogenesis and Pheromone Production
3.2.1. Exposure to Pheromones Primes the Hypothalamic-Pituitary-Gonadal Axis
3.2.2. Gonadal Hormones Induce Gametogenesis and Pheromone Production
3.2.3. Hypothetic Signaling Pathway Inducing Physiological Effects in Response to Pheromone Exposure
3.2.4. Impacts on Reproduction
3.3. Section Summary
4. Neuronal Mechanisms Induced by Odor Detection
4.1. Olfactomotor Circuitry in Lampreys
4.1.1. Olfactory Organs and Receptors
4.1.2. Olfactory Bulb Circuitry
4.1.3. Projections to the Posterior Tuberculum
4.1.4. Common Descending Locomotor Pathway
4.2. Modulation of the Olfactomotor Circuitry
4.2.1. Neuromodulation in the Olfactory System
4.2.2. External (Environmental) Signals Modulate Olfactory Behavior
4.2.3. Internal (Hormonal) Signals Modulate Olfactory Behavior
Modulation of Olfactory Behavior by Gonadotropin-Releasing Hormones
Modulation of Olfactory Behavior by Gonadal Hormones
4.3. Section Summary
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOO | Accessory olfactory organ |
| DkPES | 3,12-diketo-4,6-petromyzonene-24-sulfate |
| FSH | Follicle-stimulating hormone |
| GABA | γ-aminobutyric acid |
| GnIH | Gonadotropin-inhibitory hormones |
| GnRH | Gonadotropin-releasing hormone |
| HPG | Hypothalamic-pituitary-gonadal |
| lGnRH | Lamprey gonadotropin-releasing hormone |
| LH | Luteinizing hormone |
| LPal | Lateral pallium |
| medOB | Medial olfactory bulb |
| MLR | Mesencephalic locomotor region |
| MOB | Main olfactory bulb |
| MOE | Main olfactory epithelium |
| OB | Olfactory bulb |
| OR | Odorant receptor |
| PT | Posterior tuberculum |
| PZS | Petromyzonol sulfate |
| RS | Reticulospinal |
| TAAR | Trace amine-associated receptor-like |
| V1R | Vomeronasal type 1 receptor |
| V2R | Vomeronasal type 2 receptor |
| 15α-P | 15α-hydroxyprogesterone |
| 3kPZS | 3-keto petromyzonol sulfate |
| 3sPZS | Petromyzonol tetrasulfate |
References
- Almeida, P.R.; Quintella, B.R.; Dias, N.M. Movement of radio-tagged anadromous sea lamprey during the spawning migration in the River Mondego (Portugal). Hydrobiologia 2002, 483, 1–8. [Google Scholar] [CrossRef]
- Binder, T.R.; McDonald, D.G. Is there a role for vision in the behaviour of sea lampreys (Petromyzon marinus) during their upstream spawning migration? Can. J. Fish. Aquat. Sci. 2007, 64, 1403–1412. [Google Scholar] [CrossRef]
- McCann, E.L.; Johnson, N.S.; Hrodey, P.J.; Pangle, K.L. Characterization of sea lamprey stream entry using dual-frequency identification sonar. Trans. Am. Fish. Soc. 2018, 147, 514–524. [Google Scholar] [CrossRef]
- Piavis, G.W. Embryology. In The Biology of Lampreys; Hardisty, M.W., Potter, I.C., Eds.; Academic Press: London, UK, 1971; Volume 1, pp. 361–400. [Google Scholar] [CrossRef]
- Zielinski, B.S.; Fredricks, K.; McDonald, R.; Zaidi, A.U. Morphological and electrophysiological examination of olfactory sensory neurons during the early developmental prolarval stage of the sea lamprey Petromyzon marinus L. J. Neurocytol. 2005, 34, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Vandenbossche, J.; Seelye, J.G.; Zielinski, B.S. The morphology of the olfactory epithelium in larval, juvenile and upstream migrant stages of the sea lamprey, Petromyzon marinus. Brain Behav. Evol. 1995, 45, 19–24. [Google Scholar] [CrossRef]
- Hardisty, M.W.; Potter, I.C. The behaviour, ecology and growth of larval lampreys. In The Biology of Lampreys; Hardisty, M.W., Potter, I.C., Eds.; Academic Press: London, UK, 1971; Volume 1, pp. 85–125. [Google Scholar] [CrossRef]
- Sutton, T.M.; Bowen, S.H. Significance of organic detritus in the diet of larval lampreys in the Great Lakes basin. Can. J. Fish. Aquat. Sci. 1994, 51, 2380–2387. [Google Scholar] [CrossRef]
- Enequist, P. Das bachneunauge als ökologische modifikation des flussneunauges. Über die fluss–und bachneunaugen Schwedens; vorläufige mitteilung. Ark. Zool. 1937, 29, 1–22. [Google Scholar]
- Morman, R.H.; Cuddy, D.W.; Rugen, P.C. Factors influencing the distribution of sea lamprey (Petromyzon marinus) in the Great Lakes. Can. J. Fish. Aquat. Sci. 1980, 37, 1811–1826. [Google Scholar] [CrossRef]
- Dawson, H.A.; Quintella, B.R.; Almeida, P.R.; Treble, A.J.; Jolley, J.C. The ecology of larval and metamorphosing lampreys. In Lampreys: Biology, Conservation and Control; Docker, M.F., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 75–137. [Google Scholar] [CrossRef]
- Smith, D.M.; Welsh, S.A.; Turk, P.J. Available benthic habitat type may influence predation risk in larval lampreys. Ecol. Freshw. Fish. 2012, 21, 160–163. [Google Scholar] [CrossRef]
- Derosier, A.L.; Jones, M.L.; Scribner, K.T. Dispersal of sea lamprey larvae during early life: Relevance for recruitment dynamics. Environ. Biol. Fish. 2007, 78, 271–284. [Google Scholar] [CrossRef]
- Wagner, C.M.; Kierczynski, K.E.; Hume, J.B.; Luhring, T.M. Exposure to a putative alarm cue reduces downstream drift in larval sea lamprey Petromyzon marinus in the laboratory. J. Fish. Biol. 2016, 89, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Perrault, K.; Imre, I.; Brown, G.E. Behavioural response of larval sea lamprey (Petromyzon marinus) in a laboratory environment to potential damage-released chemical alarm cues. Can. J. Zool. 2014, 92, 443–447. [Google Scholar] [CrossRef]
- Kats, L.B.; Dill, L.M. The scent of death: Chemosensory assessment of predation risk by prey animals. Ecoscience 1998, 5, 361–394. [Google Scholar] [CrossRef]
- Ayotte, J.L.; Imre, I. Larval sea lampreys (Petromyzon marinus) do not emigrate from a risky habitat under semi-natural conditions. Can. Field-Nat. 2016, 130, 49–52. [Google Scholar] [CrossRef]
- De Filippi, F. Cenni sui Pesci d’aqua Dolce Della Lombardia; Tipografia Bernardoni: Milano, Italy, 1844. [Google Scholar]
- Youson, J.H. Morphology and physiology of lamprey metamorphosis. Can. J. Fish. Aquat. Sci. 1980, 37, 1687–1710. [Google Scholar] [CrossRef]
- Vandenbossche, J.; Youson, J.H.; Pohlman, D.; Wong, E.; Zielinski, B.S. Metamorphosis of the olfactory organ of the sea lamprey (Petromyzon marinus L.): Morphological changes and morphometric analysis. J. Morphol. 1997, 231, 41–52. [Google Scholar] [CrossRef]
- Davis, R.M. Parasitism by newly-transformed anadromous sea lampreys on landlocked salmon and other fishes in a coastal Maine lake. T Am. Fish. Soc. 1967, 96, 11–16. [Google Scholar] [CrossRef]
- Potter, I.C.; Beamish, F.W. The freshwater biology of adult anadromous sea lampreys Petromyzon marinus. J. Zool. 1977, 181, 113–130. [Google Scholar] [CrossRef]
- Silva, S.; Servia, M.J.; Vieira-Lanero, R.; Barca, S.; Cobo, F. Life cycle of the sea lamprey Petromyzon marinus: Duration of and growth in the marine life stage. Aquat. Biol. 2013, 18, 59–62. [Google Scholar] [CrossRef]
- Johnson, N.S.; Miehls, S.M.; Haro, A.J.; Wagner, C.M. Push and pull of downstream moving juvenile sea lamprey (Petromyzon marinus) exposed to chemosensory and light cues. Conserv. Physiol. 2019, 7, coz080. [Google Scholar] [CrossRef]
- Kleerekoper, H.; Sibakin, K. An investigation of the electrical “spike” potentials produced by the sea lamprey (Petromyzon marinus) in the water surrounding the head region. J. Fish. Res. Board. Can. 1956, 13, 375–383. [Google Scholar] [CrossRef]
- Kleerekoper, H.; Sibakin, K. Spike potentials produced by the sea lamprey (Petromyzon marinus) in the water surrounding the head region. Nature 1956, 178, 490–491. [Google Scholar] [CrossRef]
- Kleerekoper, H.; Mogensen, J. Role of olfaction in the orientation of Petromyzon marinus. I. Response to a single amine in prey’s body odor. Physiol. Zool. 1963, 36, 347–360. [Google Scholar] [CrossRef]
- Li, W.; Sorensen, P.W. The olfactory sensitivity of sea lamprey to amino acids is specifically restricted to arginine (abstract). Chem. Senses 1992, 17, 658. [Google Scholar] [CrossRef]
- Zielinski, B.S.; Osahan, J.K.; Hara, T.J.; Hosseini, M.; Wong, E. Nitric oxide synthase in the olfactory mucosa of the larval sea lamprey (Petromyzon marinus). J. Comp. Neurol. 1996, 365, 18–26. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.J.; Mai, K.S.; Tian, L.X. Advance in researches on arginine requirement for fish: A review. J. Fish. China 2004, 28, 450–459. [Google Scholar]
- Halver, J.E.; DeLong, D.C.; Mertz, E.T. Nutrition of salmonoid fishes: V. Classification of essential amino acids for Chinook salmon. J. Nutr. 1957, 63, 95–105. [Google Scholar] [CrossRef]
- Kaushik, S.J.; Luquet, P.; Blanc, D. Usefulness of feeding protein and non-protein calories apart in studies on energy-protein interrelationships in rainbow trout. Ann. Zootech. 1981, 30, 3–11. [Google Scholar] [CrossRef]
- Ketola, H.G. Requirement for dietary lysine and arginine by fry of rainbow trout. J. Anim. Sci. 1983, 56, 101–107. [Google Scholar] [CrossRef]
- Wilson, R.P.; Halver, J.E. Protein and amino acid requirements of fishes. Annu. Rev. Nutr. 1986, 6, 225–244. [Google Scholar] [CrossRef]
- Hardisty, M.W.; Potter, I.C. The Biology of Lampreys; Academic Press: London, UK, 1971; Volume 1, p. 423. [Google Scholar] [CrossRef]
- Larsen, L.O. Physiology of adult lampreys, with special regard to natural starvation, reproduction, and death after spawning. Can. J. Fish. Aquat. Sci. 1980, 37, 1762–1779. [Google Scholar] [CrossRef]
- Hussakof, L. The spawning habits of the sea lamprey, Petromyzon marinus. Am. Nat. 1912, 46, 729–740. [Google Scholar] [CrossRef]
- Bergstedt, R.A.; Seelye, J.G. Evidence for lack of homing by sea lampreys. T Am. Fish. Soc. 1995, 124, 235–239. [Google Scholar] [CrossRef]
- Bryan, M.B.; Zalinski, D.; Filcek, K.B.; Libants, S.V.; Li, W.; Scribner, K.T. Patterns of invasion and colonization of the sea lamprey (Petromyzon marinus) in North America as revealed by microsatellite genotypes. Mol. Ecol. 2005, 14, 3757–3773. [Google Scholar] [CrossRef]
- Waldman, J.; Grunwald, C.; Wirgin, I. Sea lamprey Petromyzon marinus: An exception to the rule of homing in anadromous fishes. Biol. Lett. 2008, 4, 659–662. [Google Scholar] [CrossRef]
- Nordeng, H. Is the local orientation of anadromous fishes determined by pheromones? Nature 1971, 233, 411–413. [Google Scholar] [CrossRef]
- Vrieze, L.A.; Bergstedt, R.A.; Sorensen, P.W. Olfactory-mediated stream-finding behavior of migratory adult sea lamprey (Petromyzon marinus). Can. J. Fish. Aquat. Sci. 2011, 68, 523–533. [Google Scholar] [CrossRef]
- Vrieze, L.A.; Sorensen, P.W. Laboratory assessment of the role of a larval pheromone and natural stream odor in spawning stream localization by migratory sea lamprey (Petromyzon marinus). Can. J. Fish. Aquat. Sci. 2001, 58, 2374–2385. [Google Scholar] [CrossRef]
- Wagner, C.M.; Twohey, M.B.; Fine, J.M. Conspecific cueing in the sea lamprey: Do reproductive migrations consistently follow the most intense larval odour? Anim. Behav. 2009, 78, 593–599. [Google Scholar] [CrossRef]
- Meckley, T.D.; Wagner, C.M.; Gurarie, E. Coastal movements of migrating sea lamprey (Petromyzon marinus) in response to a partial pheromone added to river water: Implications for management of invasive populations. Can. J. Fish. Aquat. Sci. 2014, 71, 533–544. [Google Scholar] [CrossRef]
- Meckley, T.D.; Gurarie, E.; Miller, J.R.; Wagner, C.M. How fishes find the shore: Evidence for orientation to bathymetry from the non-homing sea lamprey. Can. J. Fish. Aquat. Sci. 2017, 74, 2045–2058. [Google Scholar] [CrossRef]
- Wagner, C.M.; Jones, M.L.; Twohey, M.B.; Sorensen, P.W. A field test verifies that pheromones can be useful for sea lamprey (Petromyzon marinus) control in the Great Lakes. Can. J. Fish. Aquat. Sci. 2006, 63, 475–479. [Google Scholar] [CrossRef]
- Webster, D.R.; Weissburg, M.J. The hydrodynamics of chemical cues among aquatic organisms. Annu. Rev. Fluid. Mech. 2009, 41, 73–90. [Google Scholar] [CrossRef]
- Johnson, N.S.; Muhammad, A.; Thompson, H.T.; Choi, J.; Li, W. Sea lamprey orient toward a source of a synthesized pheromone using odor-conditioned rheotaxis. Behav. Ecol. Sociobiol. 2012, 66, 1557–1567. [Google Scholar] [CrossRef]
- Vrieze, L.A.; Bjerselius, R.; Sorensen, P.W. Importance of the olfactory sense to migratory sea lampreys Petromyzon marinus seeking riverine spawning habitat. J. Fish. Biol. 2010, 76, 949–964. [Google Scholar] [CrossRef]
- Haslewood, G.A.D.; Tökés, L. Comparative studies of bile salts. Bile salts of the lamprey Petromyzon marinus L. Biochem. J. 1969, 114, 179–184. [Google Scholar] [CrossRef]
- Li, W.; Scott, A.P.; Siefkes, M.J.; Yan, H.; Liu, Q.; Yun, S.S.; Gage, D.A. Bile acid secreted by male sea lamprey that acts as a sex pheromone. Science 2002, 296, 138–141. [Google Scholar] [CrossRef]
- Moore, H.H.; Schleen, L.P. Changes in spawning runs of sea lamprey (Petromyzon marinus) in selected streams of Lake Superior after chemical control. Can. J. Fish. Aquat. Sci. 1980, 37, 1851–1860. [Google Scholar] [CrossRef]
- Teeter, J.H. Pheromone communication in sea lampreys (Petromyzon marinus): Implications for population management. Can. J. Fish. Aquat. Sci. 1980, 37, 2123–2132. [Google Scholar] [CrossRef]
- Fine, J.M.; Sorensen, P.W. Production and fate of the sea lamprey migratory pheromone. Fish. Physiol. Biochem. 2010, 36, 1013–1020. [Google Scholar] [CrossRef]
- Sorensen, P.W.; Vrieze, L.A.; Fine, J.M. A multi-component migratory pheromone in the sea lamprey. Fish. Physiol. Biochem. 2003, 28, 253–257. [Google Scholar] [CrossRef]
- Sorensen, P.W.; Vrieze, L.A. The chemical ecology and potential application of the sea lamprey migratory pheromone. J. Great Lakes Res. 2003, 29, 66–84. [Google Scholar] [CrossRef]
- Polkinghorne, C.N.; Olson, J.M.; Gallaher, D.G.; Sorensen, P.W. Larval sea lamprey release two unique bile acids** to the water at a rate sufficient to produce detectable riverine pheromone plumes. Fish. Physiol. Biochem. 2001, 24, 15–30. [Google Scholar] [CrossRef]
- Li, W.; Sorensen, P.W.; Gallaher, D.D. The olfactory system of migratory adult sea lamprey (Petromyzon marinus) is specifically and acutely sensitive to unique bile acids released by conspecific larvae. J. Gen. Physiol. 1995, 105, 569–587. [Google Scholar] [CrossRef]
- Bjerselius, R.; Li, W.; Teeter, J.H.; Seelye, J.G.; Johnsen, P.B.; Maniak, P.J.; Grant, G.C.; Polkinghorne, C.N.; Sorensen, P.W. Direct behavioral evidence that unique bile acids released by larval sea lamprey (Petromyzon marinus) function as a migratory pheromone. Can. J. Fish. Aquat. Sci. 2000, 57, 557–569. [Google Scholar] [CrossRef]
- Johnson, N.S.; Siefkes, M.J.; Wagner, C.M.; Dawson, H.A.; Wang, H.; Steeves, T.B.; Twohey, M.B.; Li, W. A synthesized mating pheromone component increases adult sea lamprey (Petromyzon marinus) trap capture in management scenarios. Can. J. Fish. Aquat. Sci. 2013, 70, 1101–1108. [Google Scholar] [CrossRef]
- Brant, C.O.; Li, K.; Johnson, N.S.; Li, W. A pheromone outweighs temperature in influencing migration of sea lamprey. R. Soc. Open Sci. 2015, 2, 150009. [Google Scholar] [CrossRef]
- Brant, C.O.; Johnson, N.S.; Li, K.; Buchinger, T.J.; Li, W. Female sea lamprey shift orientation toward a conspecific chemical cue to escape a sensory trap. Behav. Ecol. 2016, 27, 810–819. [Google Scholar] [CrossRef]
- Fine, J.M.; Vrieze, L.A.; Sorensen, P.W. Evidence that petromyzontid lampreys employ a common migratory pheromone that is partially comprised of bile acids. J. Chem. Ecol. 2004, 30, 2091–2110. [Google Scholar] [CrossRef]
- Fine, J.M.; Sorensen, P.W. Biologically relevant concentrations of petromyzonol sulfate, a component of the sea lamprey migratory pheromone, measured in stream water. J. Chem. Ecol. 2005, 31, 2205–2210. [Google Scholar] [CrossRef]
- Li, W.; Sorensen, P.W. Highly independent olfactory receptor sites for naturally occurring bile acids in the sea lamprey, Petromyzon marinus. J. Comp. Physiol. A 1997, 180, 429–438. [Google Scholar] [CrossRef]
- Fine, J.M.; Sorensen, P.W. Isolation and biological activity of the multi-component sea lamprey migratory pheromone. J. Chem. Ecol. 2008, 34, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, P.W.; Fine, J.M.; Dvornikovs, V.; Jeffrey, C.S.; Shao, F.; Wang, J.; Vrieze, L.A.; Anderson, K.R.; Hoye, T.R. Mixture of new sulfated steroids functions as a migratory pheromone in the sea lamprey. Nat. Chem. Biol. 2005, 1, 324–328. [Google Scholar] [CrossRef]
- Hoye, T.R.; Dvornikovs, V.; Fine, J.M.; Anderson, K.R.; Jeffrey, C.S.; Muddiman, D.C.; Shao, F.; Sorensen, P.W.; Wang, J. Details of the structure determination of the sulfated steroids PSDS and PADS: New components of the sea lamprey (Petromyzon marinus) migratory pheromone. J. Org. Chem. 2007, 72, 7544–7550. [Google Scholar] [CrossRef]
- Sorensen, P.W.; Hoye, T.R. A critical review of the discovery and application of a migratory pheromone in an invasive fish, the sea lamprey Petromyzon marinus L. J. Fish. Biol. 2007, 71, 100–114. [Google Scholar] [CrossRef]
- Meckley, T.D.; Wagner, C.M.; Luehring, M.A. Field evaluation of larval odor and mixtures of synthetic pheromone components for attracting migrating sea lampreys in rivers. J. Chem. Ecol. 2012, 38, 1062–1069. [Google Scholar] [CrossRef]
- Li, K.; Brant, C.O.; Huertas, M.; Hur, S.K.; Li, W. Petromyzonin, a hexahydrophenanthrene sulfate isolated from the larval sea lamprey (Petromyzon marinus L.). Org. Lett. 2013, 15, 5924–5927. [Google Scholar] [CrossRef]
- Li, K.; Huertas, M.; Brant, C.O.; Chung-Davidson, Y.W.; Bussy, U.; Hoye, T.R.; Li, W. (+)- and (−)-petromyroxols: Antipodal tetrahydrofurandiols from larval sea lamprey (Petromyzon marinus L.) that elicit enantioselective olfactory responses. Org. Lett. 2015, 17, 286–289. [Google Scholar] [CrossRef]
- Li, K.; Brant, C.O.; Bussy, U.; Pinnamaneni, H.; Patel, H.; Hoye, T.R.; Li, W. Iso-petromyroxols: Novel dihydroxylated tetrahydrofuran enantiomers from sea lamprey (Petromyzon marinus). Molecules 2015, 20, 5215–5222. [Google Scholar] [CrossRef]
- Li, K.; Brant, C.O.; Huertas, M.; Hessler, E.J.; Mezei, G.; Scott, A.M.; Hoye, T.R.; Li, W. Fatty-acid derivative acts as a sea lamprey migratory pheromone. Proc. Natl. Acad. Sci. USA 2018, 115, 8603–8608. [Google Scholar] [CrossRef]
- Johnson, N.S.; Buchinger, T.J.; Li, W. Reproductive ecology of lampreys. In Lampreys: Biology, Conservation and Control; Docker, M.F., Ed.; Springer: Dordrecht, The Netherlands, 2015; Volume 37, pp. 265–303. [Google Scholar] [CrossRef]
- Renaud, C.B. Lampreys of the World. An Annotated and Illustrated Catalogue of Lamprey Species Known to Date; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Buchinger, T.J.; Wang, H.; Li, W.; Johnson, N.S. Evidence for a receiver bias underlying female preference for a male mating pheromone in sea lamprey. Proc. Biol. Sci. 2013, 280, 20131966. [Google Scholar] [CrossRef] [PubMed]
- Gaudron, S.M.; Lucas, M.C. First evidence of attraction of adult river lamprey in the migratory phase to larval odour. J. Fish. Biol. 2006, 68, 640–644. [Google Scholar] [CrossRef]
- Yun, S.S.; Wildbill, A.J.; Siefkes, M.J.; Moser, M.L.; Dittman, A.H.; Corbett, S.C.; Li, W.; Close, D.A. Identification of putative migratory pheromones from Pacific lamprey (Lampetra tridentata). Can. J. Fish. Aquat. Sci. 2011, 68, 2194–2203. [Google Scholar] [CrossRef]
- Ferrari, M.C.O.; Wisenden, B.D.; Chivers, D.P. Chemical ecology of predator–prey interactions in aquatic ecosystems: A review and prospectus. Can. J. Zool. 2010, 88, 698–724. [Google Scholar] [CrossRef]
- Siefkes, M.J. Use of Physiological Knowledge to Control the Invasive Sea Lamprey (Petromyzon marinus) in the Laurentian Great Lakes. Conserv. Physiol. 2017, 5, cox031. [Google Scholar] [CrossRef]
- Surface, H.A. Removal of Lampreys from the Interior Waters of New York; Fourth Annual Report, Commission of Fisheries, Game and Forests of the State of New York; Wynkoop Hallenbeck Crawford Co.: Albany, NY, USA, 1899; pp. 191–245. [Google Scholar]
- Sjöberg, K. Time-related predator/prey interactions between birds and fish in a northern Swedish river. Oecologia 1989, 80, 1–10. [Google Scholar] [CrossRef]
- Hume, J.B.; Wagner, C.M. A death in the family: Sea lamprey (Petromyzon marinus) avoidance of confamilial alarm cues diminishes with phylogenetic distance. Ecol. Evol. 2018, 8, 3751–3762. [Google Scholar] [CrossRef]
- Boulêtreau, S.; Carry, L.; Meyer, E.; Filloux, D.; Menchi, O.; Mataix, V.; Santoul, F. High predation of native sea lamprey during spawning migration. Sci. Rep. 2020, 10, 6122. [Google Scholar] [CrossRef]
- Wagner, C.M.; Bals, J.D.; Byford, G.J.; Scott, A.M.; Feder, M.E. Olfactory sensitivity and threat-sensitive responses to alarm cue in an invasive fish. Biol. Invasions 2023, 25, 3083–3101. [Google Scholar] [CrossRef]
- Pietrzakowski, R.; Imre, I.; Brown, G.E. The behavioural response of migratory sea lamprey (Petromyzon marinus) to potential damage-released larval and migratory chemical alarm cues. J. Great Lakes Res. 2013, 39, 234–238. [Google Scholar] [CrossRef]
- Mensch, E.L.; Dissanayake, A.A.; Nair, M.G.; Wagner, C.M. The effect of putrescine on space use and activity in sea lamprey (Petromyzon marinus). Sci. Rep. 2022, 12, 17400. [Google Scholar] [CrossRef] [PubMed]
- Luhring, T.M.; Meckley, T.D.; Johnson, N.S.; Siefkes, M.J.; Hume, J.B.; Wagner, C.M. A semelparous fish continues upstream migration when exposed to alarm cue, but adjusts movement speed and timing. Anim. Behav. 2016, 121, 41–51. [Google Scholar] [CrossRef]
- Wagner, C.M.; Stroud, E.M.; Meckley, T.D. A deathly odor suggests a new sustainable tool for controlling a costly invasive species. Can. J. Fish. Aquat. Sci. 2011, 68, 1157–1160. [Google Scholar] [CrossRef]
- Bals, J.D.; Wagner, C.M. Behavioral responses of sea lamprey (Petromyzon marinus) to a putative alarm cue derived from conspecific and heterospecific sources. Behaviour 2012, 149, 901–923. [Google Scholar] [CrossRef]
- Hume, J.B.; Meckley, T.D.; Johnson, N.S.; Luhring, T.M.; Siefkes, M.J.; Wagner, C.M. Application of a putative alarm cue hastens the arrival of invasive sea lamprey (Petromyzon marinus) at a trapping location. Can. J. Fish. Aquat. Sci. 2015, 72, 1799–1806. [Google Scholar] [CrossRef]
- Di Rocco, R.T.; Johnson, N.S.; Brege, L.; Imre, I.; Brown, G.E. Sea lamprey avoid areas scented with conspecific tissue extract in Michigan streams. Fish. Manag. Ecol. 2016, 23, 548–560. [Google Scholar] [CrossRef]
- Di Rocco, R.T.; Imre, I.; Johnson, N.S.; Brown, G.E. Behavioural response of adult sea lamprey (Petromyzon marinus) to predator and conspecific alarm cues: Evidence of additive effects. Hydrobiologia 2016, 767, 279–287. [Google Scholar] [CrossRef]
- Hume, J.B.; Luhring, T.M.; Wagner, C.M. Push, pull, or push–pull? An alarm cue better guides sea lamprey towards capture devices than a mating pheromone during the reproductive migration. Biol. Invasions 2020, 22, 2129–2142. [Google Scholar] [CrossRef]
- Di Rocco, R.T.; Belanger, C.F.; Imre, I.; Brown, G.E.; Johnson, N.S. Daytime avoidance of chemosensory alarm cues by adult sea lamprey (Petromyzon marinus). Can. J. Fish. Aquat. Sci. 2014, 71, 824–830. [Google Scholar] [CrossRef]
- Cochran, P.A. Predation on lampreys. In Biology, Management, and Conservation of Lampreys in North America, 2009; Brown, L.R., Chase, S.D., Mesa, M.G., Beamish, R.J., Moyle, P.B., Eds.; Symposium 72; American Fisheries Society: Bethesda, MD, USA, 2009; pp. 139–151. [Google Scholar]
- Byford, G.J.; Wagner, C.M.; Hume, J.B.; Moser, M.L. Do native Pacific lamprey and invasive sea lamprey share an alarm cue? Implications for use of a natural repellent to guide imperiled Pacific lamprey into fishways. N. Am. J. Fish. Manag. 2016, 36, 1090–1096. [Google Scholar] [CrossRef]
- Imre, I.; Di Rocco, R.T.; Belanger, C.F.; Brown, G.E.; Johnson, N.S. The behavioural response of adult Petromyzon marinus to damage-released alarm and predator cues. J. Fish. Biol. 2014, 84, 1490–1502. [Google Scholar] [CrossRef] [PubMed]
- Tilden, J. An account of a singular property of lamprey eels. Mem. Am. Acad. Sci. 1809, 46, 335–336. [Google Scholar]
- Ferrero, D.M.; Lemon, J.K.; Fluegge, D.; Pashkovski, S.L.; Korzan, W.J.; Datta, S.R.; Spehr, M.; Fendt, M.; Liberles, S.D. Detection and avoidance of a carnivore odor by prey. Proc. Natl. Acad. Sci. USA 2011, 108, 11235–11240. [Google Scholar] [CrossRef] [PubMed]
- Imre, I.; Di Rocco, R.T.; Brown, G.E.; Johnson, N.S. Habituation of adult sea lamprey repeatedly exposed to damage-released alarm and predator cues. Environ. Biol. Fish. 2016, 99, 613–620. [Google Scholar] [CrossRef]
- Imre, I.; Di Rocco, R.T.; McClure, H.; Johnson, N.S.; Brown, G.E. Migratory-stage sea lamprey Petromyzon marinus stop responding to conspecific damage-released alarm cues after 4 h of continuous exposure in laboratory conditions. J. Fish. Biol. 2017, 90, 1297–1304. [Google Scholar] [CrossRef]
- Wagner, C.M.; Bals, J.D.; Hanson, M.E.; Scott, A.M. Attenuation and recovery of an avoidance response to a chemical antipredator cue in an invasive fish: Implications for use as a repellent in conservation. Conserv. Physiol. 2022, 10, coac019. [Google Scholar] [CrossRef]
- Mensch, E.L.; Dissanayake, A.A.; Nair, M.G.; Wagner, C.M. Sea lamprey alarm cue comprises water- and chloroform- soluble components. J. Chem. Ecol. 2022, 48, 704–717. [Google Scholar] [CrossRef]
- Dissanayake, A.A.; Wagner, C.M.; Nair, M.G. Chemical characterization of lipophilic constituents in the skin of migratory adult sea lamprey from the Great Lakes region. PLoS ONE 2016, 11, e0168609. [Google Scholar] [CrossRef]
- Dissanayake, A.A.; Wagner, C.M.; Nair, M.G. Nitrogenous compounds characterized in the deterrent skin extract of migratory adult sea lamprey from the Great Lakes region. PLoS ONE 2019, 14, e0217417. [Google Scholar] [CrossRef]
- Cooke, M.; Leeves, N.; White, C. Time profile of putrescine, cadaverine, indole and skatole in human saliva. Arch. Oral Biol. 2003, 48, 323–327. [Google Scholar] [CrossRef]
- Yao, M.; Rosenfeld, J.; Attridge, S.; Sidhu, S.; Aksenov, V.; Rollo, C.D. The ancient chemistry of avoiding risks of predation and disease. Evol. Biol. 2009, 36, 267–281. [Google Scholar] [CrossRef]
- Applegate, V.C. Natural History of the Sea Lamprey, Petromyzon Marinus, in Michigan; United States Department of the Interior Special Scientific Report Fisheries; University of Michigan: Ann Arbor, MI, USA, 1950; Volume 55, pp. 1–237. [Google Scholar]
- Manion, P.J.; Hanson, L.H. Spawning behavior and fecundity of lampreys from the upper three Great Lakes. Can. J. Fish. Aquat. Sci. 1980, 37, 1635–1640. [Google Scholar] [CrossRef]
- Johnson, N.S.; Yun, S.S.; Thompson, H.T.; Brant, C.O.; Li, W. A synthesized pheromone induces upstream movement in female sea lamprey and summons them into traps. Proc. Natl. Acad. Sci. USA 2009, 106, 1021–1026. [Google Scholar] [CrossRef]
- Johnson, N.S.; Yun, S.S.; Buchinger, T.J.; Li, W. Multiple functions of a multi-component mating pheromone in sea lamprey Petromyzon marinus. J. Fish. Biol. 2012, 80, 538–554. [Google Scholar] [CrossRef]
- Buchinger, T.J.; Scott, A.M.; Fissette, S.D.; Brant, C.O.; Huertas, M.; Li, K.; Johnson, N.S.; Li, W. A pheromone antagonist liberates female sea lamprey from a sensory trap to enable reliable communication. Proc. Natl. Acad. Sci. USA 2020, 117, 7284–7289. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sargent, P.A.; Fisher, M.M.; Youson, J.H. Periductal fibrosis and lipocytes (fat-storing cells or Ito cells) during biliary atresia in the lamprey. Hepatology 1986, 6, 54–59. [Google Scholar] [CrossRef]
- Siefkes, M.J.; Scott, A.P.; Zielinski, B.S.; Yun, S.S.; Li, W. Male sea lampreys, Petromyzon marinus L., excrete a sex pheromone from gill epithelia. Biol. Reprod. 2003, 69, 125–132. [Google Scholar] [CrossRef]
- Brant, C.O.; Chung-Davidson, Y.W.; Li, K.; Scott, A.M.; Li, W. Biosynthesis and release of pheromonal bile salts in mature male sea lamprey. BMC Biochem. 2013, 14, 30. [Google Scholar] [CrossRef]
- Tamrakar, S.; Huerta, B.; Chung-Davidson, Y.W.; Li, W. Plasma metabolomic profiles reveal sex- and maturation-dependent metabolic strategies in sea lamprey (Petromyzon marinus). Metabolomics 2022, 18, 90. [Google Scholar] [CrossRef]
- Buchinger, T.J.; Li, K.; Huertas, M.; Baker, C.F.; Jia, L.; Hayes, M.C.; Li, W.; Johnson, N.S. Evidence for partial overlap of male olfactory cues in lampreys. J. Exp. Biol. 2017, 220, 497–506. [Google Scholar] [CrossRef]
- Buchinger, T.J.; Bussy, U.; Li, K.; Jia, L.; Baker, C.F.; Buchinger, E.G.; Zhe, Z.; Johnson, N.S.; Li, W. Intra- and interspecific variation in production of bile acids that act as sex pheromones in lampreys. Physiol. Biochem. Zool. 2019, 92, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Walaszczyk, E.J.; Johnson, N.S.; Steibel, J.P.; Li, W. Effects of sex pheromones and sexual maturation on locomotor activity in female sea lamprey (Petromyzon marinus). J. Biol. Rhythm. 2013, 28, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Walaszczyk, E.J.; Goheen, B.B.; Steibel, J.P.; Li, W. Differential effects of sex pheromone compounds on adult female sea lamprey (Petromyzon marinus) locomotor patterns. J. Biol. Rhythm. 2016, 31, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Fissette, S.D.; Buchinger, T.J.; Tamrakar, S.; Scott, A.M.; Li, W. Sensory trap leads to reliable communication without a shift in nonsexual responses to the model cue. Behav. Ecol. 2024, 35, arae006. [Google Scholar] [CrossRef]
- Siefkes, M.J.; Li, W. Electrophysiological evidence for detection and discrimination of pheromonal bile acids by the olfactory epithelium of female sea lampreys (Petromyzon marinus). J. Comp. Physiol. A 2004, 190, 193–199. [Google Scholar] [CrossRef]
- Johnson, N.S.; Luehring, M.A.; Siefkes, M.J.; Li, W. Mating pheromone reception and induced behavior in ovulating female sea lampreys. N. Am. J. Fish. Manag. 2006, 26, 88–96. [Google Scholar] [CrossRef]
- Scott, A.M.; Johnson, N.S.; Siefkes, M.J.; Li, W. Synergistic behavioral antagonists of a sex pheromone reduce reproduction of invasive sea lamprey. Iscience 2023, 26, 107744. [Google Scholar] [CrossRef]
- Li, W. Olfactory Biology of Adult Sea Lamprey (Petromyzon marinus). Ph.D. Thesis, University of Minnesota, Saint Paul, MN, USA, 1994. [Google Scholar]
- Siefkes, M.J.; Winterstein, S.R.; Li, W. Evidence that 3-keto petromyzonol sulphate specifically attracts ovulating female sea lamprey, Petromyzon marinus. Anim. Behav. 2005, 70, 1037–1045. [Google Scholar] [CrossRef]
- Johnson, N.S.; Lewandoski, S.A.; Alger, B.J.; O’Connor, L.; Bravener, G.; Hrodey, P.J.; Huerta, B.; Barber, J.; Li, W.; Wagner, C.M. Behavioral responses of sea lamprey to varying application rates of a synthesized pheromone in diverse trapping scenarios. J. Chem. Ecol. 2020, 46, 233–249. [Google Scholar] [CrossRef]
- Moore, H.H.; Braem, R.A. Distribution of fishes in US streams tributary to Lake Superior. United States Dep. Inter. Spec. Sci. Rep. Fish. 1965, 516, 1–61. [Google Scholar]
- Hanson, L.H.; Manion, P.J. Chemosterilization of the sea lamprey (Petromyzon marinus). Great Lakes Fish. Comm. Tech. Rep. 1978, 29, 1–15. [Google Scholar]
- Hanson, L.H.; Manion, P.J. Sterility method of pest control and its potential role in an integrated sea lamprey (Petromyzon marinus) control program. Can. J. Fish. Aquat. Sci. 1980, 37, 2108–2117. [Google Scholar] [CrossRef]
- Case, B. Spawning behaviour of the chestnut lamprey (Ichthyomyzon castaneus). J. Fish. Res. Board. Can. 1970, 27, 1872–1874. [Google Scholar] [CrossRef]
- Morman, R.H. Distribution and ecology of lampreys in the lower peninsula of Michigan, 1957–1975. Great Lakes Fish. Comm. Tech. Rep. 1979, 33, 1–59. [Google Scholar]
- Brant, C.O.; Huertas, M.; Li, K.; Li, W. Mixtures of two bile alcohol sulfates function as a proximity pheromone in sea lamprey. PLoS ONE 2016, 11, e0149508. [Google Scholar] [CrossRef]
- Scott, A.M.; Zhang, Z.; Jia, L.; Li, K.; Zhang, Q.; Dexheimer, T.S.; Ellsworth, E.; Ren, J.; Chung-Davidson, Y.W.; Zu, Y.; et al. Spermine in semen of male sea lamprey acts as a sex pheromone. PLoS Biol. 2019, 17, e3000332. [Google Scholar] [CrossRef]
- Luehring, M.A.; Wagner, C.M.; Li, W. The efficacy of two synthesized sea lamprey sex pheromone components as a trap lure when placed in direct competition with natural male odors. Biol. Invasions 2011, 13, 1589–1597. [Google Scholar] [CrossRef]
- Li, K.; Brant, C.O.; Siefkes, M.J.; Kruckman, H.G.; Li, W. Characterization of a novel bile alcohol sulfate released by sexually mature male sea lamprey (Petromyzon marinus). PLoS ONE 2013, 8, e68157. [Google Scholar] [CrossRef]
- Johnson, N.S.; Tix, J.A.; Hlina, B.L.; Wagner, C.M.; Siefkes, M.J.; Wang, H.; Li, W. A sea lamprey (Petromyzon marinus) sex pheromone mixture increases trap catch relative to a single synthesized component in specific environments. J. Chem. Ecol. 2015, 41, 311–321. [Google Scholar] [CrossRef]
- Li, K.; Siefkes, M.J.; Brant, C.O.; Li, W. Isolation and identification of petromyzestrosterol, a polyhydroxysteroid from sexually mature male sea lamprey (Petromyzon marinus L.). Steroids 2012, 77, 806–810. [Google Scholar] [CrossRef]
- Li, K.; Scott, A.M.; Riedy, J.J.; Fissette, S.D.; Middleton, Z.E.; Li, W. Three novel bile alcohols of mature male sea lamprey (Petromyzon marinus) act as chemical cues for conspecifics. J. Chem. Ecol. 2017, 43, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Scott, A.M.; Brant, C.O.; Fissette, S.D.; Riedy, J.J.; Hoye, T.R.; Li, W. Bile salt-like dienones having a novel skeleton or a rare substitution pattern function as chemical cues in adult sea lamprey. Org. Lett. 2017, 19, 4444–4447. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Scott, A.M.; Fissette, S.D.; Buchinger, T.J.; Riedy, J.J.; Li, W. Petromylidenes A(-)C: 2-alkylidene bile salt derivatives isolated from sea lamprey (Petromyzon marinus). Mar. Drugs 2018, 16, 308. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.S.; Yun, S.S.; Li, W. Investigations of novel unsaturated bile salts of male sea lamprey as potential chemical cues. J. Chem. Ecol. 2014, 40, 1152–1160. [Google Scholar] [CrossRef]
- Tabor, C.W.; Tabor, H. Polyamines. Annu. Rev. Biochem. 1984, 53, 749–790. [Google Scholar] [CrossRef]
- Jia, L.; Li, S.; Dai, W.; Guo, L.; Xu, Z.; Scott, A.M.; Zhang, Z.; Ren, J.; Zhang, Q.; Dexheimer, T.S.; et al. Convergent olfactory trace amine-associated receptors detect biogenic polyamines with distinct motifs via a conserved binding site. J. Biol. Chem. 2021, 297, 101268. [Google Scholar] [CrossRef]
- Fissette, S.D.; Buchinger, T.J.; Tamrakar, S.; Li, W. Female sea lamprey use seminal pheromones to discriminate among potential mates. Anim. Behav. 2024, 215, 153–162. [Google Scholar] [CrossRef]
- Fissette, S.D.; Buchinger, T.J.; Wagner, C.M.; Johnson, N.S.; Scott, A.M.; Li, W. Progress towards integrating an understanding of chemical ecology into sea lamprey control. J. Great Lakes Res. 2021, 47, S660–S672. [Google Scholar] [CrossRef]
- Li, K.; Buchinger, T.J.; Li, W. Discovery and characterization of natural products that act as pheromones in fish. Nat. Prod. Rep. 2018, 35, 501–513. [Google Scholar] [CrossRef]
- Johnson, N.S.; Li, W. Understanding behavioral responses of fish to pheromones in natural freshwater environments. J. Comp. Physiol. A 2010, 196, 701–711. [Google Scholar] [CrossRef]
- Chung-Davidson, Y.W.; Wang, H.; Siefkes, M.J.; Bryan, M.B.; Wu, H.; Johnson, N.S.; Li, W. Pheromonal bile acid 3-ketopetromyzonol sulfate primes the neuroendocrine system in sea lamprey. BMC Neurosci. 2013, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Fissette, S.D.; Bussy, U.; Huerta, B.; Buchinger, T.J.; Li, W. Evidence that male sea lamprey increase pheromone release after perceiving a competitor. J. Exp. Biol. 2020, 223, jeb226647. [Google Scholar] [CrossRef] [PubMed]
- Chung-Davidson, Y.W.; Bussy, U.; Fissette, S.D.; Scott, A.M.; Li, W. Bile acid production is life-stage and sex-dependent and affected by primer pheromones in the sea lamprey. J. Exp. Biol. 2021, 224, jeb229476. [Google Scholar] [CrossRef]
- Bryan, M.B.; Scott, A.P.; Li, W. Sex steroids and their receptors in lampreys. Steroids 2008, 73, 1–12. [Google Scholar] [CrossRef]
- Sower, S.A. Landmark discoveries in elucidating the origins of the hypothalamic-pituitary system from the perspective of a basal vertebrate, sea lamprey. Gen. Comp. Endocrinol. 2018, 264, 3–15. [Google Scholar] [CrossRef]
- Kanda, S. Evolution of the regulatory mechanisms for the hypothalamic-pituitary-gonadal axis in vertebrates–hypothesis from a comparative view. Gen. Comp. Endocrinol. 2019, 284, 113075. [Google Scholar] [CrossRef]
- Rekwot, P.I.; Ogwu, D.; Oyedipe, E.O.; Sekoni, V.O. The role of pheromones and biostimulation in animal reproduction. Anim. Reprod. Sci. 2001, 65, 157–170. [Google Scholar] [CrossRef]
- Freamat, M.; Sower, S.A. Integrative neuro-endocrine pathways in the control of reproduction in lamprey: A brief review. Front. Endocrinol. 2013, 4, 151. [Google Scholar] [CrossRef]
- Sower, S.A.; Freamat, M.; Kavanaugh, S.I. The origins of the vertebrate hypothalamic-pituitary-gonadal (HPG) and hypothalamic-pituitary-thyroid (HPT) endocrine systems: New insights from lampreys. Gen. Comp. Endocrinol. 2009, 161, 20–29. [Google Scholar] [CrossRef]
- Sherwood, N.M.; Sower, S.A.; Marshak, D.R.; Fraser, B.A.; Brownstein, M.J. Primary structure of gonadotropin-releasing hormone from lamprey brain. J. Biol. Chem. 1986, 261, 4812–4819. [Google Scholar] [CrossRef]
- Kavanaugh, S.I.; Nozaki, M.; Sower, S.A. Origins of gonadotropin-releasing hormone (GnRH) in vertebrates: Identification of a novel GnRH in a basal vertebrate, the sea lamprey. Endocrinology 2008, 149, 3860–3869. [Google Scholar] [CrossRef] [PubMed]
- Sower, S.A.; Chiang, Y.C.; Lovas, S.; Conlon, J.M. Primary structure and biological activity of a third gonadotropin-releasing hormone from lamprey brain. Endocrinology 1993, 132, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Oztan, N.; Gorbman, A. The hypophysis and hypothalamo-hypophyseal neurosecretory system of larval lampreys and their responses to light. J. Morphol. 1960, 106, 243–261. [Google Scholar] [CrossRef]
- Sterba, G. Distribution of nerve cells with secretory-like granules in Petromyzontes. Nature 1962, 193, 400–401. [Google Scholar] [CrossRef]
- Crim, J.W.; Urano, A.; Gorbman, A. Immunocytochemical studies of luteinizing hormone-releasing hormone in brains of agnathan fishes. I. Comparisons of adult Pacific lamprey (Entosphenus tridentata) and the Pacific hagfish (Eptatretus stouti). Gen. Comp. Endocrinol. 1979, 37, 294–305. [Google Scholar] [CrossRef]
- Crim, J.W.; Urano, A.; Gorbman, A. Immunocytochemical studies of luteinizing hormone-releasing hormone in brains of agnathan fishes. II. Patterns of immunoreactivity in larval and maturing Western brook lamprey (Lampetra richardsoni). Gen. Comp. Endocrinol. 1979, 38, 290–299. [Google Scholar] [CrossRef]
- Nozaki, M.; Kobayashi, H. Distribution of LHRH-like substance in the vertebrate brain as revealed by immunohistochemistry. Arch. Histol. Jpn. 1979, 42, 201–219. [Google Scholar] [CrossRef]
- Kim, Y.S.; Stumpf, W.E.; Reid, F.A.; Sar, M.; Selzer, M.E. Estrogen target cells in the forebrain of river lamprey, Ichthyomyzon unicuspis. J. Comp. Neurol. 1980, 191, 607–613. [Google Scholar] [CrossRef]
- Kim, Y.S.; Stumpf, W.E.; Sar, M.; Reid, F.A.; Selzer, M.E.; Epple, A.W. Autoradiographic studies of estrogen target cells in the forebrain of larval lamprey, Petromyzon marinus. Brain Res. 1981, 210, 53–60. [Google Scholar] [CrossRef]
- King, J.C.; Sower, S.A.; Anthony, E.L. Neuronal systems immunoreactive with antiserum to lamprey gonadotropin-releasing hormone in the brain of Petromyzon marinus. Cell Tissue Res. 1988, 253, 1–8. [Google Scholar] [CrossRef]
- Tobet, S.A.; Nozaki, M.; Youson, J.H.; Sower, S.A. Distribution of lamprey gonadotropin-releasing hormone-III (GnRH-III) in brains of larval lampreys (Petromyzon marinus). Cell Tissue Res. 1995, 279, 261–270. [Google Scholar] [CrossRef]
- Nozaki, M.; Ominato, K.; Gorbman, A.; Sower, S.A. The distribution of lamprey GnRH-III in brains of adult sea lampreys (Petromyzon marinus). Gen. Comp. Endocrinol. 2000, 118, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.L.; MacIntyre, J.K.; Tobet, S.A.; Trudeau, V.L.; MacEachern, L.; Rubin, B.S.; Sower, S.A. The spatial relationship of gamma-aminobutyric acid (GABA) neurons and gonadotropin-releasing hormone (GnRH) neurons in larval and adult sea lamprey, Petromyzon marinus. Brain Behav. Evol. 2002, 60, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Root, A.R.; Nucci, N.V.; Sanford, J.D.; Rubin, B.S.; Trudeau, V.L.; Sower, S.A. In situ characterization of gonadotropin- releasing hormone-I, -III, and glutamic acid decarboxylase expression in the brain of the sea lamprey, Petromyzon marinus. Brain Behav. Evol. 2005, 65, 60–70. [Google Scholar] [CrossRef]
- Van Gulick, E.R.; Marquis, T.J.; Sower, S.A. Co-localization of three gonadotropin-releasing hormone transcripts in larval, parasitic, and adult sea lamprey brains. Gen. Comp. Endocrinol. 2018, 264, 84–93. [Google Scholar] [CrossRef]
- Sower, S.A. The reproductive hypothalamic-pituitary axis in lampreys. In Lampreys: Biology, Conservation and Control; Docker, M.F., Ed.; Springer: Dordrecht, The Netherlands, 2015; Volume 1, pp. 305–373. [Google Scholar] [CrossRef]
- Silver, M.R.; Nucci, N.V.; Root, A.R.; Reed, K.L.; Sower, S.A. Cloning and characterization of a functional type II gonadotropin-releasing hormone receptor with a lengthy carboxy-terminal tail from an ancestral vertebrate, the sea lamprey. Endocrinology 2005, 146, 3351–3361. [Google Scholar] [CrossRef]
- Silver, M.R.; Sower, S.A. Functional characterization and kinetic studies of an ancestral lamprey GnRH-III selective type II GnRH receptor from the sea lamprey, Petromyzon marinus. J. Mol. Endocrinol. 2006, 36, 601–610. [Google Scholar] [CrossRef]
- Joseph, N.T.; Aquilina-Beck, A.; MacDonald, C.; Decatur, W.A.; Hall, J.A.; Kavanaugh, S.I.; Sower, S.A. Molecular cloning and pharmacological characterization of two novel GnRH receptors in the lamprey (Petromyzon marinus). Endocrinology 2012, 153, 3345–3356. [Google Scholar] [CrossRef]
- Sower, S.A.; Decatur, W.A.; Joseph, N.T.; Freamat, M. Evolution of vertebrate GnRH receptors from the perspective of a basal vertebrate. Front. Endocrinol. 2012, 3, 140. [Google Scholar] [CrossRef]
- Knox, C.J.; Boyd, S.K.; Sower, S.A. Characterization and localization of gonadotropin-releasing hormone receptors in the adult female sea lamprey, Petromyzon marinus. Endocrinology 1994, 134, 492–498. [Google Scholar] [CrossRef]
- Hall, J.A.; Decatur, W.A.; Daukss, D.M.; Hayes, M.K.; Marquis, T.J.; Morin, S.J.; Kelleher, T.F.; Sower, S.A. Expression of three GnRH receptors in specific tissues in male and female sea lampreys Petromyzon marinus at three distinct life stages. Front. Neurosci. 2013, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Gazourian, L.; Deragon, K.L.; Chase, C.F.; Pati, D.; Habibi, H.R.; Sower, S.A. Characteristics of GnRH binding in the gonads and effects of lamprey GnRH-I and -III on reproduction in the adult sea lamprey. Gen. Comp. Endocrinol. 1997, 108, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, H.; Sower, S.A. The dawn and evolution of hormones in the adenohypophysis. Gen. Comp. Endocrinol. 2006, 148, 3–14. [Google Scholar] [CrossRef]
- Sower, S.A.; Moriyama, S.; Kasahara, M.; Takahashi, A.; Nozaki, M.; Uchida, K.; Dahlstrom, J.M.; Kawauchi, H. Identification of sea lamprey GTHbeta-like cDNA and its evolutionary implications. Gen. Comp. Endocrinol. 2006, 148, 22–32. [Google Scholar] [CrossRef]
- Sower, S.A.; Decatur, W.A.; Hausken, K.N.; Marquis, T.J.; Barton, S.L.; Gargan, J.; Freamat, M.; Wilmot, M.; Hollander, L.; Hall, J.A.; et al. Emergence of an ancestral glycoprotein hormone in the pituitary of the sea lamprey, a basal vertebrate. Endocrinology 2015, 156, 3026–3037. [Google Scholar] [CrossRef]
- Chung-Davidson, Y.W.; Wang, H.; Bryan, M.B.; Wu, H.; Johnson, N.S.; Li, W. An anti-steroidogenic inhibitory primer pheromone in male sea lamprey (Petromyzon marinus). Gen. Comp. Endocrinol. 2013, 189, 24–31. [Google Scholar] [CrossRef]
- Chung-Davidson, Y.W.; Bussy, U.; Fissette, S.D.; Huerta, B.; Li, W. Waterborne pheromones modulate gonadotropin-inhibitory hormone levels in sea lamprey (Petromyzon marinus). Gen. Comp. Endocrinol. 2020, 288, 113358. [Google Scholar] [CrossRef]
- Gazourian, L.; Evans, E.L.; Hanson, L.H.; Chase, C.F.; Sower, S.A. The effects of lamprey GnRH-I,-III and analogs on steroidogenesis in the sea lamprey (Petromyzon marinus). Aquaculture 2000, 188, 147–165. [Google Scholar] [CrossRef]
- Sower, S.A. Neuroendocrine control of reproduction in lampreys. Fish. Physiol. Biochem. 1990, 8, 365–374. [Google Scholar] [CrossRef]
- Sower, S.A.; Kawauchi, H. Update: Brain and pituitary hormones of lampreys. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 129, 291–302. [Google Scholar] [CrossRef]
- Sower, S.A. The endocrinology of reproduction in lampreys and applications for male lamprey sterilization. J. Great Lakes Res. 2003, 29, 50–65. [Google Scholar] [CrossRef]
- Youson, J.H.; Sower, S.A. Concentration of gonadotropin-releasing hormone in the brain during metamorphosis in the lamprey, Petromyzon marinus. J. Exp. Zool. 1991, 259, 399–404. [Google Scholar] [CrossRef]
- Sower, S.A.; Dickhoff, W.W.; Gorbman, A.; Rivier, J.E.; Vale, W.W. Ovulatory and steroidal responses in the lamprey following administration of salmon gonadotropin and agonistic and antagonistic analogues of gonadotropin-releasing hormone. Can. J. Zool. 1983, 61, 2653–2659. [Google Scholar] [CrossRef]
- Sower, S.A.; King, J.A.; Millar, R.P.; Sherwood, N.M.; Marshak, D.R. Comparative biological properties of lamprey gonadotropin-releasing hormone in vertebrates. Endocrinology 1987, 120, 773–779. [Google Scholar] [CrossRef]
- Sower, S.A. Effects of lamprey gonadotropin-releasing hormone and analogs on steroidogenesis and spermiation in male sea lampreys. Fish. Physiol. Biochem. 1989, 7, 101–107. [Google Scholar] [CrossRef]
- Deragon, K.L.; Sower, S.A. Effects of lamprey gonadotropin-releasing hormone-III on steroidogenesis and spermiation in male sea lampreys. Gen. Comp. Endocrinol. 1994, 95, 363–367. [Google Scholar] [CrossRef]
- Bryan, M.B.; Scott, A.P.; Cerny, I.; Young, B.A.; Li, W. 15α-hydroxyprogesterone in male sea lampreys, Petromyzon marinus L. Steroids 2004, 69, 473–481. [Google Scholar] [CrossRef]
- Young, B.A.; Bryan, M.B.; Sower, S.A.; Scott, A.P.; Li, W. 15α-hydroxytestosterone induction by GnRH I and GnRH III in Atlantic and Great Lakes sea lamprey (Petromyzon marinus L.). Gen. Comp. Endocrinol. 2004, 136, 276–281. [Google Scholar] [CrossRef]
- Bryan, M.B.; Young, B.A.; Close, D.A.; Semeyn, J.; Robinson, T.C.; Bayer, J.M.; Li, W. Comparison of synthesis of 15α-hydroxylated steroids in males of four North American lamprey species. Gen. Comp. Endocrinol. 2006, 146, 149–156. [Google Scholar] [CrossRef]
- Young, B.A.; Bryan, M.B.; Glenn, J.R.; Yun, S.S.; Scott, A.P.; Li, W. Dose-response relationship of 15α-hydroxylated sex steroids to gonadotropin-releasing hormones and pituitary extract in male sea lampreys (Petromyzon marinus). Gen. Comp. Endocrinol. 2007, 151, 108–115. [Google Scholar] [CrossRef]
- Sower, S.A.; Balz, E.; Aquilina-Beck, A.; Kavanaugh, S.I. Seasonal changes of brain GnRH-I, -II, and -III during the final reproductive period in adult male and female sea lamprey. Gen. Comp. Endocrinol. 2011, 170, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Kime, D.E. ‘Classical’ and ‘non-classical’ reproductive steroids in fish. Rev. Fish. Biol. Fish. 1993, 3, 160–180. [Google Scholar] [CrossRef]
- Tokarz, J.; Moller, G.; Hrabe de Angelis, M.; Adamski, J. Steroids in teleost fishes: A functional point of view. Steroids 2015, 103, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Katz, Y.; Dashow, L.; Epple, A.W. Circulating steroid hormones of anadromous sea lampreys under various experimental conditions. Gen. Comp. Endocrinol. 1982, 48, 261–268. [Google Scholar] [CrossRef]
- Sower, S.A.; Plisetskaya, E.; Gorbman, A. Changes in plasma steroid and thyroid hormones and insulin during final maturation and spawning of the sea lamprey, Petromyzon marinus. Gen. Comp. Endocrinol. 1985, 58, 259–269. [Google Scholar] [CrossRef]
- Fukayama, S.; Takahashi, H. Changes in serum levels of estradiol-17β and testosterone in the Japanese river lamprey, Lampetra japonica, in the course of sexual maturation. Bull. Fish. Sci. Hokkaido Univ. 1985, 36, 163–169. [Google Scholar]
- Linville, J.E.; Hanson, L.H.; Sower, S.A. Endocrine events associated with spawning behavior in the sea lamprey (Petromyzon marinus). Horm. Behav. 1987, 21, 105–117. [Google Scholar] [CrossRef]
- Bolduc, T.G.; Sower, S.A. Changes in brain gonadotropin-releasing hormone, plasma estradiol 17-β, and progesterone during the final reproductive cycle of the female sea lamprey, Petromyzon marinus. J. Exp. Zool. 1992, 264, 55–63. [Google Scholar] [CrossRef]
- Botticelli, C.R.; Hisaw, F.L., Jr.; Roth, W.D. Estradiol-17-β, estrone, and progesterone in the ovaries of lamprey (Petromyzon marinus). Proc. Soc. Exp. Biol. Med. 1963, 114, 255–257. [Google Scholar] [CrossRef]
- Weisbart, M.; Youson, J.H. Steroid formation in the larval and parasitic adult sea lamprey, Petromyzon marinus L. Gen. Comp. Endocrinol. 1975, 27, 517–526. [Google Scholar] [CrossRef]
- Weisbart, M.; Dickhoff, W.W.; Gorbman, A.; Idler, D.R. The presence of steroids in the sera of the Pacific hagfish, Eptatretus stouti, and the sea lamprey, Petromyzon marinus. Gen. Comp. Endocrinol. 1980, 41, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Dashow, L.; Katz, Y.; Trachtman, M.S.; Epple, A.W. Plasma steroids in the ammocoete of Petromyzon marinus. Gen. Comp. Endocrinol. 1984, 55, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Bryan, M.B.; Scott, A.P.; Li, W. The sea lamprey (Petromyzon marinus) has a receptor for androstenedione. Biol. Reprod. 2007, 77, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Seiler, K.; Seiler, R.; Ackermann, W.; Claus, R. Estimation of steroids and steroid metabolism in a lower vertebrate (Lampetra planeri Bloch). Acta Zool. 1985, 66, 145–150. [Google Scholar] [CrossRef]
- Mesa, M.G.; Bayer, J.M.; Bryan, M.B.; Sower, S.A. Annual sex steroid and other physiological profiles of Pacific lampreys (Entosphenus tridentatus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 155, 56–63. [Google Scholar] [CrossRef]
- Pickering, A.D. Effects of gonadectomy, oestradiol and testosterone on the migrating river lamprey, Lampetra fluviatilis (L). Gen. Comp. Endocrinol. 1976, 28, 473–480. [Google Scholar] [CrossRef]
- Sower, S.A.; Plisetskaya, E.; Gorbman, A. Steroid and thyroid hormone profiles following a single injection of partly purified salmon gonadotropin or GnRH analogues in male and female sea lamprey. J. Exp. Zool. 1985, 235, 403–408. [Google Scholar] [CrossRef]
- Ho, S.M.; Press, D.; Liang, L.C.; Sower, S.A. Identification of an estrogen receptor in the testis of the sea lamprey, Petromyzon marinus. Gen. Comp. Endocrinol. 1987, 67, 119–125. [Google Scholar] [CrossRef]
- Fahien, C.M.; Sower, S.A. Relationship between brain gonadotropin-releasing hormone and final reproductive period of the adult male sea lamprey, Petromyzon marinus. Gen. Comp. Endocrinol. 1990, 80, 427–437. [Google Scholar] [CrossRef]
- Barannikova, I.A. Features of hormonal regulation of the reproduction of lamprey, Lampetra fluviatilis, during the final period of the sexual cycle. J. Ichthyol. 1995, 35, 184–197. [Google Scholar]
- Mewes, K.R.; Latz, M.; Golla, H.; Fischer, A. Vitellogenin from female and estradiol-stimulated male river lampreys (Lampetra fluviatilis L.). J. Exp. Zool. 2002, 292, 52–72. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.O. Effects of testosterone and oestradiol on gonadectomized and intact male and female river lampreys (Lampetra fluviatilis (L.) Gray). Gen. Comp. Endocrinol. 1974, 24, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Kime, D.E.; Rafter, J.J. Biosynthesis of 15-hydroxylated steroids by gonads of the river lamprey, Lampetra fluviatilis, in vitro. Gen. Comp. Endocrinol. 1981, 44, 69–76. [Google Scholar] [CrossRef]
- Kime, D.E.; Callard, G.V. Formation of 15α-hydroxylated androgens by the testis and other tissues of the sea lamprey, Petromyzon marinus, in vitro. Gen. Comp. Endocrinol. 1982, 46, 267–270. [Google Scholar] [CrossRef]
- Lowartz, S.M.; Petkam, R.; Renaud, R.; Beamish, F.W.; Kime, D.E.; Raeside, J.; Leatherland, J.F. Blood steroid profile and in vitro steroidogenesis by ovarian follicles and testis fragments of adult sea lamprey, Petromyzon marinus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003, 134, 365–376. [Google Scholar] [CrossRef]
- Lowartz, S.M.; Renaud, R.L.; Beamish, F.W.; Leatherland, J.F. Evidence for 15α- and 7α-hydroxylase activity in gonadal tissue of the early-life stages of sea lampreys, Petromyzon marinus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 138, 119–127. [Google Scholar] [CrossRef]
- Bryan, M.B.; Scott, A.P.; Cerny, I.; Yun, S.S.; Li, W. 15α-Hydroxytestosterone produced in vitro and in vivo in the sea lamprey, Petromyzon marinus. Gen. Comp. Endocrinol. 2003, 132, 418–426. [Google Scholar] [CrossRef]
- Kime, D.E.; Larsen, L.O. Effect of gonadectomy and hypophysectomy on plasma steroid levels in male and female lampreys (Lampetra fluviatilis, L.). Gen. Comp. Endocrinol. 1987, 68, 189–196. [Google Scholar] [CrossRef]
- Bryan, M.B.; Chung-Davidson, Y.W.; Ren, J.; Bowman, S.; Scott, A.P.; Huertas, M.; Connolly, M.P.; Li, W. Evidence that progestins play an important role in spermiation and pheromone production in male sea lamprey (Petromyzon marinus). Gen. Comp. Endocrinol. 2015, 212, 17–27. [Google Scholar] [CrossRef]
- Wang, H.; Bussy, U.; Chung-Davidson, Y.W.; Li, W. Ultra-performance liquid chromatography tandem mass spectrometry for simultaneous determination of natural steroid hormones in sea lamprey (Petromyzon marinus) plasma and tissues. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1009, 170–178. [Google Scholar] [CrossRef]
- Chung-Davidson, Y.W.; Bussy, U.; Fissette, S.D.; Li, W. Sex-dependent pheromonal effects on steroid hormone levels in sea lampreys (Petromyzon marinus). Gen. Comp. Endocrinol. 2020, 299, 113608. [Google Scholar] [CrossRef] [PubMed]
- Thornton, J.W. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc. Natl. Acad. Sci. USA 2001, 98, 5671–5676. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.E.; Asnaashari, P.; Chang, D.J.; McDonnell, S. 3D models of lamprey progesterone receptor complexed with progesterone, 7α-hydroxy-progesterone and 15α-hydroxy-progesterone. Steroids 2011, 76, 169–176. [Google Scholar] [CrossRef]
- Yokoi, T.; Ohmichi, M.; Tasaka, K.; Kimura, A.; Kanda, Y.; Hayakawa, J.; Tahara, M.; Hisamoto, K.; Kurachi, H.; Murata, Y. Activation of the luteinizing hormone β promoter by gonadotropin-releasing hormone requires c-Jun NH2-terminal protein kinase. J. Biol. Chem. 2000, 275, 21639–21647. [Google Scholar] [CrossRef]
- Harris, D.; Bonfil, D.; Chuderland, D.; Kraus, S.; Seger, R.; Naor, Z. Activation of MAPK cascades by GnRH: ERK and Jun N-terminal kinase are involved in basal and GnRH-stimulated activity of the glycoprotein hormone LHβ-subunit promoter. Endocrinology 2002, 143, 1018–1025. [Google Scholar] [CrossRef]
- Bonfil, D.; Chuderland, D.; Kraus, S.; Shahbazian, D.; Friedberg, I.; Seger, R.; Naor, Z. Extracellular signal-regulated kinase, Jun N-terminal kinase, p38, and c-Src are involved in gonadotropin-releasing hormone-stimulated activity of the glycoprotein hormone follicle-stimulating hormone β-subunit promoter. Endocrinology 2004, 145, 2228–2244. [Google Scholar] [CrossRef]
- Haisenleder, D.J.; Burger, L.L.; Walsh, H.E.; Stevens, J.; Aylor, K.W.; Shupnik, M.A.; Marshall, J.C. Pulsatile gonadotropin-releasing hormone stimulation of gonadotropin subunit transcription in rat pituitaries: Evidence for the involvement of Jun N-terminal kinase but not p38. Endocrinology 2008, 149, 139–145. [Google Scholar] [CrossRef]
- Burger, L.L.; Haisenleder, D.J.; Aylor, K.W.; Marshall, J.C. Regulation of Lhb and Egr1 gene expression by GNRH pulses in rat pituitaries is both c-Jun N-terminal kinase (JNK)- and extracellular signal-regulated kinase (ERK)-dependent. Biol. Reprod. 2009, 81, 1206–1215. [Google Scholar] [CrossRef]
- Hofmann, A.F.; Hagey, L.R.; Krasowski, M.D. Bile salts of vertebrates: Structural variation and possible evolutionary significance. J. Lipid Res. 2010, 51, 226–246. [Google Scholar] [CrossRef]
- Stieger, B.; Meier, Y.; Meier, P.J. The bile salt export pump. Pflug. Arch. 2007, 453, 611–620. [Google Scholar] [CrossRef]
- Trauner, M.; Boyer, J.L. Bile salt transporters: Molecular characterization, function, and regulation. Physiol. Rev. 2003, 83, 633–671. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.Y.; Lionarons, D.A.; Hagey, L.R.; Soroka, C.J.; Mennone, A.; Boyer, J.L. Adult sea lamprey tolerates biliary atresia by altering bile salt composition and renal excretion. Hepatology 2013, 57, 2418–2426. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, B. The structure of the liver cells during the life cycle of a brook-lamprey (Lampetra zanandreai). Z. Zellforsch. Mik Ana 1965, 67, 297–318. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.Y.; Chung-Davidson, Y.W.; Wang, H.; Li, K.; Li, W. Intestinal synthesis and secretion of bile salts as an adaptation to developmental biliary atresia in the sea lamprey. Proc. Natl. Acad. Sci. USA 2012, 109, 11419–11424. [Google Scholar] [CrossRef]
- Yun, S.S.; Scott, A.P.; Siefkes, M.J.; Li, W. Development and application of an ELISA for a sex pheromone released by the male sea lamprey (Petromyzon marinus L.). Gen. Comp. Endocrinol. 2002, 129, 163–170. [Google Scholar] [CrossRef]
- Lionarons, D.A.; Boyer, J.L.; Cai, S.Y. Evolution of substrate specificity for the bile salt transporter ASBT (SLC10A2). J. Lipid Res. 2012, 53, 1535–1542. [Google Scholar] [CrossRef]
- Yun, S.S.; Scott, A.P.; Li, W. Pheromones of the male sea lamprey, Petromyzon marinus L.: Structural studies on a new compound, 3-keto allocholic acid, and 3-keto petromyzonol sulfate. Steroids 2003, 68, 297–304. [Google Scholar] [CrossRef]
- Venkatachalam, K.V. Petromyzonol sulfate and its derivatives: The chemoattractants of the sea lamprey. Bioessays 2005, 27, 222–228. [Google Scholar] [CrossRef]
- Gamage, N.; Barnett, A.; Hempel, N.; Duggleby, R.G.; Windmill, K.F.; Martin, J.L.; McManus, M.E. Human sulfotransferases and their role in chemical metabolism. Toxicol. Sci. 2006, 90, 5–22. [Google Scholar] [CrossRef]
- Siefkes, M.J.; Bergstedt, R.A.; Twohey, M.B.; Li, W. Chemosterilization of male sea lampreys (Petromyzon marinus) does not affect sex pheromone release. Can. J. Fish. Aquat. Sci. 2003, 60, 23–31. [Google Scholar] [CrossRef]
- Tsutsui, K.; Osugi, T.; Son, Y.L.; Ubuka, T. Review: Structure, function and evolution of GnIH. Gen. Comp. Endocrinol. 2018, 264, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, K.; Ubuka, T. Discovery of gonadotropin-inhibitory hormone (GnIH), progress in GnIH research on reproductive physiology and behavior and perspective of GnIH research on neuroendocrine regulation of reproduction. Mol. Cell Endocrinol. 2020, 514, 110914. [Google Scholar] [CrossRef] [PubMed]
- Osugi, T.; Ukena, K.; Sower, S.A.; Kawauchi, H.; Tsutsui, K. Evolutionary origin and divergence of PQRFamide peptides and LPXRFamide peptides in the RFamide peptide family. Insights from novel lamprey RFamide peptides. FEBS J. 2006, 273, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Osugi, T.; Daukss, D.M.; Gazda, K.; Ubuka, T.; Kosugi, T.; Nozaki, M.; Sower, S.A.; Tsutsui, K. Evolutionary origin of the structure and function of gonadotropin-inhibitory hormone: Insights from lampreys. Endocrinology 2012, 153, 2362–2374. [Google Scholar] [CrossRef]
- Tinbergen, N. On aims and methods of ethology. Z. Tierpsychol. 1963, 20, 410–433. [Google Scholar] [CrossRef]
- Rovainen, C.M. Neurobiology of lampreys. Physiol. Rev. 1979, 59, 1007–1077. [Google Scholar] [CrossRef]
- Robertson, B.; Kardamakis, A.A.; Capantini, L.; Pérez-Fernández, J.; Suryanarayana, S.M.; Wallén, P.; Stephenson-Jones, M.; Grillner, S. The lamprey blueprint of the mammalian nervous system. Prog. Brain Res. 2014, 212, 337–349. [Google Scholar] [CrossRef]
- Grillner, S.; Robertson, B. The basal ganglia over 500 million years. Curr. Biol. 2016, 26, R1088–R1100. [Google Scholar] [CrossRef]
- Ryczko, D.; Dubuc, R. Dopamine and the brainstem locomotor networks: From lamprey to human. Front. Neurosci. 2017, 11, 295. [Google Scholar] [CrossRef]
- Grillner, S. Evolution of the vertebrate motor system–from forebrain to spinal cord. Curr. Opin. Neurobiol. 2021, 71, 11–18. [Google Scholar] [CrossRef]
- Suryanarayana, S.M.; Pérez-Fernández, J.; Robertson, B.; Grillner, S. The lamprey forebrain–Evolutionary implications. Brain Behav. Evol. 2022, 96, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayana, S.M.; Robertson, B.; Grillner, S. The neural bases of vertebrate motor behaviour through the lens of evolution. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2022, 377, 20200521. [Google Scholar] [CrossRef] [PubMed]
- Ryczko, D.; Dubuc, R. Dopamine control of downstream motor centers. Curr. Opin. Neurobiol. 2023, 83, 102785. [Google Scholar] [CrossRef]
- Frost-Nylén, J.; Thompson, W.S.; Robertson, B.; Grillner, S. The basal ganglia downstream control of action–an evolutionarily conserved strategy. Curr. Neuropharmacol. 2024, 22, 1419–1430. [Google Scholar] [CrossRef]
- Nieuwenhuys, R. Deuterostome brains: Synopsis and commentary. Brain Res. Bull. 2002, 57, 257–270. [Google Scholar] [CrossRef]
- Kleerekoper, H.; van Erkel, G.A. The olfactory apparatus of Petromyzon marinus L. Can. J. Zool. 1960, 38, 209–223. [Google Scholar] [CrossRef]
- Stoddart, D.M. The Scented Ape: The Biology and Culture of Human Odour; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Nieuwenhuys, R. The brain of the lamprey in a comparative perspective. Ann. N. Y Acad. Sci. 1977, 299, 97–145. [Google Scholar] [CrossRef]
- Beauséjour, P.A.; Zielinski, B.S.; Dubuc, R. Olfactory-induced locomotion in lampreys. Cell Tissue Res. 2022, 387, 13–27. [Google Scholar] [CrossRef]
- Derjean, D.; Moussaddy, A.; Atallah, E.; St-Pierre, M.; Auclair, F.; Chang, S.; Ren, X.; Zielinski, B.S.; Dubuc, R. A novel neural substrate for the transformation of olfactory inputs into motor output. PLoS Biol. 2010, 8, e1000567. [Google Scholar] [CrossRef]
- Scott, W.B. Notes on the development of Petromyzon. J. Morphol. 1896, 1, 253–310. [Google Scholar] [CrossRef]
- Hagelin, L.O.; Johnels, A.G. On the structure and function of the accessory olfactory organ in lampreys. Acta Zool. 1955, 36, 113–125. [Google Scholar] [CrossRef]
- Ren, X.; Chang, S.; Laframboise, A.J.; Green, W.W.; Dubuc, R.; Zielinski, B.S. Projections from the accessory olfactory organ into the medial region of the olfactory bulb in the sea lamprey (Petromyzon marinus): A novel vertebrate sensory structure? J. Comp. Neurol. 2009, 516, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Chung-Davidson, Y.W.; Libants, S.V.; Nanlohy, K.G.; Kiupel, M.; Brown, C.T.; Li, W. The sea lamprey has a primordial accessory olfactory system. BMC Evol. Biol. 2013, 13, 172. [Google Scholar] [CrossRef]
- Buchinger, T.J.; Siefkes, M.J.; Zielinski, B.S.; Brant, C.O.; Li, W. Chemical cues and pheromones in the sea lamprey (Petromyzon marinus). Front. Zool. 2015, 12, 32. [Google Scholar] [CrossRef]
- Tirindelli, R.; Dibattista, M.; Pifferi, S.; Menini, A. From pheromones to behavior. Physiol. Rev. 2009, 89, 921–956. [Google Scholar] [CrossRef]
- Ubeda-Bañon, I.; Pro-Sistiaga, P.; Mohedano-Moriano, A.; Saiz-Sanchez, D.; de la Rosa-Prieto, C.; Gutierrez-Castellanos, N.; Lanuza, E.; Martinez-Garcia, F.; Martinez-Marcos, A. Cladistic analysis of olfactory and vomeronasal systems. Front. Neuroanat. 2011, 5, 3. [Google Scholar] [CrossRef]
- Berghard, A.; Dryer, L. A novel family of ancient vertebrate odorant receptors. J. Neurobiol. 1998, 37, 383–392. [Google Scholar] [CrossRef]
- Freitag, J.; Beck, A.; Ludwig, G.; von Buchholtz, L.; Breer, H. On the origin of the olfactory receptor family: Receptor genes of the jawless fish (Lampetra fluviatilis). Gene 1999, 226, 165–174. [Google Scholar] [CrossRef]
- Libants, S.V.; Carr, K.; Wu, H.; Teeter, J.H.; Chung-Davidson, Y.W.; Zhang, Z.; Wilkerson, C.; Li, W. The sea lamprey Petromyzon marinus genome reveals the early origin of several chemosensory receptor families in the vertebrate lineage. BMC Evol. Biol. 2009, 9, 180. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Dexheimer, T.S.; Ren, J.; Neubig, R.R.; Li, W. Two highly related odorant receptors specifically detect alpha-bile acid pheromones in sea lamprey (Petromyzon marinus). J. Biol. Chem. 2020, 295, 12153–12166. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Nishida, M. Evolution of trace amine associated receptor (TAAR) gene family in vertebrates: Lineage-specific expansions and degradations of a second class of vertebrate chemosensory receptors expressed in the olfactory epithelium. Mol. Biol. Evol. 2007, 24, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Dieris, M.; Kowatschew, D.; Korsching, S.I. Olfactory function in the trace amine-associated receptor family (TAARs) evolved twice independently. Sci. Rep. 2021, 11, 7807. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Dai, W.; Xu, Z.; Liang, Q.; Miller, E.T.; Li, S.; Gao, X.; Baldwin, M.W.; Chai, R.; Li, Q. Evolution of brain-expressed biogenic amine receptors into olfactory trace amine-associated receptors. Mol. Biol. Evol. 2022, 39, msac006. [Google Scholar] [CrossRef] [PubMed]
- Grus, W.E.; Zhang, J. Origin of the genetic components of the vomeronasal system in the common ancestor of all extant vertebrates. Mol. Biol. Evol. 2009, 26, 407–419. [Google Scholar] [CrossRef]
- Kowatschew, D.; Korsching, S.I. Lamprey possess both V1R and V2R olfactory receptors, but only V1Rs are expressed in olfactory sensory neurons. Chem. Senses 2022, 47, bjac007. [Google Scholar] [CrossRef]
- Laframboise, A.J.; Ren, X.; Chang, S.; Dubuc, R.; Zielinski, B.S. Olfactory sensory neurons in the sea lamprey display polymorphisms. Neurosci. Lett. 2007, 414, 277–281. [Google Scholar] [CrossRef]
- Green, W.W.; Boyes, K.C.; McFadden, C.; Daghfous, G.; Auclair, F.; Zhang, H.; Li, W.; Dubuc, R.; Zielinski, B.S. Odorant organization in the olfactory bulb of the sea lamprey. J. Exp. Biol. 2017, 220, 1350–1359. [Google Scholar] [CrossRef]
- Suryanarayana, S.M.; Pérez-Fernández, J.; Robertson, B.; Grillner, S. Olfaction in lamprey pallium revisited—Dual projections of mitral and tufted cells. Cell Rep. 2021, 34, 108596. [Google Scholar] [CrossRef]
- Green, W.W.; Basilious, A.; Dubuc, R.; Zielinski, B.S. The neuroanatomical organization of projection neurons associated with different olfactory bulb pathways in the sea lamprey, Petromyzon marinus. PLoS ONE 2013, 8, e69525. [Google Scholar] [CrossRef]
- Frontini, A.; Zaidi, A.U.; Hua, H.N.; Wolak, T.P.; Greer, C.A.; Kafitz, K.W.; Li, W.; Zielinski, B.S. Glomerular territories in the olfactory bulb from the larval stage of the sea lamprey Petromyzon marinus. J. Comp. Neurol. 2003, 465, 27–37. [Google Scholar] [CrossRef]
- Daghfous, G.; Auclair, F.; Clotten, F.; Létourneau, J.L.; Atallah, E.; Millette, J.P.; Derjean, D.; Robitaille, R.; Zielinski, B.S.; Dubuc, R. GABAergic modulation of olfactomotor transmission in lampreys. PLoS Biol. 2018, 16, e2005512. [Google Scholar] [CrossRef] [PubMed]
- Døving, K.B.; Selset, R. Behavior patterns in cod released by electrical stimulation of olfactory tract bundlets. Science 1980, 207, 559–560. [Google Scholar] [CrossRef] [PubMed]
- Kyle, A.L.; Stacey, N.E.; Peter, R.E. Ventral telencephalic lesions: Effects on bisexual behavior, activity, and olfaction in the male goldfish. Behav. Neural Biol. 1982, 36, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Münz, H.; Claas, B.; Stumpf, W.E.; Jennes, L. Centrifugal innervation of the retina by luteinizing hormone releasing hormone (LHRH)-immunoreactive telencephalic neurons in teleostean fishes. Cell Tissue Res. 1982, 222, 313–323. [Google Scholar] [CrossRef]
- Demski, L.S.; Northcutt, R.G. The terminal nerve: A new chemosensory system in vertebrates? Science 1983, 220, 435–437. [Google Scholar] [CrossRef]
- Stacey, N.E.; Kyle, A.L. Effects of olfactory tract lesions on sexual and feeding behavior in the goldfish. Physiol. Behav. 1983, 30, 621–628. [Google Scholar] [CrossRef]
- Demski, L.S.; Dulka, J.G. Functional-anatomical studies on sperm release evoked by electrical stimulation of the olfactory tract in goldfish. Brain Res. 1984, 291, 241–247. [Google Scholar] [CrossRef]
- von Bartheld, C.S.; Meyer, D.L.; Fiebig, E.; Ebbesson, S.O.E. Central connections of the olfactory bulb in the goldfish, Carassius auratus. Cell Tissue Res. 1984, 238, 475–487. [Google Scholar] [CrossRef]
- Friedrich, R.W.; Korsching, S.I. Chemotopic, combinatorial, and noncombinatorial odorant representations in the olfactory bulb revealed using a voltage-sensitive axon tracer. J. Neurosci. 1998, 18, 9977–9988. [Google Scholar] [CrossRef]
- Hara, T.J.; Zhang, C. Topographic bulbar projections and dual neural pathways of the primary olfactory neurons in salmonid fishes. Neuroscience 1998, 82, 301–313. [Google Scholar] [CrossRef]
- Hamdani, E.H.; Stabell, O.B.; Alexander, G.; Døving, K.B. Alarm reaction in the crucian carp is mediated by the medial bundle of the medial olfactory tract. Chem. Senses 2000, 25, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, E.H.; Kasumyan, A.; Døving, K.B. Is feeding behaviour in crucian carp mediated by the lateral olfactory tract? Chem. Senses 2001, 26, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, E.H.; Alexander, G.; Døving, K.B. Projection of sensory neurons with microvilli to the lateral olfactory tract indicates their participation in feeding behaviour in crucian carp. Chem. Senses 2001, 26, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Nikonov, A.A.; Caprio, J. Electrophysiological evidence for a chemotopy of biologically relevant odors in the olfactory bulb of the channel catfish. J. Neurophysiol. 2001, 86, 1869–1876. [Google Scholar] [CrossRef]
- Hamdani, E.H.; Døving, K.B. The alarm reaction in crucian carp is mediated by olfactory neurons with long dendrites. Chem. Senses 2002, 27, 395–398. [Google Scholar] [CrossRef]
- Hamdani, E.H.; Døving, K.B. Sensitivity and selectivity of neurons in the medial region of the olfactory bulb to skin extract from conspecifics in crucian carp, Carassius carassius. Chem. Senses 2003, 28, 181–189. [Google Scholar] [CrossRef]
- Hansen, A.; Rolen, S.H.; Anderson, K.; Morita, Y.; Caprio, J.; Finger, T.E. Correlation between olfactory receptor cell type and function in the channel catfish. J. Neurosci. 2003, 23, 9328–9339. [Google Scholar] [CrossRef]
- Weltzien, F.A.; Höglund, E.; Hamdani, E.H.; Døving, K.B. Does the lateral bundle of the medial olfactory tract mediate reproductive behavior in male crucian carp? Chem. Senses 2003, 28, 293–300. [Google Scholar] [CrossRef]
- Nikonov, A.A.; Finger, T.E.; Caprio, J. Beyond the olfactory bulb: An odotopic map in the forebrain. Proc. Natl. Acad. Sci. USA 2005, 102, 18688–18693. [Google Scholar] [CrossRef]
- Hamdani, E.H.; Døving, K.B. Specific projection of the sensory crypt cells in the olfactory system in crucian carp, Carassius carassius. Chem. Senses 2006, 31, 63–67. [Google Scholar] [CrossRef]
- Koide, T.; Miyasaka, N.; Morimoto, K.; Asakawa, K.; Urasaki, A.; Kawakami, K.; Yoshihara, Y. Olfactory neural circuitry for attraction to amino acids revealed by transposon-mediated gene trap approach in zebrafish. Proc. Natl. Acad. Sci. USA 2009, 106, 9884–9889. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, N.; Morimoto, K.; Tsubokawa, T.; Higashijima, S.; Okamoto, H.; Yoshihara, Y. From the olfactory bulb to higher brain centers: Genetic visualization of secondary olfactory pathways in zebrafish. J. Neurosci. 2009, 29, 4756–4767. [Google Scholar] [CrossRef]
- Miyasaka, N.; Arganda-Carreras, I.; Wakisaka, N.; Masuda, M.; Sümbül, U.; Seung, H.S.; Yoshihara, Y. Olfactory projectome in the zebrafish forebrain revealed by genetic single-neuron labelling. Nat. Commun. 2014, 5, 3639. [Google Scholar] [CrossRef]
- Eisthen, H.L. Evolution of vertebrate olfactory systems. Brain Behav. Evol. 1997, 50, 222–233. [Google Scholar] [CrossRef]
- Hamdani, E.H.; Døving, K.B. The functional organization of the fish olfactory system. Prog. Neurobiol. 2007, 82, 80–86. [Google Scholar] [CrossRef]
- Kermen, F.; Franco, L.M.; Wyatt, C.; Yaksi, E. Neural circuits mediating olfactory-driven behavior in fish. Front. Neural Circuits 2013, 7, 62. [Google Scholar] [CrossRef]
- Olivares, J.; Schmachtenberg, O. An update on anatomy and function of the teleost olfactory system. PeerJ 2019, 7, e7808. [Google Scholar] [CrossRef]
- Scalia, F.; Winans, S.S. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J. Comp. Neurol. 1975, 161, 31–55. [Google Scholar] [CrossRef]
- Kimchi, T.; Xu, J.; Dulac, C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 2007, 448, 1009–1014. [Google Scholar] [CrossRef]
- Igarashi, K.M.; Ieki, N.; An, M.; Yamaguchi, Y.; Nagayama, S.; Kobayakawa, K.; Kobayakawa, R.; Tanifuji, M.; Sakano, H.; Chen, W.R.; et al. Parallel mitral and tufted cell pathways route distinct odor information to different targets in the olfactory cortex. J. Neurosci. 2012, 32, 7970–7985. [Google Scholar] [CrossRef]
- Shpak, G.; Zylbertal, A.; Yarom, Y.; Wagner, S. Calcium-activated sustained firing responses distinguish accessory from main olfactory bulb mitral cells. J. Neurosci. 2012, 32, 6251–6262. [Google Scholar] [CrossRef] [PubMed]
- Baxi, K.N.; Dorries, K.M.; Eisthen, H.L. Is the vomeronasal system really specialized for detecting pheromones? Trends Neurosci. 2006, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Breer, H.; Fleischer, J.; Strotmann, J. The sense of smell: Multiple olfactory subsystems. Cell Mol. Life Sci. 2006, 63, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Ma, M. Encoding olfactory signals via multiple chemosensory systems. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 463–480. [Google Scholar] [CrossRef]
- Munger, S.D.; Leinders-Zufall, T.; Zufall, F. Subsystem organization of the mammalian sense of smell. Annu. Rev. Physiol. 2009, 71, 115–140. [Google Scholar] [CrossRef]
- Suárez, R.; García-González, D.; de Castro, F. Mutual influences between the main olfactory and vomeronasal systems in development and evolution. Front. Neuroanat. 2012, 6, 50. [Google Scholar] [CrossRef]
- Salazar, I.; Sanchez-Quinteiro, P.; Barrios, A.W.; López Amado, M.; Vega, J.A. Anatomy of the olfactory mucosa. In Handbook of Clinical Neurology; Doty, R.L., Ed.; Elsevier Publishing: Amsterdam, The Netherlands, 2019; Volume 164, pp. 47–65. [Google Scholar] [CrossRef]
- Suh, G.S.; Wong, A.M.; Hergarden, A.C.; Wang, J.W.; Simon, A.F.; Benzer, S.; Axel, R.; Anderson, D.J. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature 2004, 431, 854–859. [Google Scholar] [CrossRef]
- Semmelhack, J.L.; Wang, J.W. Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature 2009, 459, 218–223. [Google Scholar] [CrossRef]
- Riffell, J.A.; Lei, H.; Abrell, L.; Hildebrand, J.G. Neural basis of a pollinator’s buffet: Olfactory specialization and learning in Manduca sexta. Science 2013, 339, 200–204. [Google Scholar] [CrossRef]
- Galizia, C.G.; Rössler, W. Parallel olfactory systems in insects: Anatomy and function. Annu. Rev. Entomol. 2010, 55, 399–420. [Google Scholar] [CrossRef]
- Das Chakraborty, S.; Sachse, S. Olfactory processing in the lateral horn of Drosophila. Cell Tissue Res. 2021, 383, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernández, J.; Stephenson-Jones, M.; Suryanarayana, S.M.; Robertson, B.; Grillner, S. Evolutionarily conserved organization of the dopaminergic system in lamprey: SNc/VTA afferent and efferent connectivity and D2 receptor expression. J. Comp. Neurol. 2014, 522, 3775–3794. [Google Scholar] [CrossRef] [PubMed]
- Beauséjour, P.A.; Veilleux, J.C.; Condamine, S.; Zielinski, B.S.; Dubuc, R. Olfactory projections to locomotor control centers in the sea lamprey. Int. J. Mol. Sci. 2024, 25, 9370. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernández, J.; Kardamakis, A.A.; Suzuki, D.G.; Robertson, B.; Grillner, S. Direct dopaminergic projections from the SNc modulate visuomotor transformation in the lamprey tectum. Neuron 2017, 96, 910–924. [Google Scholar] [CrossRef]
- Baumgarten, H.G. Biogenic monoamines in the cyclostome and lower vertebrate brain. Prog. Histochem. Cyto 1972, 4, 1–90. [Google Scholar] [CrossRef]
- Pombal, M.A.; El Manira, A.; Grillner, S. Afferents of the lamprey striatum with special reference to the dopaminergic system: A combined tracing and immunohistochemical study. J. Comp. Neurol. 1997, 386, 71–91. [Google Scholar] [CrossRef]
- Ericsson, J.; Stephenson-Jones, M.; Pérez-Fernández, J.; Robertson, B.; Silberberg, G.; Grillner, S. Dopamine differentially modulates the excitability of striatal neurons of the direct and indirect pathways in lamprey. J. Neurosci. 2013, 33, 8045–8054. [Google Scholar] [CrossRef]
- Ryczko, D.; Cone, J.J.; Alpert, M.H.; Goetz, L.; Auclair, F.; Dubé, C.; Parent, M.; Roitman, M.F.; Alford, S.; Dubuc, R. A descending dopamine pathway conserved from basal vertebrates to mammals. Proc. Natl. Acad. Sci. USA 2016, 113, E2440–E2449. [Google Scholar] [CrossRef]
- Gariépy, J.F.; Missaghi, K.; Chevallier, S.; Chartré, S.; Robert, M.; Auclair, F.; Lund, J.P.; Dubuc, R. Specific neural substrate linking respiration to locomotion. Proc. Natl. Acad. Sci. USA 2012, 109, E84–E92. [Google Scholar] [CrossRef]
- Ryczko, D.; Grätsch, S.; Auclair, F.; Dubé, C.; Bergeron, S.; Alpert, M.H.; Cone, J.J.; Roitman, M.F.; Alford, S.; Dubuc, R. Forebrain dopamine neurons project down to a brainstem region controlling locomotion. Proc. Natl. Acad. Sci. USA 2013, 110, E3235–E3242. [Google Scholar] [CrossRef]
- Ryczko, D.; Grätsch, S.; Schläger, L.; Keuyalian, A.; Boukhatem, Z.; Garcia, C.; Auclair, F.; Büschges, A.; Dubuc, R. Nigral glutamatergic neurons control the speed of locomotion. J. Neurosci. 2017, 37, 9759–9770. [Google Scholar] [CrossRef] [PubMed]
- Ryczko, D.; Grätsch, S.; Alpert, M.H.; Cone, J.J.; Kasemir, J.; Ruthe, A.; Beauséjour, P.A.; Auclair, F.; Roitman, M.F.; Alford, S.; et al. Descending dopaminergic inputs to reticulospinal neurons promote locomotor movements. J. Neurosci. 2020, 40, 8478–8490. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.H.; Ménard, A.; Pombal, M.A.; Grillner, S. Forebrain dopamine depletion impairs motor behavior in lamprey. Eur. J. Neurosci. 2008, 27, 1452–1460. [Google Scholar] [CrossRef]
- Heier, P. Fundamental principles in the structure of the brain. A study of the brain of Petromyzon fluviatilis. Acta Anat 1948, 8, 3–213. [Google Scholar]
- Northcutt, R.G.; Puzdrowski, R.L. Projections of the olfactory bulb and nervus terminalis in the silver lamprey. Brain Behav. Evol. 1988, 32, 96–107. [Google Scholar] [CrossRef]
- Polenova, O.A.; Vesselkin, N.P. Olfactory and nonolfactory projections in the river lamprey (Lampetra fluviatilis) telencephalon. J. Hirnforsch. 1993, 34, 261–279. [Google Scholar]
- Beauséjour, P.A.; Auclair, F.; Daghfous, G.; Ngovandan, C.; Veilleux, D.; Zielinski, B.S.; Dubuc, R. Dopaminergic modulation of olfactory-evoked motor output in sea lampreys (Petromyzon marinus L.). J. Comp. Neurol. 2020, 528, 114–134. [Google Scholar] [CrossRef]
- Suryanarayana, S.M.; Robertson, B.; Wallén, P.; Grillner, S. The lamprey pallium provides a blueprint of the mammalian layered cortex. Curr. Biol. 2017, 27, 3264–3277e3265. [Google Scholar] [CrossRef]
- Ocaña, F.M.; Suryanarayana, S.M.; Saitoh, K.; Kardamakis, A.A.; Capantini, L.; Robertson, B.; Grillner, S. The lamprey pallium provides a blueprint of the mammalian motor projections from cortex. Curr. Biol. 2015, 25, 413–423. [Google Scholar] [CrossRef]
- Northcutt, R.G.; Wicht, H. Afferent and efferent connections of the lateral and medial pallia of the silver lamprey. Brain Behav. Evol. 1997, 49, 1–19. [Google Scholar] [CrossRef]
- Nieuwenhuys, R.; Nicholson, C. Lampreys, petromyzontoidea. In The Central Nervous System of Vertebrates; Nieuwenhuys, R., ten Donkelaar, H.J., Nicholson, C., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1998; Volume 1, pp. 397–495. [Google Scholar] [CrossRef]
- Lopes, G.; Kampff, A.R. Cortical control: Learning from the lamprey. Curr. Biol. 2015, 25, R203–R205. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayana, S.M.; Pérez-Fernández, J.; Robertson, B.; Grillner, S. The evolutionary origin of visual and somatosensory representation in the vertebrate pallium. Nat. Ecol. Evol. 2020, 4, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Sirota, M.G.; Viana Di Prisco, G.; Dubuc, R. Stimulation of the mesencephalic locomotor region elicits controlled swimming in semi-intact lampreys. Eur. J. Neurosci. 2000, 12, 4081–4092. [Google Scholar] [CrossRef]
- Grätsch, S.; Auclair, F.; Demers, O.; Auguste, E.; Hanna, A.; Büschges, A.; Dubuc, R. A brainstem neural substrate for stopping locomotion. J. Neurosci. 2019, 39, 1044–1057. [Google Scholar] [CrossRef]
- Dubuc, R.; Brocard, F.; Antri, M.; Fénelon, K.; Gariépy, J.F.; Smetana, R.W.; Ménard, A.; Le Ray, D.; Viana Di Prisco, G.; Pearlstein, E.; et al. Initiation of locomotion in lampreys. Brain Res. Rev. 2008, 57, 172–182. [Google Scholar] [CrossRef]
- Le Ray, D.; Juvin, L.; Ryczko, D.; Dubuc, R. Supraspinal control of locomotion: The mesencephalic locomotor region. Prog. Brain Res. 2011, 188, 51–70. [Google Scholar] [CrossRef]
- Ryczko, D.; Dubuc, R. The multifunctional mesencephalic locomotor region. Curr. Pharm. Des. 2013, 19, 4448–4470. [Google Scholar] [CrossRef]
- Grätsch, S.; Büschges, A.; Dubuc, R. Descending control of locomotor circuits. Curr. Opin. Physiol. 2019, 8, 94–98. [Google Scholar] [CrossRef]
- Ryczko, D. The mesencephalic locomotor region: Multiple cell types, multiple behavioral roles, and multiple implications for disease. Neuroscientist 2024, 30, 347–366. [Google Scholar] [CrossRef]
- Noga, B.R.; Whelan, P.J. The mesencephalic locomotor region: Beyond locomotor control. Front. Neural Circuits 2022, 16, 884785. [Google Scholar] [CrossRef]
- Brocard, F.; Dubuc, R. Differential contribution of reticulospinal cells to the control of locomotion induced by the mesencephalic locomotor region. J. Neurophysiol. 2003, 90, 1714–1727. [Google Scholar] [CrossRef] [PubMed]
- Le Ray, D.; Brocard, F.; Bourcier-Lucas, C.; Auclair, F.; Lafaille, P.; Dubuc, R. Nicotinic activation of reticulospinal cells involved in the control of swimming in lampreys. Eur. J. Neurosci. 2003, 17, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Smetana, R.W.; Alford, S.; Dubuc, R. Muscarinic receptor activation elicits sustained, recurring depolarizations in reticulospinal neurons. J. Neurophysiol. 2007, 97, 3181–3192. [Google Scholar] [CrossRef] [PubMed]
- Smetana, R.W.; Juvin, L.; Dubuc, R.; Alford, S. A parallel cholinergic brainstem pathway for enhancing locomotor drive. Nat. Neurosci. 2010, 13, 731–738. [Google Scholar] [CrossRef]
- Drew, T.; Dubuc, R.; Rossignol, S. Discharge patterns of reticulospinal and other reticular neurons in chronic, unrestrained cats walking on a treadmill. J. Neurophysiol. 1986, 55, 375–401. [Google Scholar] [CrossRef]
- Perreault, M.C.; Drew, T.; Rossignol, S. Activity of medullary reticulospinal neurons during fictive locomotion. J. Neurophysiol. 1993, 69, 2232–2247. [Google Scholar] [CrossRef]
- Buchanan, J.T.; Brodin, L.; Dale, N.; Grillner, S. Reticulospinal neurones activate excitatory amino acid receptors. Brain Res. 1987, 408, 321–325. [Google Scholar] [CrossRef]
- Ohta, Y.; Grillner, S. Monosynaptic excitatory amino acid transmission from the posterior rhombencephalic reticular nucleus to spinal neurons involved in the control of locomotion in lamprey. J. Neurophysiol. 1989, 62, 1079–1089. [Google Scholar] [CrossRef]
- Grillner, S.; Wallén, P.; Saitoh, K.; Kozlov, A.; Robertson, B. Neural bases of goal-directed locomotion in vertebrates—An overview. Brain Res. Rev. 2008, 57, 2–12. [Google Scholar] [CrossRef]
- Viana Di Prisco, G.; Pearlstein, E.; Le Ray, D.; Robitaille, R.; Dubuc, R. A cellular mechanism for the transformation of a sensory input into a motor command. J. Neurosci. 2000, 20, 8169–8176. [Google Scholar] [CrossRef]
- Wickelgren, W.O. Physiological and anatomical characteristics of reticulospinal neurones in lamprey. J. Physiol. 1977, 270, 89–114. [Google Scholar] [CrossRef] [PubMed]
- Wickelgren, W.O. Post-tetanic potentiation, habituation and facilitation of synaptic potentials in reticulospinal neurones of lamprey. J. Physiol. 1977, 270, 115–131. [Google Scholar] [CrossRef]
- Stowers, L.; Logan, D.W. Sexual dimorphism in olfactory signaling. Curr. Opin. Neurobiol. 2010, 20, 770–775. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, H.; Yang, L. Transcriptome analysis of zebrafish olfactory epithelium reveal sexual differences in odorant detection. Genes 2020, 11, 592. [Google Scholar] [CrossRef]
- Meléndez-Ferro, M.; Pérez-Costas, E.; Rodríguez-Muñoz, R.; Gómez-López, M.P.; Anadón, R.; Rodicio, M.C. GABA immunoreactivity in the olfactory bulbs of the adult sea lamprey Petromyzon marinus L. Brain Res. 2001, 893, 253–260. [Google Scholar] [CrossRef]
- Pierre, J.; Rio, J.P.; Mahouche, M.; Repérant, J. Catecholamine systems in the brain of cyclostomes, the lamprey, Lampetra fluviatilis. In Phylogeny and Development of Catecholamine Systems in the CNS of Vertebrates; Smeets, W.J.A.J., Reiner, A., Eds.; Cambridge University Press: Cambridge, UK, 1994; pp. 7–19. [Google Scholar]
- Pierre, J.; Mahouche, M.; Suderevskaya, E.I.; Repérant, J.; Ward, R. Immunocytochemical localization of dopamine and its synthetic enzymes in the central nervous system of the lamprey Lampetra fluviatilis. J. Comp. Neurol. 1997, 380, 119–135. [Google Scholar] [CrossRef]
- Pierre-Simons, J.; Repérant, J.; Mahouche, M.; Ward, R. Development of tyrosine hydroxylase-immunoreactive systems in the brain of the larval lamprey Lampetra fluviatilis. J. Comp. Neurol. 2002, 447, 163–176. [Google Scholar] [CrossRef]
- Abalo, X.M.; Villar-Cheda, B.; Anadón, R.; Rodicio, M.C. Development of the dopamine-immunoreactive system in the central nervous system of the sea lamprey. Brain Res. Bull. 2005, 66, 560–564. [Google Scholar] [CrossRef]
- Barreiro-Iglesias, A.; Villar-Cerviño, V.; Anadón, R.; Rodicio, M.C. Dopamine and gamma-aminobutyric acid are colocalized in restricted groups of neurons in the sea lamprey brain: Insights into the early evolution of neurotransmitter colocalization in vertebrates. J. Anat. 2009, 215, 601–610. [Google Scholar] [CrossRef]
- Barreiro-Iglesias, A.; Laramore, C.; Shifman, M.I.; Anadón, R.; Selzer, M.E.; Rodicio, M.C. The sea lamprey tyrosine hydroxylase: cDNA cloning and in situ hybridization study in the brain. Neuroscience 2010, 168, 659–669. [Google Scholar] [CrossRef]
- Fernández-López, B.; Sobrido-Cameán, D.; Anadón, R.; Rodicio, M.C.; Barreiro-Iglesias, A. Restricted co-localization of glutamate and dopamine in neurons of the adult sea lamprey brain. J. Anat. 2017, 231, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernández, J.; Megías, M.; Pombal, M.A. Expression of a novel D4 dopamine receptor in the lamprey brain. Evolutionary considerations about dopamine receptors. Front. Neuroanat. 2015, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernández, J. Characterization of Y and Dopamine Receptors in Lampreys by Using “in situ” Hybridization: An evolutionary approach. Ph.D. Thesis, Universidad de Vigo, Vigo, Spain, 2013. [Google Scholar]
- Pierre, J.; Repérant, J.; Ward, R.; Vesselkin, N.P.; Rio, J.P.; Miceli, D.; Kratskin, I. The serotoninergic system of the brain of the lamprey, Lampetra fluviatilis: An evolutionary perspective. J. Chem. Neuroanat. 1992, 5, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, B.S.; Moretti, N.; Hua, H.N.; Zaidi, A.U.; Bisaillon, A.D. Serotonergic nerve fibers in the primary olfactory pathway of the larval sea lamprey, Petromyzon marinus. J. Comp. Neurol. 2000, 420, 324–334. [Google Scholar] [CrossRef]
- Abalo, X.M.; Villar-Cheda, B.; Meléndez-Ferro, M.; Pérez-Costas, E.; Anadón, R.; Rodicio, M.C. Development of the serotonergic system in the central nervous system of the sea lamprey. J. Chem. Neuroanat. 2007, 34, 29–46. [Google Scholar] [CrossRef]
- Antri, M.; Cyr, A.; Auclair, F.; Dubuc, R. Ontogeny of 5-HT neurons in the brainstem of the lamprey, Petromyzon marinus. J. Comp. Neurol. 2006, 495, 788–800. [Google Scholar] [CrossRef]
- Boyes, K.C. Serotonergic Modulation of Odour-Evoked Neural Activity in the Olfactory Bulb of the Sea Lamprey (Petromyzon marinus). Master’s Thesis, University of Windsor, Windsor, ON, Canada, 2014. Available online: https://scholar.uwindsor.ca/etd/5062 (accessed on 1 July 2025).
- Cornide-Petronio, M.E.; Anadón, R.; Barreiro-Iglesias, A.; Rodicio, M.C. Serotonin 1A receptor (5-HT1A) of the sea lamprey: cDNA cloning and expression in the central nervous system. Brain Struct. Funct. 2013, 218, 1317–1335. [Google Scholar] [CrossRef]
- Chung-Davidson, Y.W.; Wang, H.; Scott, A.M.; Li, W. Pheromone 3kPZS evokes context-dependent serotonin sexual dimorphism in the brain of the sea lamprey (Petromyzon marinus). Integr. Zool. 2015, 10, 91–101. [Google Scholar] [CrossRef]
- Potter, I.C. Ecology of larval and metamorphosing lampreys. Can. J. Fish. Aquat. Sci. 1980, 37, 1641–1657. [Google Scholar] [CrossRef]
- Almeida, P.R.; Paulo-Martins, C.; Andrade, N.O.; Quintella, B.R. Influence of the Light-Dark Cycle in the Diel Activity Rhythms of Sea Lamprey’s Ammocoetes. In Proceedings of the Fifth Conference on Fish Telemetry Held in Europe, Ustica, Italy, 9–13 June 2003; Spedicato, M.T., Lembo, G., Marmulla, G., Eds.; FAO/COISPA: Rome, Italy, 2005; pp. 225–230. [Google Scholar]
- Hansen, M.J.; Madenjian, C.P.; Slade, J.W.; Steeves, T.B.; Almeida, P.R.; Quintella, B.R. Population ecology of the sea lamprey (Petromyzon marinus) as an invasive species in the Laurentian Great Lakes and an imperiled species in Europe. Rev. Fish. Biol. Fish. 2016, 26, 509–535. [Google Scholar] [CrossRef]
- Applegate, V.C. Downstream movement of lampreys and fishes in the Carp Lake River, Michigan. United States Dep. Inter. Spec. Sci. Rep. Fish. 1961, 387, 1–71. [Google Scholar]
- Hanson, L.H.; Swink, W.D. Downstream migration of recently metamorphosed sea lampreys in the Ocqueoc River, Michigan, before and after treatment with lampricides. N. Am. J. Fish. Manag. 1989, 9, 327–331. [Google Scholar] [CrossRef]
- Swink, W.D.; Johnson, N.S. Growth and survival of sea lampreys from metamorphosis to spawning in Lake Huron. Trans. Am. Fish. Soc. 2014, 143, 380–386. [Google Scholar] [CrossRef]
- Miehls, S.M.; Holbrook, C.M.; Marsden, J.E. Diel activity of newly metamorphosed juvenile sea lamprey (Petromyzon marinus). PLoS ONE 2019, 14, e0211687. [Google Scholar] [CrossRef]
- Haro, A.J.; Miehls, S.M.; Johnson, N.S.; Wagner, C.M. Evaluation of visible light as a cue for guiding downstream migrant juvenile sea lamprey. Trans. Am. Fish. Soc. 2020, 149, 635–647. [Google Scholar] [CrossRef]
- Cochran, P.A. The daily timing of lamprey attacks. Environ. Biol. Fish. 1986, 16, 325–329. [Google Scholar] [CrossRef]
- Manion, P.J.; McLain, A.L. Biology of larval sea lampreys (Petromyzon marinus) of the 1960 year class, isolated in the Big Garlic River, Michigan, 1960–1965. Great Lakes Fish. Comm. Tech. Rep. 1971, 16, 1–35. [Google Scholar]
- Kelso, J.R.M.; Gardner, W.M. Emigration, upstream movement, and habitat use by sterile and fertile sea lampreys in three Lake Superior tributaries. N. Am. J. Fish. Manag. 2000, 20, 144–153. [Google Scholar] [CrossRef]
- Almeida, P.R.; Silva, H.T.; Quintella, B.R. The migratory behaviour of the sea lamprey Petromyzon marinus L., observed by acoustic telemetry in the River Mondego (Portugal). In Advances in Fish Telemetry; Moore, A., Russel, I., Eds.; The Centre for Environment, Fisheries and Aquaculture Science: Lowestoft, UK, 2000; pp. 99–108. [Google Scholar]
- Jellyman, D.J.; Glova, G.J.; Sykes, J.R.E. Movements and habitats of adult lamprey (Geotria australis) in two New Zealand waterways. N. Z. J. Mar. Freshw. Res. 2002, 36, 53–65. [Google Scholar] [CrossRef]
- Andrade, N.O.; Quintella, B.R.; Ferreira, J.; Pinela, S.; Póvoa, I.; Pedro, S.; Almeida, P.R. Sea lamprey (Petromyzon marinus L.) spawning migration in the Vouga river basin (Portugal): Poaching impact, preferential resting sites and spawning grounds. Hydrobiologia 2007, 582, 121–132. [Google Scholar] [CrossRef]
- Sjöberg, K. Locomotor activity of river lamprey Lampetra fluviatilis (L.) during the spawning season. Hydrobiologia 1977, 55, 265–270. [Google Scholar] [CrossRef]
- Semlitsch, R.D. Interactions between fish and salamander larvae: Costs of predator avoidance or competition? Oecologia 1987, 72, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Keitt, B.S.; Tershy, B.R.; Croll, D.A. Nocturnal behavior reduces predation pressure on black-vented shearwaters Puffinus opisthomelas. Mar. Ornithol. 2004, 32, 173–178. [Google Scholar] [CrossRef]
- Morita, Y.; Dodt, E. Slow photic responses of the isolated pineal organ of lamprey. Nova Acta Leopold. 1973, 38, 331–339. [Google Scholar]
- Pu, G.A.; Dowling, J.E. Anatomical and physiological characteristics of pineal photoreceptor cell in the larval lamprey, Petromyzon marinus. J. Neurophysiol. 1981, 46, 1018–1038. [Google Scholar] [CrossRef]
- Bolliet, V.; Ali, M.A.; Anctil, M.; Zachmann, A. Melatonin secretion in vitro from the pineal complex of the lamprey Petromyzon marinus. Gen. Comp. Endocrinol. 1993, 89, 101–106. [Google Scholar] [CrossRef]
- Samejima, M.; Shavali, S.; Tamotsu, S.; Uchida, K.; Morita, Y.; Fukuda, A. Light- and temperature-dependence of the melatonin secretion rhythm in the pineal organ of the lamprey, Lampetra japonica. Jpn. J. Physiol. 2000, 50, 437–442. [Google Scholar] [CrossRef]
- Tamotsu, S.; Morita, Y. Photoreception in pineal organs of larval and adult lampreys, Lampetra japonica. J. Comp. Physiol. A 1986, 159, 1–5. [Google Scholar] [CrossRef]
- Morita, Y.; Tabata, M.; Uchida, K.; Samejima, M. Pineal-dependent locomotor activity of lamprey, Lampetra japonica, measured in relation to LD cycle and circadian rhythmicity. J. Comp. Physiol. A 1992, 171, 555–562. [Google Scholar] [CrossRef]
- Puzdrowski, R.L.; Northcutt, R.G. Central projections of the pineal complex in the silver lamprey Ichthyomyzon unicuspis. Cell Tissue Res. 1989, 255, 269–274. [Google Scholar] [CrossRef]
- Yáñez, J.; Anadón, R.; Holmqvist, B.I.; Ekström, P. Neural projections of the pineal organ in the larval sea lamprey (Petromyzon marinus L.) revealed by indocarbocyanine dye tracing. Neurosci. Lett. 1993, 164, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Young, J.Z. The photoreceptors of lampreys I. Light-sensitive fibres in the lateral line nerves. J. Exp. Biol. 1935, 12, 229–238. [Google Scholar] [CrossRef]
- Ullén, F.; Orlovsky, G.N.; Deliagina, T.G.; Grillner, S. Role of dermal photoreceptors and lateral eyes in initiation and orientation of locomotion in lamprey. Behav. Brain Res. 1993, 54, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Deliagina, T.G.; Ullén, F.; Gonzalez, M.J.; Ehrsson, H.; Orlovsky, G.N.; Grillner, S. Initiation of locomotion by lateral line photoreceptors in lamprey: Behavioural and neurophysiological studies. J. Exp. Biol. 1995, 198, 2581–2591. [Google Scholar] [CrossRef]
- Parker, G.H. The stimulation of the integumentary nerves of fishes by light. Am. J. Physiol. 1905, 14, 413–420. [Google Scholar] [CrossRef]
- Binder, T.R.; McDonald, D.G. The role of dermal photoreceptors during the sea lamprey (Petromyzon marinus) spawning migration. J. Comp. Physiol. A 2008, 194, 921–928. [Google Scholar] [CrossRef]
- Binder, T.R.; McDonald, D.G. The role of temperature in controlling diel activity in upstream migrant sea lampreys (Petromyzon marinus). Can. J. Fish. Aquat. Sci. 2008, 65, 1113–1121. [Google Scholar] [CrossRef]
- Binder, T.R.; McLaughlin, R.L.; McDonald, D.G. Relative importance of water temperature, water level, and lunar cycle to migratory activity in spawning-phase sea lampreys in Lake Ontario. Trans. Am. Fish. Soc. 2010, 139, 700–712. [Google Scholar] [CrossRef]
- Dennis III, C.E.; Wright, A.W.; Suski, C.D. Potential for carbon dioxide to act as a non-physical barrier for invasive sea lamprey movement. J. Great Lakes Res. 2016, 42, 150–155. [Google Scholar] [CrossRef]
- Neeson, T.M.; Wiley, M.J.; Adlerstein, S.A.; Riolo, R.L. River network structure shapes interannual feedbacks between adult sea lamprey migration and larval habitation. Ecol. Model. 2011, 222, 3181–3192. [Google Scholar] [CrossRef]
- Bowers, J.M.; Li, C.Y.; Parker, C.G.; Westbrook, M.E.; Juntti, S.A. Pheromone perception in fish: Mechanisms and modulation by internal status. Integr. Comp. Biol. 2023, 63, 407–427. [Google Scholar] [CrossRef] [PubMed]
- Pottinger, T.G.; Moore, A. Characterization of putative steroid receptors in the membrane, cytosol and nuclear fractions from the olfactory tissue of brown and rainbow trout. Fish. Physiol. Biochem. 1997, 16, 45–63. [Google Scholar] [CrossRef]
- Biju, K.C.; Singru, P.S.; Schreibman, M.P.; Subhedar, N. Reproduction phase-related expression of GnRH-like immunoreactivity in the olfactory receptor neurons, their projections to the olfactory bulb and in the nervus terminalis in the female Indian major carp Cirrhinus mrigala (Ham.). Gen. Comp. Endocrinol. 2003, 133, 358–367. [Google Scholar] [CrossRef]
- Onuma, T.; Higa, M.; Ando, H.; Ban, M.; Urano, A. Elevation of gene expression for salmon gonadotropin-releasing hormone in discrete brain loci of prespawning chum salmon during upstream migration. J. Neurobiol. 2005, 63, 126–145. [Google Scholar] [CrossRef]
- Harbott, L.K.; Burmeister, S.S.; White, R.B.; Vagell, M.; Fernald, R.D. Androgen receptors in a cichlid fish, Astatotilapia burtoni: Structure, localization, and expression levels. J. Comp. Neurol. 2007, 504, 57–73. [Google Scholar] [CrossRef]
- Maruska, K.P.; Fernald, R.D. Reproductive status regulates expression of sex steroid and GnRH receptors in the olfactory bulb. Behav. Brain Res. 2010, 213, 208–217. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Hong, W.S.; Liu, D.T.; Qiu, H.T.; Zhu, Y.; Chen, S.X. Involvement of membrane progestin receptor beta (mPRbeta/Paqr8) in sex pheromone progestin-induced expression of luteinizing hormone in the pituitary of male chinese black sleeper (Bostrychus sinensis). Front. Endocrinol. 2018, 9, 397. [Google Scholar] [CrossRef]
- Demski, L.S. The evolution of neuroanatomical substrates of reproductive behavior: Sex steroid and LHRH-specific pathways including the terminal nerve. Am. Zool. 1984, 24, 809–830. [Google Scholar] [CrossRef]
- Moore, F.L.; Muske, L.E.; Propper, C.R. Regulation of reproductive behaviors in amphibians by LHRH. Ann. N. Y Acad. Sci. 1987, 519, 108–116. [Google Scholar] [CrossRef]
- Muske, L.E. Evolution of gonadotropin-releasing hormone (GnRH) neuronal systems. Brain Behav. Evol. 1993, 42, 215–230. [Google Scholar] [CrossRef]
- Kawai, T.; Oka, Y.; Eisthen, H.L. The role of the terminal nerve and GnRH in olfactory system neuromodulation. Zool. Sci. 2009, 26, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Oka, Y. Mechanisms of neuromodulation by a nonhypophysiotropic GnRH system controlling motivation of reproductive behavior in the teleost brain. J. Reprod. Dev. 2011, 57, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Umatani, C.; Oka, Y. Multiple functions of non-hypophysiotropic gonadotropin releasing hormone neurons in vertebrates. Zool. Lett. 2019, 5, 23. [Google Scholar] [CrossRef]
- Moss, R.L. Actions of hypothalamic-hypophysiotropic hormones on the brain. Annu. Rev. Physiol. 1979, 41, 617–631. [Google Scholar] [CrossRef]
- Jennes, L.; Eyigor, O.; Janovick, J.A.; Conn, P.M. Brain gonadotropin releasing hormone receptors: Localization and regulation. Recent. Prog. Horm. Res. 1997, 52, 475–491. [Google Scholar]
- Campbell, R.E.; Coolen, L.M.; Hoffman, G.E.; Hrabovszky, E. Highlights of neuroanatomical discoveries of the mammalian gonadotropin-releasing hormone system. J. Neuroendocr. 2022, 34, e13115. [Google Scholar] [CrossRef]
- Wirsig-Wiechmann, C.R.; Jennes, L. Gonadotropin-releasing hormone agonist binding in tiger salamander nasal cavity. Neurosci. Lett. 1993, 160, 201–204. [Google Scholar] [CrossRef]
- Eisthen, H.L.; Delay, R.J.; Wirsig-Wiechmann, C.R.; Dionne, V.E. Neuromodulatory effects of gonadotropin releasing hormone on olfactory receptor neurons. J. Neurosci. 2000, 20, 3947–3955. [Google Scholar] [CrossRef]
- Kawai, T.; Abe, H.; Akazome, Y.; Oka, Y. Neuromodulatory effect of GnRH on the synaptic transmission of the olfactory bulbar neural circuit in goldfish, Carassius auratus. J. Neurophysiol. 2010, 104, 3540–3550. [Google Scholar] [CrossRef]
- Wirsig-Wiechmann, C.R. Function of gonadotropin-releasing hormone in olfaction. Keio. J. Med. 2001, 50, 81–85. [Google Scholar] [CrossRef]
- Wright, G.M.; McBurney, K.M.; Youson, J.H.; Sower, S.A. Distribution of lamprey gonadotropin-releasing hormone in the brain and pituitary gland of larval, metamorphic, and adult sea lampreys, Petromyzon marinus. Can. J. Zool. 1994, 72, 48–53. [Google Scholar] [CrossRef]
- Youson, J.H.; Heinig, J.A.; Khanam, S.F.; Sower, S.A.; Kawauchi, H.; Keeley, F.W. Patterns of proopiomelanotropin and proopiocortin gene expression and of immunohistochemistry for gonadotropin-releasing hormones (lGnRH-I and III) during the life cycle of a nonparasitic lamprey: Relationship to this adult life history type. Gen. Comp. Endocrinol. 2006, 148, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Tobet, S.A.; Chickering, T.W.; Sower, S.A. Relationship of gonadotropin-releasing hormone (GnRH) neurons to the olfactory system in developing lamprey (Petromyzon marinus). J. Comp. Neurol. 1996, 376, 97–111. [Google Scholar] [CrossRef]
- Eisthen, H.L.; Northcutt, R.G. Silver lampreys (Ichthyomyzon unicuspis) lack a gonadotropin-releasing hormone- and FMRFamide-immunoreactive terminal nerve. J. Comp. Neurol. 1996, 370, 159–172. [Google Scholar] [CrossRef]
- Moore, C.R.; Price, D. The question of sex hormone antagonism. Proc. Soc. Exp. Biol. Med. 1930, 28, 38–40. [Google Scholar] [CrossRef]
- Lamport, H. Periodic changes in blood estrogen. Endocrinology 1940, 27, 673–680. [Google Scholar] [CrossRef]
- Vande Wiele, R.L.; Bogumil, J.; Dyrenfurth, I.; Ferin, M.; Jewelewicz, R.; Warren, M.; Rizkallah, T.; Mikhail, G. Mechanisms regulating the menstrual cycle in women. Recent. Prog. Horm. Res. 1970, 26, 63–103. [Google Scholar] [CrossRef]
- Gill, S.; Lavoie, H.B.; Bo-Abbas, Y.; Hall, J.E. Negative feedback effects of gonadal steroids are preserved with aging in postmenopausal women. J. Clin. Endocrinol. Metab. 2002, 87, 2297–2302. [Google Scholar] [CrossRef][Green Version]
- Shaw, N.D.; Histed, S.N.; Srouji, S.S.; Yang, J.; Lee, H.; Hall, J.E. Estrogen negative feedback on gonadotropin secretion: Evidence for a direct pituitary effect in women. J. Clin. Endocrinol. Metab. 2010, 95, 1955–1961. [Google Scholar] [CrossRef]
- McQuillan, H.J.; Clarkson, J.; Kauff, A.; Han, S.Y.; Yip, S.H.; Cheong, I.; Porteous, R.; Heather, A.K.; Herbison, A.E. Definition of the estrogen negative feedback pathway controlling the GnRH pulse generator in female mice. Nat. Commun. 2022, 13, 7433. [Google Scholar] [CrossRef]
- Money, J. Influence of hormones on sexual behavior. Annu. Rev. Med. 1965, 16, 67–82. [Google Scholar] [CrossRef] [PubMed]
- MacLusky, N.J.; Naftolin, F. Sexual differentiation of the central nervous system. Science 1981, 211, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Neural gonadal steroid actions. Science 1981, 211, 1303–1311. [Google Scholar] [CrossRef]
- Pfaff, D.W.; Vasudevan, N.; Kia, H.K.; Zhu, Y.S.; Chan, J.; Garey, J.; Morgan, M.; Ogawa, S. Estrogens, brain and behavior: Studies in fundamental neurobiology and observations related to women’s health. J. Steroid Biochem. Mol. Biol. 2000, 74, 365–373. [Google Scholar] [CrossRef]
- Pfaff, D.W. Hormone-driven mechanisms in the central nervous system facilitate the analysis of mammalian behaviours. J. Endocrinol. 2005, 184, 447–453. [Google Scholar] [CrossRef]
- Micevych, P.E.; Mermelstein, P.G.; Sinchak, K. Estradiol membrane-initiated signaling in the brain mediates reproduction. Trends Neurosci. 2017, 40, 654–666. [Google Scholar] [CrossRef]
- Oka, Y. Neural control of sexual behavior in fish. Zool. Sci. 2023, 40, 128–140. [Google Scholar] [CrossRef]
- Torres, T.; Adam, N.; Mhaouty-Kodja, S.; Naulé, L. Reproductive function and behaviors: An update on the role of neural estrogen receptors alpha and beta. Front. Endocrinol. 2024, 15, 1408677. [Google Scholar] [CrossRef]
- Inoue, S. Hormonal and circuit mechanisms controlling female sexual behavior. Front. Neural Circuits 2024, 18, 1409349. [Google Scholar] [CrossRef]
- Sower, S.A.; Baron, M.P. The interrelationship of estrogen receptor and GnRH in a basal vertebrate, the sea lamprey. Front. Endocrinol. 2011, 2, 58. [Google Scholar] [CrossRef]
- Morrell, J.I.; Pfaff, D.W. A neuroendocrine approach to brain function: Localization of sex steroid concentrating cells in vertebrate brains. Am. Zool. 1978, 18, 447–460. [Google Scholar] [CrossRef]
- Kim, Y.S.; Stumpf, W.E.; Sar, M.; Christine, M. Estrogen and androgen target cells in the brain of fishes, reptiles and birds: Phylogeny and ontogeny. Am. Zool. 1978, 18, 425–433. [Google Scholar] [CrossRef]
- Balthazart, J.; Ball, G. Brain Aromatase, Estrogens, and Behavior; Oxford University Press: Oxford, UK, 2012. [Google Scholar] [CrossRef]
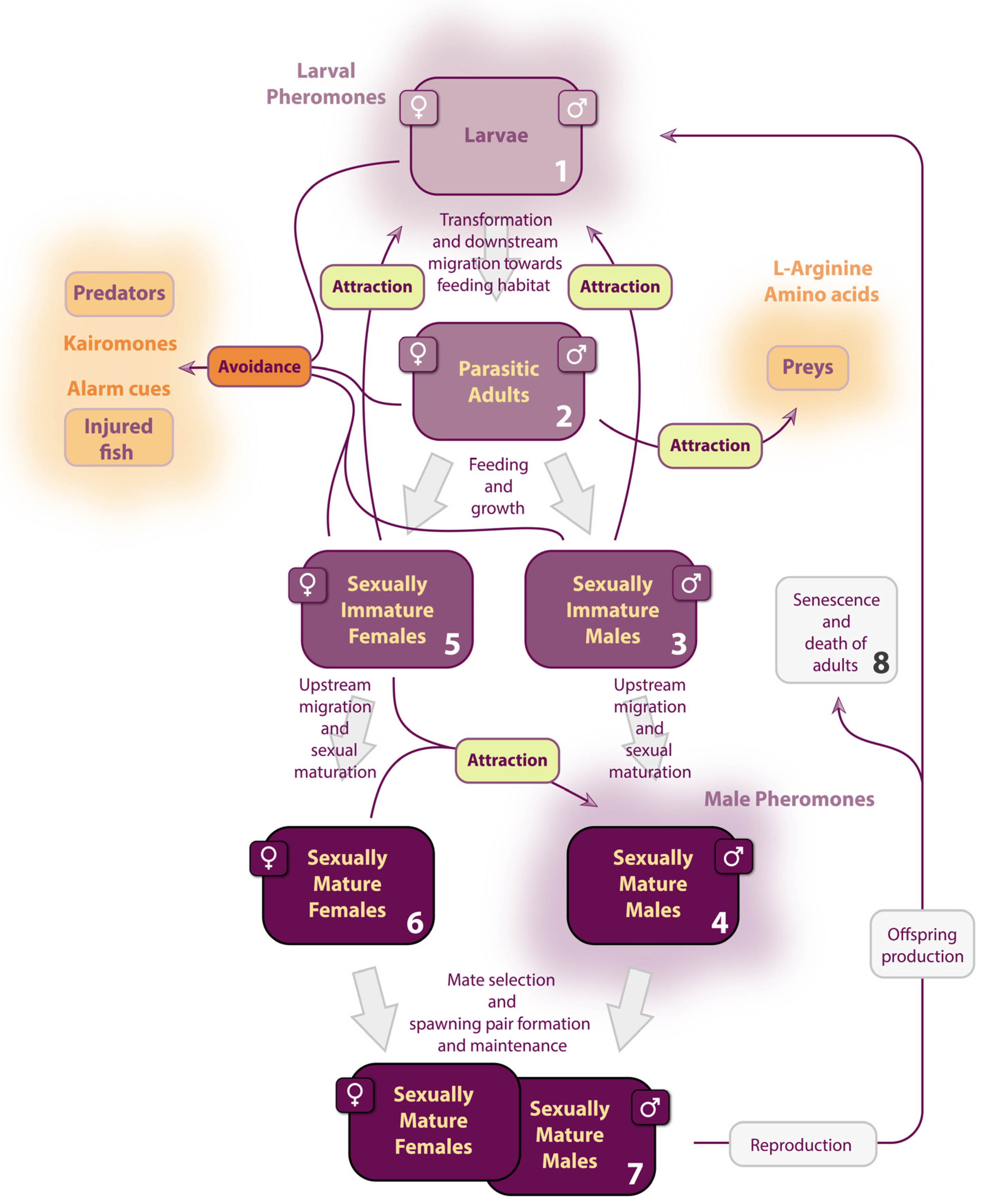
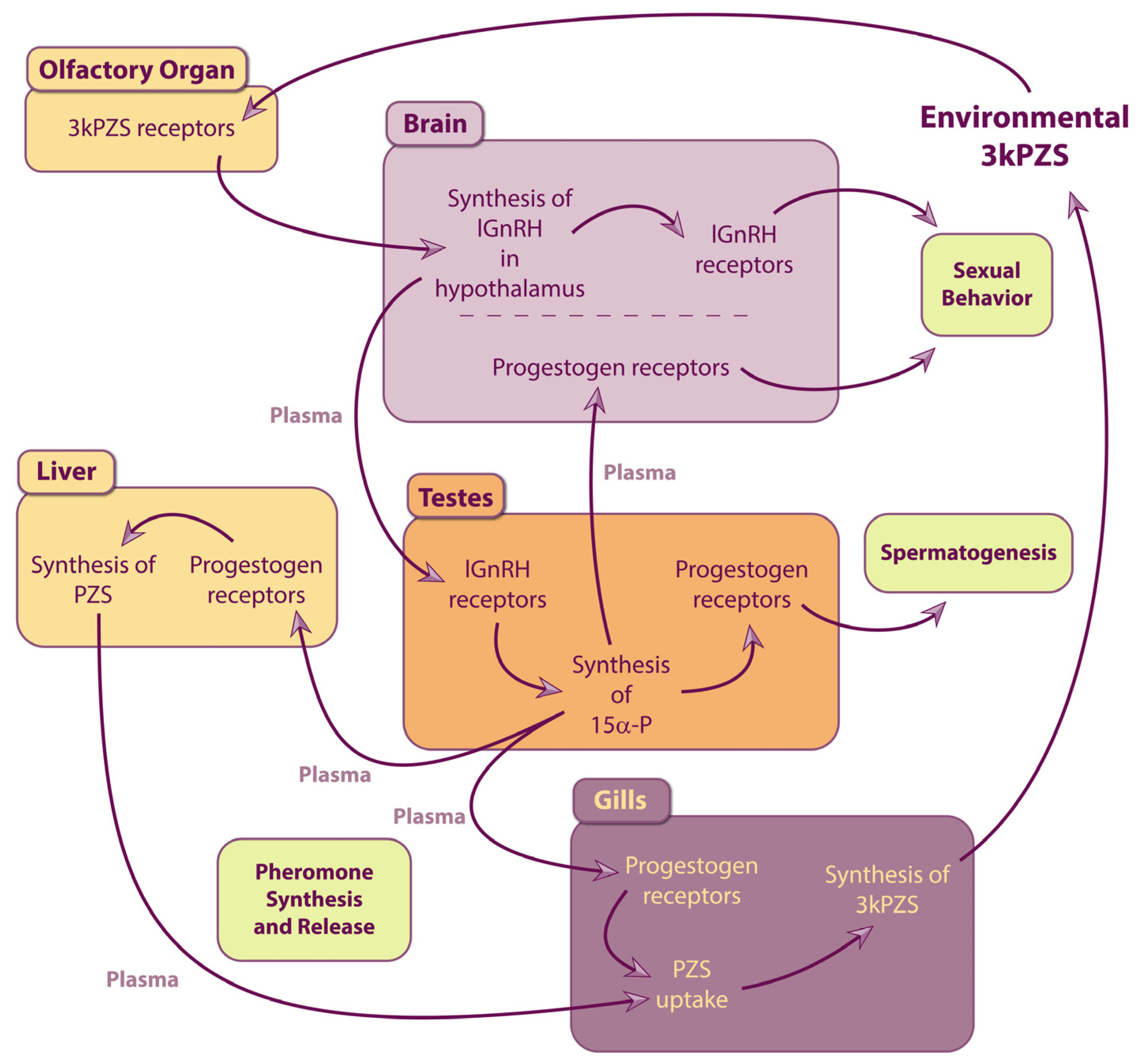

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beauséjour, P.-A.; Zielinski, B.S.; Dubuc, R. Behavioral, Endocrine, and Neuronal Responses to Odors in Lampreys. Animals 2025, 15, 2012. https://doi.org/10.3390/ani15142012
Beauséjour P-A, Zielinski BS, Dubuc R. Behavioral, Endocrine, and Neuronal Responses to Odors in Lampreys. Animals. 2025; 15(14):2012. https://doi.org/10.3390/ani15142012
Chicago/Turabian StyleBeauséjour, Philippe-Antoine, Barbara S. Zielinski, and Réjean Dubuc. 2025. "Behavioral, Endocrine, and Neuronal Responses to Odors in Lampreys" Animals 15, no. 14: 2012. https://doi.org/10.3390/ani15142012
APA StyleBeauséjour, P.-A., Zielinski, B. S., & Dubuc, R. (2025). Behavioral, Endocrine, and Neuronal Responses to Odors in Lampreys. Animals, 15(14), 2012. https://doi.org/10.3390/ani15142012





