Proteomic Analysis of Protein Ubiquitination Events in Dairy Goats with Fatty Liver
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Protein Extraction
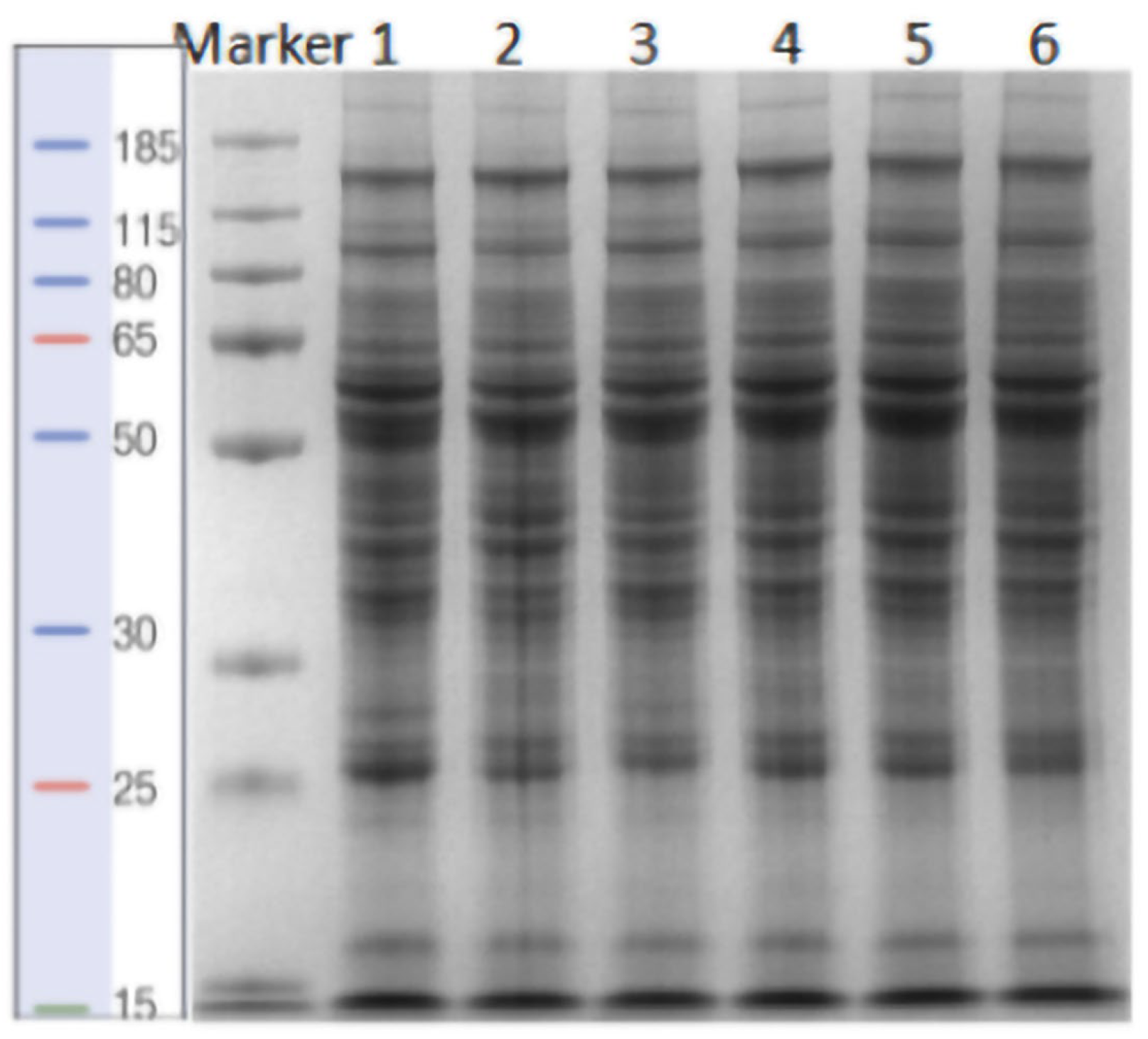
2.3. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.4. Reductive Alkylation and Enzymatic Hydrolysis
2.5. Peptide Desalination and Quantification
2.6. Enrichment of Ubiquitinated Peptides
2.7. LC-MS/MS Data Acquisition
2.8. Database Search
2.9. Bioinformatics and Statistical Analysis
2.10. Histopathological Examination
2.11. Determination of TG Levels in Liver Tissue
2.12. Determination of Non-Esterified Fatty Acids (NEFA) and β-Hydroxybutyric Acid (BHBA) Levels in the Blood
2.13. Quantitative Real-Time PCR Analysis
3. Results
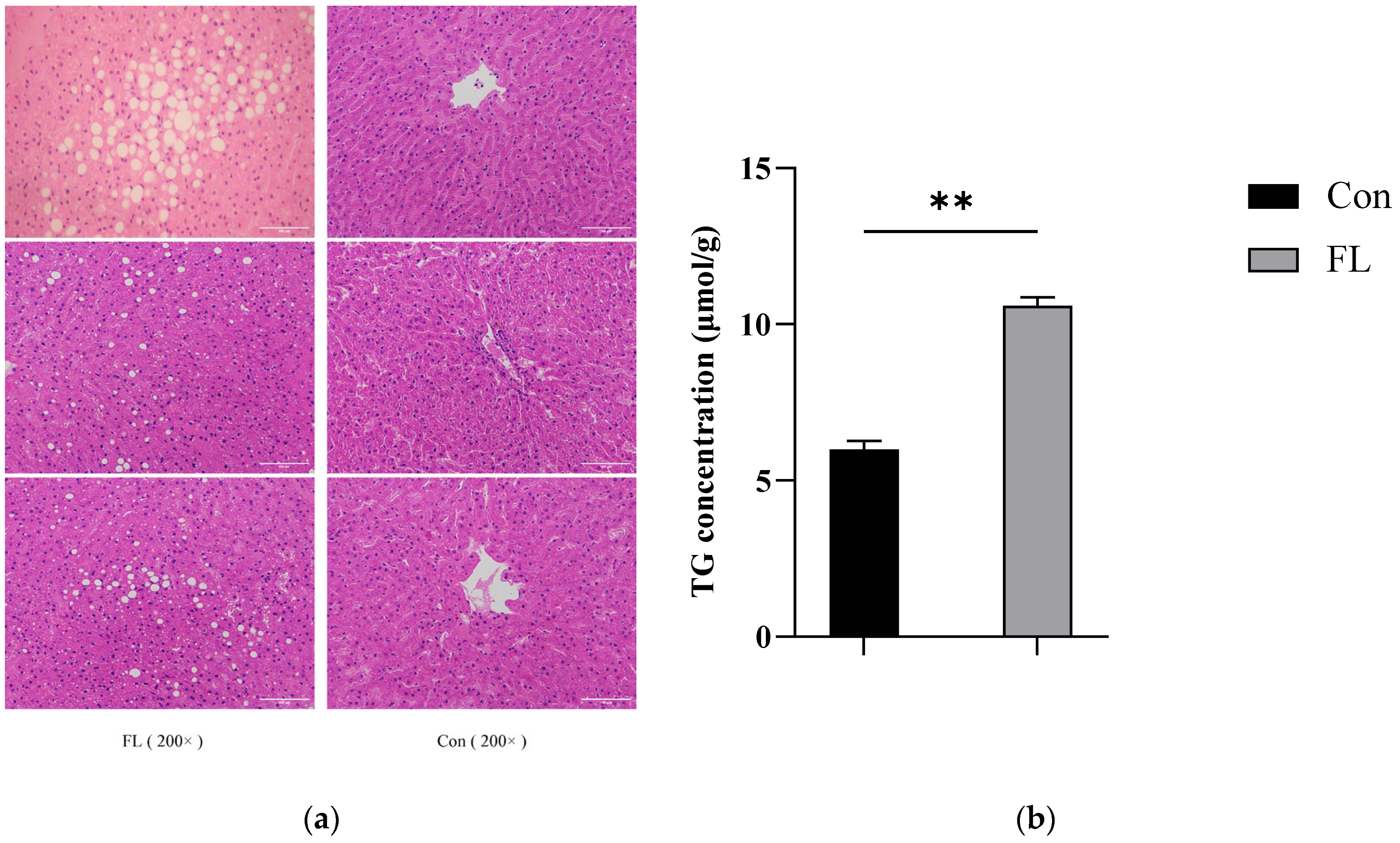
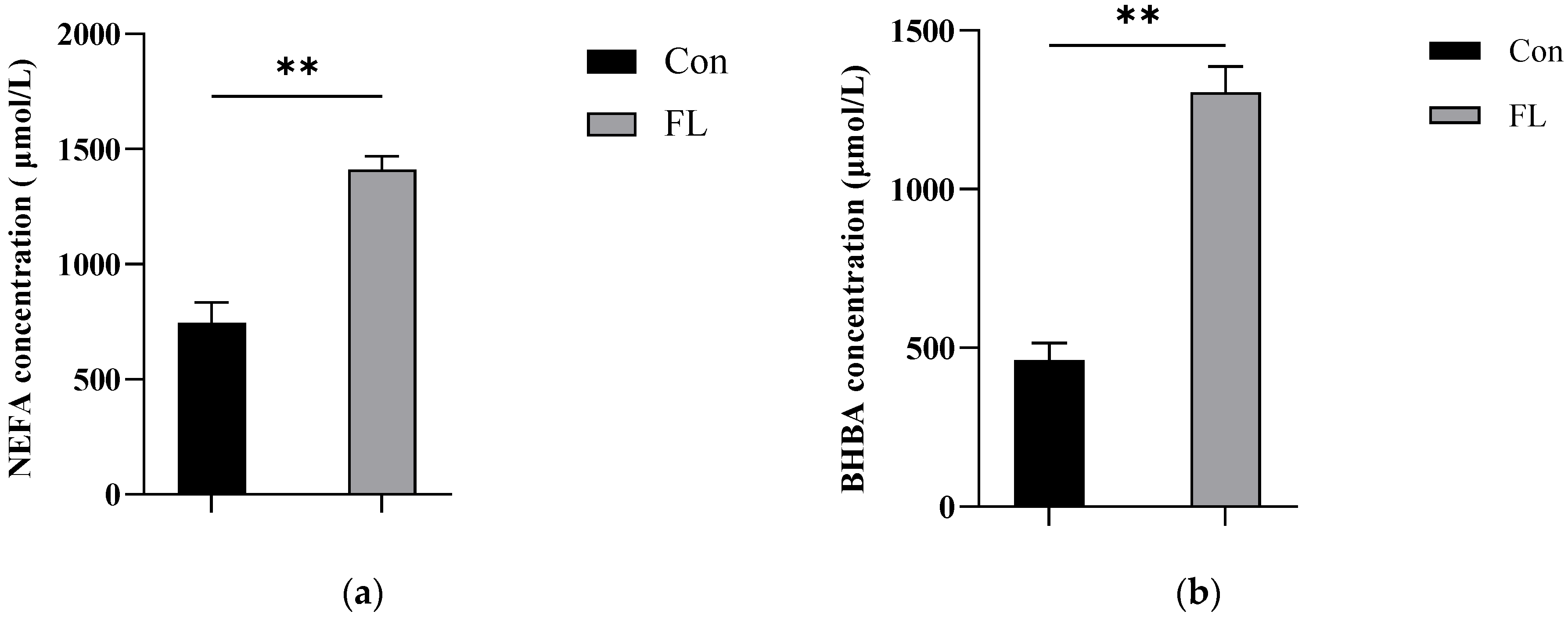
3.1. Histopathological Testing and TG Content Determination
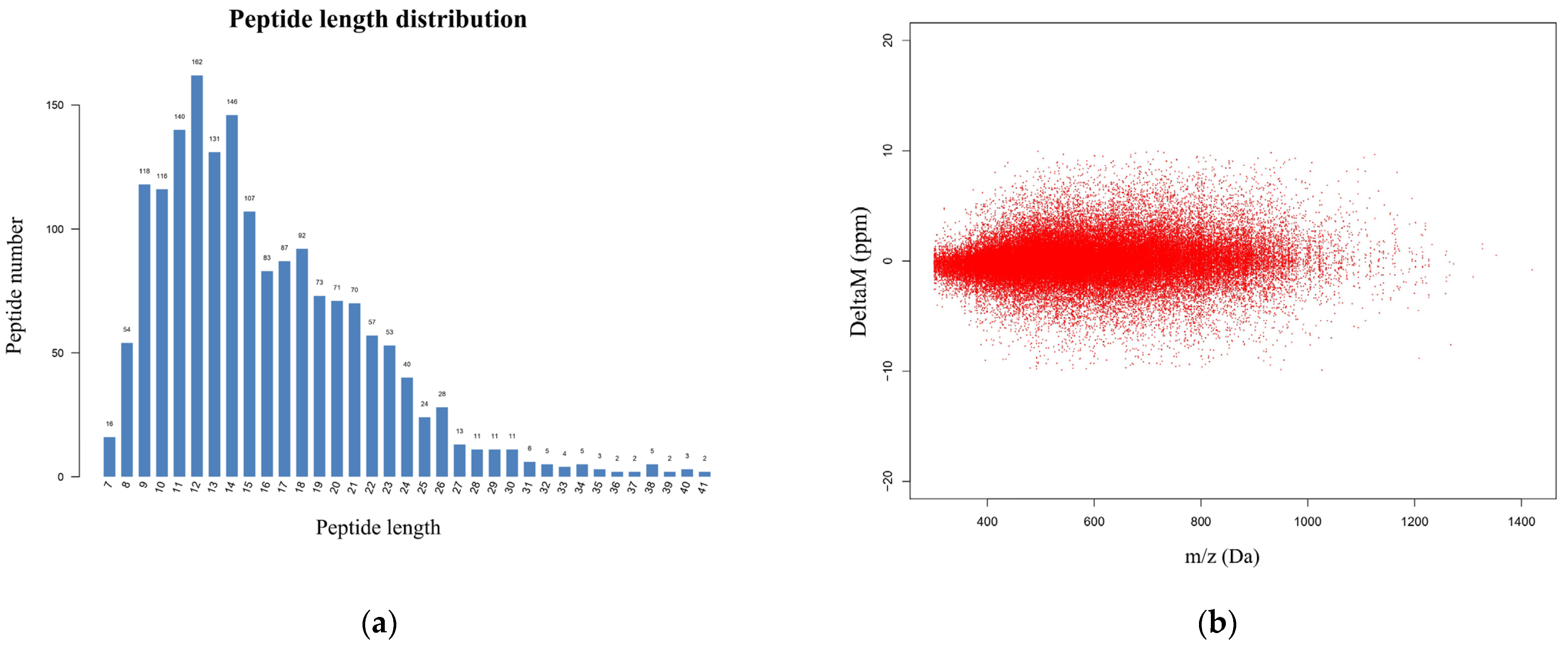
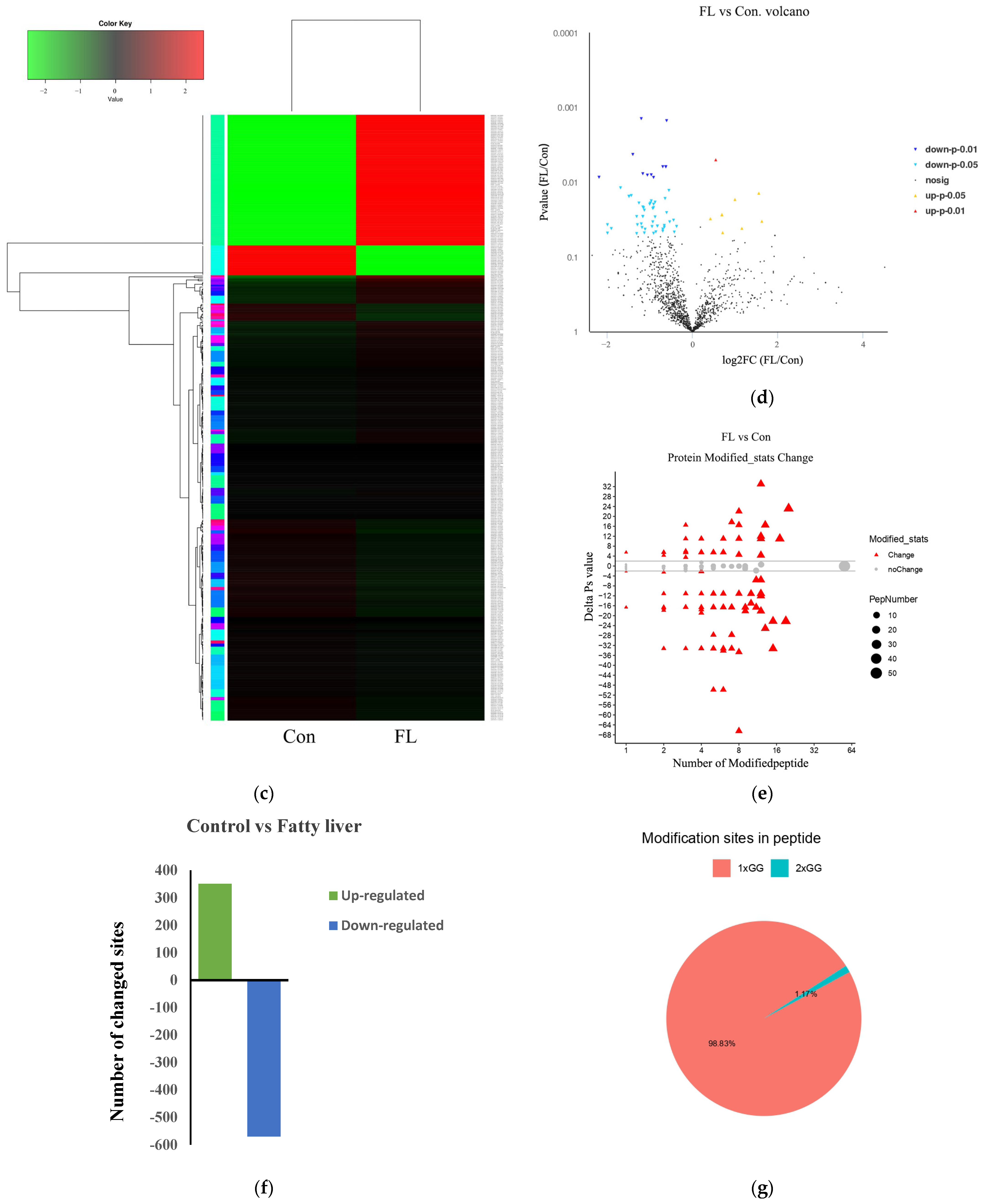
3.2. Identification of Ubiquitin-Modified Proteins
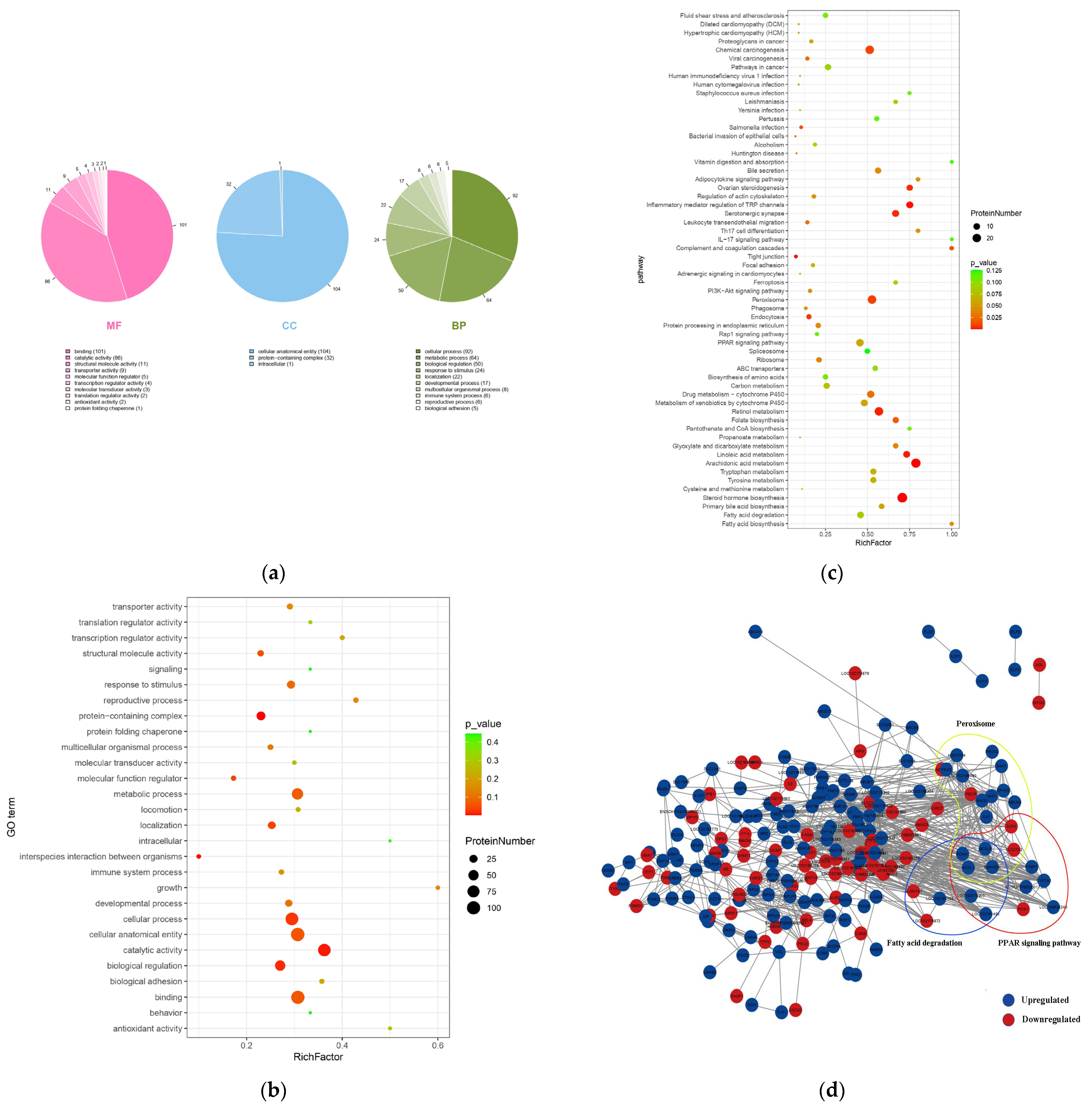
3.3. Functional Enrichment of Ubiquitinated Proteins and Protein–Interaction Networks
3.4. Motif Analysis of the Identified and Quantifiable Proteins
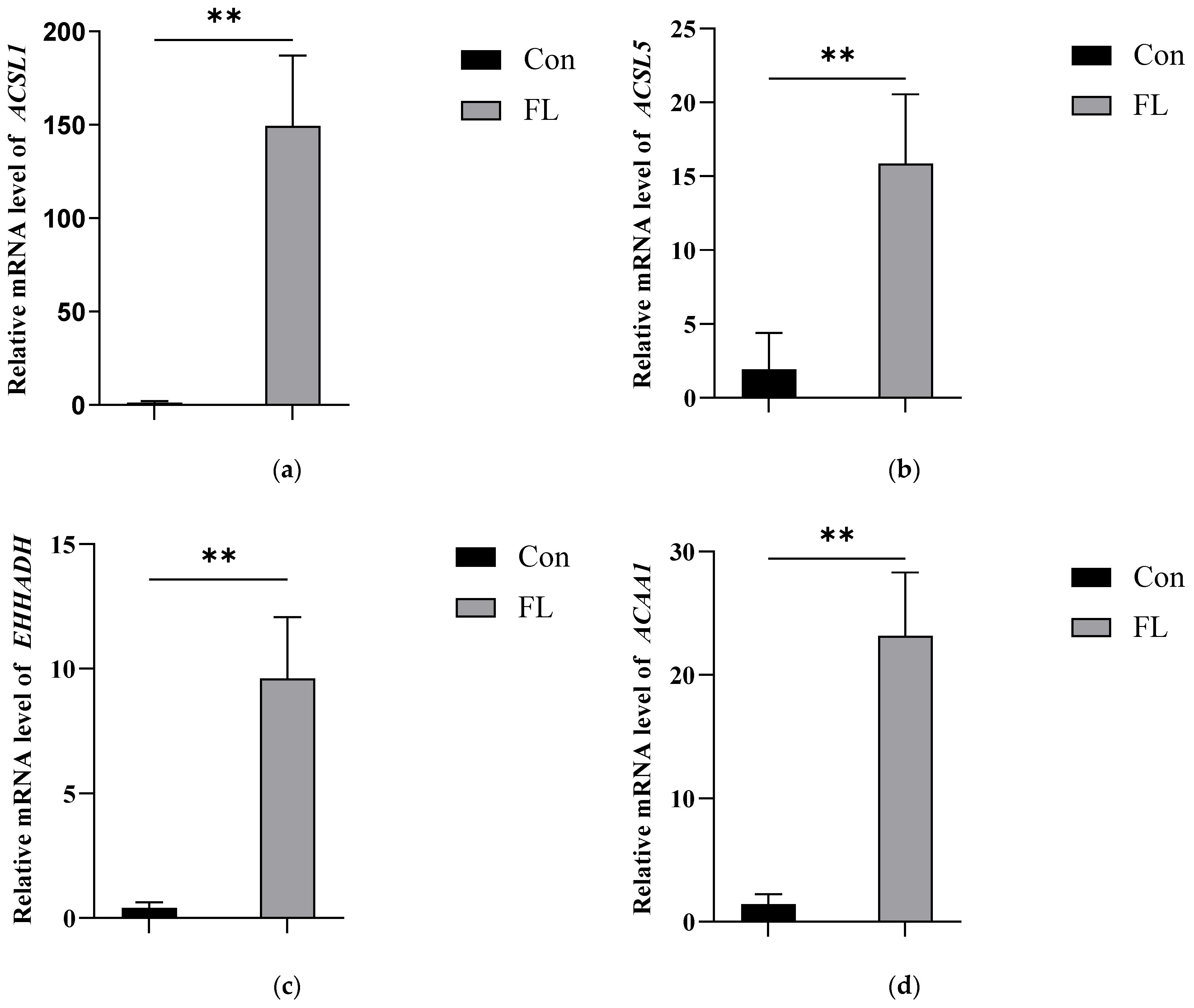
3.5. Further Analysis of Ubiquitination in Hepatic Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clark, S.; Mora García, M.B. A 100-Year Review: Advances in goat milk research. J. Dairy Sci. 2017, 100, 10026–10044. [Google Scholar] [CrossRef]
- Jiang, H.; Gong, H.; Li, Q.; Zhao, L.; Liu, B.; Gao, J.; Mao, X. Differences in proteomic profiles and immunomodulatory activity of goat and cow milk fat globule membrane. Food Chem. 2024, 455, 139885. [Google Scholar] [CrossRef]
- Pichler, M.; Damberger, A.; Schwendenwein, I.; Gasteiner, J.; Drillich, M.; Iwersen, M. Thresholds of whole-blood β-hydroxybutyrate and glucose concentrations measured with an electronic hand-held device to identify ovine hyperketonemia. J. Dairy Sci. 2014, 97, 1388–1399. [Google Scholar] [CrossRef]
- Petre, M.L.; Kontouli Pertesi, A.N.; Boulioglou, O.E.; Sarantidi, E.; Korovesi, A.G.; Kozei, A.; Katsafadou, A.I.; Tsangaris, G.T.; Trichopoulou, A.; Anagnostopoulos, A.K. Bioactive Peptides in Greek Goat Colostrum: Relevance to Human Metabolism. Foods 2024, 13, 3949. [Google Scholar] [CrossRef]
- Li, C.; Han, T.; Zhong, P.; Zhang, Y.; Zhao, T.; Wang, S.; Wang, X.; Tian, Y.; Gong, G.; Liu, Y.; et al. α2,6-linked sialylated oligosaccharides riched in goat milk alleviate food allergy by regulating the gut flora and mucin O-glycosylation. Carbohydr. Polym. 2025, 350, 123049. [Google Scholar] [CrossRef]
- Koo, S.H. Nonalcoholic fatty liver disease: Molecular mechanisms for the hepatic steatosis. Clin. Mol. Hepatol. 2013, 19, 210–215. [Google Scholar] [CrossRef]
- Fu, C.; Liu, L.; Li, F. Acetate alters the process of lipid metabolism in rabbits—ScienceDirect. Animal 2018, 12, 1895–1902. [Google Scholar] [CrossRef]
- Liu, L.; Li, C.; Fu, C.; Li, F. Dietary Niacin Supplementation Suppressed Hepatic Lipid Accumulation in Rabbits. Asian-Australas. J. Anim. Sci. 2016, 29, 1748–1755. [Google Scholar] [CrossRef]
- Liu, L.; Fu, C.; Li, F. Acetate Affects the Process of Lipid Metabolism in Rabbit Liver, Skeletal Muscle and Adipose Tissue. Animals 2019, 9, 799. [Google Scholar] [CrossRef]
- Jiang, F.; Lin, X.; Yan, Z.; Hu, Z.; Wang, Y.; Wang, Z. Effects of forage source and particle size on feed sorting, milk production and nutrient digestibility in lactating dairy cows. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1472–1481. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Yin, Z.Y.; Lin, X.Y.; Yan, Z.G.; Wang, Z.H. Effects of feeding fatty acid calcium and the interaction of forage quality on production performance and biochemical indexes in early lactation cow. J. Anim. Physiol. Anim. Nutr. 2015, 99, 899–904. [Google Scholar] [CrossRef]
- Shi, K.; Niu, F.; Zhang, Q.; Ning, C.; Yue, S.; Hu, C.; Xu, Z.; Wang, S.; Li, R.; Hou, Q.; et al. Identification of Whole-Genome Significant Single Nucleotide Polymorphisms in Candidate Genes Associated With Serum Biochemical Traits in Chinese Holstein Cattle. Front. Genet. 2020, 11, 163. [Google Scholar] [CrossRef]
- White, H. The Role of TCA Cycle Anaplerosis in Ketosis and Fatty Liver in Periparturient Dairy Cows. Animals 2015, 5, 793–802. [Google Scholar] [CrossRef]
- Garcia, M.; Greco, L.F.; Lock, A.L.; Block, E.; Santos, J.E.P.; Thatcher, W.W.; Staples, C.R. Supplementation of essential fatty acids to Holstein calves during late uterine life and first month of life alters hepatic fatty acid profile and gene expression. J. Dairy Sci. 2016, 99, 7085–7101. [Google Scholar] [CrossRef]
- Tharwat, M.; Endoh, D.; Oikawa, S. Hepatocyte apoptosis in dairy cows with fatty infiltration of the liver. Res. Vet. Sci. 2012, 93, 1281–1286. [Google Scholar] [CrossRef]
- Yue, S.J.; Zhao, Y.Q.; Gu, X.R.; Yin, B.; Jiang, Y.L.; Wang, Z.H.; Shi, K.R. A genome-wide association study suggests new candidate genes for milk production traits in Chinese Holstein cattle. Anim. Genet. 2017, 48, 677–681. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, Y.; Lin, Z.; Miao, H. Involvement of the ubiquitin-proteasome system in the regulation of the tumor microenvironment and progression. Genes Dis. 2025, 12, 101240. [Google Scholar] [CrossRef]
- Cockram, P.E.; Kist, M.; Prakash, S.; Chen, S.-H.; Wertz, I.E.; Vucic, D. Ubiquitination in the regulation of inflammatory cell death and cancer. Cell Death Differ. 2021, 28, 591–605. [Google Scholar] [CrossRef]
- Huang, Y.; Kong, Y.; Shen, B.; Li, B.; Loor, J.J.; Tan, P.; Wei, B.; Mei, L.; Zhang, Z.; Zhao, C.; et al. Untargeted metabolomics and lipidomics to assess plasma metabolite changes in dairy goats with subclinical hyperketonemia. J. Dairy Sci. 2023, 106, 3692–3705. [Google Scholar] [CrossRef]
- Nakayama, K.I.; Nakayama, K. Ubiquitin ligases: Cell-cycle control and cancer. Nat. Rev. Cancer 2006, 6, 369–381. [Google Scholar] [CrossRef]
- Vucic, D.; Dixit, V.M.; Wertz, I.E. Ubiquitylation in apoptosis: A post-translational modification at the edge of life and death. Nat. Rev. Mol. Cell Biol. 2011, 12, 439. [Google Scholar] [CrossRef]
- Sadowski, M.; Sarcevic, B. Mechanisms of mono- and poly-ubiquitination: Ubiquitination specificity depends on compatibility between the E2 catalytic core and amino acid residues proximal to the lysine. Cell Div. 2010, 5, 19. [Google Scholar] [CrossRef]
- Badmus, O.O.; Hillhouse, S.A.; Anderson, C.D.; Hinds, T.D.; Stec, D.E. Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): Functional analysis of lipid metabolism pathways. Clin. Sci. 2022, 136, 1347–1366. [Google Scholar] [CrossRef]
- Weller, S.; Gould, S.J.; Valle, D. Peroxisome Biogenesis Disorders. Annu. Rev. Genom. Hum. Genet. 2003, 4, 165–211. [Google Scholar] [CrossRef]
- Pan, Y.; Li, Y.; Fan, H.; Cui, H.; Chen, Z.; Wang, Y.; Jiang, M.; Wang, G. Roles of the peroxisome proliferator-activated receptors (PPARs) in the pathogenesis of hepatocellular carcinoma (HCC). Biomed. Pharmacother. 2024, 177, 117089. [Google Scholar] [CrossRef]
- Hlady, R.A.; Zhao, X.; El Khoury, L.Y.; Wagner, R.T.; Luna, A.; Pham, K.; Pyrosopoulos, N.T.; Jain, D.; Wang, L.; Liu, C.; et al. Epigenetic heterogeneity hotspots in human liver disease progression. Hepatology 2025, 81, 1197–1210. [Google Scholar] [CrossRef]
- Philipp, W.; Karin, N.; Christa, K.; Rosemarie, W. Association of an ACSL1 gene variant with polyunsaturated fatty acids in bovine skeletal muscle. BMC Genet. 2011, 12, 96. [Google Scholar]
- Liang, Y.; Gao, Q.; Zhang, Q.; Arbab, A.A.I.; Li, M.; Yang, Z.; Karrow, N.A.; Mao, Y. Polymorphisms of the ACSL1 Gene Influence Milk Production Traits and Somatic Cell Score in Chinese Holstein Cows. Animals 2020, 10, 2282. [Google Scholar] [CrossRef]
- Beatty, A.; Singh, T.; Tyurina, Y.Y.; Tyurin, V.A.; Samovich, S.; Nicolas, E.; Maslar, K.; Zhou, Y.; Cai, K.Q.; Tan, Y.; et al. Ferroptotic cell death triggered by conjugated linolenic acids is mediated by ACSL1. Nat. Commun. 2021, 12, 2244. [Google Scholar] [CrossRef]
- Zabielski, P.; Imierska, M.; Roszczyc-Owsiejczuk, K.; Kuźmicki, M.; Rogalski, P.; Daniluk, J.; Błachnio-Zabielska, A.U. The Role of Acyl-CoA Synthetase 1 in Bioactive Lipid Accumulation and the Development of Hepatic Insulin Resistance. Nutrients 2024, 16, 1003. [Google Scholar] [CrossRef]
- Bu, S.Y.; Mashek, D.G. Hepatic long-chain acyl-CoA synthetase 5 mediates fatty acid channeling between anabolic and catabolic pathways. J. Lipid Res. 2010, 51, 3270–3280. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Abidi, P.; Kim, A.; Chen, W.; Huang, T.T.; Kraemer, F.B.; Liu, J. Transcriptional Activation of Hepatic ACSL3 and ACSL5 by Oncostatin M Reduces Hypertriglyceridemia Through Enhanced β-Oxidation. Arter. Thromb. Vasc. Biol. 2007, 27, 2198–2205. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.Z.; Althagafi, I.I.; Shamshad, H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur. J. Med. Chem. 2019, 166, 502–513. [Google Scholar] [CrossRef]
- Long, B.; Muhamad, R.; Yan, G.; Yu, J.; Fan, Q.; Wang, Z.; Li, X.; Purnomoadi, A.; Achmadi, J.; Yan, X. Quantitative proteomics analysis reveals glutamine deprivation activates fatty acid beta-oxidation pathway in HepG2 cells. Amino Acids 2016, 48, 1297–1307. [Google Scholar] [CrossRef]
- Yang, G.; Sun, S.; He, J.; Wang, Y.; Ren, T.; He, H.; Gao, J. Enoyl-CoA hydratase/3-hydroxyacyl CoA dehydrogenase is essential for the production of DHA in zebrafish. J. Lipid Res. 2023, 64, 100326. [Google Scholar] [CrossRef]
- Houten, S.M.; Denis, S.; Argmann, C.A.; Jia, Y.; Ferdinandusse, S.; Reddy, J.K.; Wanders, R.J.A. Peroxisomal L-bifunctional enzyme (Ehhadh) is essential for the production of medium-chain dicarboxylic acids. J. Lipid Res. 2012, 53, 1296–1303. [Google Scholar] [CrossRef]
- Ding, J.; Loizides-Mangold, U.; Rando, G.; Zoete, V.; Michielin, O.; Reddy, J.K.; Wahli, W.; Riezman, H.; Thorens, B. The Peroxisomal Enzyme L-PBE Is Required to Prevent the Dietary Toxicity of Medium-Chain Fatty Acids. Cell Rep. 2013, 5, 248–258. [Google Scholar] [CrossRef]
- Yunlan, L.; Xinxin, L.; Lin, N.; Qingshan, L. Proteomics Analysis Reveals an Important Role for the PPAR Signaling Pathway in DBDCT-Induced Hepatotoxicity Mechanisms. Molecules 2017, 22, 1113. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Cao, Y.; Xiao, C.; Liu, Y.; Jin, H.; Cao, Y. Effect of the ACAA1 Gene on Preadipocyte Differentiation in Sheep. Front. Genet. 2021, 12, 649140. [Google Scholar] [CrossRef]
- Deng, T.; Wu, J.; Abdel-Shafy, H.; Wang, X.; Lv, H.; Shaukat, A.; Zhou, X.; Zhou, Y.; Sun, H.; Wei, P.; et al. Comparative Genomic Analysis of the Thiolase Family and Functional Characterization of the Acetyl-Coenzyme A Acyltransferase-1 Gene for Milk Biosynthesis and Production of Buffalo and Cattle. J. Agric. Food Chem. 2023, 71, 3325–3337. [Google Scholar] [CrossRef]
- Lisowski, P.; Kościuczuk, E.M.; Gościk, J.; Pierzchała, M.; Rowińska, B.; Zwierzchowski, L. Hepatic transcriptome profiling identifies differences in expression of genes associated with changes in metabolism and postnatal growth between Hereford and Holstein-Friesian bulls. Anim. Genet. 2014, 45, 288–292. [Google Scholar] [CrossRef] [PubMed]
| Time (min) | B (%) |
|---|---|
| 0 | 5 |
| 80 | 23 |
| 99 | 29 |
| 108 | 35 |
| 111 | 48 |
| 112 | 100 |
| 120 | Stop |
| Item | Value |
|---|---|
| ProteomeDiscoverer version | 2.4 |
| Protein Database | uniport-taxonomy-9925.unique.fasta |
| Cys alkylation | Iodoacetamide |
| Dynamic Midification | Oxidation (M), Acetyl (Protein N-Terminus), Acetyl(K), GG(K, S, T) |
| Static Modification | Carbamidomethyl (C) |
| Enzyme Name | Trypsin (Full) |
| Max. Missed Cleavage Sites | 2 |
| Precursor Mass Tolorance | 10 ppm |
| Gene | Primer (5′–3′) | |
|---|---|---|
| Capra hircus ACSL5 | F | CGAAAATGGACTCTTGAC |
| R | ACTCCTGGGTGTTCTCAT | |
| Capra hircus ACSL1 | F | GGTTGACTTCCGGCAGTACG |
| R | CCAGGTCGCATGGAGGCT | |
| Capra hircus EHHADH | F | AGCGTGTCTTTGCTGAAC |
| R | AGAATCGGCTGGGAATAA | |
| Capra hircus ACAA1 | F | TGACGACAAGGGCACAGA |
| R | CTTAAAGGCGGGCTTCAG | |
| Capra hircus Actin | F | CTCAGAGCAAGAGAGGCAT |
| R | CTCGTTGTAGAAGGTGTGGT |
| Motif | Motif Core | Foreground | Background | Fold Increase | ||
|---|---|---|---|---|---|---|
| Matches | Size | Matches | Size | |||
| xGxxxx_K_Gxxxxx | 10.55 | 20 | 1624 | 96 | 22,977 | 2.9 |
| xxxxxx_K_Gxxxxx | 6.07 | 135 | 1604 | 1253 | 22,881 | 1.5 |
| xxxGxG_K_xxxxxx | 6.89 | 22 | 1469 | 126 | 21,628 | 2.6 |
| xxxxxx_K_xxxxxR | 3.64 | 106 | 1447 | 1106 | 21,502 | 1.4 |
| xxxxxx_K_xxxRxx | 3.13 | 91 | 1341 | 91 | 20,396 | 1.4 |
| xxxxxx_K_xxGxxx | 2.72 | 108 | 1250 | 108 | 19,418 | 1.3 |
| xxxxIx_K_xxxxxx | 2.58 | 85 | 1142 | 85 | 18,156 | 1.4 |
| xxxxxL_K_xxxxxx | 3.13 | 147 | 1057 | 147 | 17,168 | 1.3 |
| xxGxxx_K_xxxxxx | 3.22 | 75 | 910 | 75 | 15,326 | 1.5 |
| xxIxxx_K_xxxxxx | 2.38 | 60 | 835 | 60 | 14,471 | 1.4 |
| xQxxxx_K_xxxxxx | 2.21 | 49 | 775 | 49 | 13,743 | 1.5 |
| Protein | PepNumber | Delta p-Value (FL/Con) | Ubiquitination Sites |
|---|---|---|---|
| ACSL1 | 8 | −34.62495976 | K448; K284; K416; K572; K407; K377; K237; K532; K243 |
| EHHADH | 15 | −22.19208675 | K330; K250; K222; K38; K591; K577; K584; K362; K171; K165; K535; K532; K700; K280; K318 |
| ACAA1 | 3 | −16.61 | K395; K292; K198 |
| ACSL5 | 3 | −16.61 | K412; K536; K616 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Liu, Z.; Zhang, Y.; Meng, Y.; Song, X.; Li, J.; Zhang, Y.; Zhao, J.; Du, L.; Deng, Q. Proteomic Analysis of Protein Ubiquitination Events in Dairy Goats with Fatty Liver. Animals 2025, 15, 2010. https://doi.org/10.3390/ani15142010
Zhu Y, Liu Z, Zhang Y, Meng Y, Song X, Li J, Zhang Y, Zhao J, Du L, Deng Q. Proteomic Analysis of Protein Ubiquitination Events in Dairy Goats with Fatty Liver. Animals. 2025; 15(14):2010. https://doi.org/10.3390/ani15142010
Chicago/Turabian StyleZhu, Yuli, Zhenhua Liu, Yuming Zhang, Yao Meng, Xunuo Song, Jinyu Li, Yue Zhang, Junkang Zhao, Liyin Du, and Qinghua Deng. 2025. "Proteomic Analysis of Protein Ubiquitination Events in Dairy Goats with Fatty Liver" Animals 15, no. 14: 2010. https://doi.org/10.3390/ani15142010
APA StyleZhu, Y., Liu, Z., Zhang, Y., Meng, Y., Song, X., Li, J., Zhang, Y., Zhao, J., Du, L., & Deng, Q. (2025). Proteomic Analysis of Protein Ubiquitination Events in Dairy Goats with Fatty Liver. Animals, 15(14), 2010. https://doi.org/10.3390/ani15142010






