Angiogenin Ameliorates Endometritis by Inhibiting NLRP3 Inflammasome Activation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Design, and Sampling
2.2. Isolation and Treatment of Mouse Endometrial Epithelial Cells
2.3. Histological and Immunohistochemical Staining
2.4. Protein Extraction and Western Blot Analysis
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Myeloperoxidase (MPO) Activity Assay
2.7. EdU
2.8. Statistical Analysis
3. Results
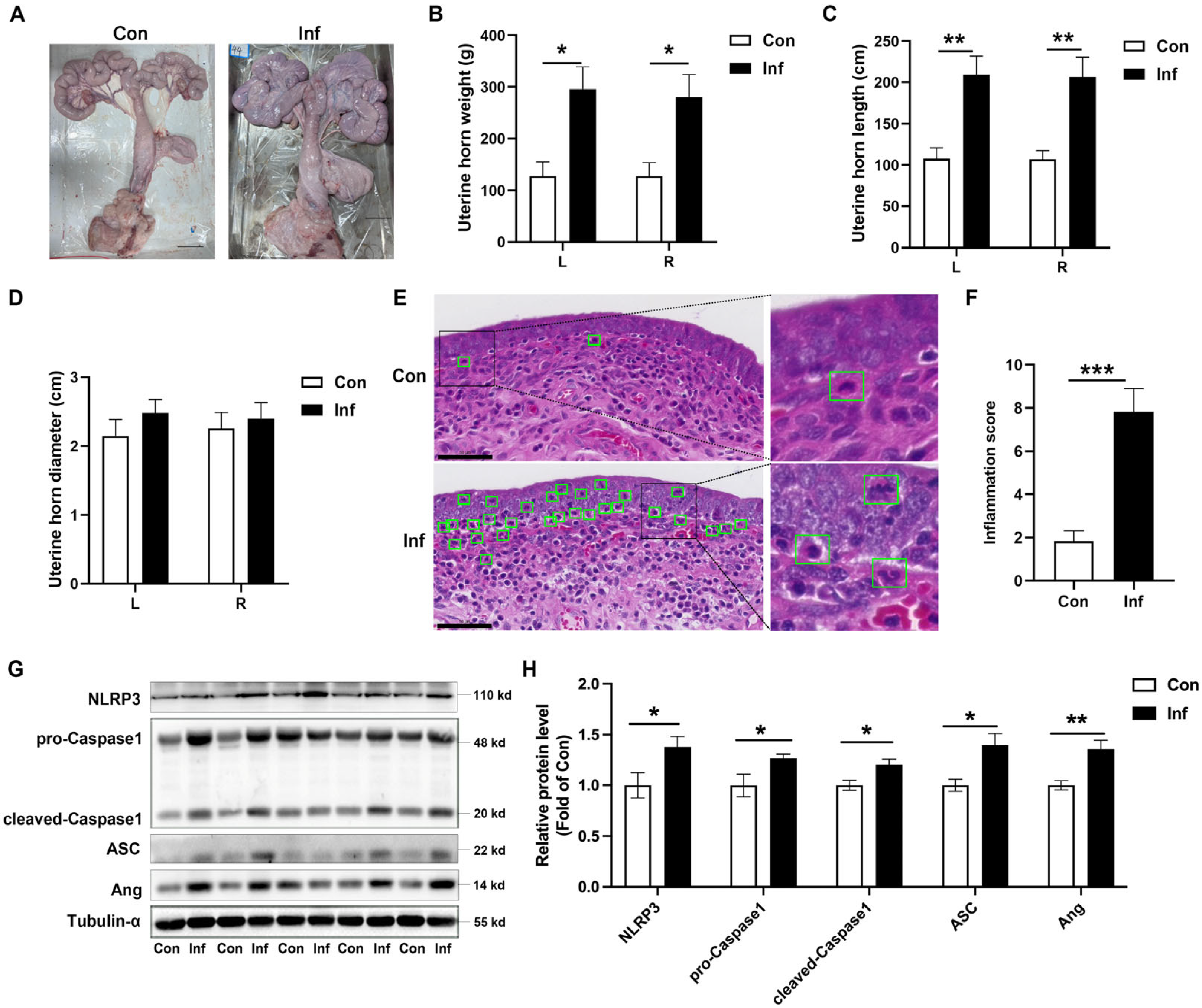
3.1. NLRP3 Inflammasome Is Activated and Ang Is Upregulated in Porcine Endometritis
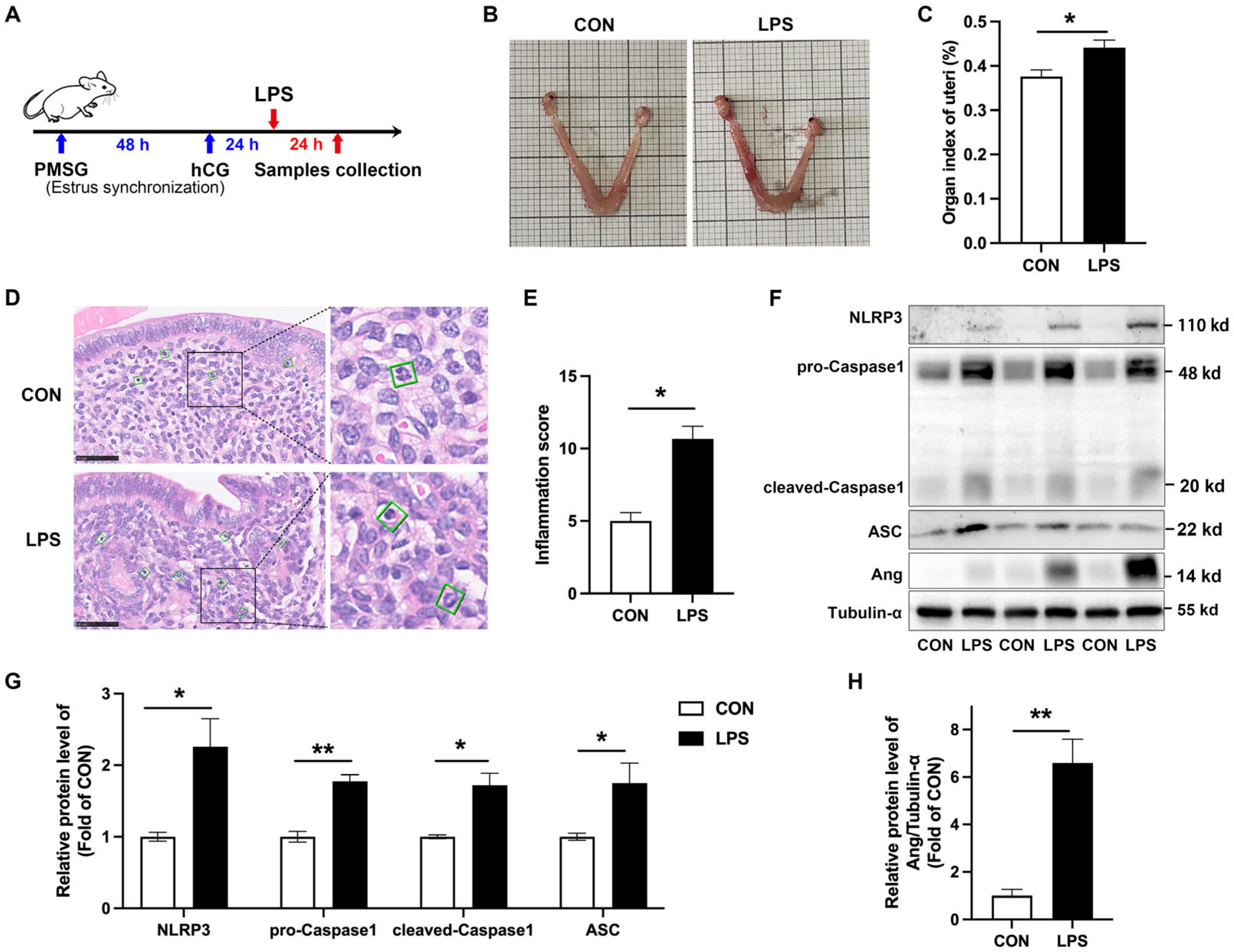
3.2. NLRP3 Inflammasome Is Activated and Ang Is Upregulated in LPS-Induced Murine Endometritis
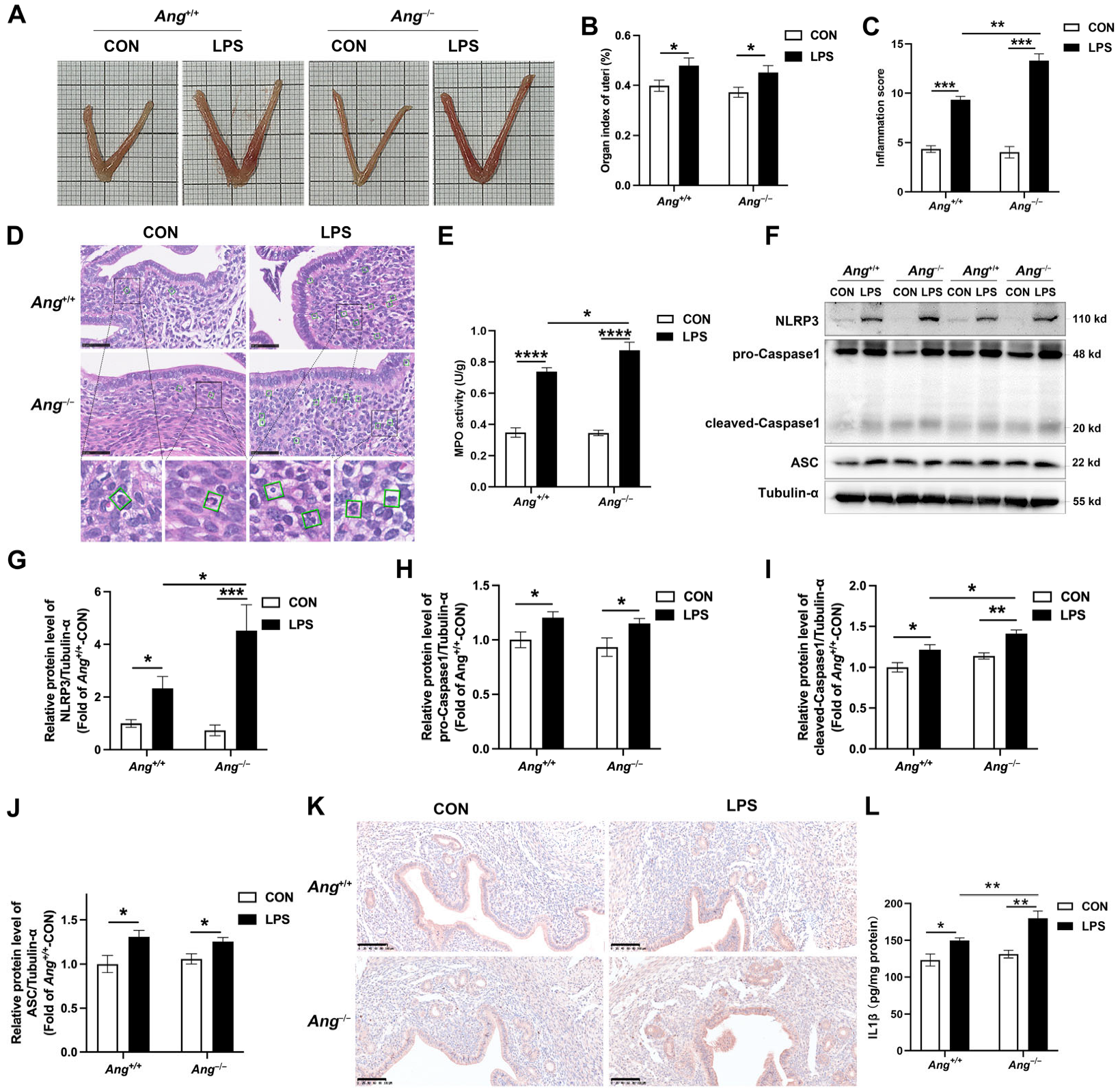
3.3. Ang Deficiency Aggravates LPS-Induced Murine Endometritis
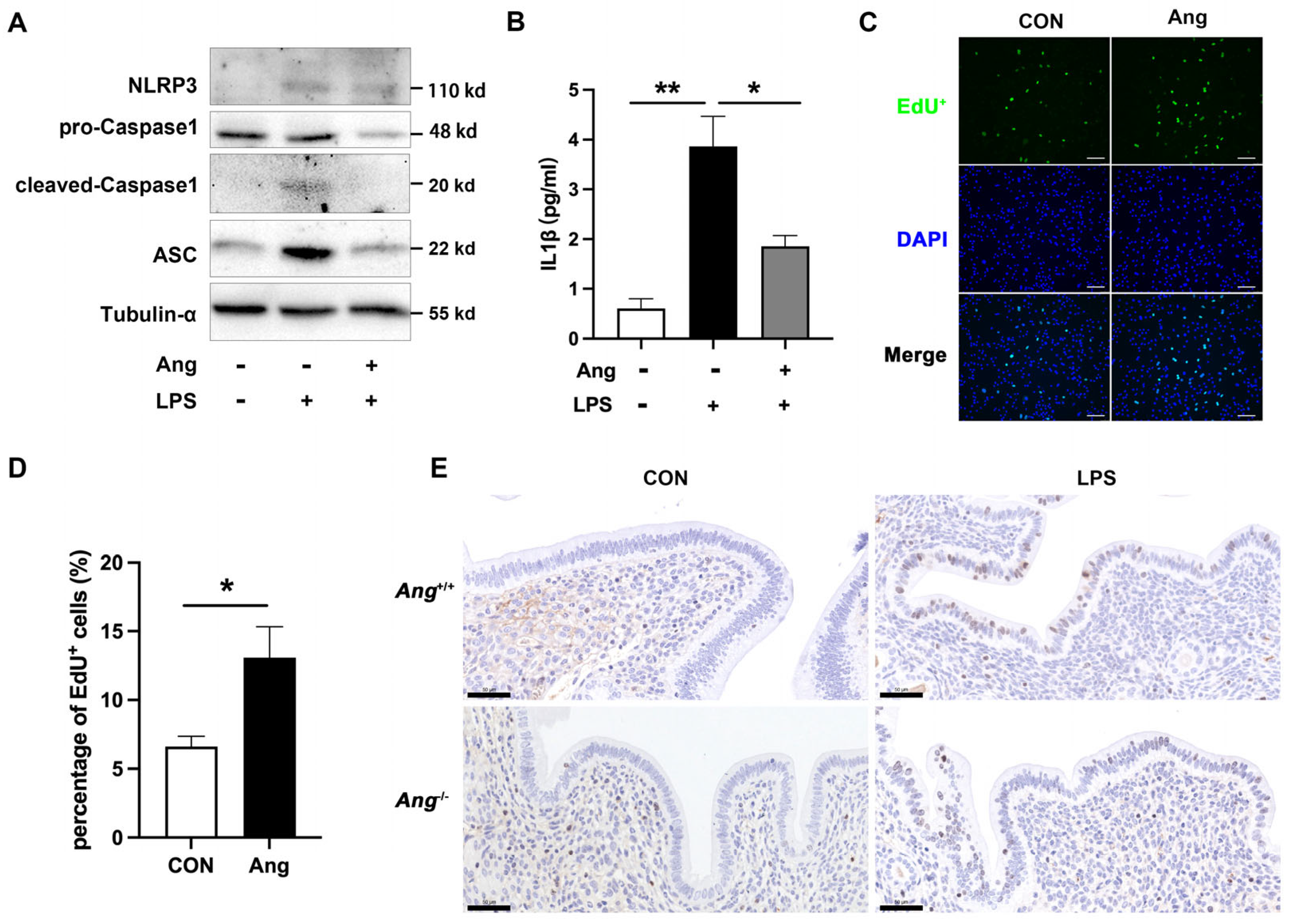
3.4. Ang Maintains the Endometrial Health via Promoting the Proliferation of Mouse Endometrial Epithelial Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindsay, C.V.; Potter, J.A.; Grimshaw, A.A.; Abrahams, V.M.; Tong, M. Endometrial responses to bacterial and viral infection: A scoping review. Hum. Reprod. Update 2023, 29, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Jana, B.; Całka, J.; Andronowska, A.; Mówińska, A.; Witek, K.; Palus, K. Noradrenaline and adrenoreceptors are involved in the regulation of prostaglandin I2 production in the porcine endometrium after experimentally induced inflammation. Int. J. Mol. Sci. 2024, 25, 6313. [Google Scholar] [CrossRef] [PubMed]
- Di, M.; Zhang, Q.; Wang, J.; Xiao, X.; Huang, J.; Ma, Y.; Yang, H.; Li, M. Epigallocatechin-3-gallate (EGCG) attenuates inflammatory responses and oxidative stress in lipopolysaccharide (LPS)-induced endometritis via silent information regulator transcript-1 (SIRT1)/nucleotide oligomerization domain (NOD)-like receptor pyrin domain-containing 3 (NLRP3) pathway. J. Biochem. Mol. Toxicol. 2022, 36, e23203. [Google Scholar] [CrossRef]
- Liu, N.; Shan, Q.; Wu, X.; Xu, L.; Li, Y.; Wang, J.; Wang, X.; Zhu, Y. Phenotypic characteristics, antimicrobial susceptibility and virulence genotype features of Trueperella pyogenes associated with endometritis of dairy cows. Int. J. Mol. Sci. 2024, 25, 3974. [Google Scholar] [CrossRef]
- Xu, J.; Nunez, G. The NLRP3 inflammasome: Activation and regulation. Trends Biochem. Sci. 2023, 48, 331–344. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, Y.; Ma, J.; Wang, S.; Ma, R.; Ge, X.; Zhao, W.; Xue, T.; Chen, L.; Yao, B. NLRP3 promotes endometrial receptivity by inducing epithelial–mesenchymal transition of the endometrial epithelium. Mol. Hum. Reprod. 2021, 27, gaab056. [Google Scholar] [CrossRef]
- Zhu, D.; Zou, H.; Liu, J.; Wang, J.; Ma, C.; Yin, J.; Peng, X.; Li, D.; Yang, Y.; Ren, Y.; et al. Inhibition of HMGB1 ameliorates the maternal-fetal interface destruction in unexplained recurrent spontaneous abortion by suppressing pyroptosis activation. Front. Immunol. 2021, 12, 782792. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, G.; Ma, X.; Zhang, T.; Guo, S.; Zhou, Q.; Yang, C. MiR-495–3p attenuates cell pyroptosis and endometritis through inhibiting the activation of NLRP3 inflammasome in bovine. Mol. Immunol. 2023, 163, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, S.; Cai, J.; Sun, X.; Li, C.; Tan, M.; He, B. Bile acids ameliorates lipopolysaccharide-induced endometritis in mice by inhibiting NLRP3 inflammasome activation. Life Sci. 2023, 331, 122062. [Google Scholar] [CrossRef]
- Bai, R.; Sun, D.; Chen, M.; Shi, X.; Luo, L.; Yao, Z.; Liu, Y.; Ge, X.; Gao, X.; Hu, G.F.; et al. Myeloid cells protect intestinal epithelial barrier integrity through the angiogenin/plexin-B2 axis. EMBO J. 2020, 39, e103325. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.T.; Lee, S.J.; Kim, J.C. The Anti-inflammatory effects of angiogenin in an endotoxin induced uveitis in rats. Int. J. Mol. Sci. 2020, 21, 413. [Google Scholar] [CrossRef]
- Hooper, L.V.; Stappenbeck, T.S.; Hong, C.V.; Gordon, J.I. Angiogenins: A new class of microbicidal proteins involved in innate immunity. Nat. Immunol. 2003, 4, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Li, C.; Liu, S.; Tan, M.; Sun, Y.; Sun, X.; Yang, M.; He, B. Angiogenin-mediated tsRNAs control inflammation and metabolic disorder by regulating NLRP3 inflammasome. Cell Death Differ. 2024, 31, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.A.; Verselis, S.J.; Fett, J.W. Angiogenin is regulated in vivo as an acute phase protein. Biochem. Biophys. Res. Commun. 1998, 26, 480–483. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, K.W.; Min, K.-M.; Kim, K.-W.; Chang, S.-I.; Kim, J.C. Angiogenin reduces immune inflammation via inhibition of TANK-binding kinase 1 expression in human corneal fibroblast cells. Mediat. Inflamm. 2014, 2014, 861435. [Google Scholar] [CrossRef]
- Yao, Z.; Bai, R.; Liu, W.; Liu, Y.; Zhou, W.; Xu, Z.; Sheng, J. Activation of angiogenin expression in macrophages by lipopolysaccharide via the TLR4/NF-κB pathway in colitis. Acta Biochim. Biophys. Sin. 2024, 25, 857–865. [Google Scholar] [CrossRef]
- Vriens, J.; Hennes, A.; De Clercq, K. Isolation of mouse endometrial epithelial and stromal cells for in vitro decidualization. J. Vis. Exp. 2017, 2, 55168. [Google Scholar] [CrossRef]
- Saleh, R.O.; Salahdin, O.D.; Ahmad, I.; Bansal, P.; Kaur, H.; Deorari, M.; Hjazi, A.; Abosaoda, M.K.; Mohammed, I.H.; Jawad, M.A. An updated study of the relationship between bacterial infections and women’s immune system, focusing on bacterial compositions with successful pregnancy. J. Reprod. Immunol. 2024, 165, 104283. [Google Scholar] [CrossRef]
- Li, J.; Wei, J.; Chen, S.; Wang, X.; Chen, J.; Zeng, D.; Fan, L. Prevalence and risk factors for chronic endometritis in patients with adenomyosis and infertility: A retrospective cohort study. BMC Womens Health 2024, 24, 403. [Google Scholar] [CrossRef]
- Coll, R.C.; Schroder, K.; Pelegrin, P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 2022, 43, 653–668. [Google Scholar] [CrossRef]
- Ren, W.; Sun, Y.; Zhao, L.; Shi, X. NLRP3 inflammasome and its role in autoimmune diseases: A promising therapeutic target. Biomed. Pharmacother. 2024, 175, 116679. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Arioz, B.I.; Tastan, B.; Tarakcioglu, E.; Tufekci, K.U.; Olcum, M.; Ersoy, N.; Bagriyanik, A.; Genc, K.; Genc, S. Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 Pathway. Front. Immunol. 2019, 10, 1511. [Google Scholar] [CrossRef]
- He, M.; Chiang, H.H.; Luo, H.; Zheng, Z.; Qiao, Q.; Wang, L.; Tan, M.; Ohkubo, R.; Mu, W.C.; Zhao, S.; et al. An acetylation switch of the NLRP3 inflammasome regulates aging-associated chronic inflammation and insulin resistance. Cell Metab. 2020, 31, 580–591.e5. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, D.; Wang, J.; Guo, J.; Li, Y.; Cao, Y.; Zhang, N.; Fu, Y. Melatonin inhibits endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in lipopolysaccharide-induced endometritis in mice. Int. Immunopharmacol. 2018, 64, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Xu, Z. Three decades of research on angiogenin: A review and perspective. Acta Biochim. Biophys. Sin. 2016, 48, 399–410. [Google Scholar] [CrossRef]
- Noschka, R.; Gerbl, F.; Loffler, F.; Kubis, J.; Rodriguez, A.A.; Mayer, D.; Grieshober, M.; Holch, A.; Raasholm, M.; Forssmann, W.G.; et al. Unbiased identification of angiogenin as an endogenous antimicrobial protein with activity against virulent Mycobacterium tuberculosis. Front. Microbiol. 2020, 11, 618278. [Google Scholar] [CrossRef]
- Sun, D.; Bai, R.; Zhou, W.; Yao, Z.; Liu, Y.; Tang, S.; Ge, X.; Luo, L.; Luo, C.; Hu, G.-f.; et al. Angiogenin maintains gut microbe homeostasis by balancing α-Proteobacteria and Lachnospiraceae. Gut 2021, 70, 666–676. [Google Scholar] [CrossRef]
- Mishra, K.; Wadhwa, N.; Guleria, K.; Agarwal, S. ER, PR and Ki-67 expression status in granulomatous and chronic non-specific endometritis. J. Obstet. Gynaecol. Res. 2008, 34, 371–378. [Google Scholar] [CrossRef]
- Yoneda, E.; Kim, S.; Tomita, K.; Minase, T.; Kayano, M.; Watanabe, H.; Tetsuka, M.; Sasaki, M.; Iwayama, H.; Sanai, H.; et al. Evaluation of lipopolysaccharide and interleukin-6 as useful screening tool for chronic endometritis. Int. J. Mol. Sci. 2024, 25, 2017. [Google Scholar] [CrossRef]
- Liu, H.; Gu, C.; Liu, M.; Liu, G.; Wang, Y. NEK7 mediated assembly and activation of NLRP3 inflammasome downstream of potassium efflux in ventilator-induced lung injury. Biochem. Pharmacol. 2020, 177, 113998. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, X.; Liu, G. Regulation of cell proliferation and transdifferentiation compensates for ventilator-induced lung injury mediated by NLRP3 inflammasome activation. Immun. Inflamm. Dis. 2023, 11, e1062. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Sun, Y.; Yang, H.; Tan, M.; Li, C.; Lu, L.; Liu, C.; He, B. Angiogenin Ameliorates Endometritis by Inhibiting NLRP3 Inflammasome Activation. Animals 2025, 15, 2002. https://doi.org/10.3390/ani15142002
Cai J, Sun Y, Yang H, Tan M, Li C, Lu L, Liu C, He B. Angiogenin Ameliorates Endometritis by Inhibiting NLRP3 Inflammasome Activation. Animals. 2025; 15(14):2002. https://doi.org/10.3390/ani15142002
Chicago/Turabian StyleCai, Jiangxue, Yiran Sun, Hao Yang, Meiling Tan, Chenxuan Li, Lu Lu, Chenxi Liu, and Bin He. 2025. "Angiogenin Ameliorates Endometritis by Inhibiting NLRP3 Inflammasome Activation" Animals 15, no. 14: 2002. https://doi.org/10.3390/ani15142002
APA StyleCai, J., Sun, Y., Yang, H., Tan, M., Li, C., Lu, L., Liu, C., & He, B. (2025). Angiogenin Ameliorates Endometritis by Inhibiting NLRP3 Inflammasome Activation. Animals, 15(14), 2002. https://doi.org/10.3390/ani15142002




