Adaptation Strategy of the Planula Strobilation in Moon Jelly, Aurelia coerulea to Acidic Environments in Terms of Statolith Formation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
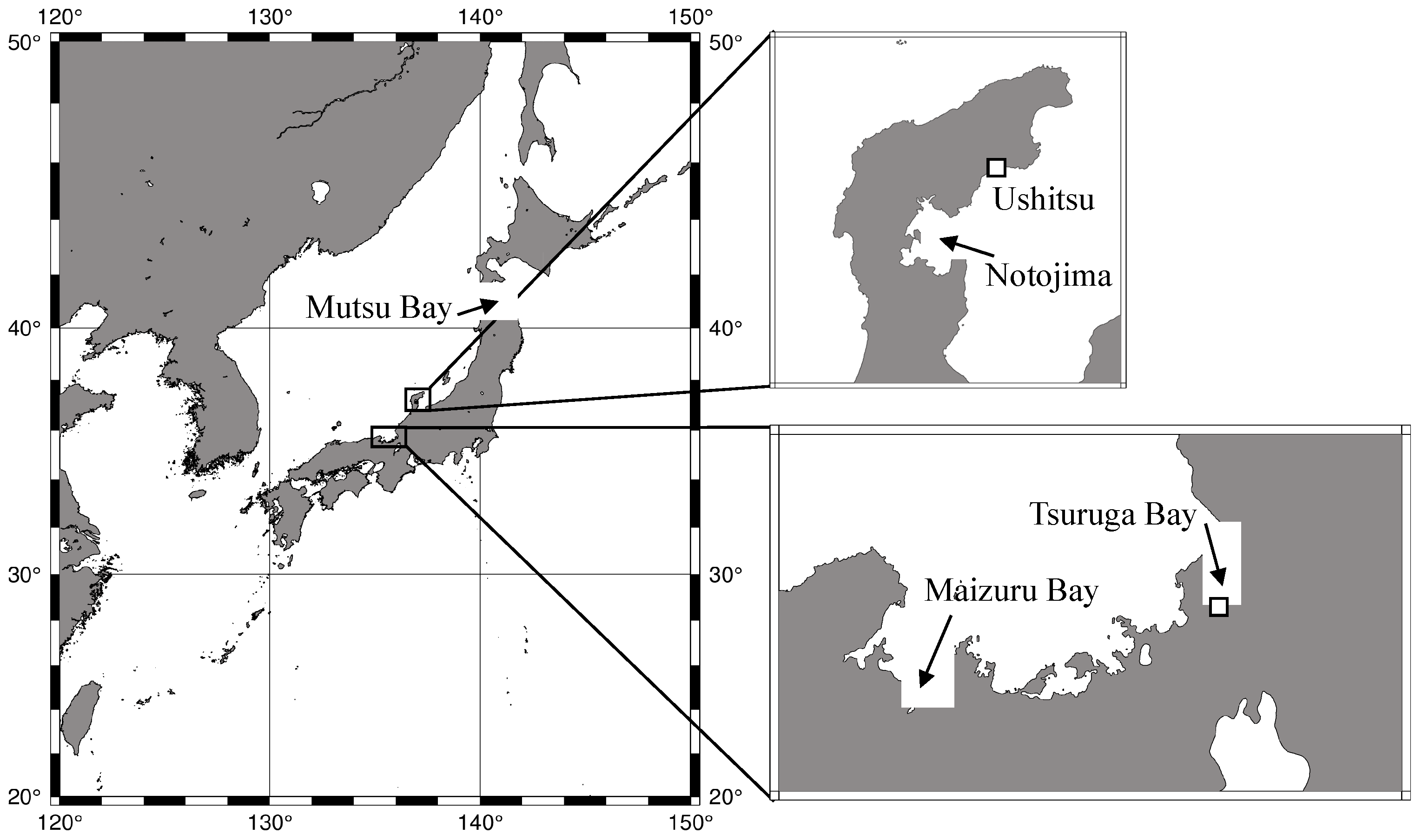
2.1. Experimental System Setup
2.2. Obtaining Polyp-Strobilated Ephyrae
2.3. Obtaining Planula-Strobilated Ephyrae
2.4. Observation of the Body Morphology of Ephyra
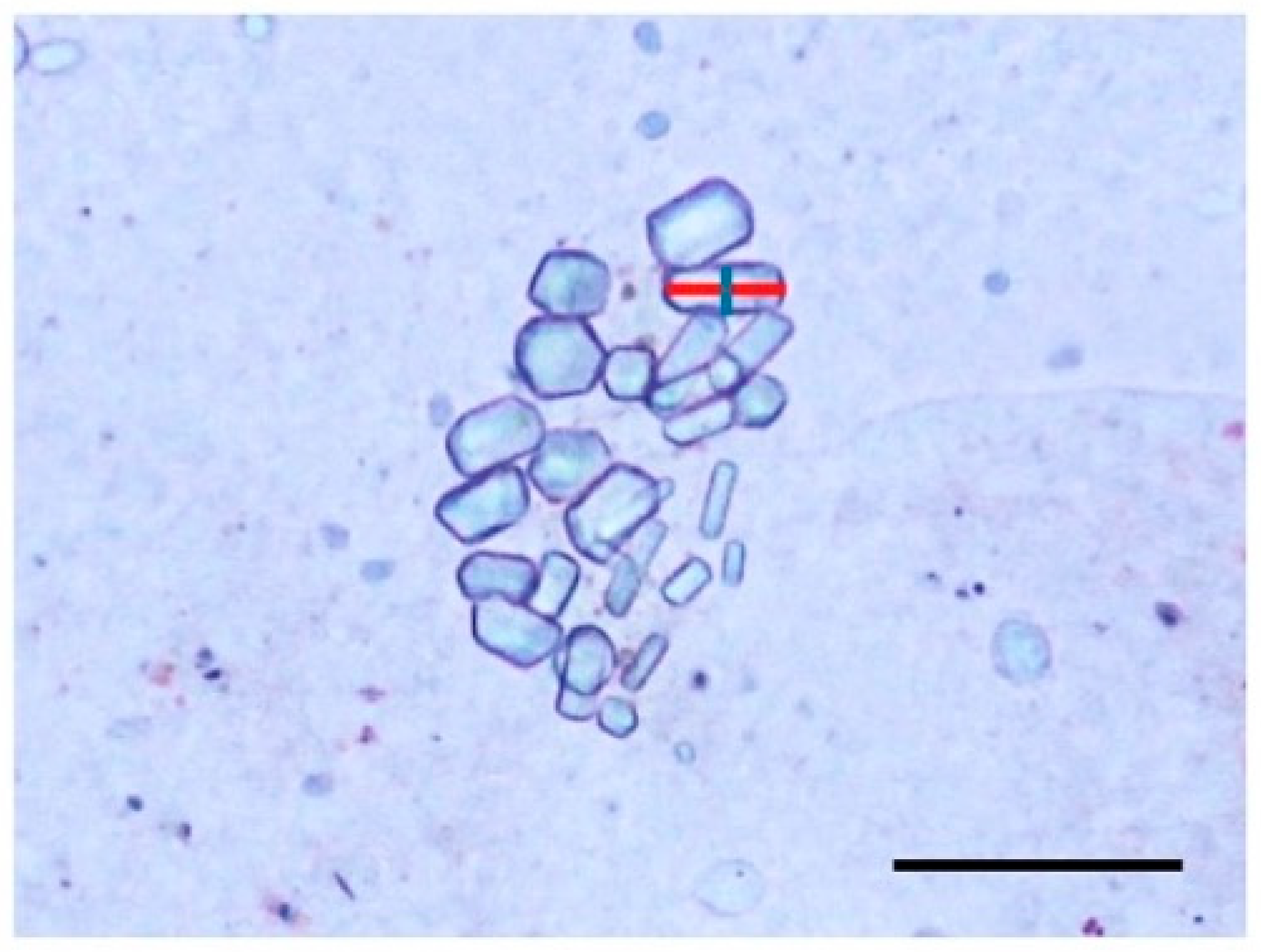
2.5. Observation of Statoliths
2.6. Statistical Analysis
3. Results
3.1. Luvene’s Test
3.2. Body Morphology of the Ephyrae
3.2.1. Results of Traditional Two-Way ANOVA
3.2.2. Polyp-Strobilated Ephyrae
3.2.3. Planula-Strobilated Ephyrae
3.3. Statoliths of the Ephyrae
3.3.1. Results of Yuen’s Trimmed Means Two-Way ANOVA
3.3.2. Polyp-Strobilated Ephyrae
3.3.3. Planula-Strobilated Ephyrae
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raven, J.; Caldeira, K.; Elderfield, H.; Hoegh-Guldberg, O.; Liss, P.; Riebesell, U.; Shepherd, J.; Turley, C.; Watson, A. Ocean Acidification Due to Increasing Atmospheric Carbon Dioxide; The Royal Society: Cambridge, UK, 2005. [Google Scholar]
- Dickson, J. Echinoderm skeletal preservation: Calcite-aragonite seas and the Mg/Ca ratio of Phanerozoic oceans. J. Sediment. Res. 2004, 74, 355–365. [Google Scholar] [CrossRef]
- Flecha, S.; Perez, F.F.; Murata, A.; Makaoui, A.; Huertas, I.E. Decadal acidification in Atlantic and Mediterranean water masses exchanging at the Strait of Gibraltar. Sci. Rep. 2019, 9, 15533. [Google Scholar] [CrossRef] [PubMed]
- Khatiwala, S.; Tanhua, T.; Mikaloff Fletcher, S.; Gerber, M.; Doney, S.C.; Graven, H.D.; Gruber, N.; McKinley, G.A.; Murata, A.; Ríos, A.F.; et al. Global ocean storage of anthropogenic carbon. Biogeosciences 2013, 10, 2169–2191. [Google Scholar] [CrossRef]
- Thor, P.; Dupont, S. Ocean Acidification. In Handbook on Marine Environment Protection: Science, Impacts and Sustainable Management; Salomon, M., Markus, T., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 375–394. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Figuerola, B.; Hancock, A.M.; Bax, N.; Cummings, V.J.; Downey, R.; Griffiths, H.J.; Smith, J.; Stark, J.S. A review and meta-analysis of potential impacts of ocean acidification on marine calcifiers from the Southern Ocean. Front. Mar. Sci. 2021, 8, 584445. [Google Scholar] [CrossRef]
- Gissi, E.; Manea, E.; Mazaris, A.D.; Fraschetti, S.; Almpanidou, V.; Bevilacqua, S.; Coll, M.; Guarnieri, G.; Lloret-Lloret, E.; Pascual, M.; et al. A review of the combined effects of climate change and other local human stressors on the marine environment. Sci. Total Environ. 2021, 755, 142564. [Google Scholar] [CrossRef]
- Hancock, A.M.; King, C.K.; Stark, J.S.; McMinn, A.; Davidson, A.T. Effects of ocean acidification on Antarctic marine organisms: A meta-analysis. Ecol. Evol. 2020, 10, 4495–4514. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.N.; Singh, G.G. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 2010, 13, 1419–1434. [Google Scholar] [CrossRef]
- Fabry, V.J.; Seibel, B.A.; Feely, R.A.; Orr, J.C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 2008, 65, 414–432. [Google Scholar] [CrossRef]
- Gazeau, F.; Alliouane, S.; Bock, C.; Bramanti, L.; López Correa, M.; Gentile, M.; Hirse, T.; Pörtner, H.-O.; Ziveri, P. Impact of ocean acidification and warming on the Mediterranean mussel (Mytilus galloprovincialis). Front. Mar. Sci. 2014, 1, 62. [Google Scholar] [CrossRef]
- Langdon, C.; Takahashi, T.; Sweeney, C.; Chipman, D.; Goddard, J.; Marubini, F.; Aceves, H.; Barnett, H.; Atkinson, M.J. Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef. Glob. Biogeochem. Cycles 2000, 14, 639–654. [Google Scholar] [CrossRef]
- Ofstad, S.; Zamelczyk, K.; Kimoto, K.; Chierici, M.; Fransson, A.; Rasmussen, T.L. Shell density of planktonic foraminifera and pteropod species Limacina helicina in the Barents Sea: Relation to ontogeny and water chemistry. PLoS ONE 2021, 16, e0249178. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Kimoto, K.; Sasaki, O.; Kano, H.; Uchida, H. Sensitivity of planktic foraminiferal test bulk density to ocean acidification. Sci. Rep. 2019, 9, 9803. [Google Scholar] [CrossRef] [PubMed]
- Michaelidis, B.; Ouzounis, C.; Paleras, A.; Pörtner, H.O. Effects of long-term moderate hypercapnia on acid-base balance and growth rate in marine mussels Mytilus galloprovincialis. Mar. Ecol. Prog. Ser. 2005, 293, 109–118. [Google Scholar] [CrossRef]
- Gattuso, J.-P.; Frankignoulle, M.; Bourge, I.; Romaine, S.; Buddemeier, R. Effect of calcium carbonate saturation of seawater on coral calcification. Glob. Planet. Change 1998, 18, 37–46. [Google Scholar] [CrossRef]
- Kleypas, J.A.; Buddemeier, R.W.; Archer, D.; Gattuso, J.-P.; Langdon, C.; Opdyke, B.N. Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 1999, 284, 118–120. [Google Scholar] [CrossRef]
- Shirayama, Y.; Thornton, H. Effect of increased atmospheric CO2 on shallow water marine benthos. J. Geophys. Res. Ocean. 2005, 110, C09S08. [Google Scholar] [CrossRef]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F.; et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Barry, J.P.; Edmunds, P.J.; Gates, R.D.; Hutchins, D.A.; Klinger, T.; Sewell, M.A. The effect of ocean acidification on calcifying organisms in marine ecosystems: An organism-to-ecosystem perspective. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 127–147. [Google Scholar] [CrossRef]
- Hammel, J.; Möckel, L.; Jahn, H.; Dries, R.; Herzen, J.; Wilde, F.; Beckman, F.; Chua, C.-M.; Jansson, F.; Konglerd, P. Effects of ocean acidification on calcifying organisms. Photon Sci. DESY Annu. 2013, 20132823. Available online: https://www.researchgate.net/publication/268075150_Effects_of_ocean_acidification_on_calcifying_organisms (accessed on 14 April 2025).
- Bille, R.; Kelly, R.; Biastoch, A.; Harrould-Kolieb, E.; Herr, D.; Joos, F.; Kroeker, K.; Laffoley, D.; Oschlies, A.; Gattuso, J.P. Taking action against ocean acidification: A review of management and policy options. Environ. Manag. 2013, 52, 761–779. [Google Scholar] [CrossRef]
- Porteus, C.S.; Roggatz, C.C.; Velez, Z.; Hardege, J.D.; Hubbard, P.C. Acidification can directly affect olfaction in marine organisms. J. Exp. Biol. 2021, 224, jeb237941. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.O.; Langenbuch, M.; Michaelidis, B. Synergistic effects of temperature extremes, hypoxia, and increases in CO2 on marine animals: From Earth history to global change. J. Geophys. Res. Ocean. 2005, 110, C09S10. [Google Scholar] [CrossRef]
- Thomas, A.; Ramkumar, A.; Shanmugam, A. CO2 acidification and its differential responses on aquatic biota—A review. Environ. Adv. 2022, 8, 100219. [Google Scholar] [CrossRef]
- Purcell, J.E.; Uye, S.; Lo, W.T. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: A review. Mar. Ecol. Prog. Ser. 2007, 350, 153–174. [Google Scholar] [CrossRef]
- Purcell, J.E.; Arai, M.N. Interactions of pelagic cnidarians and ctenophores with fish: A review. Hydrobiologia 2001, 451, 27–44. [Google Scholar] [CrossRef]
- Purcell, J.E. Jellyfish and ctenophore blooms coincide with human proliferations and environmental perturbations. Annu. Rev. Mar. Sci. 2012, 4, 209–235. [Google Scholar] [CrossRef]
- Nagai, T. Eutrophication and the increase of jellyfish population in the Seto Inland Sea. Bull. Plankton Soc. Jpn. 2005, 52, 27–31, (In Japanese with English Abstract). [Google Scholar]
- Purcell, J.E. Pelagic cnidarians and ctenophores as predators: Selective predation, feeding rates, and effects on prey populations. Ann. Inst. Oceanogr. 1997, 73, 125–137. [Google Scholar]
- Purcell, J.E.; Grover, J.J. Predation and food limitation as causes of mortality in larval herring at a spawning ground in British Columbia. Mar. Ecol. Prog. Ser. 1990, 59, 55–61. [Google Scholar] [CrossRef]
- Purcell, J.E.; Sturdevant, M.V. Prey selection and dietary overlap among zooplanktivorous jellyfish and juvenile fishes in Prince William Sound, Alaska. Mar. Ecol. Prog. Ser. 2001, 210, 67–83. [Google Scholar] [CrossRef]
- Brodeur, R.D.; Suchman, C.L.; Reese, D.C.; Miller, T.W.; Daly, E.A. Spatial overlap and trophic interactions between pelagic fish and large jellyfish in the northern California Current. Mar. Biol. 2008, 154, 649–659. [Google Scholar] [CrossRef]
- Suzuki, K.; Kumakura, E.; Endo, N.; Ishii, H.; Nogata, T. Effects of water mass structure on vertical distribution of moon jellyfish Aurelia aurita s.l. within aggregations in four Japanese coastal areas. Bull. Plankton Soc. Jpn. 2017, 64, 114–123, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Yasuda, T. Ecological studies on the jelly-fish, Aurelia aurita (L.), in Urazoko bay, Fukui Prefecture-XI. An observation on ephyra formation. Publ. Seto Mare Biol. Lab. 1975, 22, 75–80. [Google Scholar] [CrossRef]
- Yasuda, T. Studies on the Common Jelly-Fish, Aurelia aurita (Linné); Japan Fisheries Resource Conservation Association: Tokyo, Japan, 1988; p. 139. (In Japanese) [Google Scholar]
- Brusca, R.C.; Moor, W.; Shuster, S.M. Invertebtaretes, 3rd ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2016. [Google Scholar]
- Chapman, D.M.; James, R. Intraepithelial flagella in the medusa of Aurelia aurita (L.). Publ. Seto Mar. Biol. Lab. 1973, 20, 731–743. [Google Scholar] [CrossRef]
- Arai, M.N. A Functional Biology of Scyphozoa; Chapman & Hall: London, UK, 1997. [Google Scholar]
- Becker, A.; Sötje, I.; Paulmann, C.; Beckmann, F.; Donath, T.; Boese, R.; Prymak, O.; Tiemann, H.; Epple, M. Calcium sulfate hemihydrate is the inorganic mineral in statoliths of Scyphozoan medusae (Cnidaria). Dalton Trans. 2005, 8, 1545–1550. [Google Scholar] [CrossRef]
- Helm, R.R. Evolution and development of scyphozoan jellyfish. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1228–1250. [Google Scholar] [CrossRef]
- Sötje, I.; Neues, F.; Epple, M.; Ludwig, W.; Rack, A.; Gordon, M.; Boese, R.; Tiemann, H. Comparison of the statolith structures of Chironex fleckeri (Cnidaria, Cubozoa) and Periphylla periphylla (Cnidaria, Scyphozoa): A phylogenetic approach. Mar. Biol. 2011, 158, 1149–1161. [Google Scholar] [CrossRef]
- Spangenberg, D.B. Rhopalium development in Aurelia aurita ephyrae. In Coelenterate Biology: Recent Research on Cnidaria and Ctenophora; Williams, R.B., Cornelius, P.F., Hughes, R.G., Robson, E.A., Eds.; Kluwr Academic Publishers: Dordrecht, The Netherlands, 1991; pp. 45–49. [Google Scholar]
- Winans, A.K.; Purcell, J.E. Effects of pH on asexual reproduction and statolith formation of the scyphozoan, Aurelia labiata. Hydrobiologia 2010, 645, 39–52. [Google Scholar] [CrossRef]
- Tills, O.; Sun, X.; Rundle, S.D.; Heimbach, T.; Gibson, T.; Cartwright, A.; Palmer, M.; Rudin-Bitterli, T.; Spicer, J.I. Reduced pH affects pulsing behaviour and body size in ephyrae of the moon jellyfish, Aurelia aurita. J. Exp. Mar. Biol. Ecol. 2016, 480, 54–61. [Google Scholar] [CrossRef]
- Kato, K.-i.; Aochi, M.; Ozato, K. Developmental aspects of strobilation in Aurelia aurita. Publ. Seto Mare Biol. Lab. 1973, 20, 179–194. [Google Scholar] [CrossRef][Green Version]
- Spangenberg, D.B.; Beck, C.W. Calcium sulfate dihydrate statoliths in Aurelia. Trans. Am. Microsc. Soc. 1968, 87, 329–335. [Google Scholar] [CrossRef]
- Spangenberg, D.B.; Kuenning, W. SEM studies of strobilating Aurelia. In Coelenterate Ecology and Behavior; Springer: Berlin/Heidelberg, Germany, 1976; pp. 377–386. [Google Scholar]
- Scorrano, S.; Aglieri, G.; Boero, F.; Dawson, M.N.; Piraino, S. Unmasking Aurelia species in the Mediterranean Sea: An integrative morphometric and molecular approach. Zool. J. Linn. Soc. 2017, 180, 243–267. [Google Scholar]
- Haeckel, E. Metagenesis und Hypogenesis von Aurelia aurita: Ein Beitrag zur Entwickelungsgeschichte und zur Teratologie der Medusen; Gustav Fischer: Jena, Germany, 1881. [Google Scholar]
- Hirai, E. On the developmental cycles of Aurelia aurita and Dactylometra pacifica. Bull. Mar. Biol. Stat. Asamushi 1958, 9, 81. [Google Scholar]
- Kakinuma, Y. An experimental study of the life cycle and organ differentiation of Aurelia aurita Lamarck. Bull. Mar. Biol. Stat. Asamushi 1975, 15, 101–112. [Google Scholar]
- Lucas, C.H. Reproduction and life history strategies of the common jellyfish, Aurelia aurita, in relation to its ambient environment. Hydrobiologia 2001, 451, 229–246. [Google Scholar] [CrossRef]
- Miyake, H.; Ikeguchi, S.; Takayama, K.; Yoshikawa, M.; Arima, S.; Suzuki, N. On the moon jelly which develops to ephyra directly from planula. Aquabiology 2019, 41, 54–59, (In Japanese with English Abstract). [Google Scholar]
- Suzuki, K.S.; Suzuki, K.W.; Kumakura, E.; Sato, K.; Oe, Y.; Sato, T.; Sawada, H.; Masuda, R.; Nogata, Y. Seasonal alternation of the ontogenetic development of the moon jellyfish Aurelia coerulea in Maizuru Bay, Japan. PLoS ONE 2019, 14, e0225513. [Google Scholar] [CrossRef]
- Berrill, N.J. Developmental analysis of scyphomedusae. Biol. Rev. Camb. Philos. Soc. 1949, 24, 393–410. [Google Scholar] [CrossRef]
- Takauchi, S.; Miyake, H.; Hirata, N.; Nagai, M.; Suzuki, N.; Ogiso, S.; Ikeguchi, S. Morphological characteristics of ephyrae of Aurelia coerulea derived from planula strobilation. Fish. Sci. 2021, 87, 671–679. [Google Scholar] [CrossRef]
- Miyake, H.; Hashimoto, J.; Chikuchishin, M.; Miura, T. Scyphopolyps of Sanderia malayensis and Aurelia aurita attached to the tubes of vestimentiferan tube worm, Lamellibrachia satsuma, at submarine fumaroles in Kagoshima Bay. Mar. Biotechnol. 2004, 6, S174–S178. [Google Scholar]
- Fuchs, B.; Wang, W.; Graspeuntner, S.; Li, Y.; Insua, S.; Herbst, E.M.; Dirksen, P.; Bohm, A.M.; Hemmrich, G.; Sommer, F.; et al. Regulation of polyp-to-jellyfish transition in Aurelia aurita. Curr. Biol. 2014, 24, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- R_Core_Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Mair, P.; Wilcox, R. Robust statistical methods in R using the WRS2 package. Behav. Res. Methods 2020, 52, 464–488. [Google Scholar] [CrossRef]
- Kikkawa, T.; Minowa, Y.; Nakamura, Y.; Kita, J.; Ishimatsu, A. Swimming inhibition by elevated pCO2 in ephyrae of the scyphozoan jellyfish, Aurelia. Plankton Benthos Res. 2010, 5, 119–122. [Google Scholar] [CrossRef]
- Feitl, K.E.; Millett, A.F.; Colin, S.P.; Dabiri, J.O.; Costello, J.H. Functional morphology and fluid interactions during early development of the scyphomedusa Aurelia aurita. Biol. Bull. 2009, 217, 283–291. [Google Scholar] [CrossRef]
- Higgins, J.E.; Ford, M.D.; Costello, J.H. Transitions in morphology, nematocyst distribution, fluid motions, and prey capture during development of the scyphomedusa Cyanea capillata. Biol. Bull. 2008, 214, 29–41. [Google Scholar] [CrossRef]
- Guan, B.; Shen, Z.; Wu, Z.; Yang, L.; Ma, X. Effect of pH on the preparation of α-calcium sulfate hemihydrate from FGD gypsum with the hydrothermal method. J. Am. Ceram. Soc. 2008, 91, 3835–3840. [Google Scholar] [CrossRef]
- Alguero-Muniz, M.; Meunier, C.L.; Holst, S.; Alvarez-Fernandez, S.; Boersma, M. Withstanding multiple stressors: Ephyrae of the moon jellyfish (Aurelia aurita, Scyphozoa) in a high-temperature, high-CO2 and low-oxygen environment. Mar. Biol. 2016, 163, 185. [Google Scholar] [CrossRef]
- Feldmann, T.; Demopoulos, G.P. The crystal growth kinetics of alpha calcium sulfate hemihydrate in concentrated CaCl2–HCl solutions. J. Cryst. Growth 2012, 351, 9–18. [Google Scholar] [CrossRef]
- Heins, A.; Sötje, I.; Holst, S. Assessment of investigation techniques for scyphozoan statoliths, with focus on early development of the jellyfish Sanderia malayensis. Mar. Ecol. Prog. Ser. 2018, 591, 37–56. [Google Scholar] [CrossRef]
- Fu, Z.L.; Shibata, M.; Makabe, R.; Ikeda, H.; Uye, S.I. Body size reduction under starvation, and the point of no return, in ephyrae of the moon jellyfish Aurelia aurita. Mar. Ecol. Prog. Ser. 2014, 510, 255–263. [Google Scholar] [CrossRef]
- León-Cobo, M.J.; Enrique-Navarro, A.; Bartual, A.; Prieto, L. Impact of warming and acidification of the Mediterranean Sea on statolith formation of the scyphozoan jellyfish Rhizostoma pulmo Macri (1778). Mar. Environ. Res. 2024, 202, 106788. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yu, Z.; Zhen, Y.; Wang, G.; Wang, X.; Mi, T. Effect of decreasing temperature on the strobilation of Aurelia sp.1. J. Oceanol. Limnol. 2018, 36, 465–472. [Google Scholar] [CrossRef]
- Holst, S. Effects of climate warming on strobilation and ephyra production of North Sea scyphozoan jellyfish. Hydrobiologia 2012, 690, 127–140. [Google Scholar] [CrossRef]
- Purcell, J.E.; Hoover, R.A.; Schwarck, N.T. Interannual variation of strobilation by the scyphozoan Aurelia labiata in relation to polyp density, temperature, salinity, and light conditions in situ. Mar. Ecol. Prog. Ser. 2009, 375, 139–149. [Google Scholar] [CrossRef]








| Strobilation Type | Effect | Test Statistic | p Value | |||
|---|---|---|---|---|---|---|
| Polyp | Planula | |||||
| TBD (mm) | ||||||
| pH 6.8 | - | 1.37 (±0.17) | strobilation type at pH 7.8 | F = 35.12 | p < 0.001 | *** |
| pH 7.8 | 2.98 (±0.44) | 1.95 (±0.22) | strobilation type at pH 8.1 | F = 339.07 | p < 0.001 | *** |
| pH 8.1 | 3.08 (±0.14) | 1.72 (±0.17) | pH in polyp-strobilation | F = 0.28 | p = 0.61 | |
| pH in planula-strobilation | Refer to Table 3. | |||||
| CDD (mm) | ||||||
| pH 6.8 | - | 0.62 (±0.07) | strobilation type at pH 7.8 | |||
| pH 7.8 | 1.18 (±0.20) | 0.68 (±0.09) | strobilation type at pH 8.1 | F = 32.80 | p < 0.001 | *** |
| pH 8.1 | 1.28 (±0.09) | 0.61 (±0.09) | pH in polyp-strobilation | F = 261.21 | p < 0.001 | *** |
| pH in planula-strobilation | Refer to Table 3. | |||||
| CDD/TBD (%) | ||||||
| pH 6.8 | - | 45.4 (±4.8) | strobilation type at pH 7.8 | F = 21.80 | p < 0.001 | *** |
| pH 7.8 | 39.3 (±1.0) | 35.0 (±2.5) | strobilation type at pH 8.1 | F = 38.53 | p < 0.001 | *** |
| pH 8.1 | 41.5 (±1.3) | 35.3 (±2.6) | pH in polyp-strobilation | F = 11.30 | p = 0.004 | ** |
| pH in planula-strobilation | Refer to Table 3. | |||||
| TBD (mm) | p Value | CDD (mm) | p Value | CDD/TBD (%) | p Value | ||
|---|---|---|---|---|---|---|---|
| pH 6.8−pH 7.8 | p < 0.001 | *** | pH 6.8−pH 7.8 | p = 0.26 | pH 6.8−pH 7.8 | p < 0.001 | *** |
| pH 6.8−pH 8.1 | p = 0.002 | ** | pH 6.8−pH 8.1 | p = 0.97 | pH 6.8−pH 8.1 | p < 0.001 | *** |
| pH 7.8-pH 8.1 | p = 0.04 | * | pH 7.8−pH 8.1 | p = 0.17 | pH 7.8−pH 8.1 | p = 0.86 |
| Strobilation Type | |||||
|---|---|---|---|---|---|
| Polyp | Planula | Effect | p Value | ||
| No. ephyrae analyzed | |||||
| pH 7.8 | 7 | 10 | NA | ||
| pH 8.1 | 10 | 10 | NA | ||
| No. statoliths analyzed | |||||
| pH 7.8 | 600 | 536 | NA | ||
| pH 8.1 | 737 | 461 | NA | ||
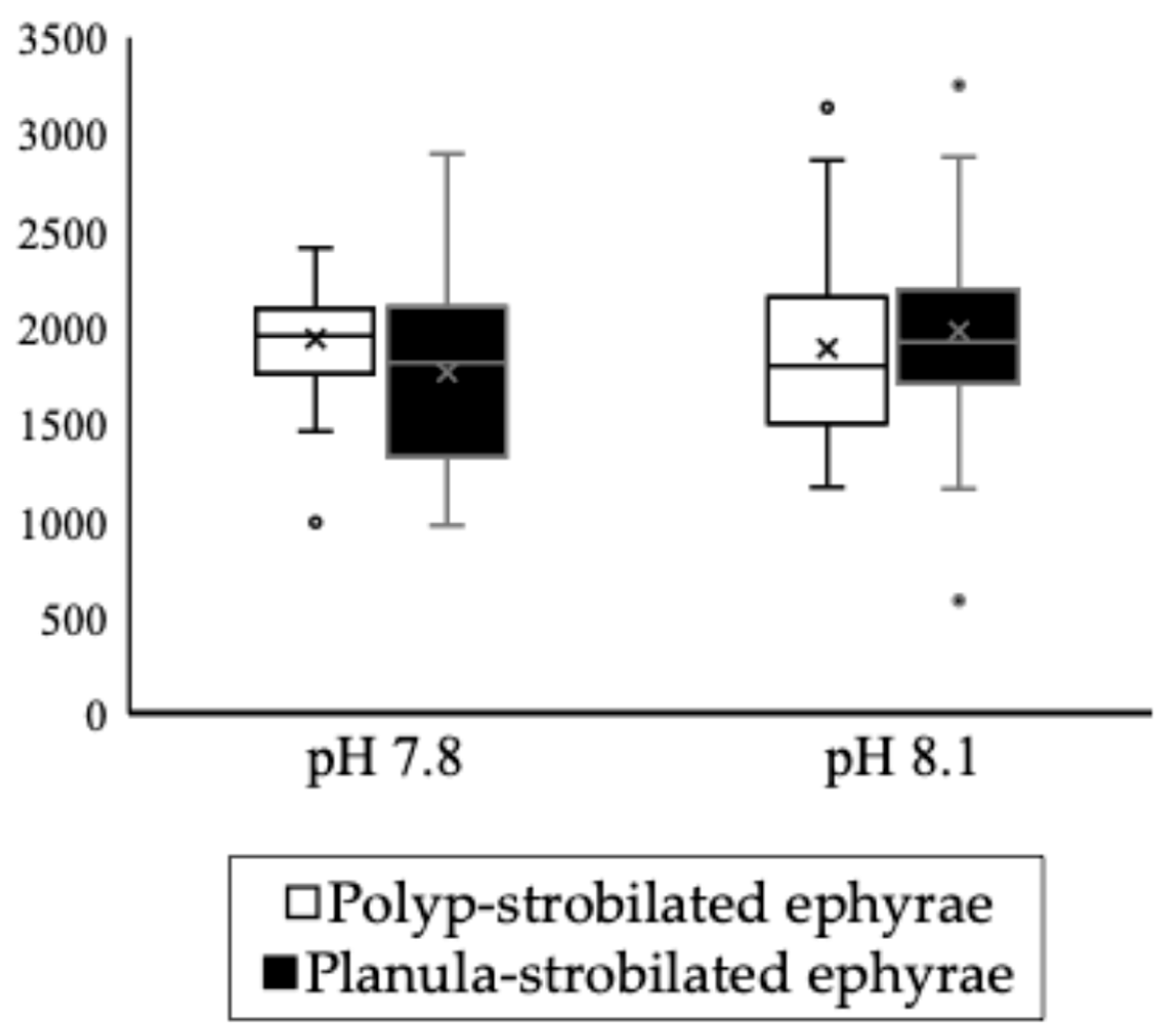
| Mean no. statoliths per statocyst | |||||
| pH 7.8 | 28.6 (±6.28) | 17.9 (±3.53) | strobilation type at pH 7.8 | p < 0.001 | *** |
| pH 8.1 | 24.6 (±3.22) | 15.4 (±3.35) | strobilation type at pH 8.1 | p < 0.001 | *** |
| pH in polyp-strobilation | p = 0.178 | ||||
| pH in planula-strobilation | p = 0.009 | ** | |||
| Mean statolith size (μm2) | |||||
| pH 7.8 | 66.2 (±10.3) | 111.0 (±20.4) | strobilation type at pH 7.8 | p < 0.001 | *** |
| pH 8.1 | 79.1 (±7.73) | 114.6 (±31.8) | strobilation type at pH 8.1 | p < 0.001 | *** |
| pH in polyp-strobilation | p = 0.003 | ** | |||
| pH in planula-strobilation | p = 0.269 | ||||
| Mean statolith aspect ratio | |||||
| pH 7.8 | 1.5 (±0.18) | 3.5 (±0.66) | strobilation type at pH 7.8 | p < 0.001 | *** |
| pH 8.1 | 2.1 (±0.28) | 3.6 (±0.72) | strobilation type at pH 8.1 | p < 0.001 | *** |
| pH in polyp-strobilation | p < 0.001 | *** | |||
| pH in planula-strobilation | p = 0.610 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, Y.; Miyake, H.; Suzuki, N.; Ogiso, S. Adaptation Strategy of the Planula Strobilation in Moon Jelly, Aurelia coerulea to Acidic Environments in Terms of Statolith Formation. Animals 2025, 15, 1999. https://doi.org/10.3390/ani15131999
Maeda Y, Miyake H, Suzuki N, Ogiso S. Adaptation Strategy of the Planula Strobilation in Moon Jelly, Aurelia coerulea to Acidic Environments in Terms of Statolith Formation. Animals. 2025; 15(13):1999. https://doi.org/10.3390/ani15131999
Chicago/Turabian StyleMaeda, Yuka, Hiroshi Miyake, Nobuo Suzuki, and Shouzo Ogiso. 2025. "Adaptation Strategy of the Planula Strobilation in Moon Jelly, Aurelia coerulea to Acidic Environments in Terms of Statolith Formation" Animals 15, no. 13: 1999. https://doi.org/10.3390/ani15131999
APA StyleMaeda, Y., Miyake, H., Suzuki, N., & Ogiso, S. (2025). Adaptation Strategy of the Planula Strobilation in Moon Jelly, Aurelia coerulea to Acidic Environments in Terms of Statolith Formation. Animals, 15(13), 1999. https://doi.org/10.3390/ani15131999






