Odontogenic Abscesses in Pet Rabbits: A Comprehensive Review of Pathogenesis, Diagnosis, and Treatment Advances
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Study Selection and Standardization Criteria
2.3. Data Aggregation for Comparative Heatmaps
3. Anatomy of Rabbit Dentition
4. Etiology and Pathogenesis
5. Clinical Presentation
6. Odontogenic Abscess Structure
7. Diagnostic Approach
8. Complications and Prognosis
9. Treatment
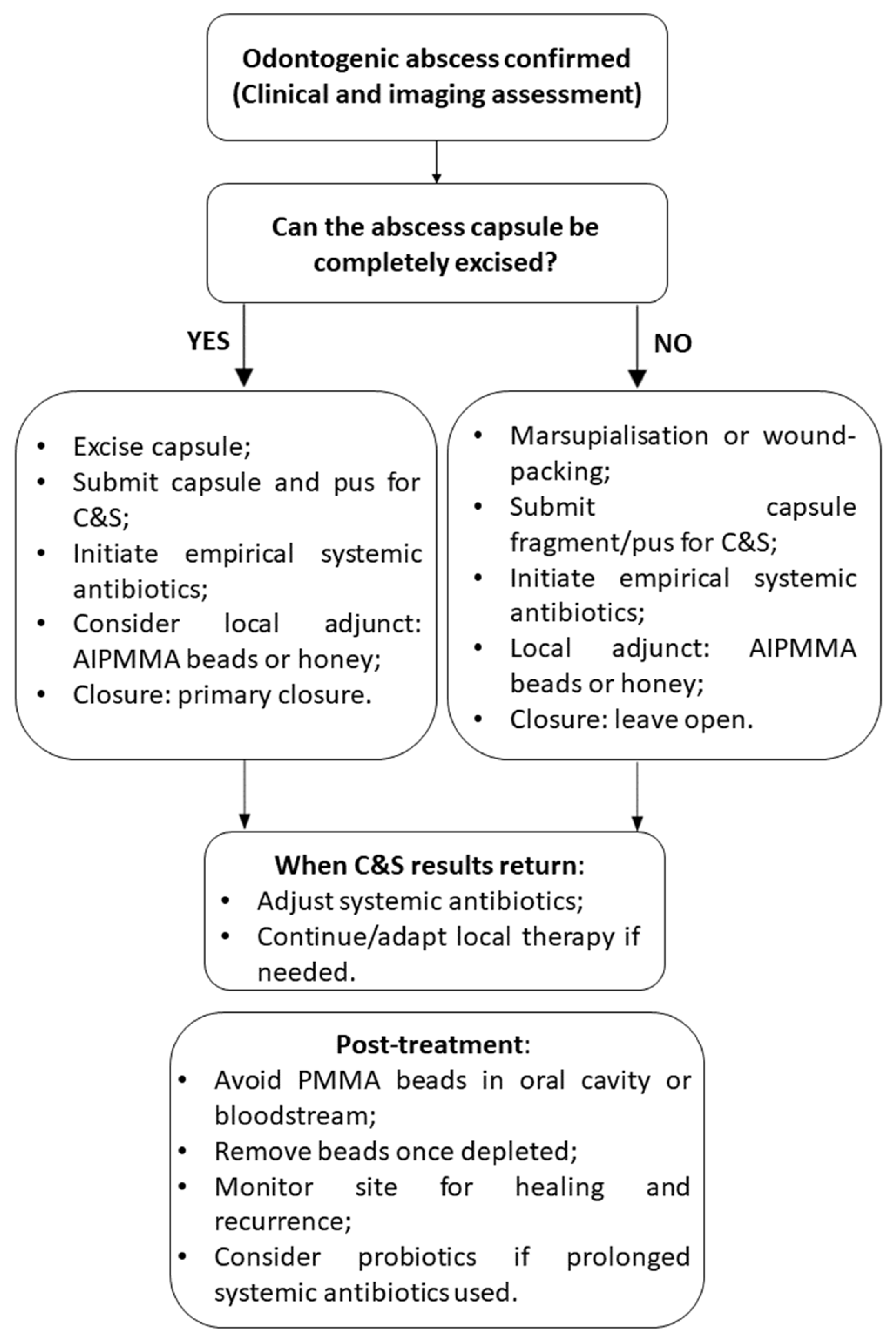
9.1. Surgical Approach
9.2. Systemic Antimicrobial Therapy
9.3. Local Antimicrobial Therapies in Rabbit Odontogenic Abscesses
9.3.1. Honey as a Topical Antibacterial Agent
9.3.2. Antibiotic-Impregnated Polymethyl Methacrylate (AIPMMA) Beads
- Gentamicin—This agent is heat-stable, commercially available in veterinary PMMA kits, and active against the Staphylococcus–Pseudomonas spectrum common to mandibular abscesses [98].
- Amikacin—This agent has broader Gram-negative coverage (including multidrug-resistant Pseudomonas) and is safe locally, even in animals with marginal renal function; the beads must lie flush against the bone because diffusion rarely exceeds 5 mm [32].
10. Practical Considerations for General Veterinary Practice
10.1. Diagnostic Prioritization Without Advanced Imaging
10.2. Optimizing Microbial Culture: Focus on Anaerobes
11. Prophylaxis in Rabbit Dental Health
- Dietary management: The cornerstone of prophylaxis is dietary fiber. Feeding a hay-based diet (timothy, meadow, or orchard grass) promotes prolonged mastication, which supports dental wear and overall oral health. Pellets should be offered in limited amounts, and muesli-type commercial mixes should be avoided due to their association with selective feeding and malocclusion. A calcium-balanced diet remains important—not because dietary hypocalcemia is a common issue in rabbits but due to the ongoing demand for calcium in continuously growing teeth and bone. However, this does not imply a need for high calcium or vitamin D intake, as excessive supplementation may lead to serious health problems such as hypervitaminosis D and renal pathology. Notably, rabbits can maintain normal serum calcium concentrations even in the presence of metabolic bone disease through compensatory mechanisms such as increased parathyroid hormone activity, which mobilizes calcium from the skeleton. This bone resorption, in turn, contributes to alveolar bone loss and dental instability [41,46,62,101].
- Regular comprehensive examinations: Every 6–12 months, rabbits should receive a full physical exam plus a conscious intraoral inspection with an otoscope or small speculum. In high-risk cases (e.g., lops, dwarfs, or rabbits with prior dental disease), stomatoscopy, a rigid endoscopic examination of the oral cavity performed under general anesthesia, provides a magnified view of cheek-tooth arcades and should be scheduled at longer intervals (e.g., every 12–18 months) or sooner if clinical signs appear [59].
- Environmental enrichment: Providing fibrous forage and appropriate items for oral activity supports a normal masticatory pattern, which is essential for even tooth wear, particularly of the cheek teeth. While chewable items such as untreated wood blocks or willow branches primarily engage the incisors, long-stemmed hay and leafy greens are critical for promoting proper lateral jaw movement and physiological attrition of the cheek teeth [46,70,102].
- Owner education: Prophylactic success hinges on owner compliance. Educating clients on the importance of nutrition, the signs of early dental disease (e.g., epiphora, weight loss), and the dangers of improper trimming (e.g., with nail clippers) is crucial [103].
- Preventing iatrogenic injury: Dental procedures must be performed by trained professionals. Improper burring or incisor trimming can result in pulp exposure, leading to infection and abscess formation [59].
12. Discussion
13. Gaps in the Literature and Research Needs
- Limited data on anaerobic bacteria: While anaerobes such as Fusobacterium spp., Peptostreptococcus spp., and Bacteroides spp. are known contributors to abscesses in other species, their detection in rabbits remains rarely reported due to limitations in anaerobic culture and diagnostic protocols. Future studies employing molecular tools (e.g., 16S rRNA sequencing, metagenomics) are needed to clarify their role in polymicrobial infections.
- Lack of standardized treatment protocols: Most rabbit abscess treatments are empirical or based on isolated case reports. There is a clear need for randomized clinical trials comparing surgical, systemic, and local therapies, as well as long-term outcome data.
- Insufficient data on long-term outcomes: Few studies track rabbits beyond the immediate post-operative period. Long-term recurrence rates, quality of life, and complication rates (e.g., from AIPMMA bead retention) need evaluation.
- Underrepresentation of regional pathogens: Most microbiological studies originate from Western Europe and North America. There is a lack of data from Asia, Eastern Europe, and developing regions, despite evidence of regional variability in bacterial isolates.
- Diagnostic imaging thresholds: The criteria for when to escalate from radiography to CT or CBCT remain unclear. Studies are needed to define cost–benefit thresholds, especially for general practitioners working in resource-limited settings.
- Manuka honey and natural therapies: Despite promising in vitro data, there is a lack of controlled in vivo trials on honey’s effects on wound healing, osteogenesis, and recurrence prevention in rabbits. More research is needed to validate dosing protocols and establish safety over prolonged treatment periods.
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández, M.; Garcias, B.; Duran, I.; Molina-López, R.A.; Darwich, L. Current Situation of Bacterial Infections and Antimicrobial Resistance Profiles in Pet Rabbits in Spain. Vet. Sci. 2023, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- DeMello, M. Rabbits Multiplying like Rabbits: The Rise in the Worldwide Popularity of Rabbits as Pets. In Companion Animals in Everyday Life: Situating Human-Animal Engagement within Cultures; Palgrave Macmillan: London, UK, 2016; pp. 91–107. [Google Scholar]
- Dobos, P.; Kulik, L.; Pongrácz, P. The Amicable Rabbit—Interactions between Pet Rabbits and Their Caregivers Based on a Questionnaire Survey. Appl. Anim. Behav. Sci. 2023, 260, 105869. [Google Scholar] [CrossRef]
- Přibylová, L.; Součková, M.; Kolářová, M.F.; Vostrá-Vydrová, H.; Chaloupková, H. Does a Stronger Bond with Pet Rabbits Equate to Better Husbandry Conditions for Them? Appl. Anim. Behav. Sci. 2024, 270, 106143. [Google Scholar] [CrossRef]
- Druce, K. Dental Disease in Rabbits—Malocclusion. Vet. Nurs. J. 2015, 30, 309–311. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Craven, H.C.; Brodbelt, D.C.; Church, D.B.; Hedley, J. Morbilidad y mortalidad de conejos domésticos (Oryctolagus cuniculus) bajo atención veterinaria primaria en Inglaterra. Recreación Vet. 2020, 186, 451. [Google Scholar] [CrossRef]
- Mäkitaipale, J.; Harcourt-Brown, F.M.; Laitinen-Vapaavuori, O. Health survey of 167 pet rabbits (Oryctolagus cuniculus) in Finland. Vet. Rec. 2015, 177, 418. [Google Scholar] [CrossRef]
- Lord, B. Dental Disease in the Rabbit Part 4: Diagnosis and Management of Odontogenic Abscesses. UK Vet. Companion Anim. 2011, 16, 42–45. [Google Scholar] [CrossRef]
- Van Caelenberg, A.; De Rycke, L.; Hermans, K.; Verhaert, L.; van Bree, H.; Gielen, I. Diagnosis of Dental Problems in Pet Rabbits (Oryctolagus cuniculus). Vlaams Diergeneeskd. Tijdschr. 2008, 77, 386–394. [Google Scholar] [CrossRef]
- Summa, N.M.; Brandão, J. Evidence-Based Advances in Rabbit Medicine. Vet. Clin. N. Am. Exot. Anim. Pract. 2017, 20, 749–771. [Google Scholar] [CrossRef]
- Schumacher, M. Measurement of clinical crown length of incisor and premolar teeth in clinically healthy rabbits. J. Vet. Dent. 2011, 28, 90–95. [Google Scholar] [CrossRef]
- Harcourt-Brown, F. Dental Disease in Pet Rabbits 3. Jaw Abscesses. Practice 2009, 31, 496–505. [Google Scholar] [CrossRef]
- Tyrrell, K.L.; Citron, D.M.; Jenkins, J.R.; Goldstein, E.J.C. Periodontal Bacteria in Rabbit Mandibular and Maxillary Abscesses. J. Clin. Microbiol. 2002, 40, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Van Caelenberg, A.I.; De Rycke, L.M.; Hermans, K.; Verhaert, L.; Van Bree, H.J.; Gielen, I.M. Comparison of Radiography and CT to Identify Changes in the Skulls of Four Rabbits with Dental Disease. J. Vet. Dent. 2011, 28, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Palma-Medel, T.; Marcone, D.; Alegría-Morán, R. Dental Disease in Rabbits (Oryctolagus cuniculus) and Its Risk Factors—A Private Practice Study in the Metropolitan Region of Chile. Animals 2023, 13, 676. [Google Scholar] [CrossRef]
- Jackson, M.A.; Burn, C.C.; Hedley, J.; Brodbelt, D.C.; O’Neill, D.G. Enfermedad dental en conejos de compañía bajo atención veterinaria primaria del Reino Unido: Frecuencia y factores de riesgo. Rec. Vet. 2024, E3993. [Google Scholar] [CrossRef]
- Peña, T.; Campoy, L.; de Matos, R. Investigation of a Maxillary Nerve Block Technique in Healthy New Zealand White Rabbits (Oryctolagus cuniculus). Am. J. Vet. Res. 2020, 81, 843–848. [Google Scholar] [CrossRef]
- Hamlin, J. Causes, Examination and Treatment of Dental Disease in Rabbits. Vet. Nurse 2013, 4, 156–166. [Google Scholar] [CrossRef]
- Capello, V.; Lennox, A.M. Small Mammal Dentistry. In Ferrets, Rabbits, and Rodents; WB Saunders: Philadelphia, PA, USA, 2012; pp. 452–471. [Google Scholar]
- Lord, B. Dental Disease in the Rabbit Part 1: Normal Dentition and Diet. Companion Anim. 2011, 16, 53–55. [Google Scholar] [CrossRef]
- Donnelly, T.M.; Vella, D. Anatomy, Physiology and Non-Dental Disorders of the Mouth of Pet Rabbits. Vet. Clin. N. Am. Exot. Anim. Pract. 2016, 19, 737–756. [Google Scholar] [CrossRef]
- Fecchio, R.; Gioso, M.A.; Bannon, K. Exotic Animals Oral and Dental Diseases. In Wiggs’s Veterinary Dentistry: Principles and Practice; Lobprise, H., Dodd, J.R., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; pp. 481–499. [Google Scholar]
- Kharitonova, M.; Bokhina, O.; Klyukin, S. Analysis of the Incidence of Dental Pathologies in Ornamental and Agricultural Rabbits. Proc. BIO Web Conf. 2022, 43, 03031. [Google Scholar] [CrossRef]
- Meredith, A.L.; Prebble, J.L.; Shaw, D.J. Impact of Diet on Incisor Growth and Attrition and the Development of Dental Disease in Pet Rabbits. J. Small Anim. Pract. 2015, 56, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Lord, B. Management of Dental Disease in Rabbits. Vet. Nurs. J. 2012, 27, 18–20. [Google Scholar] [CrossRef]
- Ewringmann, A. Keimspektrum und Antibiotikasensitivitäten bei eitrigen Zahnerkrankungen von Kaninchen. Tierärztl. Prax. Ausg. K Kleintiere Heimtiere 2017, 45, 373–383. [Google Scholar] [CrossRef]
- Böhmer, E. Changes of the Cheek Teeth. In Dentistry in Rabbits and Rodents; Wiley: Hoboken, NJ, USA, 2015; pp. 153–212. [Google Scholar]
- Jackson, M.A.; O’Neill, D.G.; Hedley, J.; Brodbelt, D.C.; Burn, C.C. Dental Disease in Rabbits under UK Primary Veterinary Care: Clinical Management and Associated Welfare Impacts. Vet. Rec. 2025, e5326, Online Ahead of Print. [Google Scholar]
- Taylor, W.M.; Beaufrère, H.; Mans, C.; Smith, D.A. Long-Term Outcome of Treatment of Dental Abscesses with a Wound-Packing Technique in Pet Rabbits: 13 Cases (1998–2007). J. Am. Vet. Med. Assoc. 2010, 237, 1444–1449. [Google Scholar] [CrossRef]
- Jekl, V.; Hauptman, K.; Knotek, Z. Quantitative and Qualitative Assessments of Intraoral Lesions in 180 Small Herbivorous Mammals. Vet. Rec. 2008, 162, 442–449. [Google Scholar] [CrossRef]
- Harcourt-Brown, F. Dental disease in pet rabbits: 1. Normal dentition, pathogenesis and aetiology. In Pract. 2009, 31, 370–379. [Google Scholar] [CrossRef]
- Böhmer, E. Abscesses. In Dentistry in Rabbits and Rodents; Wiley: Hoboken, NJ, USA, 2015; pp. 213–241. [Google Scholar]
- Harcourt-Brown, F.M. The progressive syndrome of acquired dental disease in rabbits. J. Exot. Pet. Med. 2007, 16, 146–157. [Google Scholar] [CrossRef]
- Harcourt-Brown, F. Metabolic Bone Disease as a Possible Cause of Dental Disease in Pet Rabbits; Royal College of Veterinary Surgeons: London, UK, 2006. [Google Scholar]
- Lord, B. Dental Disease in the Rabbit Part 2: Dental Disease Causes, Clinical Signs and Diagnosis. UK Vet. Companion Anim. 2011, 16, 39–42. [Google Scholar] [CrossRef]
- Meredith, A.; Flecknell, P. BSAVA Manual of Rabbit Medicine, 2nd ed.; British Small Animal Veterinary Association: Gloucester, UK, 2014. [Google Scholar]
- Benato, L. Odontogenic abscesses in pet rabbits. Vet. Rec. 2017, 181, 536. [Google Scholar] [CrossRef]
- Radziwonowicz, J.; Dzierżanowska-Góryń, D.; Albera-Łojek, A. The influence of age, sex and diet on the occurrence of dental problems in rabbits and chinchillas in amateur breeding. Rocz. Nauk. Zootech. 2021, 48, 67–81. [Google Scholar]
- Franklin, C.V.B. Lack of association between exposure to natural sunlight and dental disease in French companion rabbits. J. Vet. Med. 2017, 1, 13. [Google Scholar]
- Jekl, V.; Redrobe, S. Rabbit dental disease and calcium metabolism—The science behind divided opinions. J. Small Anim. Pract. 2013, 54, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Eckermann-Ross, C. Hormonal Regulation and Calcium Metabolism in the Rabbit. Veterinary Clinics of North America: Exotic Animal Practice 2008, 11, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Redrobe, S. Calcium metabolism in rabbits. Seminars in Avian and Exotic Pet Medicine 2002, 11, 94–101. [Google Scholar] [CrossRef]
- Petrini, D.; Puccinelli, C.; Citi, S.; Del Chicca, F. Computed Tomographic Findings Secondary to Dental Pathologies: Comparison between Rabbits and Guinea Pigs. Vet. Sci. 2023, 10, 705. [Google Scholar] [CrossRef]
- Artiles, C.A.; Sanchez-Migallon Guzman, D.; Beaufrère, H.; Phillips, K.L. Computed tomographic findings of dental disease in domestic rabbits (Oryctolagus cuniculus): 100 cases (2009–2017). J. Am. Vet. Med. Assoc. 2020, 257, 313–327. [Google Scholar] [CrossRef]
- Capello, V. Intraoral Treatment of Dental Disease in Pet Rabbits. Vet Clin North Am Exot Anim Pract. 2016, 19, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Varga, M. Abscesses. In Textbook of Rabbit Medicine, 2nd ed.; Varga, M., Ed.; Elsevier Health Sciences: Oxford, UK, 2014; pp. 249–270. [Google Scholar]
- Pešić, A.; Vejnović, B.; Mitrović, M.J.; Vučićević, M. Age and diet-related associations with acquired dental disease in pet rabbits. J. Vet. Dent. 2025, 08987564251322839, Online Ahead of Print.. [Google Scholar]
- Mosallanejad, B.; Moarrabi, A.; Avizeh, R.; Ghadiri, A. Prevalencia de maloclusión dental y elongación de la raíz en conejos domésticos de Ahvaz, Irán. Rev. Iraní Cienc. Tecnol. Vet. 2010, 2, 109–116. [Google Scholar]
- Tokashiki, E.; Rahal, S.; Melchert, A.; Gonçalves, R.; Rolim, L.; Teixeira, C. Estudio retrospectivo de las condiciones agrupadas por sistemas corporales en conejos de compañía. Rev. Med. Mascotas Exóticas 2018, 29, 207–211. [Google Scholar] [CrossRef]
- Flenghi, L.; Mazouffre, M.; Le Loc’h, A.; Le Loc’h, G.; Bulliot, C. Normal bacterial flora of the oral cavity in healthy pet rabbits (Oryctolagus cuniculus). Vet. Med. Sci. 2023, 9, 1621–1626. [Google Scholar] [CrossRef]
- Gardhouse, S.; Sanchez-Migallon Guzman, D.; Paul-Murphy, J.; Byrne, B.A.; Hawkins, M.G. Bacterial isolates and antimicrobial susceptibilities from odontogenic abscesses in rabbits: 48 cases. Vet. Rec. 2017, 181, 538. [Google Scholar] [CrossRef] [PubMed]
- Crăciun, S.; Novac, C.Ş.; Fiţ, N.I.; Bouari, C.M.; Bel, L.V.; Nadăş, G.C. Bacterial diversity in pet rabbits: Implications for public health, zoonotic risks, and antimicrobial resistance. Microorganisms 2025, 13, 653. [Google Scholar] [CrossRef] [PubMed]
- Levy, I.; Mans, C. Diagnosis and outcome of odontogenic abscesses in client-owned rabbits (Oryctolagus cuniculus): 72 cases (2011–2022). J. Am. Vet. Med. Assoc. 2024, 262, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Jekl, V.; Jeklova, E.; Hauptman, K. Radical debridement guided by advanced imaging and frequent monitoring is an effective approach for the treatment of odontogenic abscesses and jaw osteomyelitis in rabbits: A review of 200 cases (2018–2023). J. Am. Vet. Med. Assoc. 2023, 261 (Suppl. S2), S52–S61. [Google Scholar] [CrossRef]
- Mishra, M.; Arya, M.; Mamachan, M.; Agnihotri, I.; Kumar Maiti, S. Surgical management of dental abscess in two New Zealand White rabbits: A case report. Acta Sci. Vet. Sci. 2023, 5, 2582–3183. [Google Scholar] [CrossRef]
- Charland, C.; Gruaz, M.; Nixon, K.; van Praag, E. Facial Bacterial Abscesses and Dermatitis in Rabbits. Available online: http://www.mediarabbit.com/ (accessed on 14 September 2023).
- Divisha, R.; Vigneshwar, R. Surgical Management of Skin Abscesses in New Zealand White Rabbits—A Clinical Case Report. Int. J. Theor. Appl. Sci. 2021, 13, 32–36. [Google Scholar]
- Millward, L. Rabbit. In Veterinary Cytology, 1st ed.; Sharkey, L.C., Radin, M.J., Seelig, D., Eds.; John Wiley & Son: Hoboken, NJ, USA, 2021; pp. 766–781. [Google Scholar]
- Capello, V. Surgical treatment of facial abscesses and facial surgery in pet rabbits. Vet. Clin. N. Am. Exot. Anim. Pract. 2016, 19, 799–823. [Google Scholar] [CrossRef]
- Risi, E. Étapes du traitement d’un abcès dentaire chez le Lapin. Pratique Vet. 2008, 45, 16–18. [Google Scholar]
- Van Caelenberg, A.I.; De Rycke, L.M.; Hermans, K.; Verhaert, L.; Van Bree, H.J.; Gielen, I.M. Computed tomography and cross-sectional anatomy of the head in healthy rabbits. Am. J. Vet. Res. 2010, 71, 293–303. [Google Scholar] [CrossRef]
- Capello, V. Clinical technique: Treatment of periapical infections in pet rabbits and rodents. J. Exot. Pet. Med. 2008, 17, 124–131. [Google Scholar] [CrossRef]
- Regalado, A.; Legendre, L. Full-mouth intraoral radiographic survey in rabbits. J. Vet. Dent. 2017, 34, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Borawski, W.; Kiełbowicz, Z.; Kubiak-Nowak, D.; Prządka, P.; Pasternak, G. Computed tomographic findings of dental disease and secondary diseases of the head area in client-owned domestic rabbits (Oryctolagus cuniculus): 90 cases. Animals 2024, 14, 1160. [Google Scholar] [CrossRef] [PubMed]
- Sasai, H.; Iwai, H.; Fujita, D.; Seto, E.; Izumi, Y. The use of micro-computed tomography in the diagnosis of dental and oral disease in rabbits. BMC Vet. Res. 2014, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Riggs, G.G.; Arzi, B.; Cissell, D.D.; Hatcher, D.C.; Kass, P.H.; Zhen, A.; Verstraete, F.J. Clinical application of cone-beam computed tomography of the rabbit head: Part 2—Dental disease. Front. Vet. Sci. 2017, 4, 3. [Google Scholar] [CrossRef]
- Riggs, G.G.; Arzi, B.; Cissell, D.D.; Hatcher, D.C.; Kass, P.H.; Zhen, A.; Verstraete, F.J. Clinical application of cone-beam computed tomography of the rabbit head: Part 1—Normal dentition. Front. Vet. Sci. 2016, 3, 93. [Google Scholar] [CrossRef]
- Hernandez-Divers, S.J. Clinical technique: Dental endoscopy of rabbits and rodents. J. Exot. Pet. Med. 2008, 17, 87–92. [Google Scholar] [CrossRef]
- Capello, V. Diagnostic imaging of dental disease in pet rabbits and rodents. Vet. Clin. N. Am. Exot. Anim. Pract. 2016, 19, 757–782. [Google Scholar] [CrossRef]
- Crossley, D.A. Oral Biology and Disorders of Lagomorphs. Vet. Clin. N. Am. Exot. Anim. Pract. 2003, 6, 629–659. [Google Scholar] [CrossRef]
- Bozkan, Z.; Aybak, E. Diagnosis and treatment of retrobulbar abscess in a White New Zealand rabbit (Oryctolagus cuniculus L.). Turk. J. Vet. Res. 2025, 9, 69–73. [Google Scholar] [CrossRef]
- Martínez-Jiménez, D.; Hernández-Divers, S.J.; Dietrich, U.M.; Williams, C.O.; Blasier, M.W.; Wilson, H.; Frank, P.M. Endosurgical treatment of a retrobulbar abscess in a rabbit. J. Am. Vet. Med. Assoc. 2007, 230, 868–872. [Google Scholar] [CrossRef]
- Czubaj, N.; Kliszcz, J.; Sobczyński, J.; Gralak, A. Unusual bilateral retrobulbar abscess in the rabbit. In Proceedings of the BSAVA Congress 2019, Birmingham, UK, 5 April 2019. [Google Scholar]
- Capello, V. Novel diagnostics and surgical techniques for treatment of difficult facial abscesses in pet rabbits. In Proceedings of the North American Veterinary Conference, Orlando, FL, USA, 16–20 January 2016; pp. 1685–1689. [Google Scholar]
- Lord, B. Dental disease in the rabbit. Part 3: Treatment and prognosis of dental disease. Companion Anim. 2011, 16, 46–49. [Google Scholar] [CrossRef]
- Lennox, A.; Capello, V.; Legendre, L. Small mammal dentistry. In Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery, 4th ed.; Quesenberry, K.E., Orcutt, C.J., Mans, C., Eds.; Elsevier: St. Louis, MO, USA, 2021. [Google Scholar]
- Hedley, J. Antibiotic usage in rabbits and rodents. In Pract. 2018, 40, 230–237. [Google Scholar] [CrossRef]
- Verstraete, F.J.M.; Osofsky, A. Dentistry in pet rabbits. Compend. Contin. Educ. Pract. Vet. 2005, 27, 671–684. [Google Scholar]
- Duangurai, T.; Siengsanan-Lamont, J.; Bumrungpun, C.; Kaewmongkol, G.; Areevijittrakul, L.; Sirinarumitr, T.; Fenwick, S.G.; Kaewmongkol, S. Identification of uncultured bacteria from abscesses of exotic pet animals using broad-range nested 16S rRNA polymerase chain reaction and Sanger sequencing. Vet. World. 2019, 12, 1546–1553. [Google Scholar] [CrossRef]
- Legendre, L. Treatment of oral abscesses in rodents and lagomorphs. J. Vet. Dent. 2011, 28, 30–33. [Google Scholar]
- Zaheer, O.; Ludwig, L.; Gardhouse, S.; Foster, R. Diagnosis, treatment, and characterization with advanced diagnostic imaging of an oral ectopic elodontoma in a pet rabbit (Oryctolagus cuniculus). J. Exot. Pet. Med. 2021, 37, 28–31. [Google Scholar] [CrossRef]
- Rosenthal, K. Therapeutic contraindications in exotic pets. Semin. Avian Exot. Pet. Med. 2004, 13, 44–48. [Google Scholar] [CrossRef]
- Cope, I. Selecting the right antimicrobial for use in pet species. Vet. Rec. 2016, 179, 329–330. [Google Scholar] [CrossRef]
- Benato, L.; Hastie, P.; O’Shaughnessy, P.; Murray, J.A.; Meredith, A. Effects of probiotic Enterococcus faecium and Saccharomyces cerevisiae on the faecal microflora of pet rabbits. J. Small Anim. Pract. 2014, 55, 442–446. [Google Scholar] [CrossRef]
- Cardoso, S.; Loc’h, A.; Marques, I.; Almeida, A.; Sousa, S.; Saavedra, M.; Anastácio, S.; Silveira, E. Unveiling the emergence of multidrug-resistant pathogens in exotic pets from France: A comprehensive study. One Health Implement. Res. 2023, 3, 161–176. [Google Scholar] [CrossRef]
- Barbosa, C.K.; Teixeira, V.N.; Pimpão, C.T. Antibiotic usage patterns in exotic pets: A study in Curitiba, Paraná, Brazil. Open Vet. J. 2023, 13, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Puvaca, N. Antimicrobial Resistance and Treatment in Companion, Food and Exotic Animals. Antibiotics 2022, 11, 1360. [Google Scholar] [CrossRef] [PubMed]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Vacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Balázs, V.L.; Nagy-Radványi, L.; Bencsik-Kerekes, E.; Koloh, R.; Szabó, D.; Kocsis, B.; Kocsis, M.; Farkas, Á. Antibacterial and Antibiofilm Effect of Unifloral Honeys against Bacteria Isolated from Chronic Wound Infections. Microorganisms 2023, 11, 509. [Google Scholar] [CrossRef]
- Durham, M.E.; Elfenbein, J.R. Evaluation of vaporized hydrogen peroxide sterilization on the in vitro efficacy of meropenem-impregnated polymethyl methacrylate beads. Am. J. Vet. Res. 2019, 80, 45–50. [Google Scholar] [CrossRef]
- Hajizadeh, F.; Derakhshan, B.; Peimani, A.; Abbasi, Z. Effect of topical honey on mandibular bone defect healing in rats. J. Contemp. Dent. Pract. 2018, 19, 47–51. [Google Scholar]
- Herjuno, P.; Wibowo, M.D.; Susilo, D.H. The Effectiveness of Manuka Honey in Increasing Osteoblasts and Chondrocytes and Reducing Osteoclasts in the Healing Process of Rabbit Mandibular Symphysis Fractures. Bali Med. J. 2025, 14, 38–40. [Google Scholar] [CrossRef]
- Loncaric, I.; Künzel, F. Sequence type 398 meticillin-resistant Staphylococcus aureus infection in a pet rabbit. Vet. Dermatol. 2013, 24, 10. [Google Scholar] [CrossRef]
- Onyango, L.A.; Liang, J. Manuka honey as a non-antibiotic alternative against Staphylococcus spp. and their small colony variant (SCVs) phenotypes. Front. Cell. Infect. Microbiol. 2024, 14, 1380289. [Google Scholar] [CrossRef]
- Onuoha, E.O.; Adekunle, A.A.; Ajike, S.O.; Gbotolorun, O.M.; Adeyemo, W.L. Effect of manuka honey socket dressing on postoperative sequelae and complications following third molar extraction: A randomized controlled study. J. Craniomaxillofac. Surg. 2023, 51, 252–260. [Google Scholar] [CrossRef]
- Saputri, R.A.H.; Massie, G.C.; Gatera, V.A.; Boesoirie, S.F. The application of honey in wound care of raw surface at spontaneous rupture submandibular abscess that extends to submental and right neck: A case report. Int. J. Surg. Case Rep. 2022, 90, 106672. [Google Scholar] [CrossRef] [PubMed]
- Minarikova, A.; Hauptman, K.; Knotek, Z.; Jekl, V. Microbial flora of odontogenic abscesses in pet guinea pigs. Vet. Rec. 2016, 179, 331. [Google Scholar] [CrossRef]
- Hartley, M.P.; Sanderson, S. Use of antibiotic impregnated polymethylmethacrylate beads for the treatment of chronic mandibular osteomyelitis in a Bennett’s wallaby (Macropus rufogriseus rufogriseus). Aust. Vet. J. 2003, 81, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.L.N.; Kazakos, G.M.; Pardali, D.; Patsikas, M.N.; Komnenou, A.T. Surgical management of orbital abscesses in domestic rabbits (Oryctolagus cuniculus): A report of seven cases. J. Hell. Vet. Med. Soc. 2020, 71, 2248–2260. [Google Scholar] [CrossRef]
- Hanssen, A.D.; Spangehl, M.J. Practical applications of antibiotic-loaded bone cement for treatment of infected joint replacements. Clin. Orthop. Relat. Res. 2004, 427, 79–85. [Google Scholar] [CrossRef]
- Rosenthal, K.L.; Quesenberry, K.E.; Carpenter, J.W. Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery, 3rd ed.; Saunders: Philadelphia, PA, USA, 2011. [Google Scholar]
- Poggiagliolmi, S.; Crowell-Davis, S.; Alworth, L.; Harvey, S. Environmental enrichment of New Zealand White rabbits living in laboratory cages. J. Vet. Behav. Clin. Appl. Res. 2011, 6, 343–350. [Google Scholar] [CrossRef]
- Edgar, J.L.; Mullan, S.M. Knowledge and attitudes of 52 UK pet rabbit owners at the point of sale. Vet. Rec. 2011, 168, 353. [Google Scholar] [CrossRef]
- Conway, R.E.; Burton, M.; Mama, K.; Rao, S.; Kendall, L.V.; Desmarchelier, M.; Sadar, M.J. Behavioral and Physiologic Effects of a Single Dose of Oral Gabapentin in Rabbits (Oryctolagus cuniculus). Top. Companion Anim. Med. 2023, 53–54, 100779. [Google Scholar] [CrossRef]
- Varela, K.; Brown, J.A.; Lipton, B.; Dunn, J.; Stanek, D.; Behravesh, C.B.; Chapman, H.; Conger, T.H.; Vanover, T.; Edling, T.; et al. A review of zoonotic disease threats to pet owners: A compendium of measures to prevent zoonotic diseases associated with non-traditional pets: Rodents and other small mammals, reptiles, amphibians, backyard poultry, and other selected animals. Vector Borne Zoonotic Dis. 2022, 22, 303–360. [Google Scholar] [CrossRef]
- Rahman, M.T.; Sobur, M.A.; Islam, M.S.; Ievy, S.; Hossain, M.J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic diseases: Etiology, impact, and control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef]
- Zanen, L.A.; Kusters, J.G.; Overgaauw, P.A.M. Zoonotic risks of sleeping with pets. Pathogens 2022, 11, 1149. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Hill, F.; Elsohaby, I. Retrospective analysis of antimicrobial resistance in bacterial pathogens from pet rabbits in Hong Kong, 2019–2022. J. Vet. Diagn. Investig. 2024, 36, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Jelinski, D.C.; Orsel, K.; Weese, J.S.; Conly, J.M.; Julien, D.A. Antibacterial treatment for exotic species, backyard ruminants and small flocks: A narrative review highlighting barriers to effective and appropriate antimicrobial treatment. BMC Vet. Res. 2022, 18, 220. [Google Scholar] [CrossRef]
- Schwab, M.; Brockmann, M.; Stumpf, P.; Pfabe, J.; Müller, E.; Pees, M.; Marschang, R.E. Isolation of aerobic bacteria from abscesses and wounds in rabbits and antibiotic susceptibility testing of Staphylococcus spp. and Pseudomonas spp. isolates. J. Exot. Pet. Med. 2024, 49, 41–47. [Google Scholar] [CrossRef]
- Nadăș, G.C.; Novac, C.Ș.; Matei, I.A.; Bouari, C.M.; Gal, Z.M.; Tamas-Krumpe, O.M.; Macri, A.M.; Fiț, N.I. Prevalence of antimicrobial resistant bacteria from conjunctival flora in an eye infection prone breed (Saint Bernard). Molecules. 2021, 26, 2219. [Google Scholar] [CrossRef]
- Robinson, T.P.; Bu, D.P.; Carrique-Mas, J.; Fèvre, E.M.; Gilbert, M.; Grace, D.; Hay, S.I.; Jiwakanon, J.; Kakkar, M.; Kariuki, S.; et al. Antibiotic resistance is the quintessential One Health issue. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 377–380. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guardabassi, L.; Schwarz, S.; Lloyd, D.H. Pet animals as reservoirs of antimicrobial-resistant bacteria. J. Antimicrob. Chemother. 2004, 54, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Guardabassi, L.; Jensen, L.B.; Kruse, H. (Eds.) Guide to Antimicrobial Use in Animals; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Emerson, J.A.; Whittington, J.K.; Allender, M.C.; Mitchell, M.A. Effects of ultraviolet radiation produced from artificial lights on serum 25-hydroxyvitamin D concentration in captive domestic rabbits (Oryctolagus cuniculi). Am. J. Vet. Res. 2014, 75, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Mäkitaipale, J.; Opsomer, H.; Steiner, R.; Riond, B.; Liesegang, A.; Clauss, M.; Hatt, J.-M. Serum vitamin D concentrations in rabbits (Oryctolagus cuniculus) are more affected by UVB irradiation of food than irradiation of animals. Vet. J. 2024, 306, 106149. [Google Scholar] [CrossRef]
- Molitor, L.E.; Rockwell, K.; Gould, A.; Mitchell, M.A. Effects of Short-Duration Artificial Ultraviolet B Exposure on 25-Hydroxyvitamin D3 Concentrations in Domestic Rabbits (Oryctolagus cuniculus). Animals 2023, 13, 1307. [Google Scholar] [CrossRef]

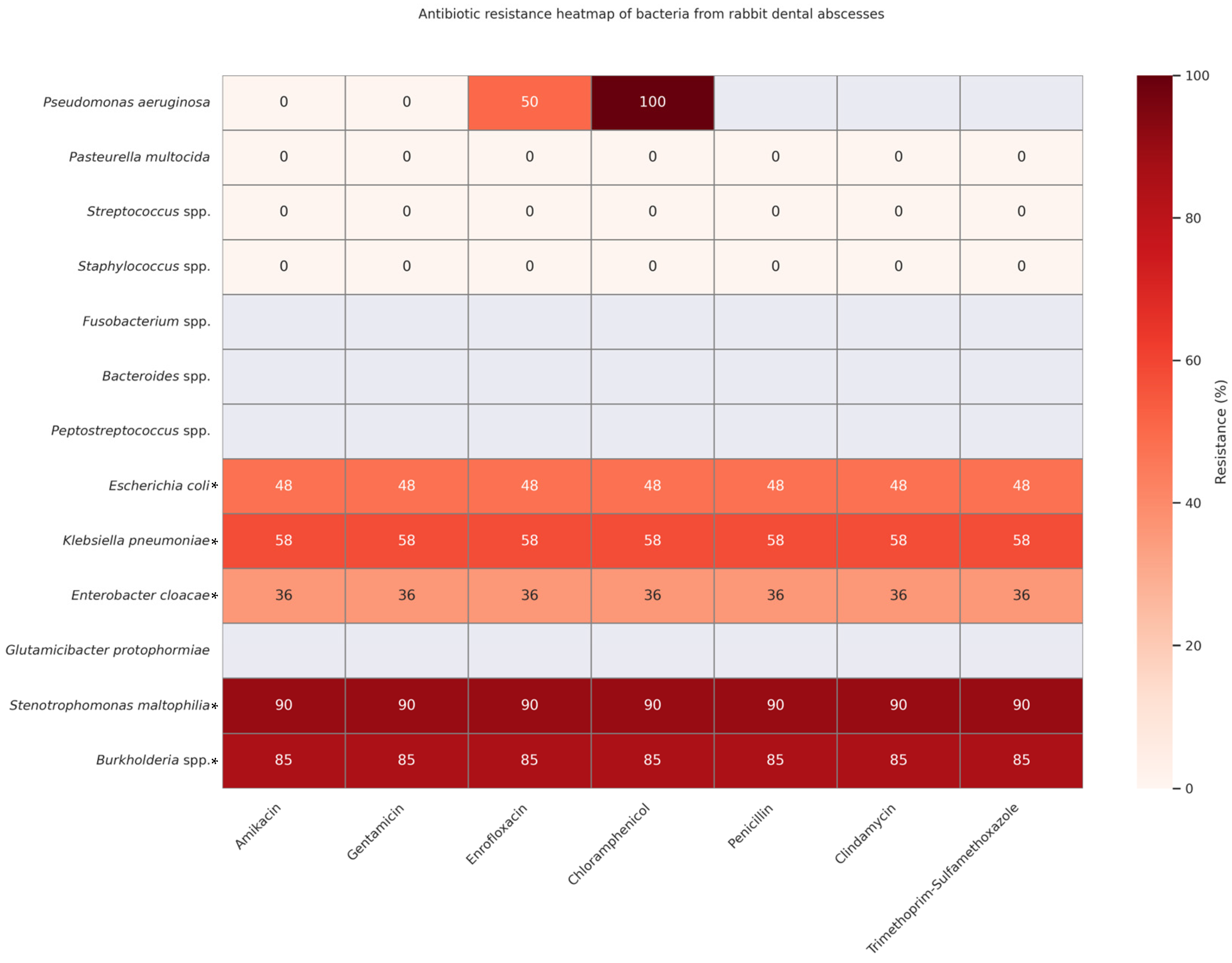
| Antibiotic | Compatible with AIPMMA? | Remarks |
|---|---|---|
| Gentamicin | Yes | Heat-stable. Released via concentration gradient, achieving higher local levels than systemic levels. Effective against Gram-negatives. Common in mandibular infections [32,98]. |
| Amikacin | Yes | Heat-stable. Effective against Gram−, including Pseudomonas spp. Ideal for local use due to systemic nephrotoxicity [32,98]. |
| Tobramycin | Yes | Heat-stable. Similar spectrum to gentamicin. Used in both human and veterinary medicine [32]. |
| Neomycin | Yes | Heat-stable. Narrower spectrum but useful against Gram−. Toxic systemically and, hence, good for local use [98]. |
| Cefazolin | Yes | Heat-stable. Active against Gram+; stable during polymerization [32]. |
| Ceftazidime | Yes | Heat-stable. Broad-spectrum, including Pseudomonas spp. Suitable for co-infections [32]. |
| Ceftiofur | Yes | Heat-stable. Commonly used in veterinary medicine. Active against Gram− bacteria [32]. |
| Lincomycin | Yes | Heat-stable. Similar spectrum to clindamycin. Useful against anaerobes, with similar risks for gut flora [98]. |
| Clindamycin | Yes, with caution | Heat-stable. Good local release; active against anaerobes. Risk of enterotoxemia in rabbits if ingested accidentally [56,98]. |
| Enrofloxacin | No | Heat-labile. Degrades during polymerization. Not recommended [32]. |
| Penicillins | No | Heat-labile (e.g., ampicillin, penicillin G). Inactivated during PMMA polymerization [32]. |
| Metronidazole | No (partially) | Heat-labile. Microencapsulated versions under research; not compatible in standard form [100]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crăciun, S.; Nadăş, G.C. Odontogenic Abscesses in Pet Rabbits: A Comprehensive Review of Pathogenesis, Diagnosis, and Treatment Advances. Animals 2025, 15, 1994. https://doi.org/10.3390/ani15131994
Crăciun S, Nadăş GC. Odontogenic Abscesses in Pet Rabbits: A Comprehensive Review of Pathogenesis, Diagnosis, and Treatment Advances. Animals. 2025; 15(13):1994. https://doi.org/10.3390/ani15131994
Chicago/Turabian StyleCrăciun, Smaranda, and George Cosmin Nadăş. 2025. "Odontogenic Abscesses in Pet Rabbits: A Comprehensive Review of Pathogenesis, Diagnosis, and Treatment Advances" Animals 15, no. 13: 1994. https://doi.org/10.3390/ani15131994
APA StyleCrăciun, S., & Nadăş, G. C. (2025). Odontogenic Abscesses in Pet Rabbits: A Comprehensive Review of Pathogenesis, Diagnosis, and Treatment Advances. Animals, 15(13), 1994. https://doi.org/10.3390/ani15131994








