Simple Summary
Maintaining fish health is essential for the growth of sustainable aquaculture. One emerging strategy is the use of postbiotics as functional feed additives. In this study, we evaluated the dietary inclusion of postbiotics derived from Vibrio proteolyticus in juvenile gilthead seabream. Fish were fed either a standard diet or a postbiotic-supplemented diet for 62 days. At the end of the feeding period, we examined their intestinal health, the composition of gut bacteria, and their immune response. Fish that received the postbiotic diet showed a healthy intestinal structure and changes in the bacterial community that favored beneficial microbes while reducing potentially harmful ones. Additionally, these fish had lower levels of inflammation-related gene activity, suggesting a more balanced immune status. After being exposed to a lipopolysaccharide (LPS) challenge, they also showed increased expression of a gene associated with maintaining gut integrity. Overall, this study shows that postbiotics from V. proteolyticus DCF12.2 can support intestinal health and help regulate immune responses in farmed fish.
Abstract
The use of postbiotics for dietary fortification in aquaculture is gaining increasing attention due to their potential immunomodulatory and gut health benefits. In this study, we evaluated the effects of postbiotics derived from Vibrio proteolyticus DCF12.2 on intestinal histology, microbiota composition, and the expression of genes related to epithelial integrity and inflammation in juvenile gilthead seabream (Sparus aurata). Fish were fed either a control (CRTL) diet or the postbiotic-supplemented diet (VP) for 62 days. At the end of the feeding trial, a lipopolysaccharide (LPS) challenge was conducted to evaluate the immune response in fish. Histological analysis revealed a healthy mucosa in both groups, though fish fed the VP diet reduced fold height and mucosal layer thickness, alongside a significant increase in goblet cells. Microbiota profiling indicated higher alpha diversity and significant shifts in community composition in the VP group, including enrichment of potentially beneficial genera (Pseudomonas, Sphingomonas) and depletion of opportunistic taxa (Enterococcus, Stenotrophomonas). After the feeding trial, fish fed the VP diet exhibited downregulation of pro-inflammatory markers (tnfα, cox2). Following LPS challenge, cdh1—a key epithelial adhesion protein required for maintaining intestinal barrier integrity—expression was upregulated significantly in the VP group, suggesting enhanced epithelial resilience. These findings demonstrate that dietary fortification with V. proteolyticus-derived postbiotics supports mucosal health as well as modulates the intestinal microbiota and immune responses in gilthead seabream juveniles, offering a promising strategy for functional aquafeed development in sustainable aquaculture.
1. Introduction
Fish serve as a vital component of the human diet, providing high-quality protein, essential fatty acids, and minerals necessary for growth and overall health [1,2]. In the context of aquaculture, it is important to evaluate various zootechnical, biological, and biochemical parameters, including growth performance [3]. Beyond these traditional metrics, recent advances have shifted attention toward nutritional strategies that not only support growth but also bolster fish health and farm sustainability. In this regard, functional feed additives have emerged as an innovative strategy to enhance sustainability, disease resistance, and resource optimization in aquaculture [4]. Among these additives, probiotics, which are live microorganisms that improve growth and confer health benefits to the host [5,6], have been widely included in aquafeeds [7,8].
Our research group previously identified Vibrio proteolyticus DCF12.2, isolated from healthy wedge sole (Dicologlossa cuneata), as a promising probiotic candidate due to its ability to enhance the immune response in fish, including the induction of cross-reactive antibody responses against fish pathogens [9,10]. This strain also exhibited other beneficial attributes, such as pathogen inhibition, non-virulence towards fish, resilience under storage conditions, and diverse hydrolytic activities (lecithinase, gelatinase, caseinase, amylase, and lipase), which could contribute to improving nutrient absorption in fish [9]. Moreover, it remained viable after passing through the fish gastrointestinal tract [10] and demonstrated protective effects against experimental infections with Photobacterium damselae subsp. piscicida and Vibrio harveyi [10], reinforcing its potential as a preventive strategy against fish diseases after dietary administration.
Nevertheless, the use of live probiotics poses certain risks, including horizontal gene transfer and the potential spread of antibiotic resistance, which may compromise their safety and efficacy [11]. To address these issues, the use of postbiotics has emerged as a promising alternative. In this sense, the International Scientific Association for Probiotics and Prebiotics (ISAPP) convened a panel that defined postbiotics as a “preparation of inanimate microorganisms and/or their components that confers a health benefit to the host” [12].
Postbiotic production traditionally relies on synthetic culture media formulated from refined substrates, which drives up costs and compromises sustainability, while energy-intensive processes further hinder large-scale use in aquafeeds [13]. Conversely, bacterial culture parameters—such as nutrient source, pH, and oxygen availability—can markedly influence the composition, stability, and bioactivity of the resulting postbiotic preparations [14]. Thus, in order to overcome these economic and environmental barriers, optimizing production using cost-effective agro-industrial by-products as culture substrates is critical for enhancing sustainability, efficacy, stability, and scalability of postbiotic production [15].
For instance, dietary supplementation with a cell-free extract derived from Lactobacillus plantarum significantly improved growth performance and stress resistance in white shrimp (Penaeus vannamei) [16]. Similarly, postbiotics derived from Bacillus pumilus have been shown to exert beneficial effects on the intestinal microbiota of grouper (Epinephelus coioides) [17] and digestive enzyme activity in gilthead seabream (Sparus aurata) [18], highlighting their potential for gut health improvement. These findings support the use of postbiotics as a promising strategy in aquafeeds, offering benefits in growth, immunity, and disease resistance when properly integrated [19].
Despite the well-characterized probiotic properties of V. proteolyticus DCF12.2, its potential as a postbiotic remains largely unexplored. Preliminary evaluations of the extracellular products (ECPs) produced by this strain under several culture conditions have revealed promising in vitro bioactivities, including stimulation of cellular proliferation, antibacterial and antibiofilm effects against fish pathogens, and enzymatic hydrolysis of both nutritional and antinutritional compounds. These effects were particularly evident when ECPs were obtained from V. proteolyticus DCF12.2 cultured in aquafeed-based media at 15 °C for 48 h. However, the in vivo effects of these postbiotic preparations within the fish gastrointestinal tract—a key organ for nutrient absorption and immune defense—remain unknown [20].
To address this gap, the present study evaluates the influence of dietary administration of a postbiotic obtained from V. proteolyticus DCF12.2 on gilthead seabream juveniles, focusing on its effects on intestinal histology, intestinal microbiota, and gene expression. Furthermore, at the end of the feeding trial, a lipopolysaccharide (LPS) challenge was conducted to analyze if the dietary administration of ECPs enhanced the fish’s immune response.
2. Materials and Methods
2.1. Bacterial Strain and Extracellular Product (ECPs) Extraction
V. proteolyticus DCF12.2 [9], originally isolated from healthy wedge sole (D. cuneata), was first cultured on tryptic soy agar (TSA; Oxoid Ltd., Basingstoke, UK) supplemented with 1.5% NaCl. After incubation at 23 °C for 24 h, one or two colonies were transferred to 50 mL of tryptic soy broth (TSB; Oxoid Ltd., Basingstoke, UK) with 1.5% NaCl and incubated at 23 °C for 12 h under shaking conditions (80 rpm) to reach the early stationary phase (approx. 109 CFU mL−1).
ECPs were obtained by culturing the strain on a medium containing experimental aquafeed (160 g L−1) and agar (1.5%), following the method described by Liu [21] with modifications [14]. The aquafeed, provided by LifeBioencapsulation S.L., was previously characterized in detail [14]. Briefly, it was composed of the following ingredients (g/100 g): fishmeal, (10), soybean protein concentrate (15), wheat gluten (17), pea protein concentrate (5), soybean meal (20), wheat meal (14.14), fish oil (7), soybean oil (4.5), rapeseed oil (4.5), vitamins and minerals (1), vitamin C (0.05), vitamin E (0.5), and monocalcium phosphate (1.3).
After incubation, bacterial cells were harvested using 2 mL of sterile phosphate-buffered saline (PBS, pH 7.2), centrifuged (10,000× g, 20 min, 4 °C), and the resulting supernatants were sequentially filtered through 0.45 µm and 0.2 µm membrane filters (Merck Millipore, Billerica, MA, USA) to obtain cell-free ECPs. Protein concentration was quantified using the Qubit™ Protein Assay Kit and Qubit 2.0 fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). ECPs were aliquoted and stored at −80 °C until use.
2.2. Experimental Diets
Two experimental diets were elaborated and produced at the Ceimar-Universidad de Almería facilities (Servicio de Piensos Experimentales): (i) a control diet (CTRL diet) mimicking the ingredient composition of commercial diets for gilthead seabream, including 20% fishmeal and 9.7% fish oil; (ii) a diet fortified with the ECPs solution (10 mL kg−1, ECP protein concentration 900 µg mL−1) applied to the feed pellets after cold-extrusion by using a vacuum fat coater (VP diet). The ingredients were first homogenized in a 10 L mixer, then finely ground with a hammer mill (UPZ 100, Hosokawa-Alpine, Augsburg, Germany) to 0.5 mm. The diets were cold-extruded in a two-screw extruder (Evolum 25, Clextral, France), fitted with 2 or 3 mm die holes. The extruder barrel consisted of four sections, and the temperature profile in each segment (from inlet to outlet) was 40, 40, 45, and 45 °C, respectively. Pellets were dried at 27 °C in a drying chamber (Airfrio, Almería, Spain) and cooled to room temperature. The ECP solution was applied to the diets the next day using a Pegasus PG-10VC LAB vacuum coater (Dinnissen, Sevenum, The Netherlands). Ingredient composition and proximate analysis of the diets are shown in Table 1. Proximate analysis of feeds was determined according to AOAC [22] procedures for dry matter and ash. Crude protein (N × 6.25) was determined by elemental analysis using a Fisons EA 1108 analyzer (Fisons Instruments, Beverly, MA, USA). Total lipid content was quantified following the procedure described by Folch et al. [23] using chloroform/methanol (2:1 v/v) as solvent.

Table 1.
Ingredient and chemical composition (% dry basis) of the experimental diets used in the feeding trial.
2.3. Feeding Trial
Juvenile gilthead seabream (S. aurata) (32.7 ± 5.2 g) were obtained from a commercial hatchery (CUPIBAR, Chiclana de la Frontera, Cádiz, Spain) and acclimated to the experimental facilities at the Servicios Centrales de Investigación en Cultivos Marinos (SCI-CM, CASEM, University of Cádiz, Puerto Real, Cádiz; Spanish Operational Code REGA ES11028000312). Fish were maintained for 2 weeks in an open-flow seawater system under controlled conditions: temperature (19 °C), salinity (37 ppt), and natural photoperiod from January to March 2024 (36°31′45″ N, 6°11′31″ W). After acclimation, fish were randomly distributed into six 400 L tanks (n = 30 fish/tank; initial density 4.00 ± 0.02 kg m−3) and fed one of two experimental diets for 62 days: a control diet (CTRL diet) or a diet supplemented with ECPs from V. proteolyticus DCF12.2 (VP diet), each in triplicate. Fish were fed six times a week manually to apparent satiety twice daily. Diet identity was blinded to the personnel performing the feeding; feeds were labeled using color codes to eliminate observer bias.
All experimental procedures were performed in accordance with European Directive 2010/63/EU and Spanish legislation (RD 53/2013) regarding animal experimentation. Approval was granted by the Ethics and Animal Welfare Committee of the University of Cádiz and the Andalusian Regional Government (Junta de Andalucía, reference number 3/11/21/173).
2.4. Immunological Challenge
At the end of the feeding trial, six fish per tank (n = 18 per group) were randomly selected for a lipopolysaccharide (LPS) challenge. Prior to handling, fish were anesthetized with 2-phenoxyethanol (0.3 mL L−1). Fish from the CTRL group were intraperitoneally injected with either 0.1 mL of sterile saline (n = 3 per tank, n = 9 per group) or 0.1 mL of LPS (50 μg mL−1, Sigma-Aldrich, Madrid, Spain, #L4005) (n = 3 per tank, n = 9 per dietary treatment). The same procedure was applied to fish from the VP group (n = 3 per tank, n = 9 per treatment).
2.5. Fish Sampling
At the end of the 62-day feeding period, five fish per tank (n = 15 per dietary treatment) were randomly selected, fasted for 24 h, and euthanized with an overdose of 2-phenoxyethanol (1 mL L−1). Immediately after dissection, the abdominal cavity was opened, and the entire intestine was carefully removed. Whole intestines were preserved in DNA/RNA Shield (ZYMO Research) and stored at −80 °C for subsequent gene expression and intestinal microbiota analyses. Additionally, 1 cm sections of the proximal intestine from three fish per tank (n = 9 per dietary group) were excised and fixed for histological evaluation (see Section 2.6 and Section 2.7).
Following the intraperitoneal injection with saline or LPS solution, all challenged fish were sampled 72 h post-inoculation. The whole intestine was collected and stored at −80 °C for gene expression analysis.
2.6. Intestine Histology
Intestinal samples were fixed for 24 h in phosphate-buffered formalin (4% v/v, pH 7.2) at room temperature, and then dehydrated and embedded in paraffin following standard histological procedures. Transverse sections (5 μm) were cut to encompass the intestinal lumen. Slides were stained using hematoxylin and eosin (H&E) and examined under an Olympus IX51 light microscope equipped with a CC12 digital camera (Olympus Soft Imaging Solutions GmbH, Münster, Germany). Morphometric analysis was performed using ImageJ software (version 1.45; National Institutes of Health Image software, Bethesda, MD, USA). For each sample (9 fish per diet), 10 measurements per fish were recorded, assessing mucosal fold length, enterocyte height, lamina propria thickness, and goblet cell density (number per 100 μm of mucosal fold). These parameters were selected due to their sensitivity to dietary changes, particularly plant-derived ingredients [24].
2.7. Ultrastructural Study of the Intestinal Mucosa
At the end of the feeding trial, intestinal samples were collected for scanning (SEM) electron microscopy analysis. Tissues were fixed in 4% formaldehyde in phosphate buffer (pH 7.2) for 24 h at room temperature. They were then rinsed and passed through an ethanol gradient for dehydration. Samples were dried at the critical point using ethanol as the intermediate fluid and CO2 as the transition fluid (critical point dryer CDP 030, Leica Microsystems, Madrid, Spain). Dried samples were mounted on aluminum stubs, secured with colloidal graphite (PELCO Colloidal Graphite, Ted Pella Inc., Redding, CA, USA), and coated with gold using an SCD 005 Sputter Coater (Leica Microsystems, Madrid, Spain). SEM observations were conducted using a HITACHI S-3500 scanning electron microscope (Hitachi High Technologies Corporation, Tokyo, Japan). Digital images were processed with UTHSCSA ImageTool (University of Texas Health Sciences Center, San Antonio, TX, USA). SEM image data were used to estimate the apical area of enterocytes (EA) according to Vizcaíno et al. [25].
2.8. Microbiota Analysis from the Fish Gut
DNA was extracted from intestinal samples (n = 12 per dietary group) using a saline precipitation protocol [26], with modifications described by Tapia-Paniagua et al. [27]. A blank control using ddH2O was included to monitor contamination. DNA concentration was measured fluorometrically with the Qubit™ dsDNA HS Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA), while purity and integrity were assessed using a NanoDrop™ One UV–Vis Spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and 1% agarose gel electrophoresis, respectively.
The V3–V4 region of the 16S rRNA gene was amplified using the primers 5′-CCTACGGGNGGCWGCAG-3′ and 5′-GACTACHVGGGTATCTAATCC-3′ [28] and sequenced on an Illumina MiSeq platform (2 × 300 bp paired-end reads) at the Ultrasequencing Service of Novogene Europe (Munich, Germany).
Raw reads were quality-checked using FastQC (v0.11.9) [29]. Data processing, including trimming, error correction, and taxonomic assignment, was performed using the DADA2 pipeline with the SILVA v138 database [30], using a 99% similarity cutoff. Downstream microbiota analyses were conducted using the phyloseq and vegan packages in R [31,32]. Alpha diversity was assessed by calculating observed richness, Shannon, and Simpson diversity indices. Beta diversity was evaluated using non-metric multidimensional scaling (NMDS) based on Bray–Curtis dissimilarities. Amplicon sequence variants (ASVs) with fewer than 10 reads in at least 10% of the samples were removed. Taxonomic classification was reported at the phylum and genus levels.
Functional predictions of the microbial community were inferred using PICRUSt2 (v2.5) based on 16S rRNA gene data (https://github.com/picrust/picrust2/wiki, accessed on 20 October 2024).
2.9. Gene Expression Evaluation
Total RNA was extracted from the intestinal tissues of six fish per experimental group from the feeding trial using the GeneJET RNA Purification Kit (#K0732, Thermo Scientific), following the manufacturer’s protocol. The same procedure was applied to intestinal samples from fish injected with saline solution or LPS (n = 6 per group). RNA concentration was measured at 260 nm using a NanoDrop ND-1000 spectrophotometer, and RNA integrity was confirmed by agarose gel electrophoresis. Samples were stored at −80 °C until further use. To remove genomic DNA contamination, total RNA was treated with DNase I (Roche Diagnostics GmbH, Mannheim, Germany) following the manufacturer’s instructions. Reverse transcription was performed using the qScript cDNA Kit (Quanta BioSciences, Gaithersburg, MD, USA) with 1 μg of total RNA, and the resulting cDNA was stored at −20 °C.
Quantitative PCR (qPCR) was used to evaluate the relative expression of genes related to intestinal permeability and integrity—cadherin 1 (cdh1), cadherin 17 (cdh17), integrin β6 (itgb6), occludin (ocln), and zonula occludens-1 (tjp1)—as well as pro-inflammatory markers tumor necrosis factor α (tnfα) and cyclooxygenase-2 (cox2) (Table 2). Expression levels were normalized using two reference genes: elongation factor 1α (ef1α) and glyceraldehyde 3-phosphate dehydrogenase (gapdh) (Table 2).

Table 2.
List of genes studied in this work.
qPCR reactions were conducted in triplicate using a C1000 Touch™ thermal cycler with a CFX96™ optical module (Bio-Rad Laboratories, Madrid, Spain). Each reaction (10 μL final volume) contained 5 μL of GoTaq® qPCR Master Mix (Promega Co., Madison, WI, USA), 0.5 μL each of forward and reverse primers (10 μM), 1 μL of cDNA, and 3 μL of nuclease-free water. qPCR cycling conditions followed the protocol described by Cerezo-Ortega et al. [36]. Amplification threshold cycle (Cq) values above 40 were considered negative. Relative mRNA expression was calculated using the 2−ΔΔCq method [37], with normalization based on the geometric mean of the two reference genes and expression levels relative to the corresponding control group.
2.10. Statistical Analysis
Statistical differences in microbiota alpha diversity were determined using Student’s t-test, while differences in beta diversity between treatments were assessed via PERMANOVA. Predicted metabolic pathways were analyzed using the ALDEx2 tool following PICRUSt2 recommendations. Significantly different pathways were identified based on ALDEx2 “effect” size cutoffs of 0.5.
Histological parameters, taxonomic composition, and gene expression levels following the feeding trial were compared between experimental groups using Student’s t-test. For the experimental challenge, differences between groups were analyzed using two-way ANOVA followed by Tukey’s post hoc test. All data are expressed as mean ± standard deviation (SD), and differences were considered statistically significant at p ≤ 0.05.
All statistical analyses were performed using GraphPad Prism 9 (version 9.3.0; GraphPad Software, La Jolla, CA, USA).
3. Results
No fish mortality was recorded during the experimental period. Although growth performance was not a primary objective of this study, final body weight did not differ significantly between groups (70.1 ± 1.6 g in the CTRL group and 69.2 ± 2.1 g in the VP group; p > 0.05).
3.1. Effect of ECPs on Intestinal Histology and Ultrastructure
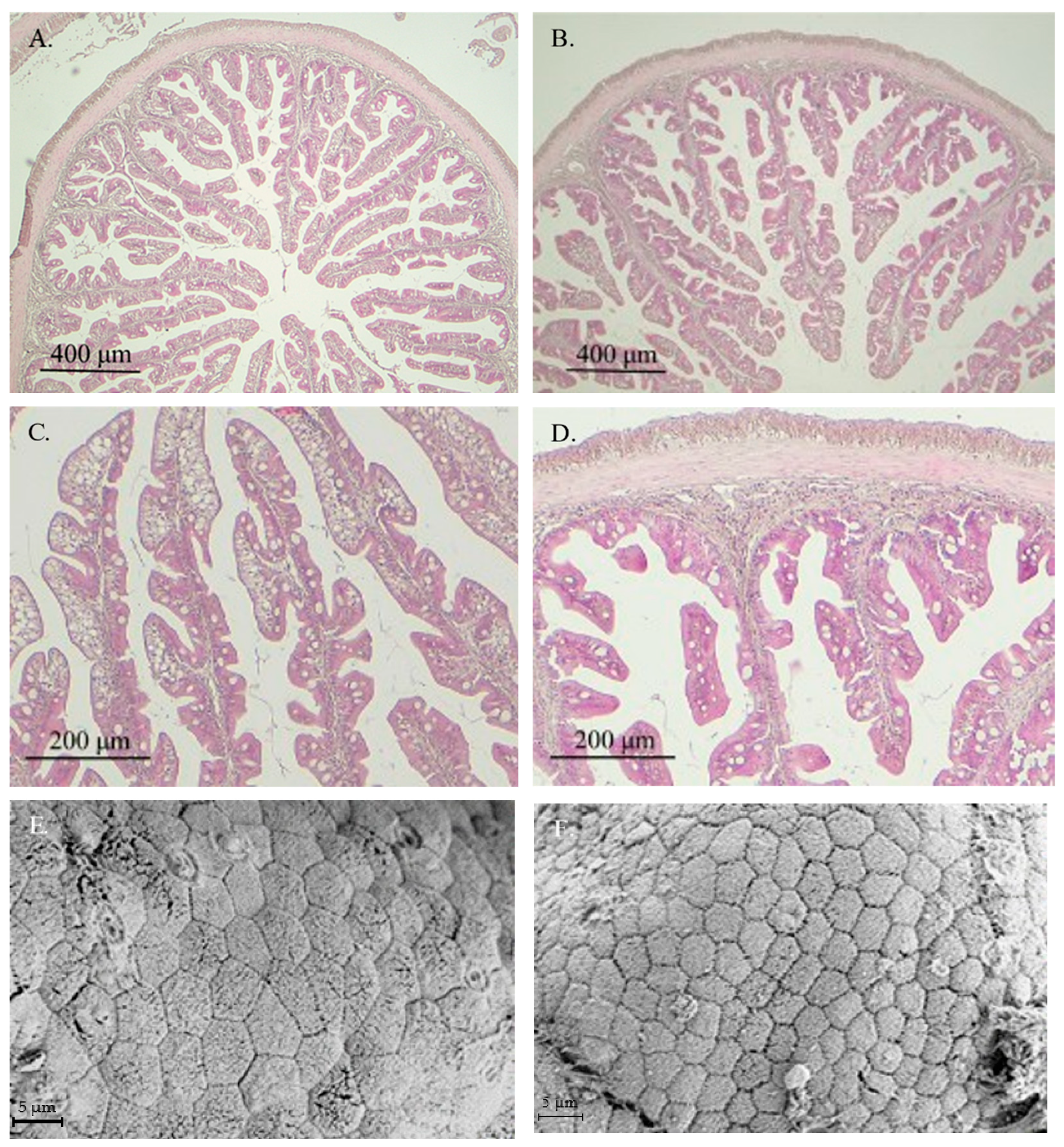
In general, healthy intestinal mucosa was observed in fish from both experimental groups, with no histological alterations or signs of enteritis in specimens receiving the VP diet (Figure 1B–D). Ultrastructural analysis showed similar healthy intestinal mucosa in fish fed both experimental diets (Figure 1E,F).
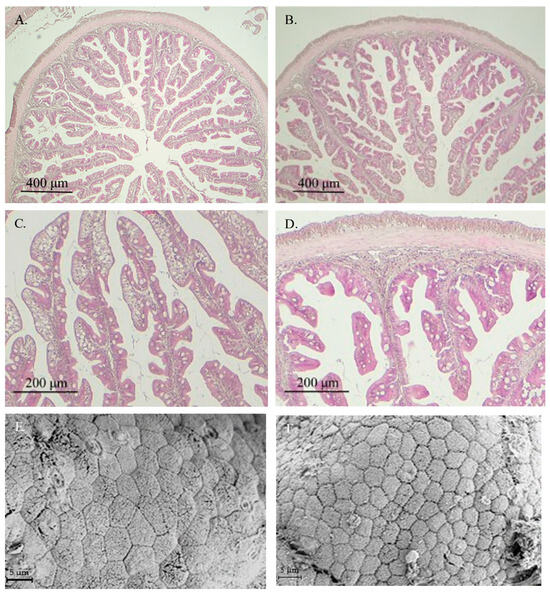
Figure 1.
Images from light microscopy (A–D) and scanning electron microscopy (E,F) of the anterior intestinal region of gilthead seabream juveniles fed with CTRL (A,C,E) or VP (B,D,F) diets. No significant differences were observed between fish receiving both aquafeeds. CTRL: control diet; VP: ECPs of V. proteolyticus diet.
The histomorphometric data derived from those images are presented in Table 3. Fish fed the VP diet exhibited significantly shorter intestinal folds, reduced enterocyte height, and decreased thickness of the lamina propria, muscular layer, and submucosa compared to those fed the CT diet. In contrast, the number of goblet cells enhanced significantly in the VP group. Additionally, the apical area of the enterocytes was significantly larger in fish from the CT group than in those fed the VP diet.

Table 3.
Histomorphometric analysis of the intestinal mucosa in juvenile gilthead seabream fed with the experimental diets.
3.2. Effect of ECPs on Intestinal Microbiota
No significant differences were observed in the number of observed ASVs between dietary groups (Table 4). Furthermore, Shannon and Simpson’s indices were found to be significantly higher in fish fed the VP diet.

Table 4.
Alpha diversity indices in the intestine of gilthead seabream juveniles fed with the experimental diets.
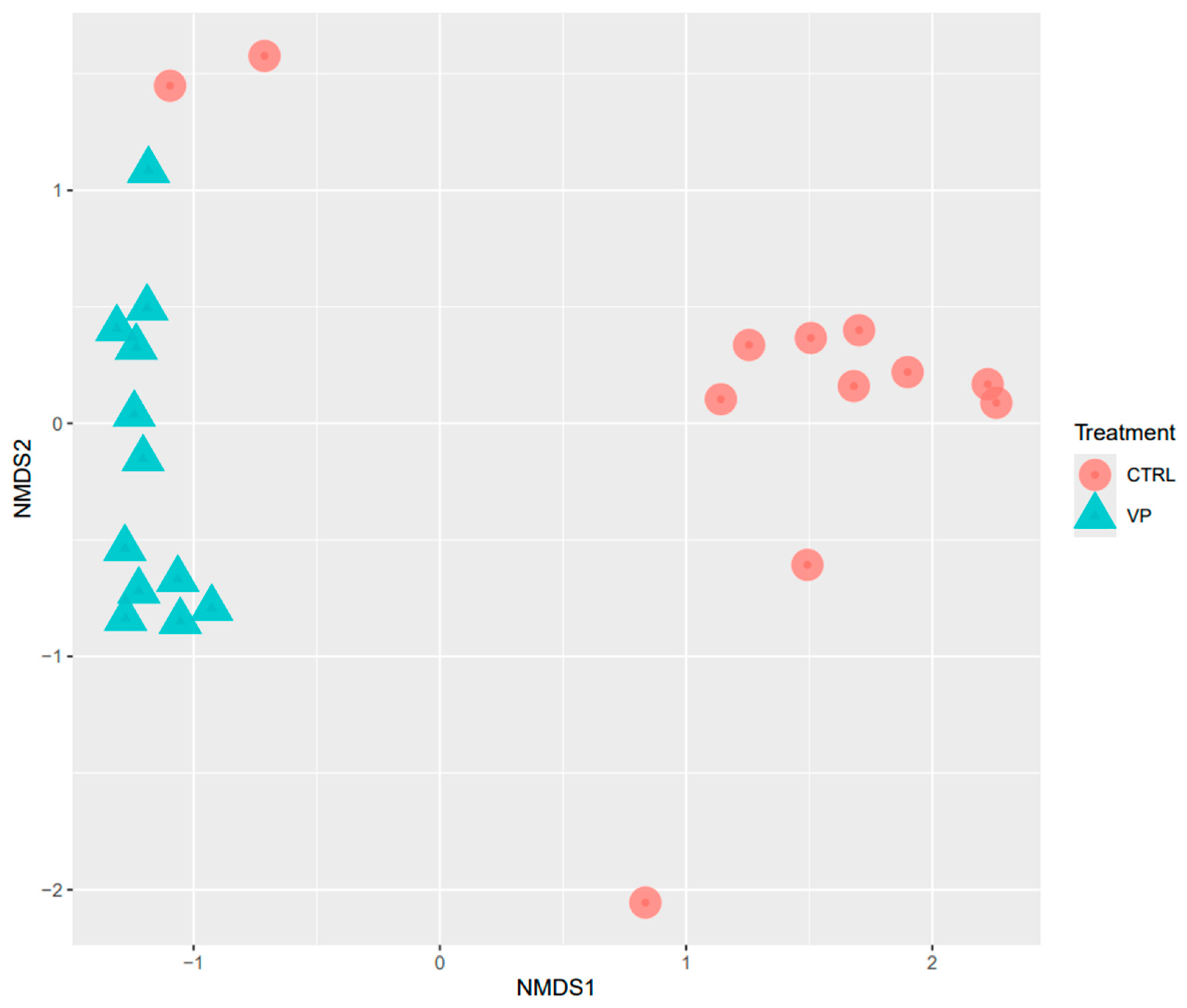
Beta diversity was analyzed using NMDS based on Bray–Curtis distances (Figure 2). The NMDS plot revealed statistically different clustering of microbial communities according to the dietary treatments (PERMANOVA, p = 0.001).
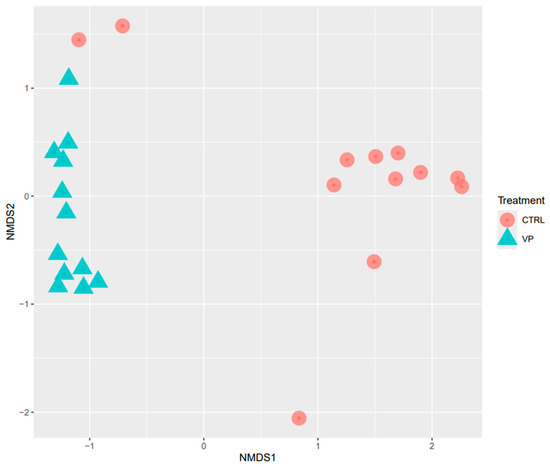
Figure 2.
NMDS analysis of the intestine of gilthead seabream juveniles fed with the experimental diets. Codes are: CTRL: control diet (red circle); VP: ECPs of V. proteolyticus diet (blue triangle).
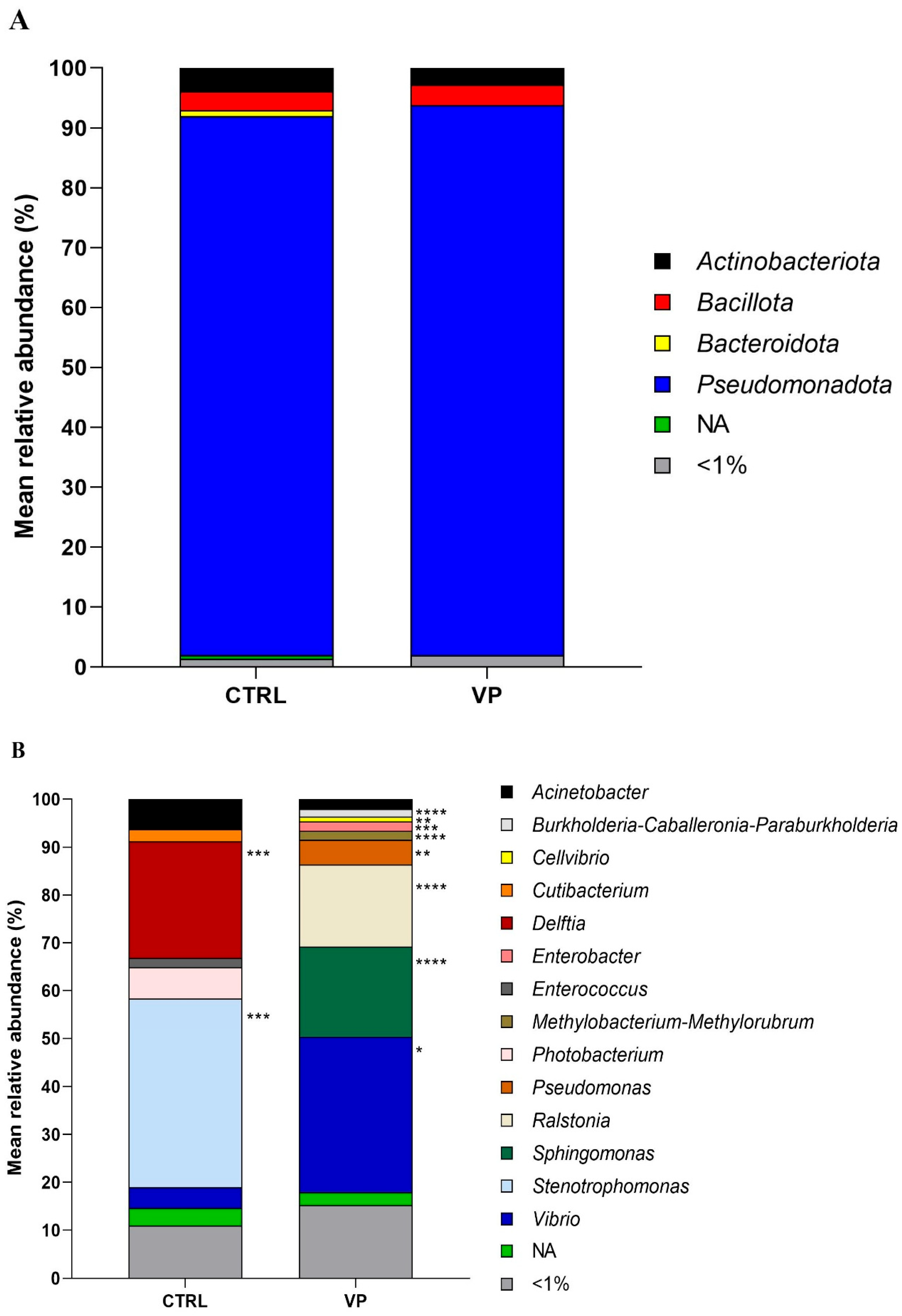
The relative abundance of the most prevalent gut microbes at the phylum and genus levels is shown in Figure 3. The predominant phylum detected in fish from both dietary groups was Pseudomonadota, followed by Actinobacteriota and Bacillota. Although their relative abundances varied slightly depending on the diet, no significant differences were observed (Figure 3A).
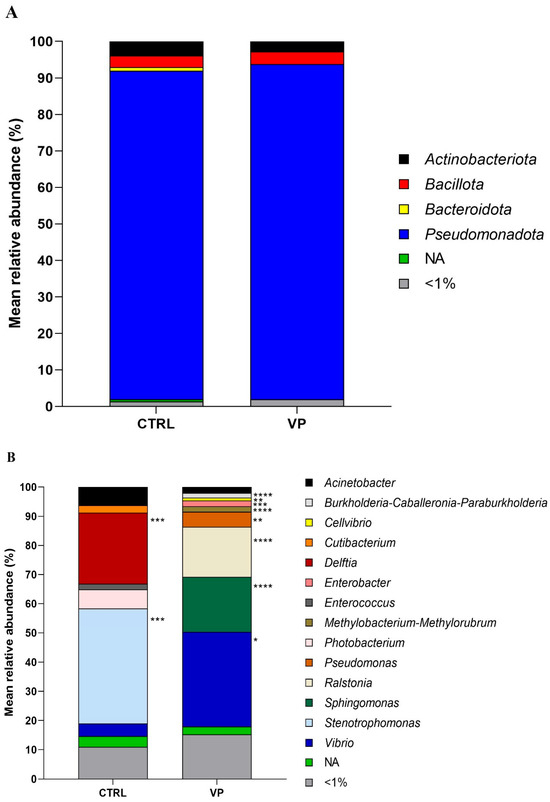
Figure 3.
Relative abundance (%) of the taxa at the phylum (A) and genus (B) taxonomical categories in the intestine of gilthead seabream juveniles fed with the experimental diets. Codes are CTRL: control diet; VP: ECPs of V. proteolyticus diet. NA: Not assigned. Asterisks indicate significant differences between experimental groups (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001).
At the genus level (Figure 3B), Delftia, Enterococcus, and Stenotrophomonas were abundant in the CTRL group but were completely absent in the VP group. In contrast, Burkholderia-Caballeronia-Paraburkholderia, Cellvibrio, and Methylobacterium-Methylorubrum were exclusively detected in the VP group. Among the shared genera, Pseudomonas, Ralstonia, Sphingomonas, and Vibrio showed significantly higher relative abundances in the VP group compared to the CTRL group. Additionally, Acinetobacter, Cutibacterium, and Photobacterium exhibited a slight, although not statistically significant, reduction in fish fed the VP diet.
Furthermore, predicted metabolic functionality did not show significant differences between the two dietary groups (Figure S1).
3.3. Influence of ECPs on Intestinal Gene Expression
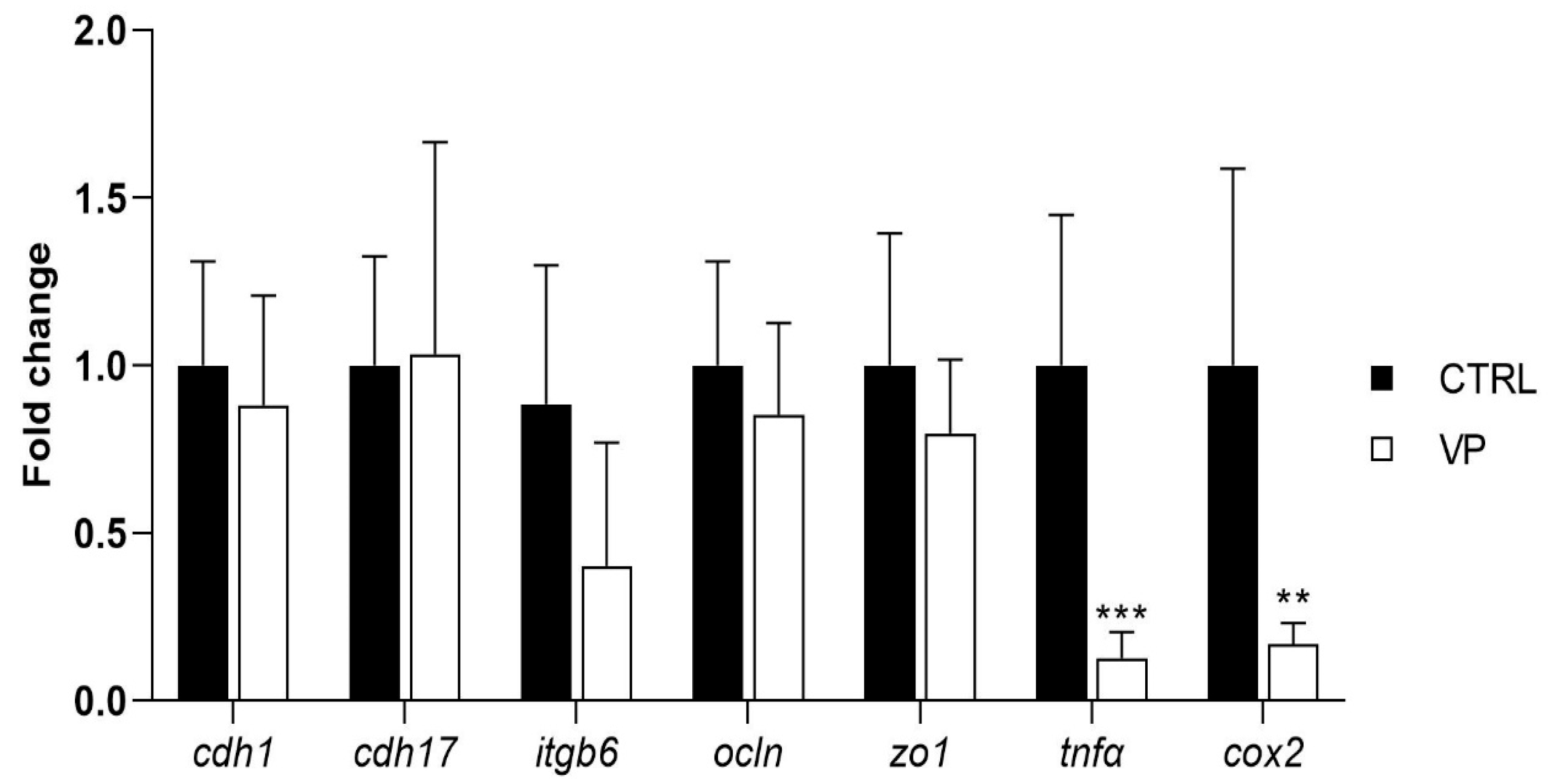
The expression levels of genes related to intestinal permeability and integrity (cdh1, cdh17, itgb6, ocln, and zo1) did not show significant differences between dietary treatments (Figure 4). However, fish fed the VP diet exhibited a downregulation of the pro-inflammatory markers tnfα and cox2 compared to the CTRL group (Figure 4).
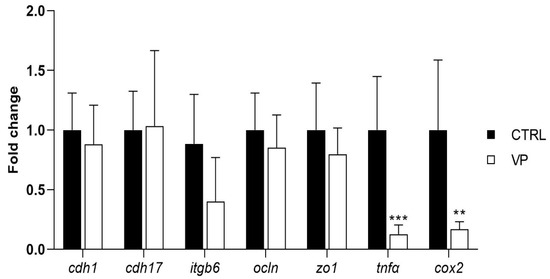
Figure 4.
Relative quantification of selected gene expression in the intestine of gilthead seabream juveniles fed with the experimental diets. Data were normalized with ef1α and gadph transcription levels and expressed as mean ± SD (n = 6) of fold change. Codes are CTRL: control diet; VP: ECPs of V. proteolyticus diet. Student’s t-test was used. Asterisks indicate significant differences between experimental groups (** p < 0.01; *** p < 0.001).
3.4. Effect of LPS Challenge on Intestinal Gene Expression
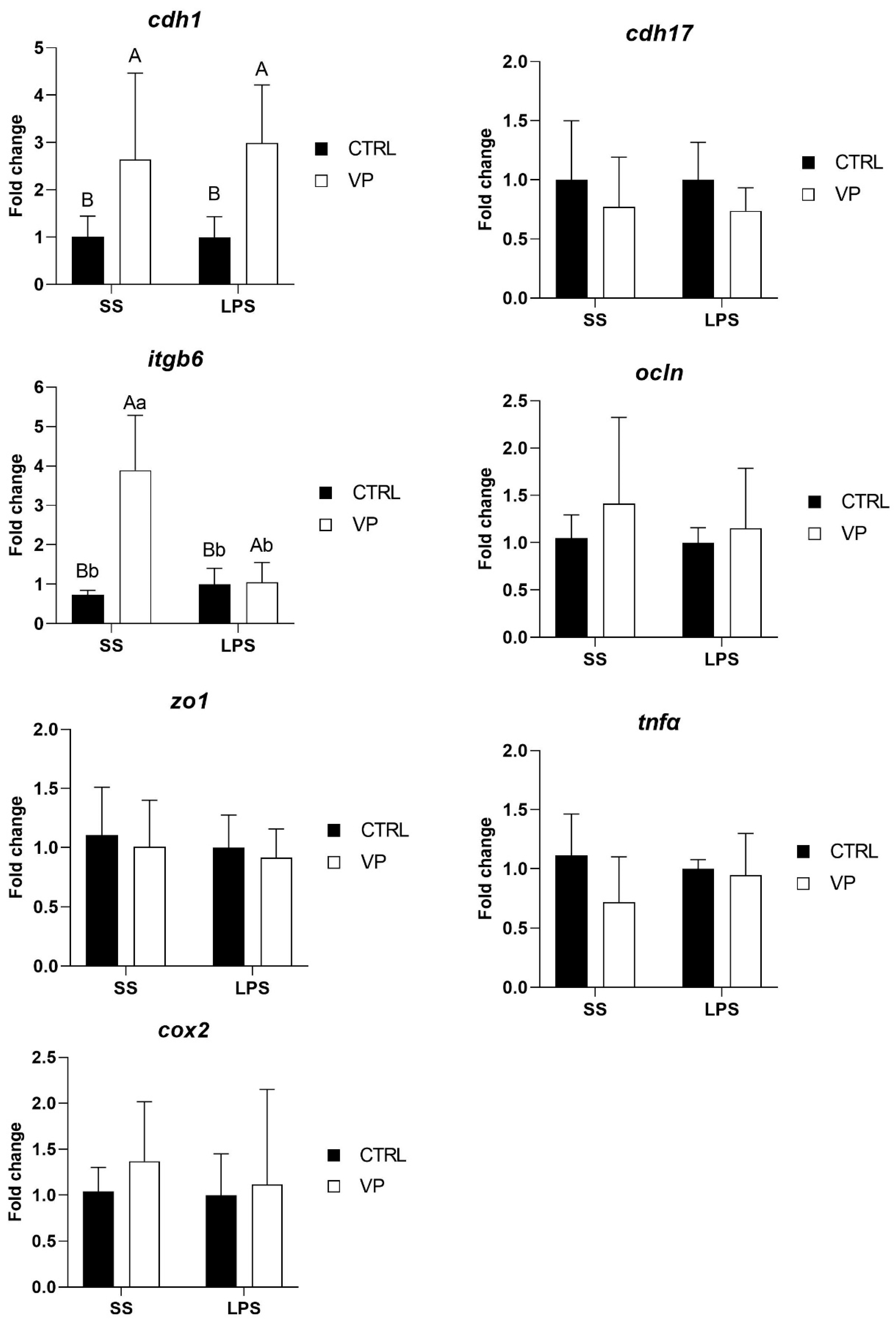
Following the LPS challenge, cdh1 transcription level was affected by the diet, being significantly higher in fish fed the VP diet, but not by the challenge (Figure 5, Table 5). Furthermore, the itgb6 expression level was upregulated significantly in fish from the VP group injected with saline solution. No significant differences were observed in the transcription levels of cdh17, ocln, zo1, tnfα, and cox2 between dietary groups following the challenge.
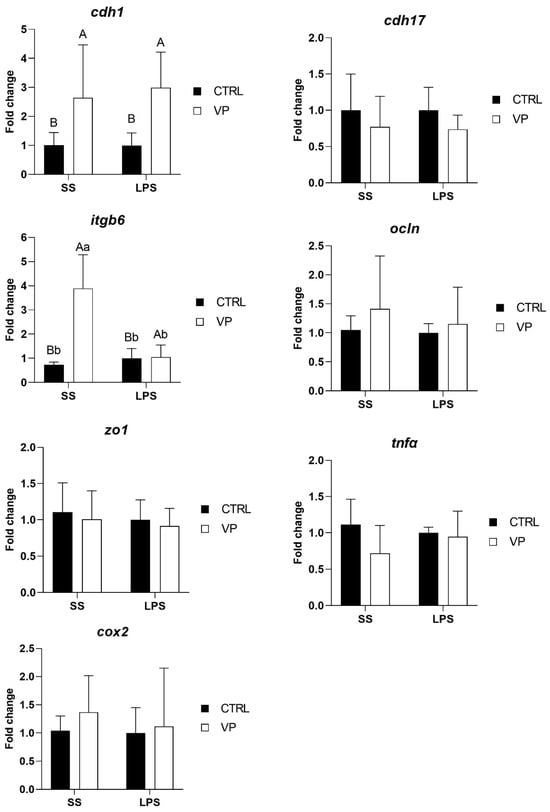
Figure 5.
Relative quantification of selected gene expression in the intestine of gilthead seabream juveniles fed with the experimental diets and subjected to experimental challenge. Data were normalized with ef1α and gadph transcription levels and expressed as mean ± SD (n = 6) of fold change. Codes are CTRL: control diet; VP: ECPs of V. proteolyticus diet; SS: challenge with Saline solution; LPS: challenge with LPS. Differences between groups were analyzed using two-way ANOVA, followed by Tukey’s post hoc test, and were considered statistically significant at p ≤ 0.05. Different uppercase letters indicate significant differences due to the diet, and lowercase letters indicate significant differences due to the LPS challenge.

Table 5.
Statistical parameters (p-value) obtained from two-way ANOVA analysis of fish fed the experimental diets subjected to the LPS challenge. Asterisks indicate significant differences (p < 0.05).
4. Discussion
The use of postbiotics—non-viable microbial cells or their metabolic byproducts that confer health benefits to the host—has gained growing interest in aquaculture as a safe and effective alternative to traditional probiotics. In the present study, we evaluated the effects of dietary supplementation with extracellular products (ECPs) derived from Vibrio proteolyticus grown on a medium containing experimental aquafeed and agar, focusing on their impact on intestinal histology, microbiota composition, and gene expression in juvenile Sparus aurata. This specific culture condition was selected due to its ability to produce V. proteolyticus ECPs that promote cellular proliferation, have antibacterial and antibiofilm activity against fish pathogens, and display a variety of enzymes to hydrolyse nutritional and antinutritional compounds. Although growth performance was not the primary objective, both experimental diets were well tolerated, and no significant differences in final body weight were observed between groups.
Histological assessment revealed that fish fed the VP diet exhibited a well-preserved mucosal structure in the anterior intestinal region, with no apparent signs of enteritis or tissue damage, suggesting the absence of any deleterious effects associated with postbiotic administration. However, a significant reduction in several mucosal parameters, including fold height, enterocyte height, and the thickness of the lamina propria, muscular layer, and submucosa, was observed in the VP group compared to the control. These reductions might be interpreted as a potential compromise in the absorptive surface area [38,39]. Nonetheless, some authors have described such morphological changes as regulatory adjustments in response to functional aquafeeds or microbial-derived compounds, rather than pathological alterations (see review by De Marco et al. [40]).
In this context, the VP diet significantly enhanced the number of goblet cells in the intestinal mucosa. Goblet cells play a central role in maintaining mucosal integrity by secreting mucins that form the protective mucus layer over the epithelium [41]. An increase in goblet cell density is often considered a marker of enhanced mucosal protection and improved epithelial defense, especially under microbial stimulation or in response to immunomodulatory interventions [42]. Additionally, the reduced apical area of enterocytes observed in the VP group may reflect subtle alterations in membrane dynamics or nutrient absorption potential. However, since no differences were observed in fish growth performance, these histological changes do not appear to negatively affect nutrient assimilation and may instead represent a shift toward a more compact and regulated epithelial profile.
The dietary fortification with ECPs significantly influenced the intestinal microbial diversity and composition. Alpha diversity, as reflected by the Shannon and Simpson indices, was significantly higher in the VP group. This observation is consistent with previous studies reporting enhanced alpha diversity in fish fed postbiotic-supplemented diets [43,44]. High microbial diversity is generally associated with improved gut health, metabolic resilience, and enhanced resistance to pathogen colonization in fish [45,46]. According to this idea, the inclusion of ECPs in the aquafeed can be beneficial by improving the physiological state of the fish. Furthermore, beta diversity analysis revealed significant differences in microbial community between dietary groups, confirming the modulatory effect of the VP postbiotic diet on the intestinal microbiota of these individuals.
At the phylum level, the microbiota was dominated by Pseudomonadota, Actinobacteriota, and Bacillota in both groups, consistent with previous studies in gilthead seabream [36,47,48]. However, more detailed taxonomic analysis at the genus level revealed marked differences. In the control group, Delftia, Enterococcus, and Stenotrophomonas were abundant but completely absent in the VP group. Interestingly, some Enterococcus strains are known as opportunistic or pathogenic potential bacteria, and their reduction may be favorable in the context of fish health [49,50].
In contrast, the VP diet promoted the exclusive presence of Burkholderia-Caballeronia-Paraburkholderia, Cellvibrio, and Methylobacterium-Methylorubrum. Members of the Burkholderia-Caballeronia-Paraburkholderia group are known for their broad-spectrum antimicrobial activity [51] and their role in bioremediation due to their ability to degrade aromatic compounds [52]. Cellvibrio species are able to degrade complex polysaccharides such as cellulose, xylan, and pectin [53], while Methylobacterium-Methylorubrum has been associated with enhanced energy metabolism [54]. These shifts suggest a selective pressure exerted by the postbiotic, fostering microbial taxa with metabolic capacities adapted for complex substrate utilization or beneficial interactions with the intestinal mucosa.
Moreover, the significant enrichment of Pseudomonas, Ralstonia, Sphingomonas, and Vibrio in the VP group is notable. Although some Vibrio species are known to be pathogenic to fish [55,56], others (including V. proteolyticus) have been shown to exhibit probiotic-like properties or immune stimulation potential in fish [10,57]. Pseudomonas and Sphingomonas species are known to have a wide range of metabolic capabilities, produce antimicrobial compounds, mitigate intestinal inflammation, and modulate host immunity [58,59,60,61,62]. Likewise, Ralstonia has been associated with protein digestion and absorption, as well as pathways involved in phenylalanine metabolism, ketone body synthesis and degradation, and lysine catabolism [63]. Altogether, these findings suggest that the postbiotic may promote a more dynamic and potentially beneficial microbial community.
Importantly, despite these microbial shifts, predictive metabolic functionality analyses did not reveal significant differences between dietary treatments. This indicates that while taxonomic composition was altered, the overall functional capacity of the gut microbiota remained stable. This could be due to functional redundancy, where different microbial taxa perform similar metabolic roles [64,65]. For future studies, it would be interesting to check whether these changes in the microbial structure confer protection to specimens that have received aquafeed supplemented with ECPs and are subjected to different changes typical of aquaculture practice (hypoxia, fasting, temperature changes, etc.).
The expression levels of genes associated with intestinal integrity (cdh1, cdh17, itgb6, ocln, and zo1) did not differ significantly between dietary groups. These genes play crucial roles in maintaining epithelial cohesion, regulating paracellular permeability, and ensuring intestinal homeostasis [66,67]. It has been demonstrated in S. aurata that the alteration of such genes due to the presence of mycotoxins in the aquafeed induces a dysregulation of intestinal physiology [68]; however, this is not appreciated when including ECPs in the aquafeed. The absence of changes at the level of intestinal integrity biomarkers is in agreement with histological analysis, suggesting that the inclusion of V. proteolyticus extracellular products did not compromise the structural integrity of the intestinal barrier. The stability in their expression levels suggests that the VP diet did not induce gut barrier dysfunction, a key concern when evaluating new dietary additives.
Interestingly, fish fed the VP diet exhibited significant downregulation of tnfα and cox2, two key genes involved in inflammation. tnfα (tumor necrosis factor-alpha) is a pro-inflammatory cytokine that plays a central role in initiating immune responses and is commonly upregulated during intestinal stress or pathogenic challenges [69,70]. cox2 (cyclooxygenase-2) is an enzyme responsible for the synthesis of prostaglandins, which mediate inflammatory responses in the gut [71]. The lower transcription levels of these genes in fish fed the VP diet suggest a reduction in basal inflammation status, which may indicate a beneficial immunomodulatory effect of the dietary treatment.
This reduction in transcription levels of key inflammatory genes may be due to specific changes in the intestinal microbiota. As mentioned above, Stenotrophomonas and Delftia, both detected in the CTRL group, were completely absent in the VP group. Both genera have been implicated in pro-inflammatory responses [72,73]. Their disappearance in fish fed the VP diet could indicate that the ECPs from V. proteolyticus may exert a selective pressure on the gut microbiota, limiting the presence of potentially pro-inflammatory bacteria. This microbial shift, in turn, could contribute to the observed downregulation of inflammatory markers, highlighting a possible microbiota-immune axis modulated by the dietary postbiotic.
Notably, the decrease in tnfα and cox2 expression without alterations in tight junction or adhesion-related genes (see above) may imply that ECPs incorporated into the diet contribute to maintaining gut homeostasis by reducing unnecessary inflammatory signaling. Chronic intestinal inflammation can lead to tissue damage, increased permeability, and impaired nutrient absorption [74]. Therefore, the observed reduction in inflammation-related gene expression could reflect a more balanced immune status, which may be advantageous for long-term gut health and overall fish performance.
After the LPS challenge, the expression of cdh1 was significantly higher in fish fed the VP diet, suggesting a potential dietary influence on epithelial integrity. cdh1 encodes E-cadherin, a key adhesion molecule essential for maintaining epithelial structure and intestinal barrier function [66]. The upregulation of cdh1 in fish fed the VP diet may indicate a protective effect of the dietary treatment, potentially enhancing epithelial resilience against stressors. Notably, this effect was attributed to the diet rather than the LPS challenge itself, suggesting a preconditioning effect of the VP diet in reinforcing intestinal epithelial stability.
Interestingly, itgb6 expression showed a complex pattern of change. Before the challenge, itgb6 expression levels were lower in VP-fed fish compared to the CTRL group, although this reduction was not statistically significant (Figure 4). However, after the challenge, itgb6 was significantly upregulated in VP-fed fish injected with saline but not in those challenged with LPS (Figure 5). itgb6 encodes integrin β6, which plays a key role in epithelial repair and immune regulation, particularly in response to tissue injury [75]. The initial non-significant reduction in itgb6 before the challenge may suggest a lower basal need for epithelial remodelling in VP-fed fish, possibly reflecting a more stable intestinal environment. The significant post-injection upregulation of itgb6 in response to saline, but not to LPS, may indicate that the VP diet modulated the epithelial response to mild perturbations while preventing excessive activation during inflammatory stimulation.
Furthermore, no significant differences were observed in the transcription levels of cdh17, ocln, zo1, tnfα, and cox2 between fish fed the CTRL and VP diets after the challenge (Figure 5). The stability in tight junction (ocln, zo1) and cadherin (cdh17) expression suggests that the VP diet did not compromise gut barrier function under inflammatory conditions. Moreover, the absence of differences in tnfα and cox2 expression post-challenge indicates that the VP diet did not exacerbate or suppress the acute inflammatory response triggered by LPS. Given that these genes were downregulated before the challenge (Figure 4), this suggests that the VP diet may have conferred a baseline anti-inflammatory effect, rather than altering the immediate immune response to LPS exposure.
5. Conclusions
In conclusion, the dietary inclusion of a postbiotic derived from V. proteolyticus DCF12.2 modulated the intestinal status of S. aurata specimens by increasing goblet cell numbers, promoting microbial diversity, reducing inflammatory gene expression, and enhancing epithelial gene responses under immune challenge. These findings highlight the immunomodulatory and gut-health-promoting potential of postbiotics as promising, stable alternatives to probiotics in aquafeeds. Further research should aim to validate their efficacy under commercial farming conditions and in response to pathogenic exposure, while optimizing postbiotic production processes for large-scale application.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani15131982/s1, Figure S1: Metabolic functionality in the intestine of gilthead seabream juveniles fed with the experimental diets. Codes are: CTRL: control diet; VP: ECPs of V. proteolyticus diet.
Author Contributions
Conceptualization, J.M.M., F.J.A.-L., J.G.-M. and M.Á.M.; methodology, O.P.-G., S.R.-B. and A.G.; software, J.G.-M.; validation, M.D.-M., I.M.C., A.G., E.M.-M. and J.G.-M.; formal analysis, O.P.-G., S.R.-B., M.D.-M., I.M.C., A.G. and J.G.-M.; data curation, I.M.C., A.G. and J.G.-M.; writing—original draft preparation, J.G.-M.; writing—review and editing, O.P.-G., S.R.-B., M.D.-M., I.M.C., A.G., E.M.-M., J.M.M., F.J.A.-L., J.G.-M., M.Á.M. and S.A.; visualization, J.G.-M.; supervision, J.M.M., F.J.A.-L., M.Á.M. and S.A.; project administration, M.Á.M. and S.A.; funding acquisition, F.J.A.-L. and M.Á.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been funded by Proyectos de Investigación #PID2020-113637RB-C22 financed by Ministerio de Ciencia e Innovación de España (MCIN), MCIN/AEI/10.13039/50110001103. The authors thank grants UNAM15-CE-3510, EQC2018-004984-P, and EQC2019-006380-P from the Service of Experimental Diets.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the Ethics and Animal Welfare Committee of the University of Cádiz and the Andalusian Regional Government (Junta de Andalucía, reference number 3/11/21/173).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors want to acknowledge the support of the Experimental Feeds Service of the University of Almeria (https://www.ual.es/universidad/serviciosgenerales/stecnicos/perifericos-convenio/piensos-experimentales) for aquafeed elaboration.
Conflicts of Interest
Francisco Javier Alarcón certifies that he is a partner in the spin-off “LifeBioencapsulation, S.L.” and declares that this circumstance has not interfered in any way with the research carried out in this study. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ISAPP | International Scientific Association for Probiotics and Prebiotics |
| UTHSCSA | University of Texas Health Sciences Center, San Antonio |
| SCI-CM | Servicios Centrales de Investigación en Cultivos Marinos |
| ECPs | Extracellular Product |
| LPS | Lipopolysaccharide |
| CRTL | Control |
| VP | Vibrio proteolyticus |
| qPCR | Quantitative PCR |
| TSA | Tryptic soy agar |
| TSB | Tryptic soy broth |
| CFU | Colony-Forming Unit |
| SEM | Scanning Electron Microscope |
| EA | Apical area of enterocytes |
| NMDS | Non-metric multidimensional scaling |
| ASVs | Amplicon sequence variants |
| Cq | Quantification Cycle |
| SD | Standard deviation |
| FL | Fold length |
| EH | Enterocyte height |
| LP | Lamina propria |
| ML | Muscular layer |
| SBL | Submucosa layer thickness |
| GC | Goblet cells |
| AE | Enterocyte apical area |
| NMDS plot | Non-metric Multidimensional Scaling plot |
| NA | Not assigned |
References
- Naeem, M.; Salam, A.; Tahir, S.S.; Rauf, N. Assessment of the essential element and toxic heavy metals in hatchery reared Oncorhynchus mykiss. Int. J. Agric. Biol. 2010, 12, 935–938. [Google Scholar]
- Naeem, M.; Salam, A.; Zuberi, A. Proximate composition of freshwater rainbow trout (Oncorhynchus mykiss) in relation to body size and condition factor from Pakistan. Pak. J. Agric. Sci. 2016, 53, 497–502. [Google Scholar]
- Ismat, N.; Ashraf, M.; Naeem, M.; Rehman, M.H.U. Effect of different feed ingredients on growth and level of intestinal enzyme secretions in juvenile Labeo rohita, Catla catla, Cirrhinus mrigala, and Hypophthalmichthys molitrix. Int. J. Aquac. 2013, 3, 85–91. [Google Scholar]
- Madhulika, M.M.; Deepti, M.; Ngasotter, S.; Gupta, S.S.; Varghese, T. Functional Feed Additives in Aquaculture to Improve Food Security. In Food Security, Nutrition and Sustainability Through Aquaculture Technologies; Sundaray, J.K., Rather, M.A., Ahmad, I., Amin, A., Eds.; Springer: Cham, Switzerland, 2025; pp. 375–396. ISBN 978-3-031-75830-0. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Bilal, S.; Ishtiaq, A.; Ghaffar, A.; Ishtiaq, T.; Naeem, M. Impact of in-feed Multispecies Probiotic Mixtures on Growth Patterns and Length-weight Relationships of Pangasianodon hypophthalmus. TSF J. Biol. 2025, 3, 27–39. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Abdel-Daim, M.M.; Van Doan, H. Probiotic application for sustainable aquaculture. Rev. Aquac. 2019, 11, 907–924. [Google Scholar] [CrossRef]
- Wan-Mohtar, W.A.A.Q.I.; Taufek, N.M.; Thiran, J.P.; Rahman, J.F.P.; Yerima, G.; Subramaniam, K.; Rowan, N. Investigations on the use of exopolysaccharide derived from mycelial extract of Ganoderma lucidum as functional feed ingredient for aquaculture-farmed red hybrid tilapia (Oreochromis sp.). Future Foods 2021, 3, 100018. [Google Scholar] [CrossRef]
- Medina, A.; Moriñigo, M.Á.; Arijo, S. Selection of putative probiotics based on antigen-antibody cross-reaction with Photobacterium damselae subsp. piscicida and Vibrio harveyi for use in Senegalese sole (Solea senegalensis). Aquac. Rep. 2020, 17, 100366. [Google Scholar] [CrossRef]
- Medina, A.; García-Márquez, J.; Moriñigo, M.Á.; Arijo, S. Effect of the potential probiotic Vibrio proteolyticus DCF12.2 on the immune system of Solea senegalensis and protection against Photobacterium damselae subsp. piscicida and Vibrio harveyi. Fishes 2023, 8, 344. [Google Scholar] [CrossRef]
- Zucko, J.; Starcevic, A.; Diminic, J.; Oros, D.; Mortazavian, A.M.; Putnik, P. Probiotic—Friend or foe? Curr. Opin. Food Sci. 2020, 32, 45–49. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Moradi, M.; Molaei, R.; Guimarães, J.T. A review on preparation and chemical analysis of postbiotics from lactic acid bacteria. Enzyme Microb. Technol. 2021, 143, 109722. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Maqueda, M.; Espinosa-Ruíz, C.; Esteban, M.Á.; Alarcón, F.J.; Tapia-Paniagua, S.T.; Balebona, M.C.; Moriñigo, M.Á. An ex vivo approach in European seabass leucocytes supports the in vitro regulation by postbiotics of Aip56 gene expression of Photobacterium damselae subsp. piscicida. Probiotics Antimicrob. Proteins 2024, 1–15. [Google Scholar] [CrossRef]
- Sharafi, H.; Divsalar, E.; Rezaei, Z.; Liu, S.Q.; Moradi, M. The potential of postbiotics as a novel approach in food packaging and biopreservation: A systematic review of the latest developments. Crit. Rev. Food Sci. Nutr. 2023, 64, 12524–12554. [Google Scholar] [CrossRef]
- Zheng, X.; Duan, Y.; Dong, H.; Zhang, J. The effect of Lactobacillus plantarum administration on the intestinal microbiota of whiteleg shrimp Penaeus vannamei. Aquaculture 2020, 526, 735331. [Google Scholar] [CrossRef]
- Yang, H.L.; Sun, Y.Z.; Hu, X.; Ye, J.-D.; Lu, K.L.; Hu, L.H.; Zhang, J.J. Bacillus pumilus SE5 originated PG and LTA tuned the intestinal TLRs/MyD88 signaling and microbiota in grouper (Epinephelus coioides). Fish Shellfish Immunol. 2019, 88, 266–271. [Google Scholar] [CrossRef] [PubMed]
- García-Márquez, J.; Díaz, A.G.; Molina-Roque, L.; Domínguez-Maqueda, M.; de las Heras, V.; Simó-Mirabet, P.; Vizcaíno, A.J.; Martos-Sitcha, J.A.; Alarcón-López, F.J.; Moriñigo, M.Á.; et al. Microalgal and Cyanobacterial Biomasses Modified the Activity of Extracellular Products from Bacillus pumilus: An In Vitro and In Vivo Assessment. Probiotics Antimicrob. Proteins 2024, 1–18. [Google Scholar] [CrossRef]
- Vinderola, G.; Sanders, M.E.; Cunningham, M.; Hill, C. Frequently Asked Questions about the ISAPP Postbiotic Definition. Front. Microbiol. 2023, 14, 1324565. [Google Scholar] [CrossRef]
- Dawood, M.A.O. Nutritional Immunity of Fish Intestines: Important Insights for Sustainable Aquaculture. Rev. Aquac. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Liu, P.V. Survey of Hemolysin Production among Species of Pseudomonads. J. Bacteriol. 1957, 74, 718–727. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; 1st revision; Association of Official Analytical Communities: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Escaffre, A.M.; Kaushik, S.; Mambrini, M. Morphometric Evaluation of Changes in the Digestive Tract of Rainbow Trout (Oncorhynchus mykiss) Due to Fish Meal Replacement with Soy Protein Concentrate. Aquaculture 2007, 273, 127–138. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; López, G.; Sáez, M.I.; Jiménez, J.A.; Barros, A.; Hidalgo, L.; Camacho-Rodríguez, J.; Martínez, T.F.; Cerón-García, M.C.; Alarcón, F.J. Effects of the Microalga Scenedesmus almeriensis as Fishmeal Alternative in Diets for Gilthead Sea Bream, Sparus aurata, Juveniles. Aquaculture 2014, 431, 34–43. [Google Scholar] [CrossRef]
- Martínez, G.; Shaw, E.M.; Carrillo, M.; Zanuy, S. Protein Salting-out Method Applied to Genomic DNA Isolation from Fish Whole Blood. Biotechniques 1998, 24, 238–239. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Paniagua, S.T.; Chabrillón, M.; Díaz-Rosales, P.; de la Banda, I.G.; Lobo, C.; Balebona, M.C.; Moriñigo, M.A. Intestinal Microbiota Diversity of the Flat Fish Solea senegalensis (Kaup, 1858) Following Probiotic Administration. Microb. Ecol. 2010, 60, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC—A Quality Control Tool for High Throughput Sequence Data. Babraham Bioinformatics. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 20 October 2024).
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Simpson, G.L.; Solymos, P.M.; Stevens, M.H.H.; Wagner, H. The Vegan Package. In Community Ecology Package: 190; 2008; Available online: https://www.researchgate.net/publication/258996522_The_vegan_Package_Community_Ecology_Package_version_113-1 (accessed on 20 October 2024).
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Pérez-Sánchez, J.; Benedito-Palos, L.; Estensoro, I.; Petropoulos, Y.; Calduch-Giner, J.A.; Browdy, C.L.; Sitjà-Bobadilla, A. Effects of Dietary NEXT ENHANCE®150 on Growth Performance and Expression of Immune and Intestinal Integrity Related Genes in Gilthead Sea Bream (Sparus aurata L.). Fish Shellfish Immunol. 2015, 44, 117–128. [Google Scholar] [CrossRef]
- Cerezuela, R.; Meseguer, J.; Esteban, M.Á. Effects of Dietary Inulin, Bacillus Subtilis and Microalgae on Intestinal Gene Expression in Gilthead Seabream (Sparus aurata L.). Fish Shellfish Immunol. 2013, 34, 843–848. [Google Scholar] [CrossRef]
- Estruch, G.; Collado, M.C.; Monge-Ortiz, R.; Tomás-Vidal, A.; Jover-Cerdá, M.; Peñaranda, D.S.; Pérez Martínez, G.; Martínez-Llorens, S. Long-Term Feeding with High Plant Protein Based Diets in Gilthead Seabream (Sparus aurata, L.) Leads to Changes in the Inflammatory and Immune Related Gene Expression at Intestinal Level. BMC Vet. Res. 2018, 14, 302. [Google Scholar] [CrossRef] [PubMed]
- Cerezo-Ortega, I.M.; Di Zeo-Sánchez, D.E.; García-Márquez, J.; Ruiz-Jarabo, I.; Sáez-Casado, M.I.; Balebona, M.C.; Moriñigo, M.A.; Tapia-Paniagua, S.T. Microbiota Composition and Intestinal Integrity Remain Unaltered after the Inclusion of Hydrolysed Nannochloropsis gaditana in Sparus aurata Diet. Sci. Rep. 2021, 11, 18779. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar]
- Debnath, S.; Saikia, S.K. Absorption of Protein in Teleosts: A Review. Fish Physiol. Biochem. 2021, 47, 313–326. [Google Scholar] [CrossRef]
- Zhang, W.; Tan, B.; Deng, J.; Dong, X.; Yang, Q.; Chi, S.; Liu, H.; Zhang, S.; Xie, S.; Zhang, H. Effects of High Level of Fermented Soybean Meal Substitution for Fish Meal on the Growth, Enzyme Activity, Intestinal Structure Protein and Immune-Related Gene Expression and Intestinal Flora in Juvenile Pearl Gentian Grouper. Aquac. Nutr. 2021, 27, 1433–1447. [Google Scholar] [CrossRef]
- De Marco, G.; Cappello, T.; Maisano, M. Histomorphological Changes in Fish Gut in Response to Prebiotics and Probiotics Treatment to Improve Their Health Status: A Review. Animals 2023, 13, 2860. [Google Scholar] [CrossRef]
- Wilson, J.M.; Castro, L.F.C. Morphological Diversity of the Gastrointestinal Tract in Fishes. Fish Physiol. 2010, 30, 1–55. [Google Scholar]
- Zhang, M.; Wu, C. The Relationship between Intestinal Goblet Cells and the Immune Response. Biosci. Rep. 2020, 40, BSR20201471. [Google Scholar] [CrossRef] [PubMed]
- Mora-Sánchez, B.; Balcázar, J.L.; Pérez-Sánchez, T. Effect of a Novel Postbiotic Containing Lactic Acid Bacteria on the Intestinal Microbiota and Disease Resistance of Rainbow Trout (Oncorhynchus mykiss). Biotechnol. Lett. 2020, 42, 1957–1962. [Google Scholar] [CrossRef]
- Pérez-Sánchez, T.; Mora-Sánchez, B.; Vargas, A.; Balcázar, J.L. Changes in Intestinal Microbiota and Disease Resistance Following Dietary Postbiotic Supplementation in Rainbow Trout (Oncorhynchus mykiss). Microb. Pathog. 2020, 142, 104060. [Google Scholar] [CrossRef]
- Infante-Villamil, S.; Huerlimann, R.; Jerry, D.R. Microbiome Diversity and Dysbiosis in Aquaculture. Rev. Aquac. 2021, 13, 1077–1096. [Google Scholar] [CrossRef]
- Yukgehnaish, K.; Kumar, P.; Sivachandran, P.; Marimuthu, K.; Arshad, A.; Paray, B.A.; Arockiaraj, J. Gut Microbiota Metagenomics in Aquaculture: Factors Influencing Gut Microbiome and Its Physiological Role in Fish. Rev. Aquac. 2020, 12, 1903–1927. [Google Scholar] [CrossRef]
- Kormas, K.A.; Meziti, A.; Mente, E.; Frentzos, A. Dietary Differences Are Reflected on the Gut Prokaryotic Community Structure of Wild and Commercially Reared Sea Bream (Sparus aurata). MicrobiologyOpen 2014, 3, 718–728. [Google Scholar] [CrossRef]
- Nikouli, E.; Meziti, A.; Antonopoulou, E.; Mente, E.; Kormas, K.A. Gut Bacterial Communities in Geographically Distant Populations of Farmed Sea Bream (Sparus aurata) and Sea Bass (Dicentrarchus labrax). Microorganisms 2018, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, B.; Wityk, P.; Gałęcka, M.; Michalik, M. The Many Faces of Enterococcus spp. Commensal, Probiotic and Opportunistic Pathogen. Microorganisms 2021, 9, 1900. [Google Scholar] [CrossRef]
- Kukułowicz, A.; Steinka, I.; Gardocka, M. Enterococcus spp. in Fish: Analysis of the Presence and Resistance in Samples from Tri-City, Poland. PLoS ONE 2024, 19, e0306826. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhu, Y.; Guo, M.; Yin, X.; Liang, M.; Lou, X.; Chen, J.; Zhou, L.; Fan, D.; Shi, L.; et al. The Potential Mechanism of BPF-Induced Neurotoxicity in Adult Zebrafish: Correlation between Untargeted Metabolomics and Gut Microbiota. Sci. Total Environ. 2022, 839, 156221. [Google Scholar] [CrossRef]
- Morya, R.; Salvachúa, D.; Thakur, I.S. Burkholderia: An Untapped but Promising Bacterial Genus for the Conversion of Aromatic Compounds. Trends Biotechnol. 2020, 38, 963–975. [Google Scholar] [CrossRef]
- Gardner, J.G. Polysaccharide Degradation Systems of the Saprophytic Bacterium Cellvibrio japonicus. World J. Microbiol. Biotechnol. 2016, 32, 121. [Google Scholar] [CrossRef]
- Lin, G.; Lu, J.; Sun, Z.; Xie, J.; Huang, J.; Su, M.; Wu, N. Characterization of Tissue-Associated Bacterial Community of Two Bathymodiolus Species from the Adjacent Cold Seep and Hydrothermal Vent Environments. Sci. Total Environ. 2021, 796, 149046. [Google Scholar] [CrossRef]
- Ina-Salwany, M.Y.; Al-saari, N.; Mohamad, A.; Mursidi, F.A.; Mohd-Aris, A.; Amal, M.N.A.; Kasai, H.; Mino, S.; Sawabe, T.; Zamri-Saad, M. Vibriosis in Fish: A Review on Disease Development and Prevention. J. Aquat. Anim. Health 2019, 31, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Regev, Y.; Davidovich, N.; Berzak, R.; Lau, S.C.K.; Scheinin, A.P.; Tchernov, D.; Morick, D. Molecular Identification and Characterization of Vibrio Species and Mycobacterium Species in Wild and Cultured Marine Fish from the Eastern Mediterranean Sea. Microorganisms 2020, 8, 863. [Google Scholar] [CrossRef]
- García-Márquez, J.; Álvarez-Torres, D.; Cerezo, I.M.; Domínguez-Maqueda, M.; Figueroa, F.L.; Alarcón, F.J.; Acién, G.; Martínez-Manzanares, E.; Abdala-Díaz, R.T.; Béjar, J.; et al. Combined Dietary Administration of Chlorella Fusca and Ethanol-Inactivated Vibrio proteolyticus Modulates Intestinal Microbiota and Gene Expression in Chelon Labrosus. Animals 2023, 13, 3325. [Google Scholar] [CrossRef]
- Hoque, F.; Jawahar Abraham, T.; Nagesh, T.S.; Kamilya, D. Pseudomonas aeruginosa FARP72 Offers Protection Against Aeromonas hydrophila Infection in Labeo rohita. Probiotics Antimicrob. Proteins 2019, 11, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Mielko, K.A.; Jabłoński, S.J.; Milczewska, J.; Sands, D.; Łukaszewicz, M.; Młynarz, P. Metabolomic Studies of Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2019, 35, 178. [Google Scholar] [CrossRef]
- Giri, S.S.; Jun, J.W.; Yun, S.; Kim, H.J.; Kim, S.G.; Kim, S.W.; Woo, K.J.; Han, S.J.; Oh, W.T.; Kwon, J.; et al. Effects of Dietary Heat-Killed Pseudomonas aeruginosa Strain VSG2 on Immune Functions, Antioxidant Efficacy, and Disease Resistance in Cyprinus carpio. Aquaculture 2020, 514, 734489. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, B.; Wang, N.; Yang, J.; Zhou, Q.; Sun, C.; Zhao, Y. Low Fish Meal Diet Supplemented with Probiotics Ameliorates Intestinal Barrier and Immunological Function of Macrobrachium Rosenbergii via the Targeted Modulation of Gut Microbes and Derived Secondary Metabolites. Front. Immunol. 2022, 13, 1074399. [Google Scholar] [CrossRef]
- Liu, W.; Zeng, J.; Suo, N.; Ke, Q.; Zhao, J.; Wang, J.; Bai, Y.; Deng, Y.; Zhou, X.; Wang, Y.; et al. Dynamic Changes in Gut Microbiota and Production Phenotypes Driven by Host Genetic Background in Large Yellow Croaker. Aquaculture 2025, 598, 741948. [Google Scholar] [CrossRef]
- Huang, Q.; Sham, R.C.; Deng, Y.; Mao, Y.; Wang, C.; Zhang, T.; Leung, K.M.Y. Diversity of Gut Microbiomes in Marine Fishes Is Shaped by Host-Related Factors. Mol. Ecol. 2020, 29, 5019–5034. [Google Scholar] [CrossRef]
- Galand, P.E.; Pereira, O.; Hochart, C.; Auguet, J.C.; Debroas, D. A Strong Link between Marine Microbial Community Composition and Function Challenges the Idea of Functional Redundancy. ISME J. 2018, 12, 2470–2478. [Google Scholar] [CrossRef]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.B.N.; Huber, J.A.; O’Connor, M.I.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A.; et al. Function and Functional Redundancy in Microbial Systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Hartsock, A.; Nelson, W.J. Adherens and Tight Junctions: Structure, Function and Connections to the Actin Cytoskeleton. Biochim. Biophys. Acta Biomembr. 2008, 1778, 660–669. [Google Scholar] [CrossRef]
- Capaldo, C.T.; Powell, D.N.; Kalman, D. Layered Defense: How Mucus and Tight Junctions Seal the Intestinal Barrier. J. Mol. Med. 2017, 95, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Barany, A.; Fuentes, J.; Martínez-Rodríguez, G.; Mancera, J.M. Aflatoxicosis Dysregulates the Physiological Responses to Crowding Densities in the Marine Teleost Gilthead Seabream (Sparus aurata). Animals 2021, 11, 753. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Secombes, C.J. The Function of Fish Cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef]
- García-Márquez, J.; Álvarez-Torres, D.; Cerezo, I.M.; Domínguez-Maqueda, M.; Acién, G.; Alarcón-López, F.J.; Figueroa, F.L.; Martínez-Manzanares, E.; Abdala-Díaz, R.T.; Béjar, J.; et al. Effects of Chlorella fusca-Supplemented Diet on Intestinal Microbiota and Gene Expression Related to Metabolism, Stress, and Immune Response in Chelon labrosus. Algal Res. 2024, 77, 103362. [Google Scholar] [CrossRef]
- Vane, J.R.; Bakhle, Y.S.; Botting, R.M. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 97–120. [Google Scholar] [CrossRef]
- Di Bonaventura, G.; Pompilio, A.; Zappacosta, R.; Petrucci, F.; Fiscarelli, E.; Rossi, C.; Piccolomini, R. Role of Excessive Inflammatory Response to Stenotrophomonas maltophilia Lung Infection in DBA/2 Mice and Implications for Cystic Fibrosis. Infect. Immun. 2010, 78, 2466–2476. [Google Scholar] [CrossRef]
- McNeill, R.M.; DeFoor, W.M.; Goller, C.C.; Ott, L.E. Delftia spp. Elicit a Pro-Inflammatory Response in Monocytes. J. Young Investig. 2015, 29, 41–48. [Google Scholar]
- Campos-Sánchez, J.C.; Esteban, M.Á. Review of Inflammation in Fish and Value of the Zebrafish Model. J. Fish Dis. 2021, 44, 123–139. [Google Scholar] [CrossRef]
- Meecham, A.; Marshall, J.F. The ITGB6 Gene: Its Role in Experimental and Clinical Biology. Gene X 2020, 5, 100023. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).