A Case Report: Post-Mortem Pathological Observations of a Fresh Dairy Cow with Type 3 Abomasal Ulcer After Sudden Death
Simple Summary
Abstract
1. Introduction and Clinical Significance
2. Case Presentation
2.1. Handling
2.2. Medical History
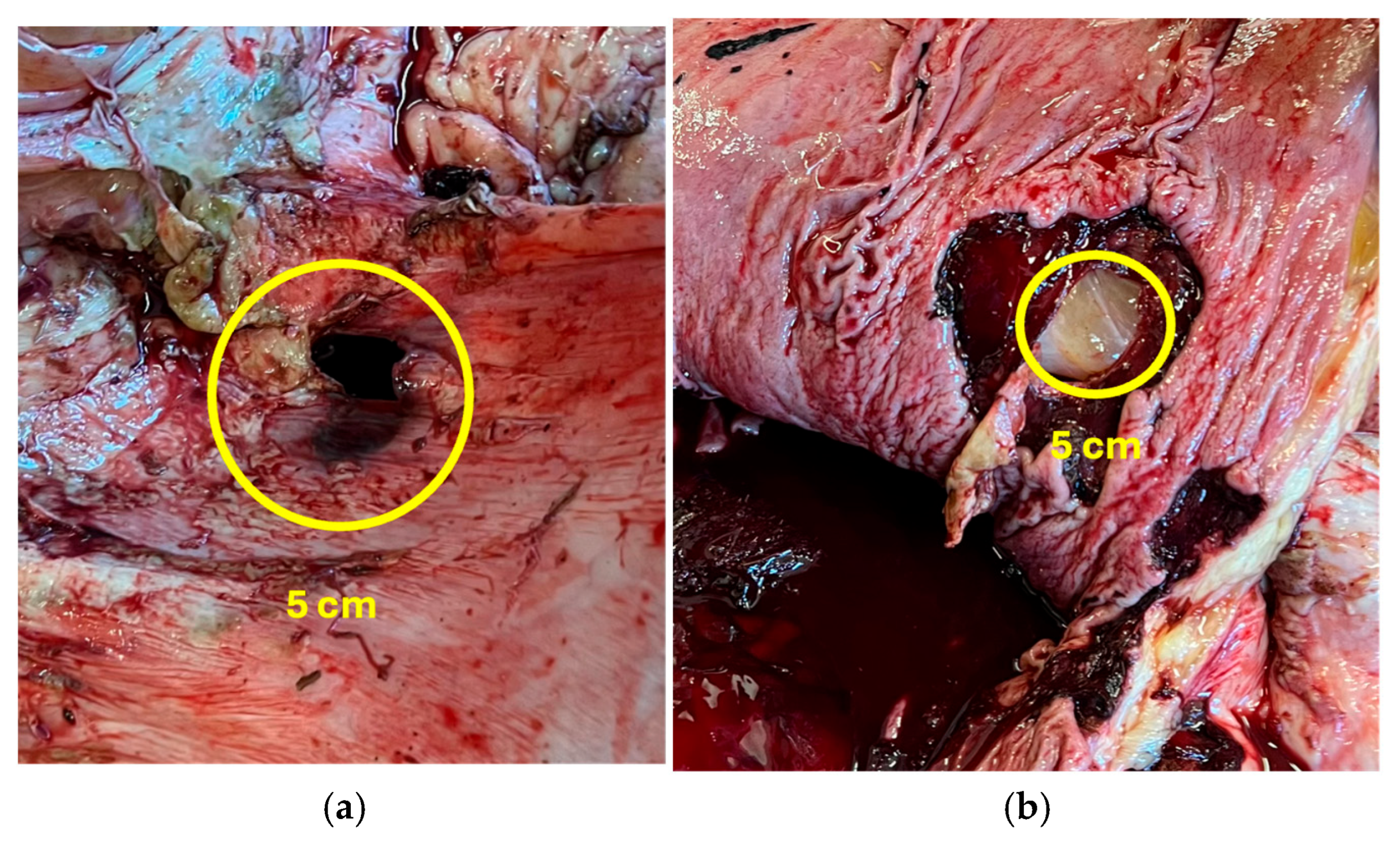
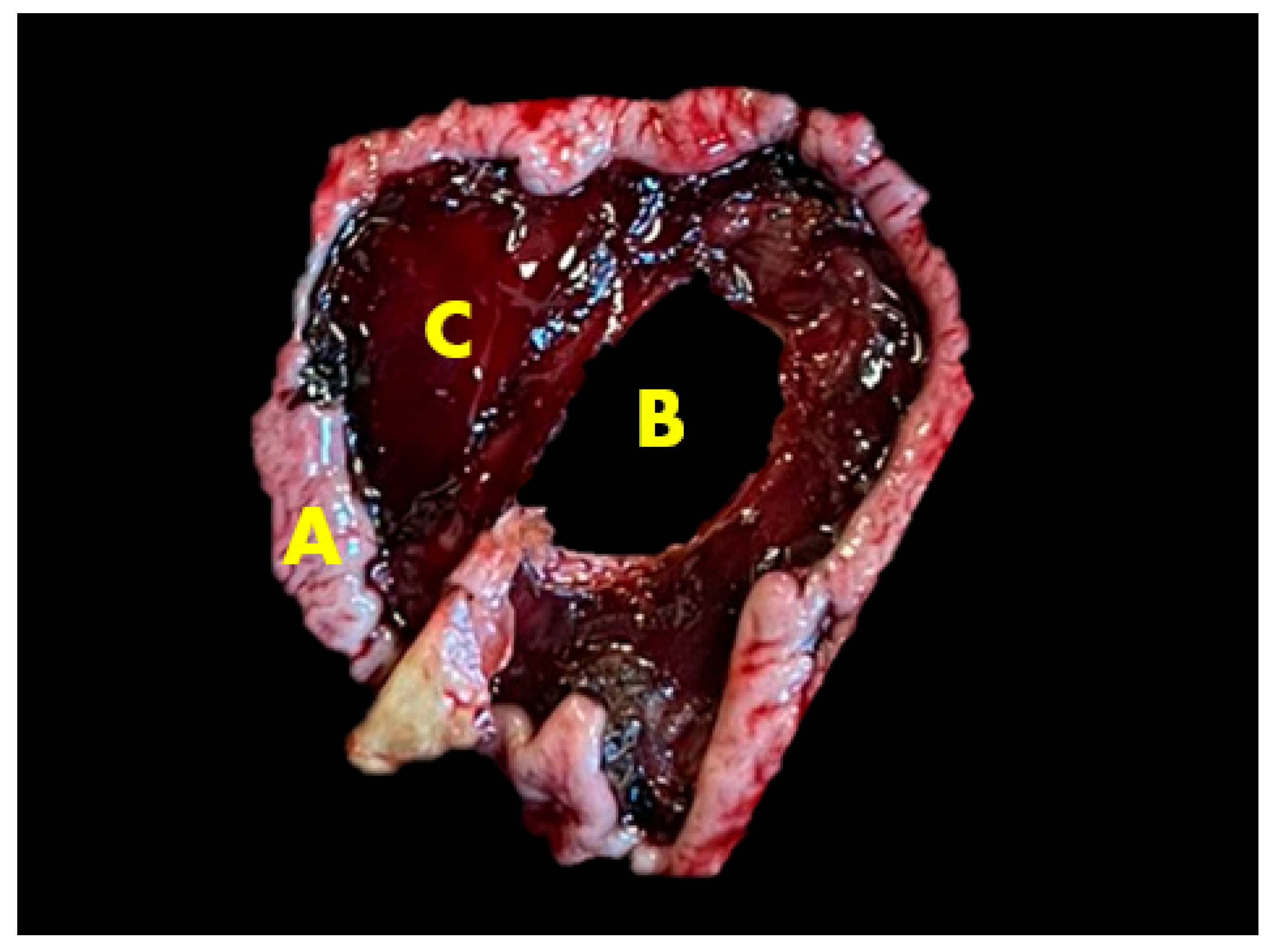
2.3. Gross Lesions
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Braun, U.; Nuss, K.; Warislohner, S.; Reif, C.; Oschlies, C.; Gerspach, C. Diagnostic reliability of clinical signs in cows with traumatic reticuloperitonitis and abomasal ulcers. BMC Vet. Res. 2020, 16, 359. [Google Scholar] [CrossRef] [PubMed]
- Swapna, P. Using AI and Machine Learning for Early Detection and Management of Cattle Diseases to Improve Livestock Health and Productivity. Int. Res. J. Educ. Technol. 2024, 6, 1815–1822. [Google Scholar] [CrossRef]
- Braun, U.; Gerspach, C.; Hilbe, M.; Devaux, D.; Reif, C. Clinical and laboratory findings in 60 cows with type-3 abomasal ulcer. Schweiz. Arch. Tierheilkd. 2019, 161, 523–531. [Google Scholar] [CrossRef]
- Gerspach, C.; Oschlies, C.; Kuratli, J.; Braun, U. Ultrasonographic documentation of type-3 abomasal ulcer in a cow with left displacement of the abomasum. Acta Vet. Scand. 2020, 62, 29. [Google Scholar] [CrossRef]
- Ducharme, N.G.; Desrochers, A.; Fubini, S.L.; Pease, A.P.; Mizer, L.A.; Walker, W.; Trent, A.M.; Roy, J.-P.; Rousseau, M.; Radcliffe, R.M.; et al. Surgery of the Bovine Digestive System. In Farm Animal Surgery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 223–343. ISBN 978-0-323-31665-1. [Google Scholar]
- Yasaswini, D.; Kumari, K.N.; Shobhamani, B.; Prameela, D.R.; Reddy, B.S.; Reddy, P.R.K. Clinical, haemato-biochemical, and ultrasonographic findings of abomasal impaction and abomasal ulcers in buffaloes. Trop. Anim. Health Prod. 2021, 53, 543. [Google Scholar] [CrossRef]
- Thomas, W. Left or Right Displaced Abomasum and Abomasal Volvulus in Cattle. 2021. Available online: https://www.merckvetmanual.com/digestive-system/diseases-of-the-abomasum/left-or-right-displaced-abomasum-and-abomasal-volvulus-in-cattle (accessed on 15 May 2025).
- Gouda, S.; Abdelaal, A.; Gomaa, M.; Elgioushy, M.; Refaai, W.; Mouncey, R.; Salem, S. Diagnostic performance of ultrasonography in clinical management of dairy cattle identified with left-sided ping sounds. J. Adv. Vet. Anim. Res. 2020, 7, 308. [Google Scholar] [CrossRef]
- Djebala, S.; Evrard, J.; Moula, N.; Gille, L.; Bayrou, C.; Eppe, J.; Casalta, H.; Sartelet, A.; Bossaert, P. Comparison between generalised peritonitis and parietal fibrinous peritonitis in cows after caesarean section. Vet. Rec. 2020, 187, 105867. [Google Scholar] [CrossRef]
- Bochniarz, M.; Hahaj-Siembida, A.; Krajewska-Wędzina, M.; Osińska, M.; Tracz, A.; Trościańczyk, A.; Brodzki, P.; Krakowski, L.; Kosior-Korzecka, U.; Nowakiewicz, A. Cytokine inflammatory response in dairy cows with mastitis caused by Streptococcus agalactiae. J. Vet. Res. 2024, 68, 115–121. [Google Scholar] [CrossRef]
- Yang, S.X.; Adams, G.P.; Palomino, J.M.; Huanca, W.F.; Lessard, C.; Rajapaksha, K.; Anzar, M. Cryopreservation of bison semen without exogenous protein in extender and its fertility potential in vitro and in vivo following fixed-time artificial insemination. Theriogenology 2020, 152, 156–164. [Google Scholar] [CrossRef]
- Hussain, S.A.; Uppal, S.K.; Sood, N.K.; Mohindroo, J. Primary type 3 abomasal ulceration in cattle and buffalo: Clinico-biochemical parameters treatment and prognostic indicators. Iran. J. Vet. Res. 2023, 24, 42–50. [Google Scholar] [CrossRef]
- Cabezas-Garcia, E.H.; Gordon, A.W.; Mulligan, F.J.; Ferris, C.P. Revisiting the Relationships between Fat-to-Protein Ratio in Milk and Energy Balance in Dairy Cows of Different Parities, and at Different Stages of Lactation. Animals 2021, 11, 3256. [Google Scholar] [CrossRef]
- Glassey, S. Legal Complexities of Entry, Rescue, Seizure and Disposal of Disaster-Affected Companion Animals in New Zealand. Animals 2020, 10, 1583. [Google Scholar] [CrossRef] [PubMed]
- Braun, U.; Reif, C.; Nuss, K.; Hilbe, M.; Gerspach, C. Clinical, laboratory and ultrasonographic findings in 87 cows with type-4 abomasal ulcer. BMC Vet. Res. 2019, 15, 100. [Google Scholar] [CrossRef]
- Vermunt, J.J. Ancillary Diagnostic Tests in Cattle: A Range of Surgical Procedures. J. Clin. Vet. Res. 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Querubino De Andrade Neto, A.; Ricardo Barboza Silva, J.; Lopes De Mendonça, C.; José Cavalcanti Souto, R.; Rose Marquez De Sá, L.; Augusto Bastos Afonso, J. Clinical and anatomopathological diagnosis in bovines affected by perforated abomasal ulcers and with comorbities. Acta Vet. Bras. 2024, 17, 67–78. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, H.; Cao, S.; Chen, J.; Qu, C.; Tang, Y.; Wang, M.; Zhu, L.; Liu, X.; Zhang, J. A Magnetic Reduced Graphene Oxide Nanocomposite: Synthesis, Characterization, and Application for High-Efficiency Detoxification of Aflatoxin B1. Toxins 2024, 16, 57. [Google Scholar] [CrossRef]
- Yasaswini, D.; Kumari, K.N.; Shobhamani, B.; Prameela, D.R.; Reddy, B.S.; Reddy, K.P.; Reddy, P.R.K. Risk factors, clinical indicators, and pathological findings of abomasal ulcers in tropical dairy buffaloes. Vet. Res. Commun. 2023, 47, 1139–1154. [Google Scholar] [CrossRef]
- Hund, A.; Wittek, T. Labmagengeschwüre beim Rind. Tierärztl. Prax. Ausg. G Großtiere Nutztiere 2017, 45, 121–128. [Google Scholar] [CrossRef]
- Tschoner, T. Methods for Pain Assessment in Calves and Their Use for the Evaluation of Pain during Different Procedures—A Review. Animals 2021, 11, 1235. [Google Scholar] [CrossRef]
- Laves, J.; Wergin, M.; Bauer, N.; Müller, S.F.; Failing, K.; Büttner, K.; Hagen, A.; Melzer, M.; Röcken, M. The effect of Traumeel LT ad us. vet. on the perioperative inflammatory response after castration of stallions: A prospective, randomized, double-blinded study. Front. Vet. Sci. 2024, 11, 1342345. [Google Scholar] [CrossRef]
- Xing, L.; Fu, L.; Cao, S.; Yin, Y.; Wei, L.; Zhang, W. The Anti-Inflammatory Effect of Bovine Bone-Gelatin-Derived Peptides in LPS-Induced RAW264.7 Macrophages Cells and Dextran Sulfate Sodium-Induced C57BL/6 Mice. Nutrients 2022, 14, 1479. [Google Scholar] [CrossRef] [PubMed]
- Savel’ev, S.A.; Mittenberg, A.G.; Aref’eva, T.I.; Oparina, T.I.; Chepanov, S.V.; Sel’kov, S.A. Peptide Composition and Identification of a Peptide with Anti-Inflammatory and Antiplatelet Properties in Cattle Prostate Extract. Pharm. Chem. J. 2024, 58, 35–43. [Google Scholar] [CrossRef]
 —an increase in the indicator. (figure created by authors with “Freeform” Version 3.5 (419.121.1)).
—an increase in the indicator. (figure created by authors with “Freeform” Version 3.5 (419.121.1)).
 —an increase in the indicator. (figure created by authors with “Freeform” Version 3.5 (419.121.1)).
—an increase in the indicator. (figure created by authors with “Freeform” Version 3.5 (419.121.1)).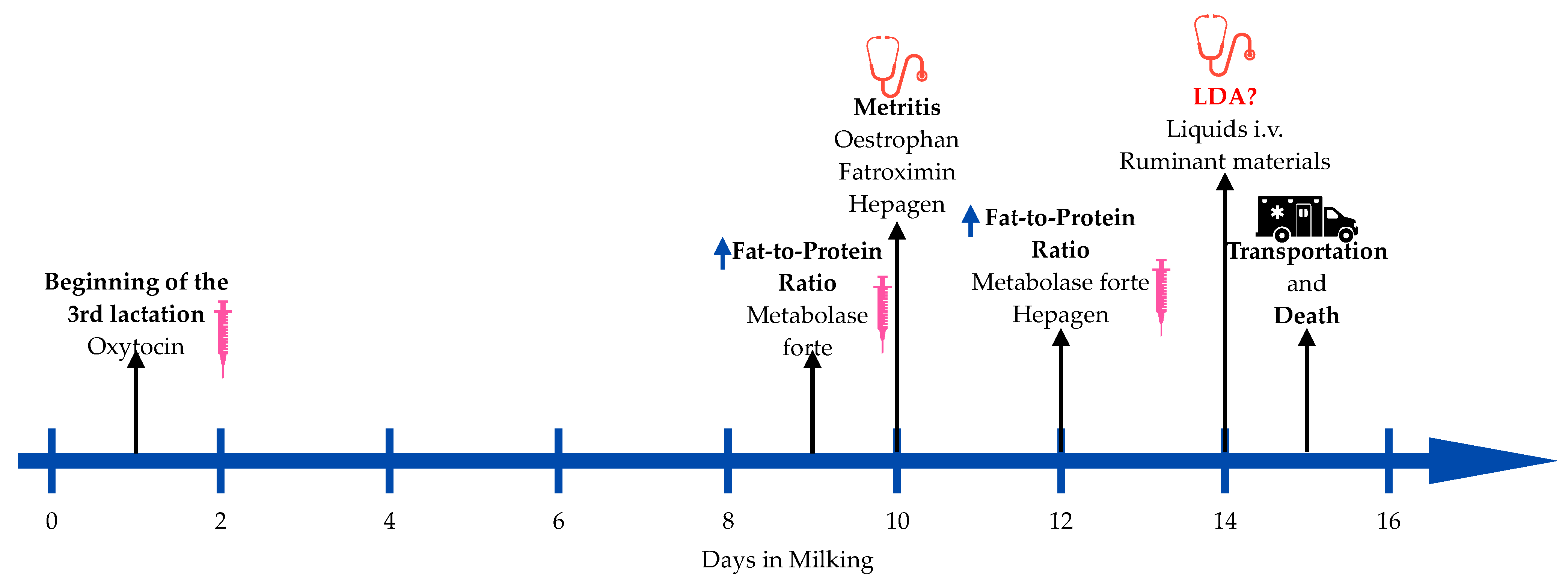
| Component of TMR * | % |
|---|---|
| Corn silage | 24% |
| Grass hay | 5% |
| Grass silage | 16% |
| Grain concentrate slurry | 50% |
| Mineral mixture | 5% |
| Parameters | % |
|---|---|
| DM Acid detergent fiber | 48 20 (%/DM) * |
| Non-fiber carbohydrates | 39 (%/DM) |
| Neutral detergent fiber | 28 (%/DM) |
| Crude protein Net energy lactation | 16 (%/DM) 1.6 (Mcal/kg) * |
| Date | Lactation Days | Rumination (min/24 h) | Milkings Per Day | Daily Production (kg) | Fat Content (%) | Protein Content (%) | Fat-to-Protein Ratio | Lactose Content (%) | Commonly Prescribed Feed (kg/Day) | Total Consumption (kg/Day) |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 September 2024 | 1 | 205 | 2 | 16.6 | 5.36 | 4.92 | 1.09 | 4.44 | 4.45 | 3.94 |
| 5 September 2024 | 2 | 263 | 4 | 24.7 | 5.36 | 4.57 | 1.17 | 4.57 | 4.71 | 4.94 |
| 6 September 2024 | 3 | 222 | 3 | 29.1 | 5.29 | 4.33 | 1.22 | 4.62 | 4.96 | 4.19 |
| 7 September 2024 | 4 | 246 | 3 | 28.4 | 5.43 | 4.1 | 1.32 | 4.65 | 5.22 | 5.54 |
| 8 September 2024 | 5 | 283 | 4 | 31.4 | 5.59 | 3.75 | 1.49 | 4.64 | 5.47 | 6.1 |
| 9 September 2024 | 6 | 165 | 2 | 30.3 | 5.52 | 3.75 | 1.47 | 4.64 | 5.73 | 3.67 |
| 10 September 2024 | 7 | 204 | 1 | 38.9 | 5.49 | 3.75 | 1.46 | 4.65 | 5.98 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šertvytytė, G.; Lembovičiūtė, G.; Rodaitis, O.; Džermeikaitė, K.; Arlauskaitė, S.; Krištolaitytė, J.; Girdauskaitė, A.; Pockevičius, A.; Rutkauskas, A.; Antanaitis, R. A Case Report: Post-Mortem Pathological Observations of a Fresh Dairy Cow with Type 3 Abomasal Ulcer After Sudden Death. Animals 2025, 15, 1969. https://doi.org/10.3390/ani15131969
Šertvytytė G, Lembovičiūtė G, Rodaitis O, Džermeikaitė K, Arlauskaitė S, Krištolaitytė J, Girdauskaitė A, Pockevičius A, Rutkauskas A, Antanaitis R. A Case Report: Post-Mortem Pathological Observations of a Fresh Dairy Cow with Type 3 Abomasal Ulcer After Sudden Death. Animals. 2025; 15(13):1969. https://doi.org/10.3390/ani15131969
Chicago/Turabian StyleŠertvytytė, Greta, Gabija Lembovičiūtė, Osvaldas Rodaitis, Karina Džermeikaitė, Samanta Arlauskaitė, Justina Krištolaitytė, Akvilė Girdauskaitė, Alius Pockevičius, Arūnas Rutkauskas, and Ramūnas Antanaitis. 2025. "A Case Report: Post-Mortem Pathological Observations of a Fresh Dairy Cow with Type 3 Abomasal Ulcer After Sudden Death" Animals 15, no. 13: 1969. https://doi.org/10.3390/ani15131969
APA StyleŠertvytytė, G., Lembovičiūtė, G., Rodaitis, O., Džermeikaitė, K., Arlauskaitė, S., Krištolaitytė, J., Girdauskaitė, A., Pockevičius, A., Rutkauskas, A., & Antanaitis, R. (2025). A Case Report: Post-Mortem Pathological Observations of a Fresh Dairy Cow with Type 3 Abomasal Ulcer After Sudden Death. Animals, 15(13), 1969. https://doi.org/10.3390/ani15131969








