Gut Fungal Community Modulates Fat Deposition in Ningxiang Pigs: Species-Specific Regulation via the Glucose–SCFAs Metabolic Axis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Feeding
2.2. Sample Collection
2.3. Serum Glucose Analysis (HPLC)
2.4. Colonic SCFA Analysis (GC-MS)
2.5. DNA Extraction and Sequencing
2.6. Bioinformatics Analysis
2.7. Statistical Analysis
3. Results
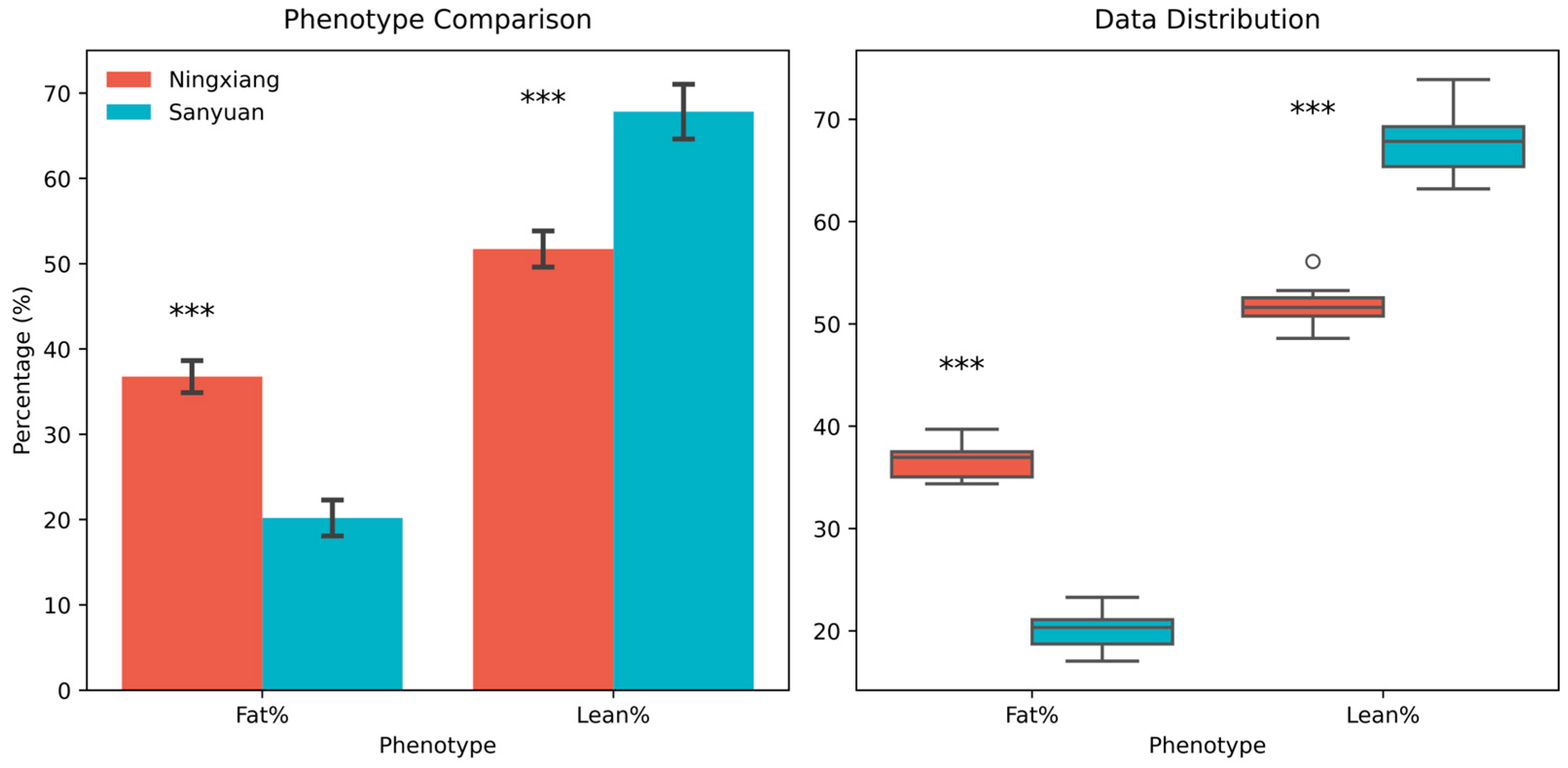
3.1. Phenotypic Differences
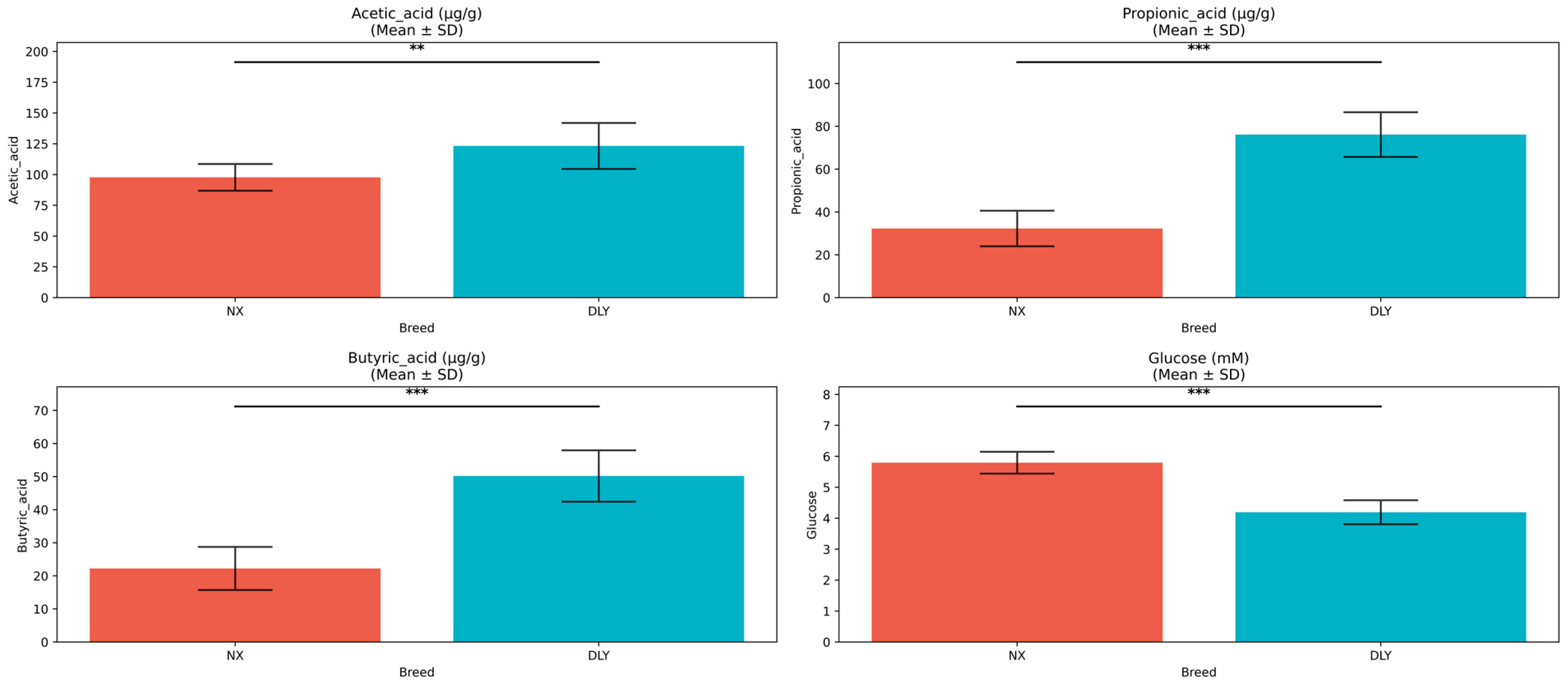
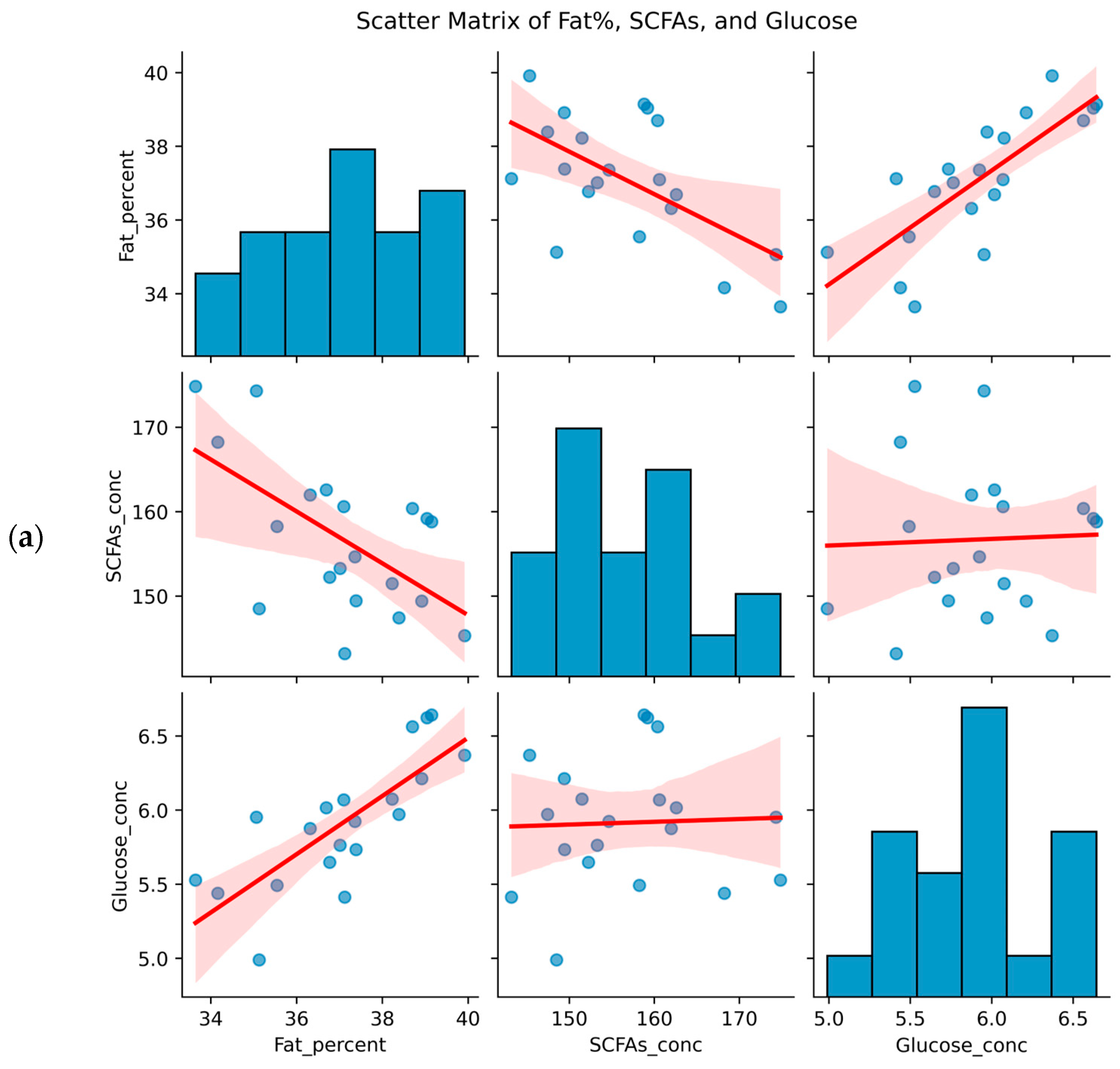
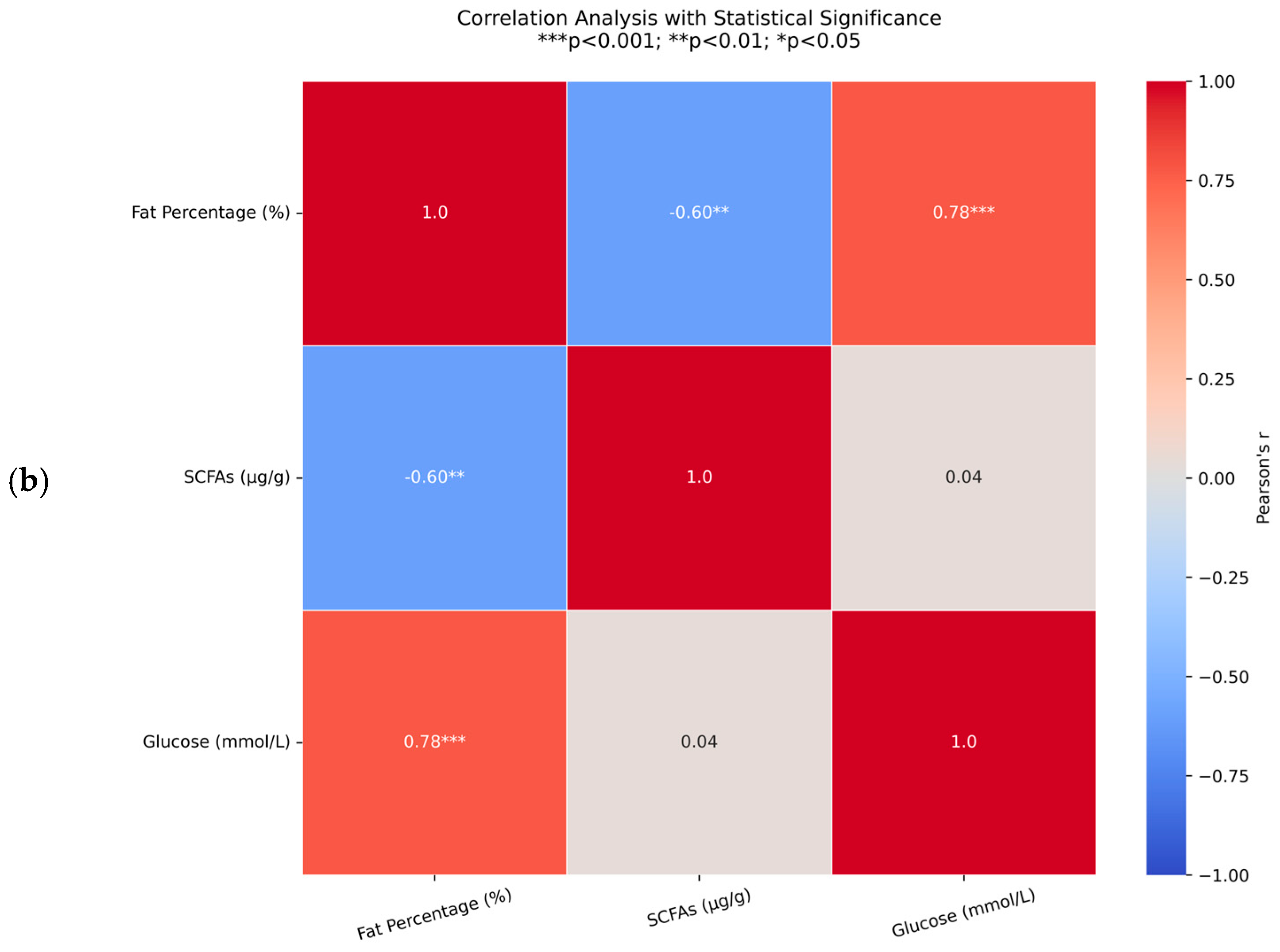
3.2. Associations Between Body Composition and Metabolites
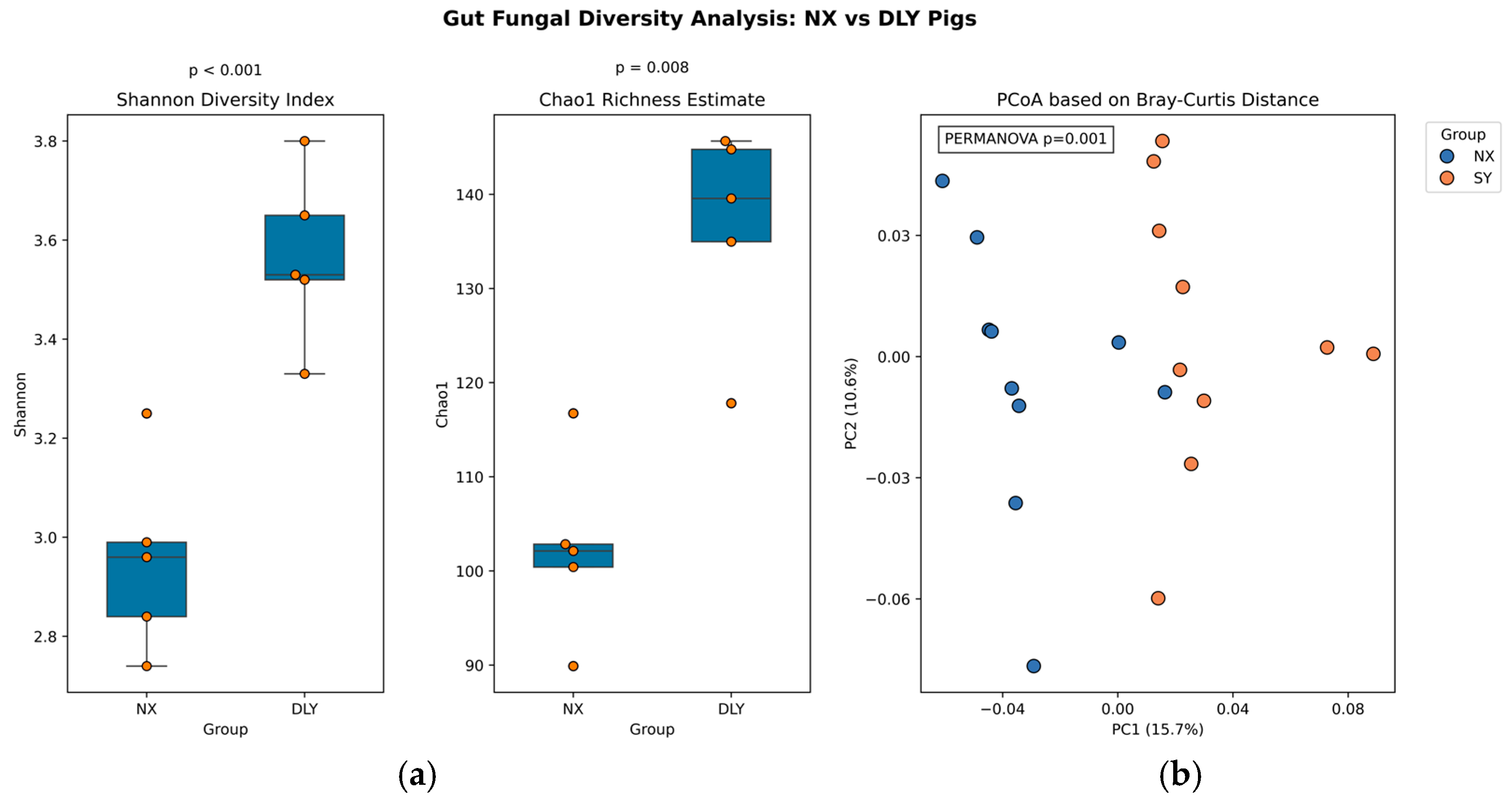
3.3. Alpha and Beta Diversity Analysis
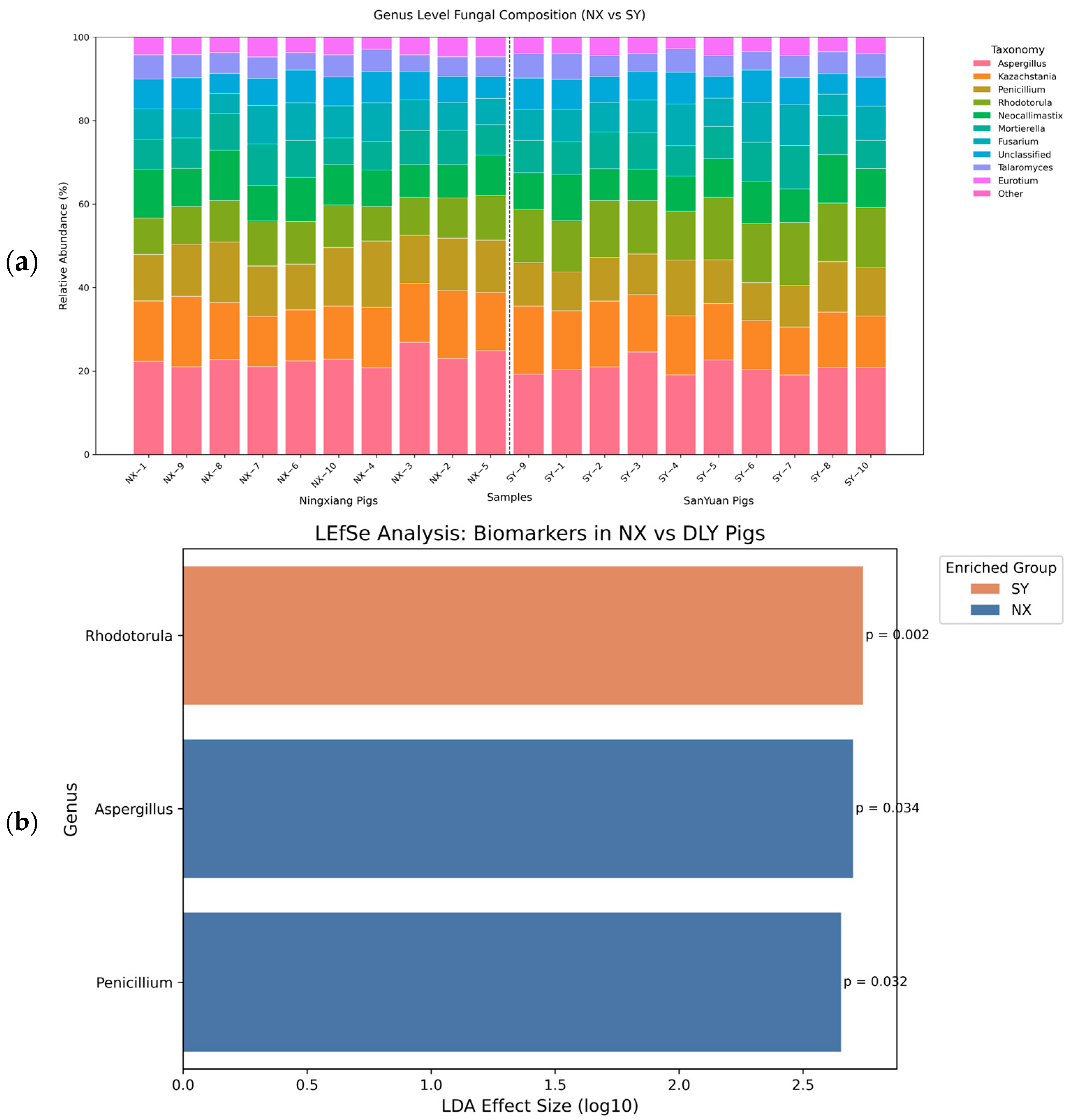
3.4. Fungal Compositional Differences
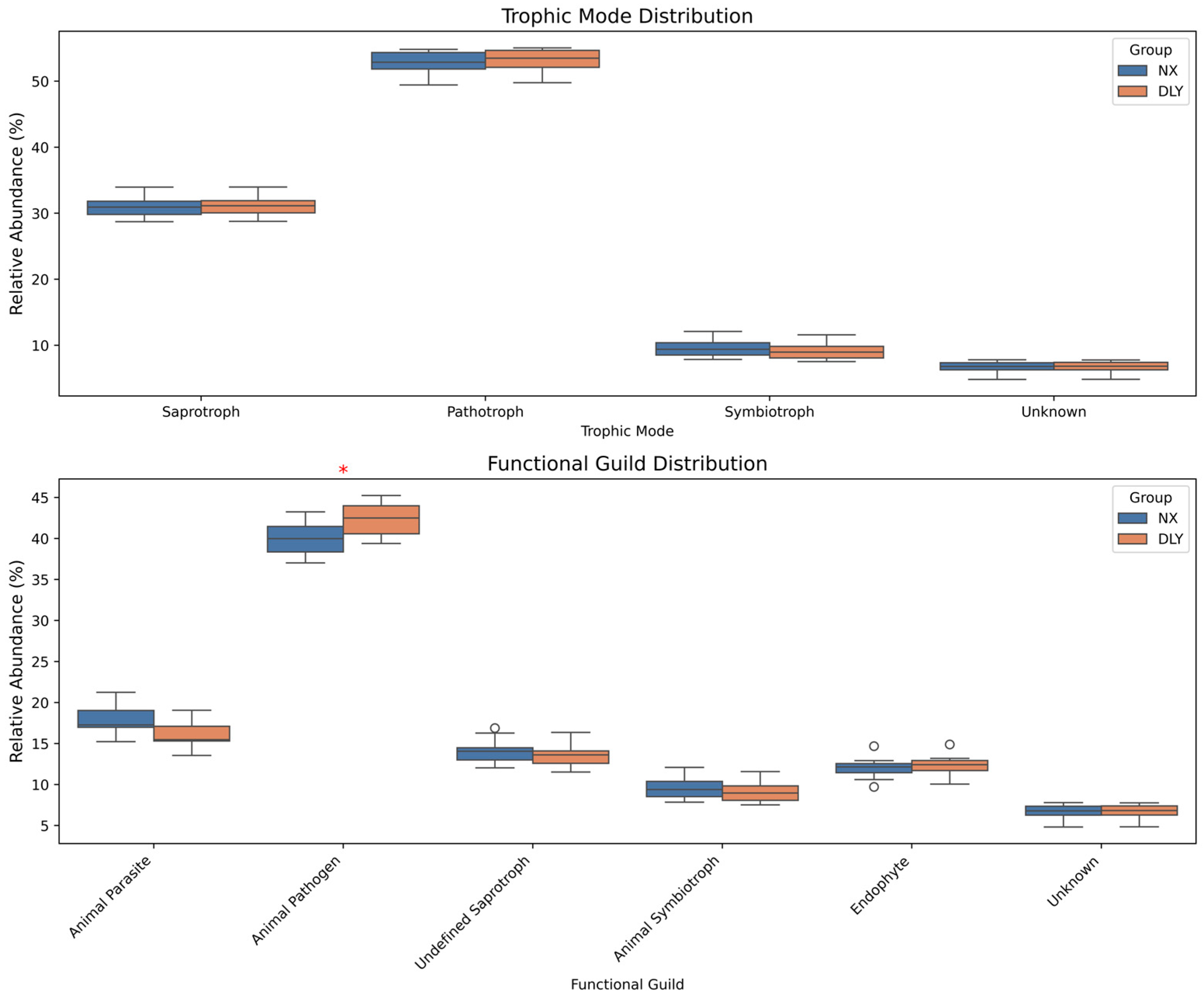
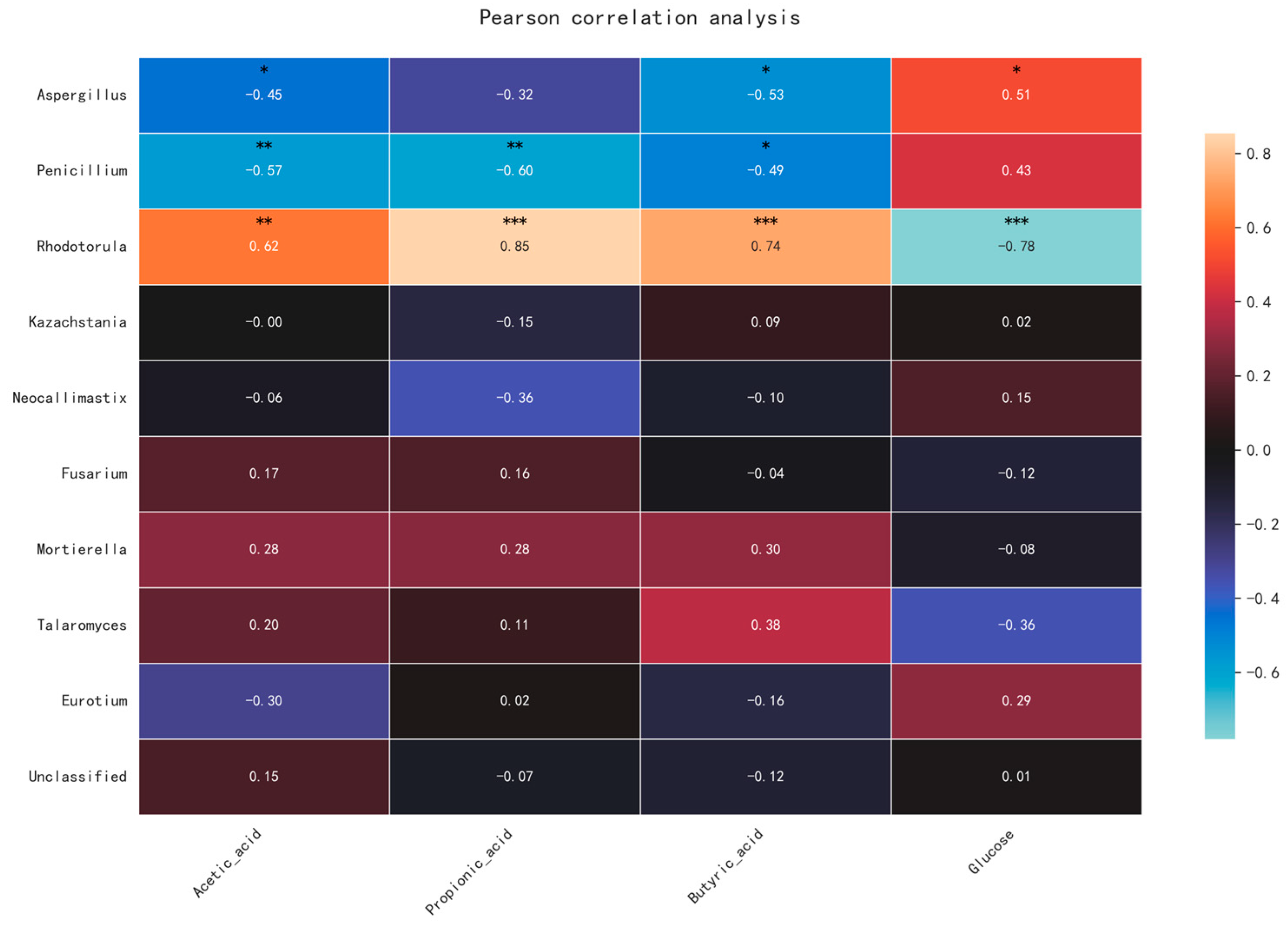
3.5. Fungal Functional Prediction and Metabolic Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bordbar, F.; Mohammadabadi, M.; Jensen, J.; Xu, L.; Li, J.; Zhang, L. Identification of Candidate Genes Regulating Carcass Depth and Hind Leg Circumference in Simmental Beef Cattle Using Illumina Bovine Beadchip and Next-Generation Sequencing Analyses. Animals 2022, 12, 1103. [Google Scholar] [CrossRef]
- Mohammadabadi, M.; Bordbar, F.; Jensen, J.; Du, M.; Guo, W. Key Genes Regulating Skeletal Muscle Development and Growth in Farm Animals. Animals 2021, 11, 835. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short Chain Fatty Acids Prevent High-fat-diet-induced Obesity in Mice by Regulating G Protein-coupled Receptors and Gut Microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef]
- Den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.J.; et al. Short-Chain Fatty Acids Protect Against High-Fat Diet–Induced Obesity via a PPARγ-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Giovanni, T.; Vincenzo, C. What are the common downstream molecular events between alcoholic and nonalcoholic fatty liver? Lipids Health Dis. 2024, 23, 41. [Google Scholar] [CrossRef]
- Milton-Laskibar, I.; Marcos-Zambrano, L.J.; Gómez-Zorita, S.; de Santa Pau, E.C.; Fernández-Quintela, A.; Martínez, J.A.; Portillo, M.P. Involvement of microbiota and short-chain fatty acids on non-alcoholic steatohepatitis when induced by feeding a hypercaloric diet rich in saturated fat and fructose. Gut Microbiome 2022, 3, e5. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, C.; Duan, C.J.; Tan, C.; Zhang, S.; Wu, J.; Liu, W.-F.; Su, T. The correlation of adipocyte size, serum insulin and leptin levels in high-fat-induced obesity mouse. China J. Mod. Med. 2013, 223, 11–14. [Google Scholar]
- Schaefer, E.J.; Gleason, J.A.; Dansinger, M.L. Dietary Fructose and Glucose Differentially Affect Lipid and Glucose Homeostasis. J. Nutr. 2009, 139, 1257S–1262S. [Google Scholar] [CrossRef]
- Deehan, E.C.; Mocanu, V.; Madsen, K.L. Effects of dietary fibre on metabolic health and obesity. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 301–318. [Google Scholar] [CrossRef]
- Wang, L.Y.; He, L.H.; Xu, L.J.; Li, S.B. Short-chain fatty acids: Bridges between diet, gut microbiota, and health. J. Gastroenterol. Hepatol. 2024, 39, 1728–1736. [Google Scholar] [CrossRef]
- Khosravi, C.; Benocci, T.; Battaglia, E.; Benoit, I.; de Vries, R.P. Sugar Catabolism in Aspergillus and Other Fungi Related to the Utilization of Plant Biomass. Adv. Appl. Microbiol. 2015, 90, 1–28. [Google Scholar]
- Glass, N.L.; Schmoll, M.; Cate, J.H.; Coradetti, S. Plant Cell Wall Deconstruction by Ascomycete Fungi. Annu. Rev. Microbiol. 2013, 67, 477–498. [Google Scholar] [CrossRef]
- Yang, L.; Bian, G.; Su, Y.; Zhu, W. Comparison of Faecal Microbial Community of Lantang, Bama, Erhualian, Meishan, Xiaomeishan, Duroc, Landrace, and Yorkshire Sows. Asian-Australas. J. Anim. Sci. 2014, 27, 898–906. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, K.; Xiang, Y.; Zhou, W.; Gui, G.; Yang, H. The fecal microbiota composition of boar Duroc, Yorkshire, Landrace and Hampshire pigs. Asian-Australas. J. Anim. Sci. 2017, 30, 1456–1463. [Google Scholar] [CrossRef]
- Doolotkeldieva, T.D.; Bobusheva, S.T. Screening of Wild-Type Fungal Isolates for Cellulolytic Activity. Microbiol. Insights 2011, 4, MBI-S6418. [Google Scholar] [CrossRef]
- Simpson, C.; Jordaan, J.; Gardiner, N.S.; Whiteley, C. Isolation, purification and characterization of a novel glucose oxidase from Penicillium sp. CBS 120262 optimally active at neutral pH. Protein Expr. Purif. 2007, 51, 260–266. [Google Scholar] [CrossRef]
- Devi, A.; Singh, A.; Kothari, R. Fungi based valorization of wheat straw and rice straw for cellulase and xylanase production. Sustain. Chem. Environ. 2024, 5, 100077. [Google Scholar] [CrossRef]
- Naher, L.; Fatin, S.N.; Sheikh, M.A.H.; Azeez, L.A.; Siddiquee, S.; Zain, N.M.; Karim, S.M.R. Cellulase Enzyme Production from Filamentous Fungi Trichoderma reesei and Aspergillus awamori in Submerged Fermentation with Rice Straw. J. Fungi 2021, 7, 868. [Google Scholar] [CrossRef]
- Clarke, S.D.; Nakamura, M.T. Lipids|Fatty Acid Structure and Synthesis. In Encyclopedia of Biological Chemistry III; Elsevier Inc.: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Scoditti, E.; Sabatini, S.; Carli, F.; Gastaldelli, A. Hepatic glucose metabolism in the steatotic liver. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 319–334. [Google Scholar] [CrossRef]
- Yang, H.; Xiang, Y.; Robinson, K.; Wang, J.; Zhang, G.; Zhao, J.; Xiao, Y. Gut Microbiota Is a Major Contributor to Adiposity in Pigs. Front. Microbiol. 2018, 9, 3045. [Google Scholar] [CrossRef]
- Hernández-Almanza, A.; Cesar Montanez, J.; Aguilar-González, M.A.; Martínez-Ávila, C.; Rodríguez-Herrera, R.; Aguilar, C.N. Rhodotorula glutinis as source of pigments and metabolites for food industry. Food Biosci. 2014, 5, 64–72. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Chen, H.; Zhou, L.; Chen, B.; Wang, M.; Zhang, D.; Han, T.L.; Zhang, H. Gut dysbiosis contributes to SCFAs reduction-associated adipose tissue macrophage polarization in gestational diabetes mellitus. Life Sci. 2024, 350, 122744. [Google Scholar] [CrossRef]
- Jeon, T.; Hwang, S.G.; Hirai, S.; Matsui, T.; Yano, H.; Kawada, T.; Lim, B.O.; Park, D.K. Red yeast rice extracts suppress adipogenesis by down-regulating adipogenic transcription factors and gene expression in 3T3-L1 cells. Life Sci. 2004, 75, 3195–3203. [Google Scholar] [CrossRef]
- Lan, Y.; Sun, Q.; Ma, Z.; Peng, J.; Zhang, M.; Wang, C.; Zhang, X.; Yan, X.; Chang, L.; Hou, X.; et al. Seabuckthorn polysaccharide ameliorates high-fat diet-induced obesity by gut microbiota-SCFAs-liver axis. Food Funct. 2022, 13, 2925–2937. [Google Scholar] [CrossRef]
- Si, X.; Shang, W.; Zhou, Z.; Strappe, P.; Wang, B.; Bird, A.; Blanchard, C. Gut Microbiome-Induced Shift of Acetate to Butyrate Positively Manages Dysbiosis in High Fat Diet. Mol. Nutr. Food Res. 2018, 62, 1700670. [Google Scholar] [CrossRef]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia spp.: A Marker of Health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, B.; Sun, J.; Liu, Z.; Chen, H.; Ge, L.; Chen, D. Short-chain Fatty Acids can Improve Lipid and Glucose Metabolism Independently of the Gut Microbiota. J. Anim. Sci. Biotechnol. 2020, 12, 1–14. [Google Scholar] [CrossRef]
- Bhagat, N.R.; Kumar, S.; Kumari, R.; Bharti, V.K. A Review on Rumen Anaerobic Fungi: Current Understanding on Carbohydrate Fermentation and Roughages Digestion in Ruminants. Appl. Biochem. Microbiol. 2023, 59, 231–249. [Google Scholar] [CrossRef]
- Thapa, S.; Mishra, J.; Arora, N.; Mishra, P.; Li, H.; O′ Hair, J.; Bhatti, S.; Zhou, S. Microbial cellulolytic enzymes: Diversity and biotechnology with reference to lignocellulosic biomass degradation. Rev. Environ. Sci. Bio/Technol. 2020, 19, 621–648. [Google Scholar] [CrossRef]
| Items | Content |
|---|---|
| Ingredient Composition † | |
| Corn | 60.0 |
| Soybean meal | 25.0 |
| Wheat bran | 8.0 |
| CaHPO4 | 2.0 |
| Limestone | 1.5 |
| NaCl | 0.3 |
| Premix 1 | 3.0 |
| Soybean Oil | 0.2 |
| Total | 100.0 |
| Nutrient levels | |
| DE, MJ/kg | 14.28 |
| CP | 16.94 |
| CF | 4.15 |
| EE | 4.72 |
| Ca | 0.68 |
| Total P | 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, P.; Wang, H.; Wang, J.; Qiu, Z.; Wang, C.; Liu, H.; Wang, Q.; Li, Y.; Yang, H. Gut Fungal Community Modulates Fat Deposition in Ningxiang Pigs: Species-Specific Regulation via the Glucose–SCFAs Metabolic Axis. Animals 2025, 15, 1887. https://doi.org/10.3390/ani15131887
Huang P, Wang H, Wang J, Qiu Z, Wang C, Liu H, Wang Q, Li Y, Yang H. Gut Fungal Community Modulates Fat Deposition in Ningxiang Pigs: Species-Specific Regulation via the Glucose–SCFAs Metabolic Axis. Animals. 2025; 15(13):1887. https://doi.org/10.3390/ani15131887
Chicago/Turabian StyleHuang, Pengfei, Hanmin Wang, Juan Wang, Zhenrong Qiu, Chunfeng Wang, Han Liu, Qiye Wang, Yali Li, and Huansheng Yang. 2025. "Gut Fungal Community Modulates Fat Deposition in Ningxiang Pigs: Species-Specific Regulation via the Glucose–SCFAs Metabolic Axis" Animals 15, no. 13: 1887. https://doi.org/10.3390/ani15131887
APA StyleHuang, P., Wang, H., Wang, J., Qiu, Z., Wang, C., Liu, H., Wang, Q., Li, Y., & Yang, H. (2025). Gut Fungal Community Modulates Fat Deposition in Ningxiang Pigs: Species-Specific Regulation via the Glucose–SCFAs Metabolic Axis. Animals, 15(13), 1887. https://doi.org/10.3390/ani15131887








