Review: Gut Microbiota—A Powerful Tool for Improving Pig Welfare by Influencing Behavior Through the Gut–Brain Axis
Simple Summary
Abstract
1. Introduction
2. Material and Methods
3. Association Between Pig Welfare and Behavior
3.1. Freedom from Hunger and Thirst
3.2. Freedom from Discomfort
3.3. Freedom from Pain, Injury, and Disease
3.4. Freedom from Fear and Distress
3.5. Freedom to Express Normal Behavior
4. Gut Microbes Influence Pig Behavior
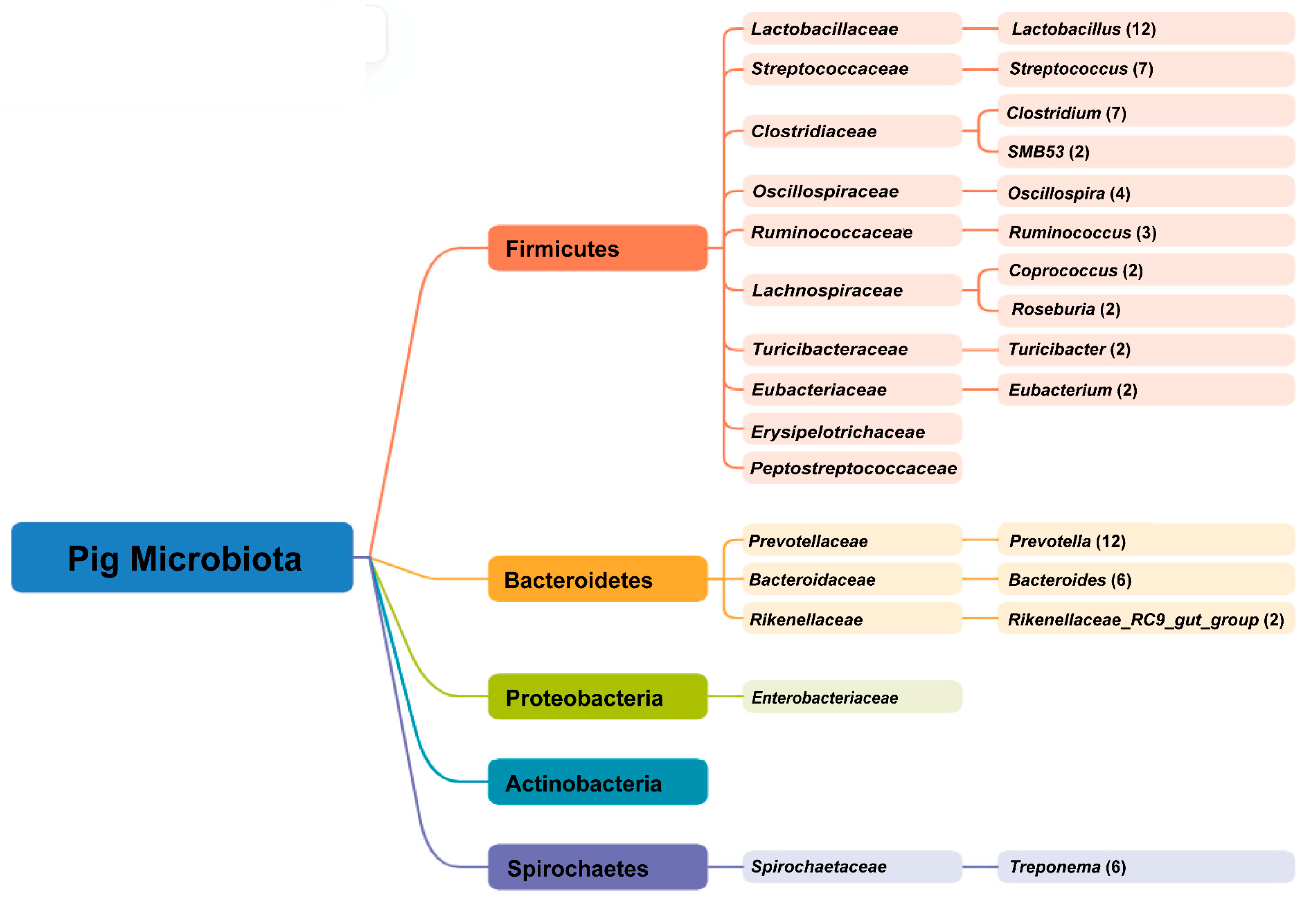
4.1. Main Microbes in the Gastrointestinal Tract of Pigs
| Research Subject | Dominant Genus | Refference |
|---|---|---|
| Fecal samples collected from 287 pigs of 17 breeds | Prevotella, Bacteroides, Clostridium, Ruminococcus and Eubacterium | [40] |
| Fecal samples collected from 244 Bamaxiang pigs and cecum content samples from 256 Erhualian pigs | Treponema, Rikenellaceae_RC9_gut_group, Prevotellaceae_NK3B31_group, and Prevotella | [41] |
| Rectum contents of 288 Duroc × Iberian pigs | Prevotella and Treponema | [42] |
| Rectum contents of 102 Diannan small-ear pigs | Streptococcus, Lachnospiraceae, Oscillospiraceae_UCG-002, and Lachnospiraceae_XPB1014_group | [36] |
| Fecal samples of 120 pigs within different breeds | Lactobacillus, Prevotella, Treponema, Oscillospira, Clostridium, Ruminococcus, Holdemania, Streptococcus, Bacteroides and Coprococcus | [33] |
| Gastrointestinal tract samples of 13 Iberian pigs | Lactobacillus and Clostridium | [43] |
| Fecal samples of 11 Tibetan pigs | Prevotella and Treponema | [44] |
| Fecal samples of 105 Jinhua pigs | Lactobacillus, Bifidobacterium, Streptococcus, Prevotella, Bacteroides, Turicibacter, Clostridium, Treponema, Allobaculum, and SMB53 | [45] |
| Fresh fecal samples of 15 Duroc × Landrace × Yorkshine pigs | Lactobacillus, Streptococcus, SMB53, Oscillospira, and Prevotella | [46] |
| Fecal samples 8 Ningxiang pigs | Lactobacillus | [47] |
| Fresh fecal samples of Duroc (n = 505), Landrace (n = 2120) and Yorkshire (n = 3754) | Streptococcus and Lactobacillus | [48] |
| Rectal content of 288 Duroc Iberian pigs | Rikenellaceae RC9 gut group, Treponema, Prevotella, and Muribaculaceae genus | [49] |
| Fecal samples of 10 Yorkshire-Landrace pigs | Clostridium, Escherichia, Megasphaera and Lactobacillus | [50] |
| Fecal samples of 274 Suhuai pigs | Lactobacillus, Clostridium, Streptococcus, unclassified p Firmicutes, Prevotella, unclassified f Lachnospiraceae, Bacteroides, Ruminococcus, Eubacterium, and Roseburia | [51] |
| Gastrointestinal tract and fecal samples of 6 Landrace × Large White pigs | Lactobacillus, Actinobacillus, Romboutsia, Escherichia-Shigella, Terripsorobacter, and Campylobacter | [52] |
| Fecal samples of 518 piglets | Prevotella and Roseburia | [53] |
| Fecal samples of Duroc (n = 6), Hampshire (n = 4), Landrace (n = 7), Yorkshir (n = 6) | Streptococcus, Prevotella Lactobacillus, Clostridium Turicibacter, Oscillospira, Coprococcus, Bacteroides and Ruminococcus | [54] |
4.2. Microbiota and Pig Behavior
4.2.1. Feeding Behavior
4.2.2. Excretion Behavior
4.2.3. Group Living Behavior
4.2.4. Fighting Behavior
4.2.5. Sexual Behavior
4.2.6. Exploratory Behavior
4.2.7. Abnormal Behavior
4.2.8. After-Effect Behavior
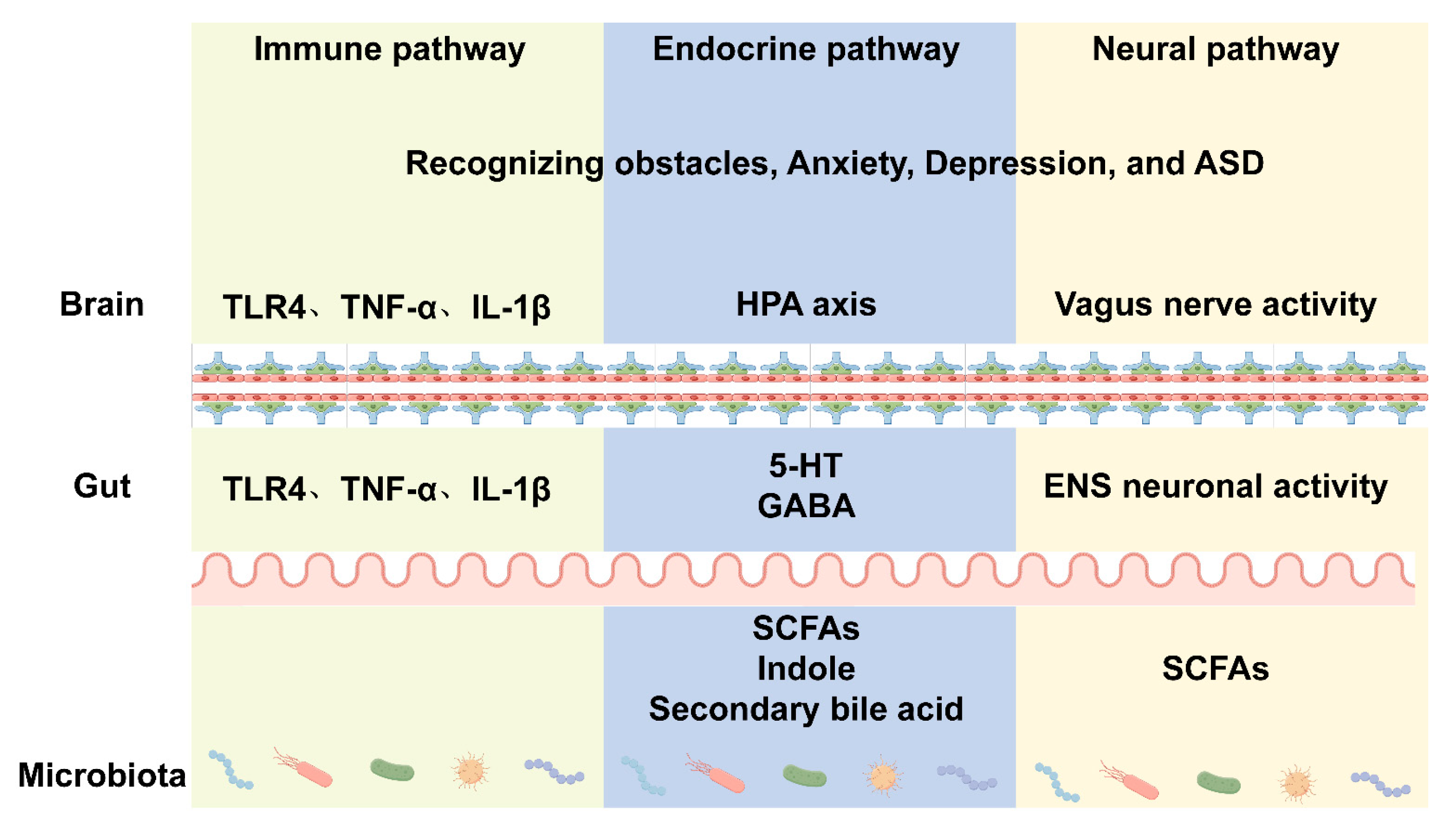
5. The Role of Microbiota in Regulating Behavior
5.1. Immune Pathway
5.2. Endocrine Pathway
5.3. Neural Pathway
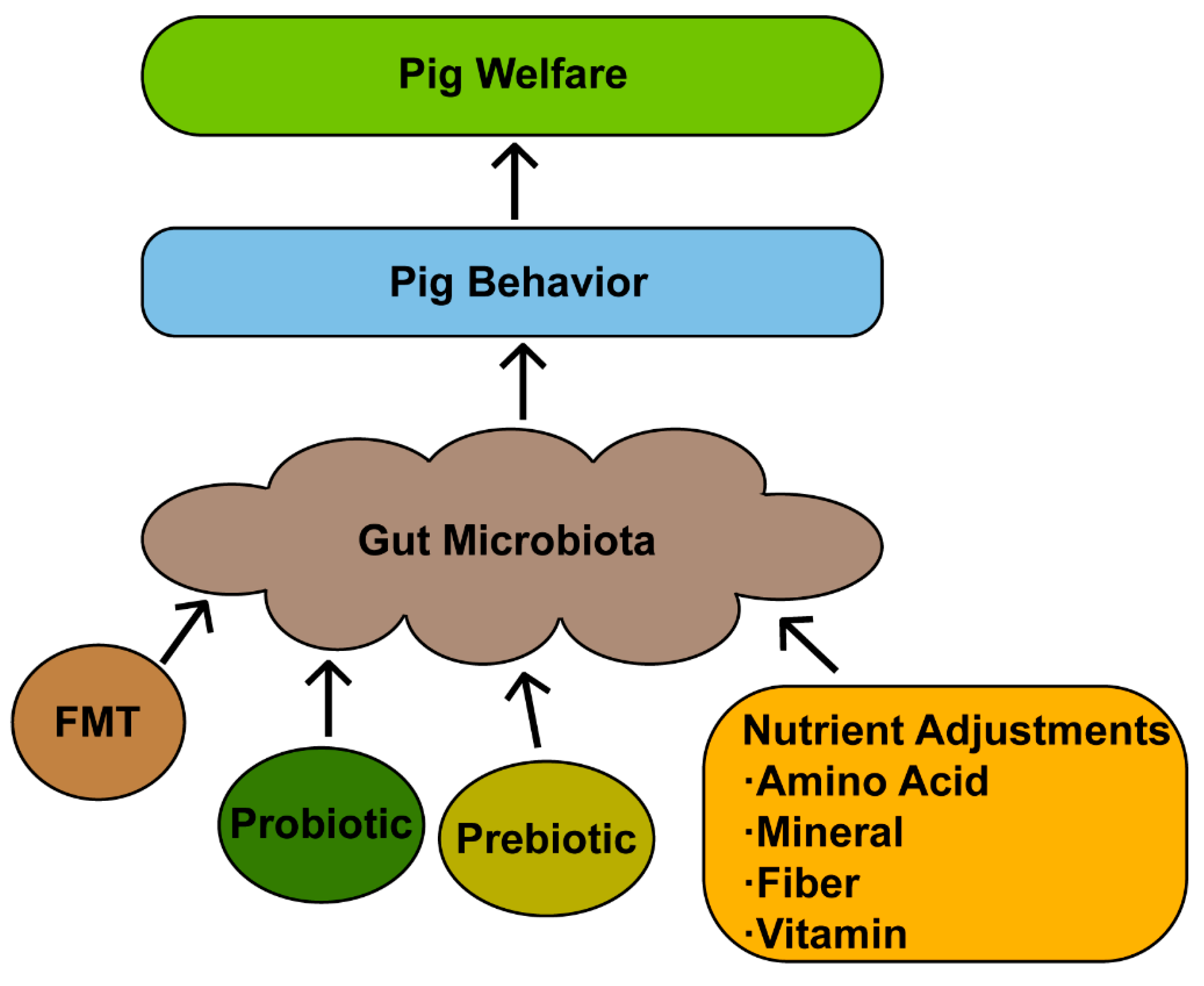
6. Approaches for Targeted Regulation of Gut Microbiota to Promote Welfare
6.1. Fecal Microbiota Transplantation
6.2. Probiotic
6.3. Prebiotic
6.4. Nutrient Adjustments
6.4.1. Amino Acid
6.4.2. Mineral
6.4.3. Fiber
6.4.4. Vitamin
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HT | 5-hydroxytryptamine |
| AHR | aryl hydrocarbon receptor |
| ASD | autism spectrum disorder |
| BBB | blood–brain barrier |
| CNS | central nervous system |
| ENS | enteric nervous system |
| FMT | fecal microbiota transplantation |
| GABA | γ-aminobutyric acid |
| GF | germ-free |
| HFD | high-fat diet |
| HPA | hypothalamic–pituitary–adrenal |
| LNAA | large neutral amino acids |
| LPS | lipopolysaccharides |
| MGBA | microbiota–gut–brain axis |
| PFC | prefrontal cortex |
| SCFA | short-chain fatty acid |
| TB | tail biting |
References
- Carrillo, G.L.; Ballard, V.A.; Glausen, T.; Boone, Z.; Teamer, J.; Hinkson, C.L.; Wohlfert, E.A.; Blader, I.J.; Fox, M.A. Toxoplasma infection induces microglia-neuron contact and the loss of perisomatic inhibitory synapses. Glia 2020, 68, 1968–1986. [Google Scholar] [CrossRef] [PubMed]
- Mifflin, K.A.; Benson, C.; Thorburn, K.C.; Baker, G.B.; Kerr, B.J. Manipulation of neurotransmitter levels has differential effects on formalin-evoked nociceptive behavior in male and female mice. J. Pain 2016, 17, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohorquez, D.V. A gut-brain neural circuit for nutrient sensory transduction. Science 2018, 361, eaat5236. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Nance, K.; Chen, S. The gut-brain axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The critical modulators regulating gut-brain axis. J. Cell Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef]
- Wu, W.L.; Adame, M.D.; Liou, C.W.; Barlow, J.T.; Lai, T.T.; Sharon, G.; Schretter, C.E.; Needham, B.D.; Wang, M.I.; Tang, W.; et al. Microbiota regulate social behaviour via stress response neurons in the brain. Nature 2021, 595, 409–414. [Google Scholar] [CrossRef]
- Zhou, M.; Fan, Y.; Xu, L.; Yu, Z.; Wang, S.; Xu, H.; Zhang, J.; Zhang, L.; Liu, W.; Wu, L.; et al. Microbiome and tryptophan metabolomics analysis in adolescent depression: Roles of the gut microbiota in the regulation of tryptophan-derived neurotransmitters and behaviors in human and mice. Microbiome 2023, 11, 145. [Google Scholar] [CrossRef]
- Zha, X.; Liu, X.; Wei, M.; Huang, H.; Cao, J.; Liu, S.; Bian, X.; Zhang, Y.; Xiao, F.; Xie, Y.; et al. Microbiota-derived Lysophosphatidylcholine alleviates Alzheimer’s disease pathology via suppressing ferroptosis. Cell Metab. 2025, 37, 169–186. [Google Scholar] [CrossRef]
- Tang, C.F.; Wang, C.Y.; Wang, J.H.; Wang, Q.N.; Li, S.J.; Wang, H.O.; Zhou, F.; Li, J.M. Short-chain fatty acids ameliorate depressive-like behaviors of high fructose-fed mice by rescuing hippocampal neurogenesis decline and blood-brain barrier damage. Nutrients 2022, 14, 1882. [Google Scholar] [CrossRef]
- Li, B.; Li, M.; Luo, Y.; Li, R.; Li, W.; Liu, Z. Engineered 5-HT producing gut probiotic improves gastrointestinal motility and behavior disorder. Front. Cell Infect. Microbiol. 2022, 12, 1013952. [Google Scholar] [CrossRef]
- Tette, F.M.; Kwofie, S.K.; Wilson, M.D. Therapeutic anti-depressant potential of microbial GABA produced by Lactobacillus rhamnosus strains for GABAergic signaling restoration and inhibition of addiction-induced HPA axis hyperactivity. Curr. Issues Mol. Biol. 2022, 44, 1434–1451. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Ryan, P.M.; Wiley, N.; Carafa, I.; Sherwin, E.; Moloney, G.; Franciosi, E.; Mandal, R.; Wishart, D.S.; Tuohy, K. Gamma-aminobutyric acid-producing lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome. Sci. Rep. 2019, 9, 16323. [Google Scholar] [CrossRef] [PubMed]
- Lyte, J.M.; Gheorghe, C.E.; Goodson, M.S.; Kelley-Loughnane, N.; Dinan, T.G.; Cryan, J.F.; Clarke, G. Gut-brain axis serotonergic responses to acute stress exposure are microbiome-dependent. Neurogastroenterol. Motil. 2020, 32, e13881. [Google Scholar] [CrossRef] [PubMed]
- Blecharz-Klin, K.; Swierczynska, M.; Piechal, A.; Wawer, A.; Joniec-Maciejak, I.; Pyrzanowska, J.; Wojnar, E.; Zawistowska-Deniziak, A.; Sulima-Celinska, A.; Mlocicki, D.; et al. Infection with intestinal helminth (Hymenolepis diminuta) impacts exploratory behavior and cognitive processes in rats by changing the central level of neurotransmitters. PLoS Pathog. 2022, 18, e1010330. [Google Scholar] [CrossRef]
- Meyer, C.J.; Cassidy, K.A.; Stahler, E.E.; Brandell, E.E.; Anton, C.B.; Stahler, D.R.; Smith, D.W. Parasitic infection increases risk-taking in a social, intermediate host carnivore. Commun. Biol. 2022, 5, 1180. [Google Scholar] [CrossRef]
- Jarrett, S.; Ashworth, C.J. The role of dietary fibre in pig production, with a particular emphasis on reproduction. J. Anim. Sci. Biotechnol. 2018, 9, 59. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales, R.J.; Schmidt, G.; Herskin, M.; et al. Welfare of pigs on farm. Eur. Food Saf. Auth. 2022, 20, e07421. [Google Scholar]
- Wegner, B.; Tenhündfeld, J.; Vogels, J.; Beumer, M.; Kamphues, J.; Hansmann, F.; Rieger, H.; Grosse Beilage, E.; Hennig-Pauka, I. Lameness in fattening pigs—Mycoplasma hyosynoviae, osteochondropathy and reduced dietary phosphorus level as three influencing factors: A case report. Porcine Health Manag. 2020, 6, 41. [Google Scholar] [CrossRef]
- Henry, M.; Shoveller, A.K.; O’Sullivan, T.L.; Niel, L.; Friendship, R. Effect of varying levels of dietary tryptophan on aggression and abnormal behavior in growing pigs. Front. Vet. Sci. 2022, 9, 849970. [Google Scholar] [CrossRef]
- Li, X.; Xiong, X.; Wu, X.; Liu, G.; Zhou, K.; Yin, Y. Effects of stocking density on growth performance, blood parameters and immunity of growing pigs. Anim. Nutr. 2020, 6, 529–534. [Google Scholar] [CrossRef]
- Cornale, P.; Macchi, E.; Miretti, S.; Renna, M.; Lussiana, C.; Perona, G.; Mimosi, A. Effects of stocking density and environmental enrichment on behavior and fecal corticosteroid levels of pigs under commercial farm conditions. J. Vet. Behav. 2015, 10, 569–576. [Google Scholar] [CrossRef]
- Daramola, J.O.; Abioja, M.O.; Onagbesan, O.M. Heat Stress Impact on Livestock Production; Springer: Berlin/Heidelberg, Germany, 2012; pp. 53–73. [Google Scholar]
- Podder, M.; Bera, S.; Naskar, S.; Sahu, D.; Mukherjee, J.; Patra, A.K. Physiological, blood-biochemical and behavioural changes of ghoongroo pigs in seasonal heat stress of a hot-humid tropical environment. Int. J. Biometeorol. 2022, 66, 1349–1364. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, M.; Poullet, N.; Beramice, D.; Dantec, L.; Canario, L.; Gourdine, J. Behavior comparison during chronic heat stress in large white and creole pigs using image-analysis. Front. Anim. Sci. 2021, 2, 784376. [Google Scholar] [CrossRef]
- Yu, J.; Chen, S.; Zeng, Z.; Xing, S.; Chen, D.; Yu, B.; He, J.; Huang, Z.; Luo, Y.; Zheng, P.; et al. Effects of cold exposure on performance and skeletal muscle fiber in weaned piglets. Animals 2021, 11, 2148. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Mun, H.S.; Yoe, H.; Yang, C.J. Monitoring of behavior using a video-recording system for recognition of salmonella infection in experimentally infected growing pigs. Animal 2015, 9, 115–121. [Google Scholar] [CrossRef]
- Benjamin, M.; Yik, S. Precision livestock farming in swine welfare: A review for swine practitioners. Animals 2019, 9, 133. [Google Scholar] [CrossRef]
- Heseker, P.; Bergmann, T.; Scheumann, M.; Traulsen, I.; Kemper, N.; Probst, J. Detecting tail biters by monitoring pig screams in weaning pigs. Sci. Rep. 2024, 14, 4523. [Google Scholar] [CrossRef]
- Briefer, E.F.; Sypherd, C.C.; Linhart, P.; Leliveld, L.; Padilla, D.L.T.M.; Read, E.R.; Guerin, C.; Deiss, V.; Monestier, C.; Rasmussen, J.H.; et al. Classification of pig calls produced from birth to slaughter according to their emotional valence and context of production. Sci. Rep. 2022, 12, 3409. [Google Scholar] [CrossRef]
- Kells, N.J. Review: The five domains model and promoting positive welfare in pigs. Animal 2022, 16 (Suppl. S2), 100378. [Google Scholar] [CrossRef]
- Godyń, D.; Nowicki, J.; Herbut, P. Effects of environmental enrichment on pig welfare—A review. Animals 2019, 9, 383. [Google Scholar] [CrossRef]
- Sherwin, E.; Bordenstein, S.R.; Quinn, J.L.; Dinan, T.G.; Cryan, J.F. Microbiota and the social brain. Science 2019, 366, eaar2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wei, S.; Chen, N.; Xiang, Y.; Wang, Y.; Jin, M. Characteristics of gut microbiota in pigs with different breeds, growth periods and genders. Microb. Biotechnol. 2022, 15, 793–804. [Google Scholar] [CrossRef]
- Hu, J.; Chen, J.; Ma, L.; Hou, Q.; Zhang, Y.; Kong, X.; Huang, X.; Tang, Z.; Wei, H.; Wang, X.; et al. Characterizing core microbiota and regulatory functions of the pig gut microbiome. ISME J. 2024, 18, wrad037. [Google Scholar] [CrossRef]
- Li, W.; Zeng, X.; Wang, L.; Yin, L.; Wang, Q.; Yang, H. Comparative analysis of gut microbiota diversity across different digestive tract sites in Ningxiang pigs. Animals 2025, 15, 936. [Google Scholar] [CrossRef]
- Lan, Q.; Lian, Y.; Peng, P.; Yang, L.; Zhao, H.; Huang, P.; Ma, H.; Wei, H.; Yin, Y.; Liu, M. Association of gut microbiota and SCFAs with finishing weight of diannan small ear pigs. Front. Microbiol. 2023, 14, 1117965. [Google Scholar] [CrossRef]
- Holman, D.B.; Brunelle, B.W.; Trachsel, J.; Allen, H.K. Meta-analysis to define a core microbiota in the swine gut. mSystems 2017, 2, e00004–e000017. [Google Scholar] [CrossRef]
- Luo, Y.; Ren, W.; Smidt, H.; Wright, A.G.; Yu, B.; Schyns, G.; McCormack, U.M.; Cowieson, A.J.; Yu, J.; He, J.; et al. Dynamic distribution of gut microbiota in pigs at different growth stages: Composition and contribution. Microbiol. Spectr. 2022, 10, e0068821. [Google Scholar] [CrossRef]
- Dong, W.; Ricker, N.; Holman, D.B.; Johnson, T.A. Meta-analysis reveals the predictable dynamic development of the gut microbiota in commercial pigs. Microbiol. Spectr. 2023, 11, e0172223. [Google Scholar] [CrossRef]
- Xiao, L.; Estelle, J.; Kiilerich, P.; Ramayo-Caldas, Y.; Xia, Z.; Feng, Q.; Liang, S.; Pedersen, A.O.; Kjeldsen, N.J.; Liu, C.; et al. A reference gene catalogue of the pig gut microbiome. Nat. Microbiol. 2016, 1, 16161. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, L.; Sun, X.; Liu, M.; Wang, Y.; Yang, B.; Ai, H.; Chen, C.; Huang, L. Assessing the relationship between the gut microbiota and growth traits in Chinese indigenous pig breeds. BMC Vet. Res. 2025, 21, 284. [Google Scholar] [CrossRef]
- Crespo-Piazuelo, D.; Migura-Garcia, L.; Estelle, J.; Criado-Mesas, L.; Revilla, M.; Castello, A.; Munoz, M.; Garcia-Casco, J.M.; Fernandez, A.I.; Ballester, M.; et al. Association between the pig genome and its gut microbiota composition. Sci. Rep. 2019, 9, 8791. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Piazuelo, D.; Estelle, J.; Revilla, M.; Criado-Mesas, L.; Ramayo-Caldas, Y.; Ovilo, C.; Fernandez, A.I.; Ballester, M.; Folch, J.M. Characterization of bacterial microbiota compositions along the intestinal tract in pigs and their interactions and functions. Sci. Rep. 2018, 8, 12727. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Luo, R.; Gong, G.; Wang, Y.; Gesang, Z.; Wang, K.; Xu, Z.; Suolang, S. Characterization of metagenome-assembled genomes and carbohydrate-degrading genes in the gut microbiota of Tibetan pig. Front. Microbiol. 2020, 11, 595066. [Google Scholar] [CrossRef]
- Yang, H.; Xiao, Y.; Wang, J.; Xiang, Y.; Gong, Y.; Wen, X.; Li, D. Core gut microbiota in Jinhua pigs and its correlation with strain, farm and weaning age. J. Microbiol. 2018, 56, 346–355. [Google Scholar] [CrossRef]
- Long, C.; Wu, J.; Tan, Z.; Wang, S. Different intestinal microbiota with growth stages of three-breed hybrid pig. Biomed. Res. Int. 2022, 2022, 5603451. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Li, Z.; Yin, J.; Tan, B.; Chen, J.; Jiang, Q.; Ma, X. Evolution of the gut microbiota and its fermentation characteristics of Ningxiang pigs at the young stage. Animals 2021, 11, 638. [Google Scholar] [CrossRef]
- Ma, J.; Chen, J.; Gan, M.; Chen, L.; Zhao, Y.; Zhu, Y.; Niu, L.; Zhang, S.; Zhu, L.; Shen, L. Gut microbiota composition and diversity in different commercial swine breeds in early and finishing growth stages. Animals 2022, 12, 1607. [Google Scholar] [CrossRef]
- Sebastia, C.; Folch, J.M.; Ballester, M.; Estelle, J.; Passols, M.; Munoz, M.; Garcia-Casco, J.M.; Fernandez, A.I.; Castello, A.; Sanchez, A.; et al. Interrelation between gut microbiota, SCFA, and fatty acid composition in pigs. mSystems 2024, 9, e0104923. [Google Scholar] [CrossRef]
- Slifierz, M.J.; Friendship, R.M.; Weese, J.S. Longitudinal study of the early-life fecal and nasal microbiotas of the domestic pig. BMC Microbiol. 2015, 15, 184. [Google Scholar] [CrossRef]
- Liu, G.; Li, P.; Hou, L.; Niu, Q.; Pu, G.; Wang, B.; Du, T.; Kim, S.W.; Niu, P.; Li, Q.; et al. Metagenomic analysis reveals new microbiota related to fiber digestion in pigs. Front. Microbiol. 2021, 12, 746717. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras, D.F.; Duniere, L.; Blanquet-Diot, S.; Forano, E. Microbiota composition and functional profiling throughout the gastrointestinal tract of commercial weaning piglets. Microorganisms 2019, 7, 343. [Google Scholar] [CrossRef] [PubMed]
- Ramayo-Caldas, Y.; Mach, N.; Lepage, P.; Levenez, F.; Denis, C.; Lemonnier, G.; Leplat, J.; Billon, Y.; Berri, M.; Dore, J.; et al. Phylogenetic network analysis applied to pig gut microbiota identifies an ecosystem structure linked with growth traits. ISME J. 2016, 10, 2973–2977. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, K.; Xiang, Y.; Zhou, W.; Gui, G.; Yang, H. The fecal microbiota composition of boar duroc, yorkshire, landrace and Hampshire pigs. Asian-Australas. J. Anim. Sci. 2017, 30, 1456–1463. [Google Scholar] [CrossRef]
- Verbeek, E.; Keeling, L.; Landberg, R.; Lindberg, J.E.; Dicksved, J. The gut microbiota and microbial metabolites are associated with tail biting in pigs. Sci. Rep. 2021, 11, 20547. [Google Scholar] [CrossRef]
- Rabhi, N.; Thibodeau, A.; Cote, J.C.; Devillers, N.; Laplante, B.; Fravalo, P.; Lariviere-Gauthier, G.; Theriault, W.P.; Faucitano, L.; Beauchamp, G.; et al. Association between tail-biting and intestinal microbiota composition in pigs. Front. Vet. Sci. 2020, 7, 563762. [Google Scholar] [CrossRef]
- Yang, H.; Huang, X.; Fang, S.; He, M.; Zhao, Y.; Wu, Z.; Yang, M.; Zhang, Z.; Chen, C.; Huang, L. Unraveling the fecal microbiota and metagenomic functional capacity associated with feed efficiency in pigs. Front. Microbiol. 2017, 8, 1555. [Google Scholar] [CrossRef]
- Yang, H.; Yang, M.; Fang, S.; Huang, X.; He, M.; Ke, S.; Gao, J.; Wu, J.; Zhou, Y.; Fu, H.; et al. Evaluating the profound effect of gut microbiome on host appetite in pigs. BMC Microbiol. 2018, 18, 215. [Google Scholar] [CrossRef]
- He, Y.; Tiezzi, F.; Howard, J.; Huang, Y.; Gray, K.; Maltecca, C. Exploring the role of gut microbiota in host feeding behavior among breeds in swine. BMC Microbiol. 2022, 22, 1. [Google Scholar] [CrossRef]
- Choudhury, R.; Middelkoop, A.; Bolhuis, J.E.; Kleerebezem, M. Exploring the association between microbiota and behaviour in suckling piglets. Sci. Rep. 2022, 12, 12322. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, X.; Zhao, S.; Sun, W.; Yan, Z.; Wang, P.; Li, S.; Huang, W.; Zhang, S.; Liu, L.; et al. Structure and function of the fecal microbiota in diarrheic neonatal piglets. Front. Microbiol. 2017, 8, 502. [Google Scholar] [CrossRef]
- Ochoteco-Asensio, J.; Zigovski, G.; Batista Costa, L.; Rio-López, R.; Clavell-Sansalvador, A.; Ramayo-Caldas, Y.; Dalmau, A. Effect on feeding behaviour and growing of being a dominant or subordinate growing pig and its relationship with the faecal microbiota. Animals 2024, 14, 1906. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fu, H.; Zhou, Y.; Yan, M.; Chen, D.; Yang, M.; Xiao, S.; Chen, C.; Huang, L. Identification of the gut microbiota biomarkers associated with heat cycle and failure to enter oestrus in gilts. Microb. Biotechnol. 2021, 14, 1316–1330. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Ke, S.; Huang, X.; Fang, S.; He, M.; Fu, H.; Chen, C.; Huang, L. Gut and vagina microbiota associated with estrus return of weaning sows and its correlation with the changes in serum metabolites. Front. Microbiol. 2021, 12, 690091. [Google Scholar] [CrossRef]
- Parois, S.P.; Eicher, S.D.; Lindemann, S.R.; Marchant, J.N. Potential improvements of the cognition of piglets through a synbiotic supplementation from 1 to 28 days via the gut microbiota. Sci. Rep. 2021, 11, 24113. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.; Li, Q.; Su, Y.; Zhu, W. Daily fluctuation of colonic microbiome in response to nutrient substrates in a pig model. NPJ Biofilms Microbiomes 2023, 9, 85. [Google Scholar] [CrossRef]
- Mahmud, M.R.; Jian, C.; Uddin, M.K.; Huhtinen, M.; Salonen, A.; Peltoniemi, O.; Venhoranta, H.; Oliviero, C. Impact of intestinal microbiota on growth performance of suckling and weaned piglets. Microbiol. Spectr. 2023, 11, e0374422. [Google Scholar] [CrossRef]
- Han, G.G.; Lee, J.; Jin, G.; Park, J.; Choi, Y.H.; Chae, B.J.; Kim, E.B.; Choi, Y. Evaluating the association between body weight and the intestinal microbiota of weaned piglets via 16s rRNA sequencing. Appl. Microbiol. Biotechnol. 2017, 101, 5903–5911. [Google Scholar] [CrossRef]
- Shen, C.; Tong, X.; Chen, R.; Gao, S.; Liu, X.; Schinckel, A.P.; Li, Y.; Xu, F.; Zhou, B. Identifying blood-based biomarkers associated with aggression in weaned pigs after mixing. Appl. Anim. Behav. Sci. 2020, 224, 104927. [Google Scholar] [CrossRef]
- Legan, T.B.; Lavoie, B.; Mawe, G.M. Direct and indirect mechanisms by which the gut microbiota influence host serotonin systems. Neurogastroenterol. Motil. 2022, 34, e14346. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, N.; Zhao, H.; Yuan, H.; Xia, D.; Lei, H. The microbiome-metabolome response in the colon of piglets under the status of weaning stress. Front. Microbiol. 2020, 11, 2055. [Google Scholar] [CrossRef]
- Diana, A.; Manzanilla, E.G.; Calderon, D.J.; Leonard, F.C.; Boyle, L.A. Do weaner pigs need in-feed antibiotics to ensure good health and welfare? PLoS ONE 2017, 12, e0185622. [Google Scholar] [CrossRef] [PubMed]
- Kobek-Kjeldager, C.; Schönherz, A.A.; Canibe, N.; Pedersen, L.J. Diet and microbiota-gut-brain axis in relation to tail biting in pigs: A review. Appl. Anim. Behav. Sci. 2022, 246, 105514. [Google Scholar] [CrossRef]
- Valros, A.; Palander, P.; Heinonen, M.; Munsterhjelm, C.; Brunberg, E.; Keeling, L.; Piepponen, P. Evidence for a link between tail biting and central monoamine metabolism in pigs (sus scrofa domestica). Physiol. Behav. 2015, 143, 151–157. [Google Scholar] [CrossRef]
- Konig, E.; Heponiemi, P.; Kivinen, S.; Rakkolainen, J.; Beasley, S.; Borman, T.; Collado, M.C.; Hukkinen, V.; Junnila, J.; Lahti, L.; et al. Fewer culturable lactobacillaceae species identified in faecal samples of pigs performing manipulative behaviour. Sci. Rep. 2024, 14, 132. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Wen, C.; van Dixhoorn, I.; Schokker, D.; Woelders, H.; Stockhofe-Zurwieden, N.; Rebel, J.; Smidt, H. Environmentally enriched housing conditions affect pig welfare, immune system and gut microbiota in early life. Anim. Microbiome 2021, 3, 52. [Google Scholar] [CrossRef]
- Aljumaah, M.R.; Bhatia, U.; Roach, J.; Gunstad, J.; Azcarate Peril, M.A. The gut microbiome, mild cognitive impairment, and probiotics: A randomized clinical trial in middle-aged and older adults. Clin. Nutr. 2022, 41, 2565–2576. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Liu, H.; Xu, H.; Liu, F.; Song, H.; Zhao, X.; Li, H. Dysregulation of ruminococcaceae and megamonas could be predictive markers for rapid progression of mild cognitive impairment. Microb. Pathog. 2023, 183, 106272. [Google Scholar] [CrossRef]
- Borre, Y.E.; Moloney, R.D.; Clarke, G.; Dinan, T.G.; Cryan, J.F. The impact of microbiota on brain and behavior: Mechanisms & therapeutic potential. Adv. Exp. Med. Biol. 2014, 817, 373–403. [Google Scholar]
- Agirman, G.; Hsiao, E.Y. SnapShot: The microbiota-gut-brain axis. Cell 2021, 184, 2524. [Google Scholar] [CrossRef]
- Zhang, P.; Yu, Y.; Qin, Y.; Zhou, Y.; Tang, R.; Wang, Q.; Li, X.; Wang, H.; Weston-Green, K.; Huang, X.; et al. Alterations to the microbiota–colon–brain axis in high-fat-diet-induced obese mice compared to diet-resistant mice. J. Nutr. Biochem. 2019, 65, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Paiva, I.; Maciel, L.M.; Silva, R.; Mendonca, I.P.; Souza, J.; Peixoto, C.A. Prebiotics modulate the microbiota-gut-brain axis and ameliorate anxiety and depression-like behavior in HFD-fed mice. Food Res. Int. 2024, 182, 114153. [Google Scholar] [CrossRef]
- Inaba, T.; Yamashiro, K.; Kurita, N.; Ueno, Y.; Miyamoto, N.; Hira, K.; Nakajima, S.; Kijima, C.; Nakaguro, R.; Urabe, T.; et al. Microbial lipopolysaccharide-induced inflammation contributes to cognitive impairment and white matter lesion progression in diet-induced obese mice with chronic cerebral hypoperfusion. CNS Neurosci. Ther. 2023, 29 (Suppl. S1), 200–212. [Google Scholar] [CrossRef]
- Pan, W.; Zhao, J.; Wu, J.; Xu, D.; Meng, X.; Jiang, P.; Shi, H.; Ge, X.; Yang, X.; Hu, M.; et al. Dimethyl itaconate ameliorates cognitive impairment induced by a high-fat diet via the gut-brain axis in mice. Microbiome 2023, 11, 30. [Google Scholar]
- Yang, X.; Yu, D.; Xue, L.; Li, H.; Du, J. Probiotics modulate the microbiota-gut-brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm. Sin. B 2020, 10, 475–487. [Google Scholar] [CrossRef]
- Magni, G.; Riboldi, B.; Ceruti, S. Modulation of glial cell functions by the gut-brain axis: A role in neurodegenerative disorders and pain transmission. Cells 2023, 12, 1612. [Google Scholar] [CrossRef]
- Leonardi, I.; Gao, I.H.; Lin, W.Y.; Allen, M.; Li, X.V.; Fiers, W.D.; De Celie, M.B.; Putzel, G.G.; Yantiss, R.K.; Johncilla, M.; et al. Mucosal fungi promote gut barrier function and social behavior via type 17 immunity. Cell 2022, 185, 831–846. [Google Scholar] [CrossRef]
- Erny, D.; Hrabe, D.A.A.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Sun, M.F.; Zhu, Y.L.; Zhou, Z.L.; Jia, X.B.; Xu, Y.D.; Yang, Q.; Cui, C.; Shen, Y.Q. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-alpha signaling pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef]
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.C.; Patel, B.; Yan, R.; Blain, M.; et al. Type i interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 2016, 22, 586–597. [Google Scholar] [CrossRef]
- Hao, W.; Ma, Q.; Wang, L.; Yuan, N.; Gan, H.; He, L.; Li, X.; Huang, J.; Chen, J. Gut dysbiosis induces the development of depression-like behavior through abnormal synapse pruning in microglia-mediated by complement c3. Microbiome 2024, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ling, Y.; Peng, Y.; Han, S.; Ren, Y.; Jing, Y.; Fan, W.; Su, Y.; Mu, C.; Zhu, W. Regulation of serotonin production by specific microbes from piglet gut. J. Anim. Sci. Biotechnol. 2023, 14, 111. [Google Scholar] [CrossRef]
- Beldowska, A.; Barszcz, M.; Dunislawska, A. State of the art in research on the gut-liver and gut-brain axis in poultry. J. Anim. Sci. Biotechnol. 2023, 14, 37. [Google Scholar] [CrossRef]
- Farzi, A.; Frohlich, E.E.; Holzer, P. Gut microbiota and the neuroendocrine system. Neurotherapeutics 2018, 15, 5–22. [Google Scholar] [CrossRef]
- Frankiensztajn, L.M.; Elliott, E.; Koren, O. The microbiota and the hypothalamus-pituitary-adrenocortical (HPA) axis, implications for anxiety and stress disorders. Curr. Opin. Neurobiol. 2020, 62, 76–82. [Google Scholar] [CrossRef]
- Huo, R.; Zeng, B.; Zeng, L.; Cheng, K.; Li, B.; Luo, Y.; Wang, H.; Zhou, C.; Fang, L.; Li, W.; et al. Microbiota modulate anxiety-like behavior and endocrine abnormalities in hypothalamic-pituitary-adrenal axis. Front. Cell Infect. Microbiol. 2017, 7, 489. [Google Scholar] [CrossRef]
- Jang, H.M.; Lee, K.E.; Lee, H.J.; Kim, D.H. Immobilization stress-induced Escherichia coli causes anxiety by inducing NF-κB activation through gut microbiota disturbance. Sci. Rep. 2018, 8, 13897. [Google Scholar] [CrossRef]
- Gao, K.; Pi, Y.; Mu, C.; Peng, Y.; Huang, Z.; Zhu, W. Antibiotics-induced modulation of large intestinal microbiota altered aromatic amino acid profile and expression of neurotransmitters in the hypothalamus of piglets. J. Neurochem. 2018, 146, 219–234. [Google Scholar] [CrossRef]
- Gao, K.; Pi, Y.; Mu, C.; Farzi, A.; Liu, Z.; Zhu, W. Increasing carbohydrate availability in the hindgut promotes hypothalamic neurotransmitter synthesis: Aromatic amino acids linking the microbiota-brain axis. J. Neurochem. 2019, 149, 641–659. [Google Scholar] [CrossRef]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1288–G1295. [Google Scholar] [CrossRef]
- Ma, T.; Jin, H.; Kwok, L.Y.; Sun, Z.; Liong, M.T.; Zhang, H. Probiotic consumption relieved human stress and anxiety symptoms possibly via modulating the neuroactive potential of the gut microbiota. Neurobiol. Stress. 2021, 14, 100294. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ouyang, J.; Hu, Z.; Yang, J.; Chu, Y.; Huang, S.; Yang, Y.; Liu, C. Intervention mechanism of repeated oral GABA administration on anxiety-like behaviors induced by emotional stress in rats. Psychiatry Res. 2019, 271, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, P.; Pan, D.; Zeng, X.; Guo, Y.; Zhao, G. Effect of adzuki bean sprout fermented milk enriched in gamma-aminobutyric acid on mild depression in a mouse model. J. Dairy Sci. 2021, 104, 78–91. [Google Scholar] [CrossRef]
- Shekarabi, A.; Qureishy, I.; Puglisi, C.H.; Dalseth, M.; Vuong, H.E. Host-microbe interactions: Communication in the microbiota-gut-brain axis. Curr. Opin. Microbiol. 2024, 80, 102494. [Google Scholar] [CrossRef]
- Yoo, B.B.; Mazmanian, S.K. The enteric network: Interactions between the immune and nervous systems of the gut. Immunity 2017, 46, 910–926. [Google Scholar] [CrossRef]
- Vicentini, F.A.; Keenan, C.M.; Wallace, L.E.; Woods, C.; Cavin, J.B.; Flockton, A.R.; Macklin, W.B.; Belkind-Gerson, J.; Hirota, S.A.; Sharkey, K.A. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome 2021, 9, 210. [Google Scholar] [CrossRef]
- Kunze, W.A.; Mao, Y.K.; Wang, B.; Huizinga, J.D.; Ma, X.; Forsythe, P.; Bienenstock, J. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J. Cell Mol. Med. 2009, 13, 2261–2270. [Google Scholar] [CrossRef]
- Gershon, M.D.; Margolis, K.G. The gut, its microbiome, and the brain: Connections and communications. J. Clin. Investig. 2021, 131, e143768. [Google Scholar] [CrossRef]
- Muller, P.A.; Schneeberger, M.; Matheis, F.; Wang, P.; Kerner, Z.; Ilanges, A.; Pellegrino, K.; Del Mármol, J.; Castro, T.B.; Furuichi, M. Microbiota modulate sympathetic neurons via a gut–brain circuit. Nature 2020, 583, 441–446. [Google Scholar] [CrossRef]
- Lyte, M.; Varcoe, J.J.; Bailey, M.T. Anxiogenic effect of subclinical bacterial infection in mice in the absence of overt immune activation. Physiol. Behav. 1998, 65, 63–68. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, L.; Chang, L.; Pu, Y.; Qu, Y.; Hashimoto, K. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl. Psychiatry 2020, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Siopi, E.; Galerne, M.; Rivagorda, M.; Saha, S.; Moigneu, C.; Moriceau, S.; Bigot, M.; Oury, F.; Lledo, P.M. Gut microbiota changes require vagus nerve integrity to promote depressive-like behaviors in mice. Mol. Psychiatry 2023, 28, 3002–3012. [Google Scholar] [CrossRef]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A.; Costa-Mattioli, M. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron 2019, 101, 246–259. [Google Scholar] [CrossRef]
- Perez-Burgos, A.; Wang, B.; Mao, Y.K.; Mistry, B.; McVey, N.K.; Bienenstock, J.; Kunze, W. Psychoactive bacteria Lactobacillus rhamnosus (JB-1) elicits rapid frequency facilitation in vagal afferents. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G211–G220. [Google Scholar] [CrossRef]
- Lind, N.M.; Moustgaard, A.; Jelsing, J.; Vajta, G.; Cumming, P.; Hansen, A.K. The use of pigs in neuroscience: Modeling brain disorders. Neurosci. Biobehav. Rev. 2007, 31, 728–751. [Google Scholar] [CrossRef]
- van der Eijk, J.A.; Rodenburg, T.B.; de Vries, H.; Kjaer, J.B.; Smidt, H.; Naguib, M.; Kemp, B.; Lammers, A. Early-life microbiota transplantation affects behavioural responses, serotonin and immune characteristics in chicken lines divergently selected on feather pecking. Sci. Rep. 2020, 10, 2750. [Google Scholar] [CrossRef]
- Hu, L.; Geng, S.; Li, Y.; Cheng, S.; Fu, X.; Yue, X.; Han, X. Exogenous fecal microbiota transplantation from local adult pigs to crossbred newborn piglets. Front. Microbiol. 2018, 8, 2663. [Google Scholar] [CrossRef]
- Hu, J.; Ma, L.; Nie, Y.; Chen, J.; Zheng, W.; Wang, X.; Xie, C.; Zheng, Z.; Wang, Z.; Yang, T.; et al. A microbiota-derived bacteriocin targets the host to confer diarrhea resistance in early-weaned piglets. Cell Host Microbe 2018, 24, 817–832. [Google Scholar] [CrossRef]
- McCormack, U.M.; Curião, T.; Metzler-Zebeli, B.U.; Wilkinson, T.; Reyer, H.; Crispie, F.; Cotter, P.D.; Creevey, C.J.; Gardiner, G.E.; Lawlor, P.G. Improvement of feed efficiency in pigs through microbial modulation via fecal microbiota transplantation in sows and dietary supplementation of inulin in offspring. Appl. Environ. Microbiol. 2019, 85, e01219–e01255. [Google Scholar] [CrossRef]
- Chen, C.; Wu, C.; Kim, Y.; Hsu, W.; Tsai, Y.; Chiu, S. Enhancing social behavior in an autism spectrum disorder mouse model: Investigating the underlying mechanisms of Lactiplantibacillus plantarum intervention. Gut Microbes 2024, 16, 2359501. [Google Scholar] [CrossRef]
- De Santa, F.; Strimpakos, G.; Marchetti, N.; Gargari, G.; Torcinaro, A.; Arioli, S.; Mora, D.; Petrella, C.; Farioli-Vecchioli, S. Effect of a multi-strain probiotic mixture consumption on anxiety and depression symptoms induced in adult mice by postnatal maternal separation. Microbiome 2024, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2022, 100, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Xiao, L.; Fu, Y.; Shao, Z.; Jing, Z.; Yuan, J.; Xie, Y.; Guo, J.; Wang, Y.; Geng, W. Neuroprotective effects of probiotics on anxiety- and depression-like disorders in stressed mice by modulating tryptophan metabolism and the gut microbiota. Food Funct. 2024, 15, 2895–2905. [Google Scholar] [CrossRef]
- Barba-Vidal, E.; Roll, V.; Castillejos, L.; Guerra-Ordaz, A.A.; Manteca, X.; Mallo, J.J.; Martin-Orue, S.M. Response to a salmonella typhimurium challenge in piglets supplemented with protected sodium butyrate or bacillus licheniformis: Effects on performance, intestinal health and behavior. Transl. Anim. Sci. 2017, 1, 186–200. [Google Scholar] [CrossRef]
- Verbeek, E.; Dicksved, J.; Keeling, L. Supplementation of lactobacillus early in life alters attention bias to threat in piglets. Sci. Rep. 2021, 11, 10130. [Google Scholar] [CrossRef]
- Pereira, M.M.C.; Andretta, I.; Franceschi, C.H.; Kipper, M.; Mariani, A.; Stefanello, T.; Carvalho, C.; Vieira, J.; Moura Rocha, L.; Ribeiro, A.M.L. Effects of multistrain probiotic supplementation on sows’ emotional and cognitive states and progeny welfare. Animals 2024, 14, 847. [Google Scholar] [CrossRef]
- Cao, G.; Tao, F.; Hu, Y.; Li, Z.; Zhang, Y.; Deng, B.; Zhan, X. Positive effects of a Clostridium butyricum-based compound probiotic on growth performance, immune responses, intestinal morphology, hypothalamic neurotransmitters, and colonic microbiota in weaned piglets. Food Funct. 2019, 10, 2926–2934. [Google Scholar] [CrossRef]
- Sonya IVANOVA, T.N. Effect of the probiotic baykal EM-1 on the growth performance, blood parameters and behavior of weaned pigs. Sci. Pap. Ser. D Anim. Sci. 2021, 64, 169–174. [Google Scholar]
- Sutkus, L.T.; Joung, S.; Hirvonen, J.; Jensen, H.M.; Ouwehand, A.C.; Mukherjea, R.; Donovan, S.M.; Dilger, R.N. Influence of 2’-fucosyllactose and Bifidobacterium longum subspecies infantis supplementation on cognitive and structural brain development in young pigs. Front. Neurosci. 2022, 16, 860368. [Google Scholar] [CrossRef]
- Fleming, S.A.; Mudd, A.T.; Hauser, J.; Yan, J.; Metairon, S.; Steiner, P.; Donovan, S.M.; Dilger, R.N. Dietary oligofructose alone or in combination with 2’-fucosyllactose differentially improves recognition memory and hippocampal mRNA expression. Nutrients 2020, 12, 2131. [Google Scholar] [CrossRef]
- Fleming, S.A.; Monaikul, S.; Patsavas, A.J.; Waworuntu, R.; Berg, B.M.; Dilger, R.N. Dietary polydextrose and galactooligosaccharide increase exploratory behavior, improve recognition memory, and alter neurochemistry in the young pig. Nutr. Neurosci. 2019, 22, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.D.; Nguyen, D.N.; Langhorn, L.; Renes, I.B.; van Elburg, R.M.; Hartog, A.; Tims, S.; van de Looij, Y.; Sangild, P.T.; Thymann, T. Synbiotics combined with glutamine stimulate brain development and the immune system in preterm pigs. J. Nutr. 2019, 149, 36–45. [Google Scholar] [CrossRef]
- Fang, J.; Guo, J.; Lao, Y.; Kang, S.G.; Huang, K.; Tong, T. L-tyrosine alleviates autism-like behavior in mice by remodeling the gut microbiota. Brain Behav. Immun. 2025, 127, 358–374. [Google Scholar] [CrossRef]
- Fang, J.; Kang, S.G.; Huang, K.; Tong, T. Integrating 16s rRNA gene sequencing and metabolomics analysis to reveal the mechanism of l-proline in preventing autism-like behavior in mice. Nutrients 2025, 17, 247. [Google Scholar] [CrossRef]
- Fang, J.; Geng, R.; Kang, S.; Huang, K.; Tong, T. Dietary l-proline supplementation ameliorates autism-like behaviors and modulates gut microbiota in the valproic acid-induced mouse model of autism spectrum disorder. Food Sci. Hum. Wellness 2024, 13, 2889–2905. [Google Scholar] [CrossRef]
- Shen, Y.B.; Voilqué, G.; Odle, J.; Kim, S.W. Dietary L-tryptophan supplementation with reduced large neutral amino acids enhances feed efficiency and decreases stress hormone secretion in nursery pigs under social-mixing stress. J. Nutr. 2012, 142, 1540–1546. [Google Scholar] [CrossRef]
- Chen, S.; Wu, X.; Wang, X.; Shao, Y.; Tu, Q.; Yang, H.; Yin, J.; Yin, Y. Responses of intestinal microbiota and immunity to increasing dietary levels of iron using a piglet model. Front. Cell Dev. Biol. 2020, 8, 603392. [Google Scholar] [CrossRef]
- Fraser, D. Mineral-deficient diets and the pig’s attraction to blood: Implications for tail-biting. Can. J. Anim. Sci. 1987, 67, 909–918. [Google Scholar] [CrossRef]
- Winther, G.; Pyndt Jørgensen, B.M.; Elfving, B.; Nielsen, D.S.; Kihl, P.; Lund, S.; Sørensen, D.B.; Wegener, G. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatr. 2015, 27, 168–176. [Google Scholar] [CrossRef]
- Hagmeyer, S.; Haderspeck, J.C.; Grabrucker, A.M. Behavioral impairments in animal models for zinc deficiency. Front. Behav. Neurosci. 2015, 8, 443. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, S.; Chen, C.; Jiang, X.; Qiu, J.; Qiu, Y.; Zhang, Y.; Wang, T.; Qin, X.; Zou, Z.; et al. Crosstalk of gut microbiota and serum/hippocampus metabolites in neurobehavioral impairments induced by zinc oxide nanoparticles. Nanoscale 2020, 12, 21429–21439. [Google Scholar] [CrossRef] [PubMed]
- Holinger, M.; Früh, B.; Stoll, P.; Kreuzer, M.; Hillmann, E. Grass silage for growing-finishing pigs in addition to straw bedding: Effects on behaviour and gastric health. Livest. Sci. 2018, 218, 50–57. [Google Scholar] [CrossRef]
- Huang, S.; Wei, J.; Yu, H.; Hao, X.; Zuo, J.; Tan, C.; Deng, J. Effects of dietary fiber sources during gestation on stress status, abnormal behaviors and reproductive performance of sows. Animals 2020, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, A.; Marchant-Forde, J.N.; Richert, B.T.; Lay, D.C. Including dietary fiber and resistant starch to increase satiety and reduce aggression in gestating sows. J. Anim. Sci. 2016, 94, 2117–2127. [Google Scholar] [CrossRef]
- Shi, H.; Ge, X.; Ma, X.; Zheng, M.; Cui, X.; Pan, W.; Zheng, P.; Yang, X.; Zhang, P.; Hu, M.; et al. A fiber-deprived diet causes cognitive impairment and hippocampal microglia-mediated synaptic loss through the gut microbiota and metabolites. Microbiome 2021, 9, 223. [Google Scholar] [CrossRef]
- Qing, W.; Chen, H.; Ma, X.; Chen, J.; Le, Y.; Chen, H.; Tong, J.; Duan, K.; Ma, D.; Ouyang, W. Gut dysbiosis-induced vitamin b6 metabolic disorder contributes to chronic stress-related abnormal behaviors in a cortisol-independent manner. Gut Microbes 2025, 17, 2447824. [Google Scholar] [CrossRef]
- Meng, J.; Li, X.; Liu, W.; Xiao, Y.; Tang, H.; Wu, Y.; Xiong, Y.; Gao, S. The role of vitamin d in the prevention and treatment of SARS-CoV-2 infection: A meta-analysis of randomized controlled trials. Clin. Nutr. 2023, 42, 2198–2206. [Google Scholar] [CrossRef]
- Meng, J.; Li, X.; Xiong, Y.; Wu, Y.; Liu, P.; Gao, S. The role of vitamin d in the prevention and treatment of tuberculosis: A meta-analysis of randomized controlled trials. Infection 2025, 53, 1129–1140. [Google Scholar] [CrossRef]
- Renteria, K.; Nguyen, H.; Koh, G.Y. The role of vitamin d in depression and anxiety disorders: A review of the literature. Nutr. Neurosci. 2024, 27, 262–270. [Google Scholar] [CrossRef]
- Tamang, M.K.; Ali, A.; Pertile, R.N.; Cui, X.; Alexander, S.; Nitert, M.D.; Palmieri, C.; Eyles, D. Developmental vitamin d-deficiency produces autism-relevant behaviours and gut-health associated alterations in a rat model. Transl. Psychiatry 2023, 13, 204. [Google Scholar] [CrossRef]
- Ribeiro, R.; Nicoli, J.R.; Santos, G.; Lima-Santos, J. Impact of vitamin deficiency on microbiota composition and immunomodulation: Relevance to autistic spectrum disorders. Nutr. Neurosci. 2021, 24, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Jiao, Y.; Zhong, H.; Tan, Y.; Yin, Y.; Liu, Y.; Liu, D.; Wu, M.; Wang, G.; Huang, J. Human-derived fecal microbiota transplantation alleviates social deficits of the BTBR mouse model of autism through a potential mechanism involving vitamin b6 metabolism. mSystems 2024, 9, e00224–e00257. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wu, M.; Su, B.; Lin, H.; Li, Q.; He, Y.; Zhong, T.; Xiao, Y.; Yu, X. Host-gut microbiota interactions: Exploring the potential role of vitamin b1 and b2 in the microbiota-gut-brain axis and anxiety, stress, and sleep quality. Nutrients 2025, 17, 1894. [Google Scholar] [CrossRef] [PubMed]
- Riboulet-Bisson, E.; Sturme, M.H.J.; Jeffery, I.B.; O’Donnell, M.M.; Neville, B.A.; Forde, B.M.; Claesson, M.J.; Harris, H.; Gardiner, G.E.; Casey, P.G.; et al. Effect of Lactobacillus salivarius bacteriocin abp118 on the mouse and pig intestinal microbiota. PLoS ONE 2012, 7, e31113. [Google Scholar] [CrossRef]
- Baldwin, B.A.; Ingram, D.L. The effects of food intake and acclimatization to temperature on behavioral thermoregulation in pigs and mice. Physiol. Behav. 1968, 3, 395–400. [Google Scholar] [CrossRef]

| Behavior | Microbiota | Influence | Reference |
|---|---|---|---|
| Feeding Behavior | Marvinbryantia, Dorea, Blautia, and Ruminococcaceae_UCG-014 | Promote pig feeding. | [59] |
| Christensenellaceae_R-7_group, Family_XIII_AD3011_group, and Ruminococcaceae_UCG-004 | Inhibition of pig feeding. | ||
| Excretion Behavior | Prevotella, Sutterella, and Campylobacter, and Clostridiaceae | Diarrhea in newborn piglets. | [61] |
| Group Living Behavior | Faecalibacterium, Peptococcus, and Oliverpabstia | Dominant pigs of cohort. | [62] |
| Holdemanella and Acetitomaculum | Subordinate pigs of cohort. | ||
| Sexual Behavior | Anaerovibrio, Succinivibrio, Treponema, Oribacterium, Faecalibacterium, and Prevotella | Successful puberty in reserve sows. | [63] |
| Bacteroidia, Lactobacillaceae, and Lactobacillus | Restoration of normal estrus in sows. | [64] | |
| Exploratory Behavior | Coprococcus 3, Eubacterium, and Coprostanoligenes | Piglets squeal in open field test. | [60] |
| Atopobium and UBA1819 | Piglets displayed nosing behaviour and total exploration behaviour. | ||
| Abnormal Behavior | Lachnospiraceae, Ruminococcaceae and Clostridiales Family XIII | Piglets show tail biting behavior. | [55] |
| Lactobacillus | Reducing the chances of pigs biting and being bitten on the tail. | [56] | |
| After-effect Behavior | Clostridium XIVa and XVIII, Faecalicoccus and Ruminococcaceae | Reducing the time pig took to successfully cross the fence barrier. | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, X.; Zheng, D.; Pang, S.; Mu, P.; Jiang, J.; Wang, X.; Yan, X.; Wu, Y.; Wang, Y. Review: Gut Microbiota—A Powerful Tool for Improving Pig Welfare by Influencing Behavior Through the Gut–Brain Axis. Animals 2025, 15, 1886. https://doi.org/10.3390/ani15131886
Jian X, Zheng D, Pang S, Mu P, Jiang J, Wang X, Yan X, Wu Y, Wang Y. Review: Gut Microbiota—A Powerful Tool for Improving Pig Welfare by Influencing Behavior Through the Gut–Brain Axis. Animals. 2025; 15(13):1886. https://doi.org/10.3390/ani15131886
Chicago/Turabian StyleJian, Xiaoying, Duo Zheng, Shengping Pang, Peiqiang Mu, Jun Jiang, Xu Wang, Xiliang Yan, Yinbao Wu, and Yan Wang. 2025. "Review: Gut Microbiota—A Powerful Tool for Improving Pig Welfare by Influencing Behavior Through the Gut–Brain Axis" Animals 15, no. 13: 1886. https://doi.org/10.3390/ani15131886
APA StyleJian, X., Zheng, D., Pang, S., Mu, P., Jiang, J., Wang, X., Yan, X., Wu, Y., & Wang, Y. (2025). Review: Gut Microbiota—A Powerful Tool for Improving Pig Welfare by Influencing Behavior Through the Gut–Brain Axis. Animals, 15(13), 1886. https://doi.org/10.3390/ani15131886





