LincRNA-MSTRG.673.2 Promotes Chicken Intramuscular Adipocyte Differentiation by Sponging miR-128-3p
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Sequencing Samples
2.2. Transcriptome Data Analysis
2.3. Primary Intramuscular Adipocyte Isolation and Culture and Induced Differentiation
2.4. Transient Transfection of Intramuscular Adipocytes
2.5. Localization Analysis of LincRNA-MSTRG.673.2 Using Fluorescence In Situ Hybridization (FISH) and Nuclear/Cytoplasmic RNA Separation
2.6. Dual Luciferase Reporter Assay
2.7. Oil-Red O Staining Triglyceride Assay Detection of Cell Differentiation
2.8. Q-PCR
2.9. Statistical Analysis
3. Result
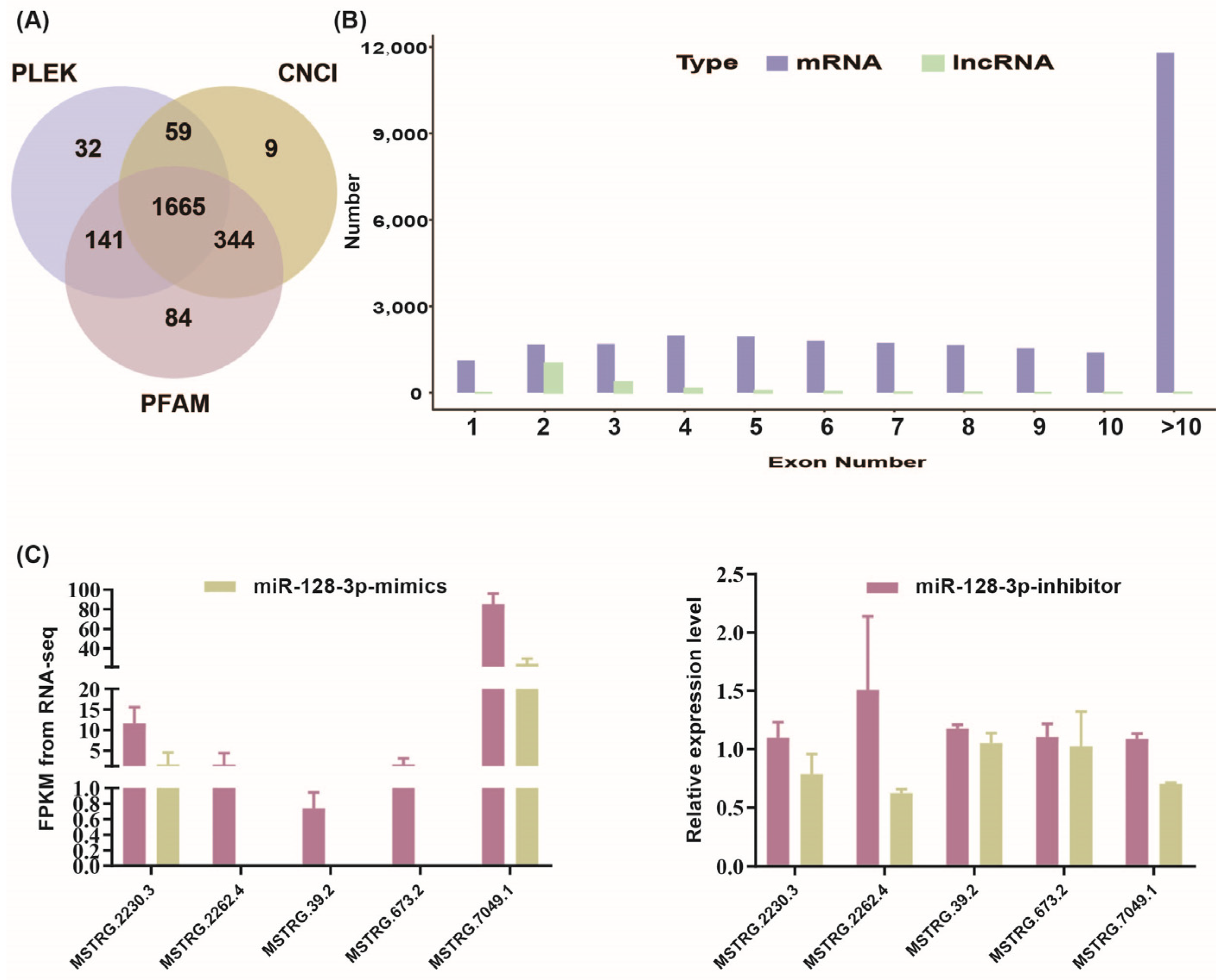
3.1. Identification and Characterization of LincRNAs in Chicken Intramuscular Adipocyte Groups Post miR-128-3p Overexpression and Interference
3.2. Analysis of Differential LincRNAs Expression and Screening for miR-128-3p Targeting LincRNAs
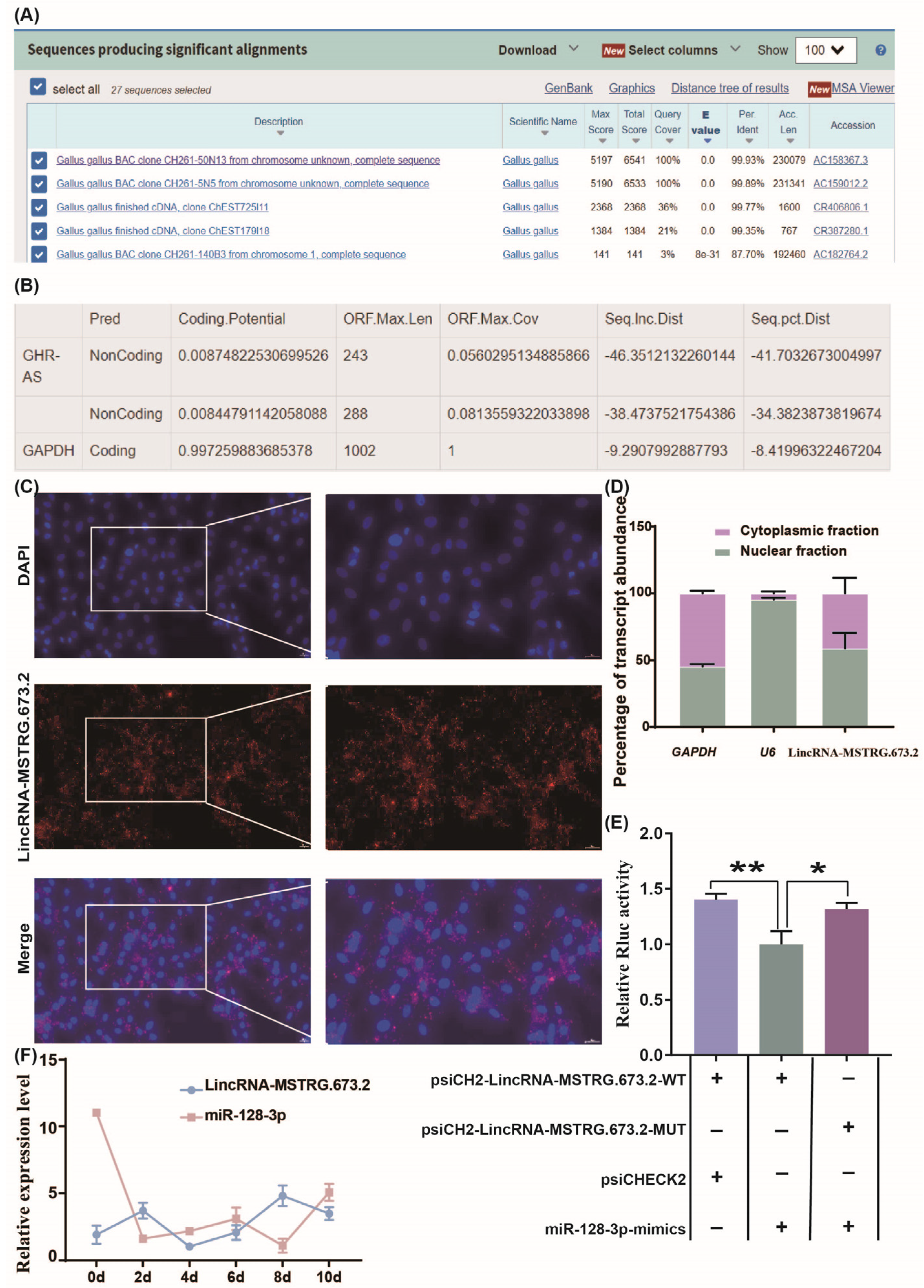
3.3. LincRNA-MSTRG.673.2 Targets Binding to miR-128-3p
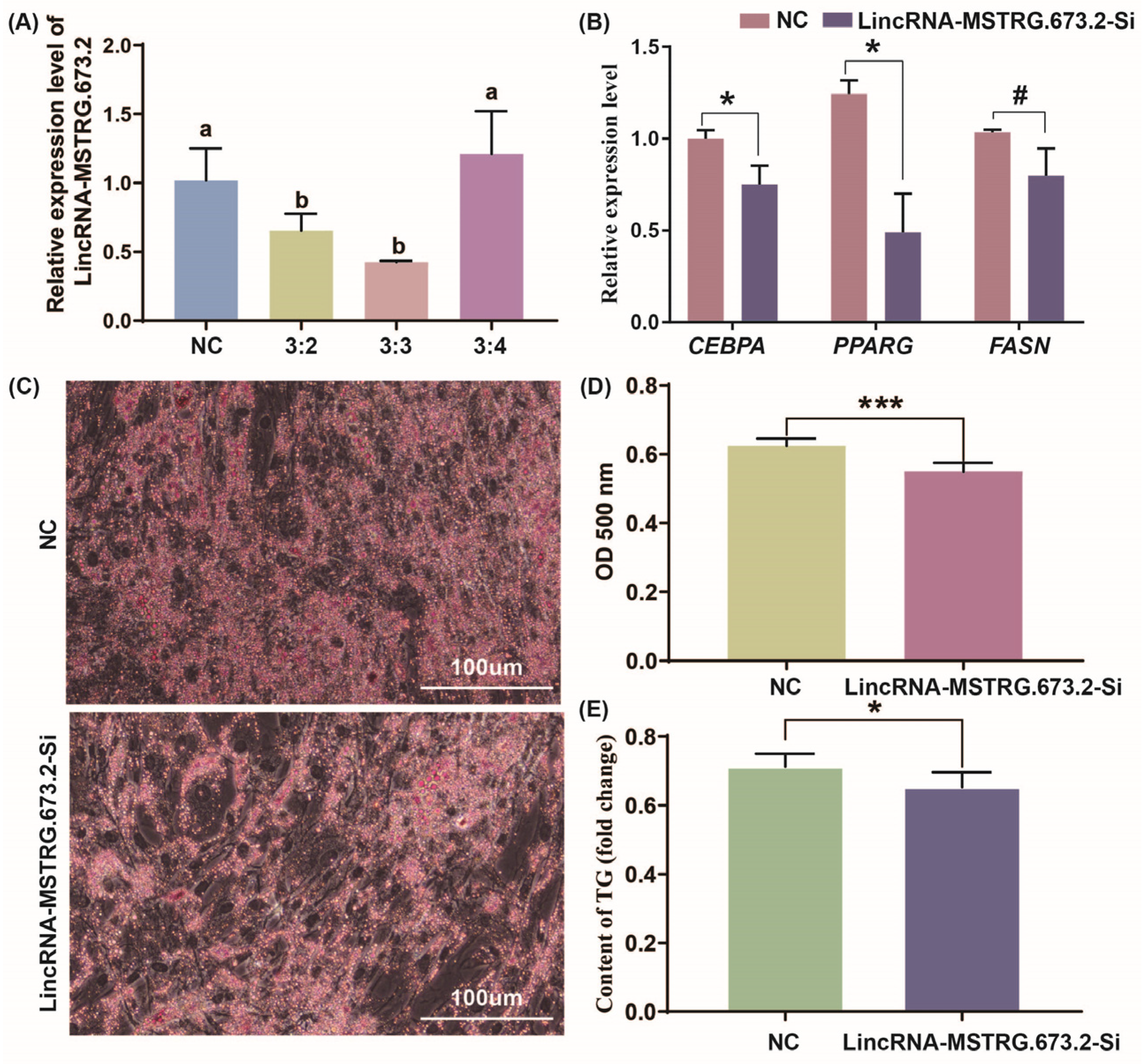
3.4. Interfering with LincRNA-MSTRG.673.2 Inhibits Lipid Deposition in Intramuscular Preadipocytes
3.5. LincRNA-MSTRG.673.2 Promoted Intramuscular Differentiation by Adsorbing miR-128-3p
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNCI | Coding–noncoding index |
| CPC | Coding potential calculator |
| IMF | Intramuscular fat |
| MiRNA | MicroRNA |
| NC group | Blank treatment group |
| M group | Mimics-treated group |
| SI group | Inhibitor-treated group |
| GO | Gene ontology |
| KEGG | Kyoto encyclopedia of genes and genomes |
| Q-PCR | Quantitative PCR |
| PBS | Phosphate-buffered saline |
| PPARG | Peroxisome proliferator-activated receptor γ |
| FABP | Fatty acid binding protein 4 |
| FASN | Fatty acid synthetase |
| CEBPA | CCAAT/enhancer-binding protein alpha |
| FDPS | Farnesyl diphosphate synthase |
References
- Acar, M.B.; Ayaz-Güner, Ş.; Di Bernardo, G.; Güner, H.; Murat, A.; Peluso, G.; Özcan, S.; Galderisi, U. Obesity induced by high-fat diet is associated with critical changes in biological and molecular functions of mesenchymal stromal cells present in visceral adipose tissue. Aging 2020, 12, 24894–24913. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Deng, Y.; Hu, X.; Ren, H.; Zhu, J.; Fu, S.; Xie, J.; Peng, Y. miR-128-3p regulates 3T3-L1 adipogenesis and lipolysis by targeting Pparg and Sertad2. J. Physiol. Biochem. 2018, 74, 381–393. [Google Scholar] [CrossRef]
- Chen, F.F.; Xiong, Y.; Peng, Y.; Gao, Y.; Qin, J.; Chu, G.Y.; Pang, W.J.; Yang, G.S. miR-425-5p Inhibits Differentiation and Proliferation in Porcine Intramuscular Preadipocytes. Int. J. Mol. Sci. 2017, 18, 2101. [Google Scholar] [CrossRef]
- Chen, Y.; Li, K.; Zhang, X.; Chen, J.; Li, M.; Liu, L. The novel long noncoding RNA lncRNA-Adi regulates adipogenesis. Stem Cells Transl. Med. 2020, 9, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.X.; Liu, R.R.; Zhao, G.P.; Zheng, M.Q.; Chen, J.L.; Wen, J. Identification of differentially expressed genes and pathways for intramuscular fat deposition in pectoralis major tissues of fast-and slow-growing chickens. BMC Genom. 2012, 13, 213. [Google Scholar] [CrossRef]
- Dong, J.; Liu, L.; Chen, L.; Xiang, Y.; Wang, Y.; Zhao, Y. The Coexistence of Bacterial Species Restructures Biofilm Architecture and Increases Tolerance to Antimicrobial Agents. Microbiol. Spectr. 2023, 11, e0358122. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Xu, Y.; Zhang, P.; Zhao, X.; Gan, M.; Li, Q.; Ma, J.; Tang, G.; Jiang, Y.; Wang, J.; et al. MicroRNA-125a-5p Affects Adipocytes Proliferation, Differentiation and Fatty Acid Composition of Porcine Intramuscular Fat. Int. J. Mol. Sci. 2018, 19, 501. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Lian, B.; Yin, X. microRNA-128-3p inhibits proliferation and accelerates apoptosis of gastric cancer cells via inhibition of TUFT1. World J. Surg. Oncol. 2023, 21, 47. [Google Scholar] [CrossRef]
- Fang, W.; Shi, C.; Wang, Y.; Song, J.; Zhang, L. microRNA-128-3p inhibits CD4+ regulatory T cells enrichment by targeting interleukin 16 in gastric cancer. Bioengineered 2022, 13, 1025–1038. [Google Scholar] [CrossRef]
- Gan, Q.; Luan, M.; Hu, M.; Liu, Z.; Zhang, Z. Functional study of CYP90A1 and ALDH3F1 gene obtained by transcriptome sequencing analysis of Brassica napus seedlings treated with brassinolide. Front. Plant Sci. 2022, 13, 1040511. [Google Scholar] [CrossRef]
- Gu, H.; Zhou, Y.; Yang, J.; Li, J.; Peng, Y.; Zhang, X.; Miao, Y.; Jiang, W.; Bu, G.; Hou, L.; et al. Targeted overexpression of PPARγ in skeletal muscle by random insertion and CRISPR/Cas9 transgenic pig cloning enhances oxidative fiber formation and intramuscular fat deposition. FASEB J. 2021, 35, e21308. [Google Scholar] [CrossRef]
- Haider, N.; Larose, L. Activation of the PDGFRα-Nrf2 pathway mediates impaired adipocyte differentiation in bone marrow mesenchymal stem cells lacking Nck1. Cell Commun. Signal. 2020, 18, 26. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, S.; Gao, X.; Hu, Y.; Zhang, Y.; Shen, Y.; Jiang, Y.; Huang, Y. Resveratrol Inhibits Proliferation and Differentiation of Porcine Preadipocytes by a Novel LincRNA-ROFM/miR-133b/AdipoQ Pathway. Foods 2022, 11, 2690. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- Khadka, V.S.; Nasu, M.; Deng, Y.; Jijiwa, M. Circulating microRNA Biomarker for Detecting Breast Cancer in High-Risk Benign Breast Tumors. Int. J. Mol. Sci. 2023, 24, 7553. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, Y.; Ye, Z.Q.; Liu, X.Q.; Zhao, S.Q.; Wei, L.; Gao, G. CPC: Assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 2007, 35, W345–W349. [Google Scholar] [CrossRef]
- Lamarre, S.; Frasse, P.; Zouine, M.; Labourdette, D.; Sainderichin, E.; Hu, G.; Le Berre-Anton, V.; Bouzayen, M.; Maza, E. Optimization of an RNA-Seq Differential Gene Expression Analysis Depending on Biological Replicate Number and Library Size. Front. Plant Sci. 2018, 9, 108. [Google Scholar] [CrossRef]
- Li, M.; Liu, T.; Cheng, W.; Jin, H.; Wang, X. A test of miR-128-3p and miR-33a-5p in serum exosome as biomarkers for auxiliary diagnosis of non-small cell lung cancer. J. Thorac. Dis. 2023, 15, 2616–2626. [Google Scholar] [CrossRef]
- Liu, H.; Li, B.; Qiao, L.; Liu, J.; Ren, D.; Liu, W. miR-340-5p inhibits sheep adipocyte differentiation by targeting ATF7. Anim. Sci. J. 2020, 91, e13462. [Google Scholar] [CrossRef]
- Long, J.K.; Dai, W.; Zheng, Y.W.; Zhao, S.P. miR-122 promotes hepatic lipogenesis via inhibiting the LKB1/AMPK pathway by targeting Sirt1 in non-alcoholic fatty liver disease. Mol. Med. 2019, 25, 26. [Google Scholar] [CrossRef]
- Luo, J.; Shen, L.; Gan, M.; Jiang, A.; Chen, L.; Ma, J.; Jin, L.; Liu, Y.; Tang, G.; Jiang, Y.; et al. Profiling of skeletal muscle tissue for long non-coding RNAs related to muscle metabolism in the QingYu pig at the growth inflection point. Anim. Biosci. 2021, 34, 1309–1320. [Google Scholar] [CrossRef]
- Miao, G.; Liu, B.; Ling, K.; Peng, T.; Zhou, E.; Xie, S.; Tan, Z. Long Noncoding RNA HCP5 Contributes to Nasopharyngeal Carcinoma Progression by Targeting MicroRNA-128-3p. J. Oncol. 2022, 2022, 5740857. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- O’Reilly, M.E.; Ho, S.; Coronel, J.; Zhu, L.; Liu, W.; Xue, C.; Kim, E.; Cynn, E.; Matias, C.V.; Soni, R.K.; et al. linc-ADAIN, a human adipose lincRNA, regulates adipogenesis by modulating KLF5 and IL-8 mRNA stability. Cell Rep. 2024, 43, 114240. [Google Scholar] [CrossRef]
- Qiu, S.; Yu, G.; Lu, X.; Domeniconi, C.; Guo, M. Isoform function prediction by Gene Ontology embedding. Bioinformatics 2022, 38, 4581–4588. [Google Scholar] [CrossRef]
- Ren, L.; Li, Q.; Hu, X.; Yang, Q.; Du, M.; Xing, Y.; Wang, Y.; Li, J.; Zhang, L. A Novel Mechanism of bta-miR-210 in Bovine Early Intramuscular Adipogenesis. Genes 2020, 11, 601. [Google Scholar] [CrossRef]
- Rzepiel, A.; Horváth, A.; Kutszegi, N.; Gézsi, A.; Sági, J.C.; Almási, L.; Egyed, B.; Lőrincz, P.; Visnovitz, T.; Kovács, G.T.; et al. MiR-128-3p as blood based liquid biopsy biomarker in childhood acute lymphoblastic leukemia. Mol. Cell. Probes 2023, 67, 101893. [Google Scholar] [CrossRef]
- Sheptulina, A.F.; Antyukh, K.Y.; Kiselev, A.R.; Mitkovskaya, N.P.; Drapkina, O.M. Possible Mechanisms Linking Obesity, Steroidogenesis, and Skeletal Muscle Dysfunction. Life 2023, 13, 1415. [Google Scholar] [CrossRef]
- Stocks, M.B.; Moxon, S.; Mapleson, D.; Woolfenden, H.C.; Mohorianu, I.; Folkes, L.; Schwach, F.; Dalmay, T.; Moulton, V. The UEA sRNA workbench: A suite of tools for analysing and visualizing next generation sequencing microRNA and small RNA datasets. Bioinformatics 2012, 28, 2059–2061. [Google Scholar] [CrossRef]
- Sun, G.R.; Zhang, M.; Sun, J.W.; Li, F.; Ma, X.F.; Li, W.T.; Han, R.L.; Li, Z.J.; Jiang, R.R.; Li, G.X.; et al. Krüppel-like factor KLF9 inhibits chicken intramuscular preadipocyte differentiation. Br. Poult. Sci. 2019, 60, 790–797. [Google Scholar] [CrossRef]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lin, X.; Zhang, Q.; Pang, Y.; Zhang, X.; Zhao, X.; Liu, D.; Yang, X. Genome-wide characterization of lncRNAs and mRNAs in muscles with differential intramuscular fat contents. Front. Vet. Sci. 2022, 9, 982258. [Google Scholar] [CrossRef] [PubMed]
- Tristan, C.A.; Hong, H.; Jethmalani, Y.; Chen, Y.; Weber, C.; Chu, P.H.; Ryu, S.; Jovanovic, V.M.; Hur, I.; Voss, T.C.; et al. Efficient and safe single-cell cloning of human pluripotent stem cells using the CEPT cocktail. Nat. Protoc. 2023, 18, 58–80. [Google Scholar] [CrossRef]
- Wang, B.; Hang, J.; Li, W.; Yuan, W. Knockdown of LncRNA DLEU2 Inhibits Cervical Cancer Progression via Targeting miR-128-3p. Onco Targets Ther. 2020, 13, 10173–10184. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Rahman, I.; Goniewicz, M.L.; Li, D. Perspectives on Epigenetics Alterations Associated with Smoking and Vaping. Function 2021, 2, zqab022. [Google Scholar] [CrossRef]
- Yang, C.; Luo, M.; Chen, Y.; You, M.; Chen, Q. MicroRNAs as Important Regulators Mediate the Multiple Differentiation of Mesenchymal Stromal Cells. Front. Cell Dev. Biol. 2021, 9, 619842. [Google Scholar] [CrossRef]
- Yi, X.; He, Z.; Tian, T.; Kou, Z.; Pang, W. LncIMF2 promotes adipogenesis in porcine intramuscular preadipocyte through sponging MiR-217. Anim. Biotechnol. 2023, 34, 268–279. [Google Scholar] [CrossRef]
- Zhang, B.; Li, H.; Li, D.; Sun, H.; Li, M.; Hu, H. Long noncoding RNA Mirt2 upregulates USP10 expression to suppress hepatic steatosis by sponging miR-34a-5p. Gene 2019, 700, 139–148. [Google Scholar] [CrossRef]
- Zhang, M.; Li, F.; Ma, X.F.; Li, W.T.; Jiang, R.R.; Han, R.L.; Li, G.X.; Wang, Y.B.; Li, Z.Y.; Tian, Y.D.; et al. Identification of differentially expressed genes and pathways between intramuscular and abdominal fat-derived preadipocyte differentiation of chickens in vitro. BMC Genom. 2019, 20, 743. [Google Scholar] [CrossRef]
- Zhang, M.; Li, F.; Sun, J.W.; Li, D.H.; Li, W.T.; Jiang, R.R.; Li, Z.J.; Liu, X.J.; Han, R.L.; Li, G.X.; et al. LncRNA IMFNCR Promotes Intramuscular Adipocyte Differentiation by Sponging miR-128-3p and miR-27b-3p. Front. Genet. 2019, 10, 42. [Google Scholar] [CrossRef]
- Zhang, T.; Ren, Z.; Mao, R.; Yi, W.; Wang, B.; Yang, H.; Wang, H.; Liu, Y. LINC00278 and BRG1: A key regulatory axis in male obesity and preadipocyte adipogenesis. Metabolism 2025, 168, 156194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, D.; Peng, Z.; Wang, R.; Li, K.; Ren, H.; Sun, X.; Du, N.; Tang, S.C. LINC00891 regulated by miR-128-3p/GATA2 axis impedes lung cancer cell proliferation, invasion and EMT by inhibiting RhoA pathway. Acta Biochim. Biophys. Sin. 2022, 54, 378–387. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Li, S.; DelProposto, J.L.; Liu, T.; Mi, L.; Porsche, C.; Peng, X.; Lumeng, C.N.; Lin, J.D. The long noncoding RNA Blnc1 orchestrates homeostatic adipose tissue remodeling to preserve metabolic health. Mol. Metab. 2018, 14, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zhang, M.; Zhang, J.; Feng, Y.; Xie, Z.; Liu, S.; Zhu, D.; Luo, Y. The gene regulatory role of non-coding RNAs in non-obstructive azoospermia. Front. Endocrinol. 2022, 13, 959487. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hong, Y.; Shang, Z.; Abuzeid, A.M.I.; Lin, J.; Li, G. The Potential Role of MicroRNA-124-3p in Growth, Development, and Reproduction of Schistosoma japonicum. Front. Cell Infect. Microbiol. 2022, 12, 862496. [Google Scholar] [CrossRef]
- Zhu, R.; Feng, X.; Wei, Y.; Guo, D.; Li, J.; Liu, Q.; Jiang, J.; Shi, D.; Huang, J. lncSAMM50 Enhances Adipogenic Differentiation of Buffalo Adipocytes With No Effect on Its Host Gene. Front. Genet. 2021, 12, 626158. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, B.; Zhu, T.; Wang, D.; Liu, C.; Liu, Y.; He, Y.; Liang, W.; Li, W.; Han, R.; et al. miR-128-3p inhibits intramuscular adipocytes differentiation in chickens by downregulating FDPS. BMC Genom. 2023, 24, 540. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Zhu, S.; He, Y.; Liang, W.; Zhu, T.; Han, R.; Li, D.; Wang, Y.; Tian, Y.; Li, G.; et al. LincRNA-MSTRG.673.2 Promotes Chicken Intramuscular Adipocyte Differentiation by Sponging miR-128-3p. Animals 2025, 15, 1879. https://doi.org/10.3390/ani15131879
Zhang B, Zhu S, He Y, Liang W, Zhu T, Han R, Li D, Wang Y, Tian Y, Li G, et al. LincRNA-MSTRG.673.2 Promotes Chicken Intramuscular Adipocyte Differentiation by Sponging miR-128-3p. Animals. 2025; 15(13):1879. https://doi.org/10.3390/ani15131879
Chicago/Turabian StyleZhang, Binbin, Shuaipeng Zhu, Yuehua He, Wenjie Liang, Tingqi Zhu, Ruili Han, Donghua Li, Yanbin Wang, Yadong Tian, Guoxi Li, and et al. 2025. "LincRNA-MSTRG.673.2 Promotes Chicken Intramuscular Adipocyte Differentiation by Sponging miR-128-3p" Animals 15, no. 13: 1879. https://doi.org/10.3390/ani15131879
APA StyleZhang, B., Zhu, S., He, Y., Liang, W., Zhu, T., Han, R., Li, D., Wang, Y., Tian, Y., Li, G., Kang, X., Li, W., & Sun, G. (2025). LincRNA-MSTRG.673.2 Promotes Chicken Intramuscular Adipocyte Differentiation by Sponging miR-128-3p. Animals, 15(13), 1879. https://doi.org/10.3390/ani15131879





