Effects of Flight Restraint and Housing Conditions on Feather Corticosterone in White Storks Under Human Care
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bird Numbers
2.2. Study Design
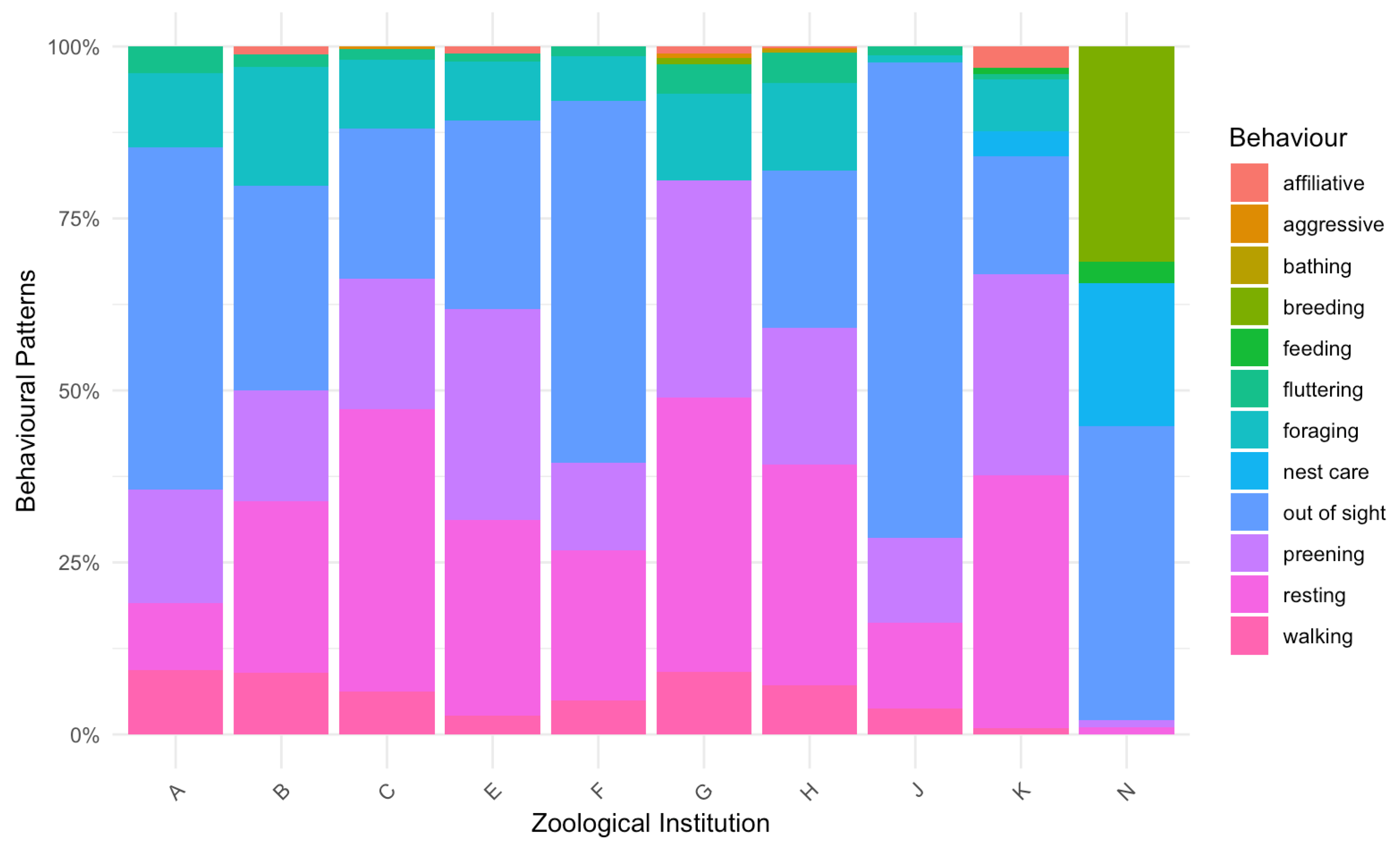
2.2.1. Behavioural Observations
2.2.2. Feather Sampling
2.3. Corticosterone Extraction and Measurement
2.4. Statistical Analysis
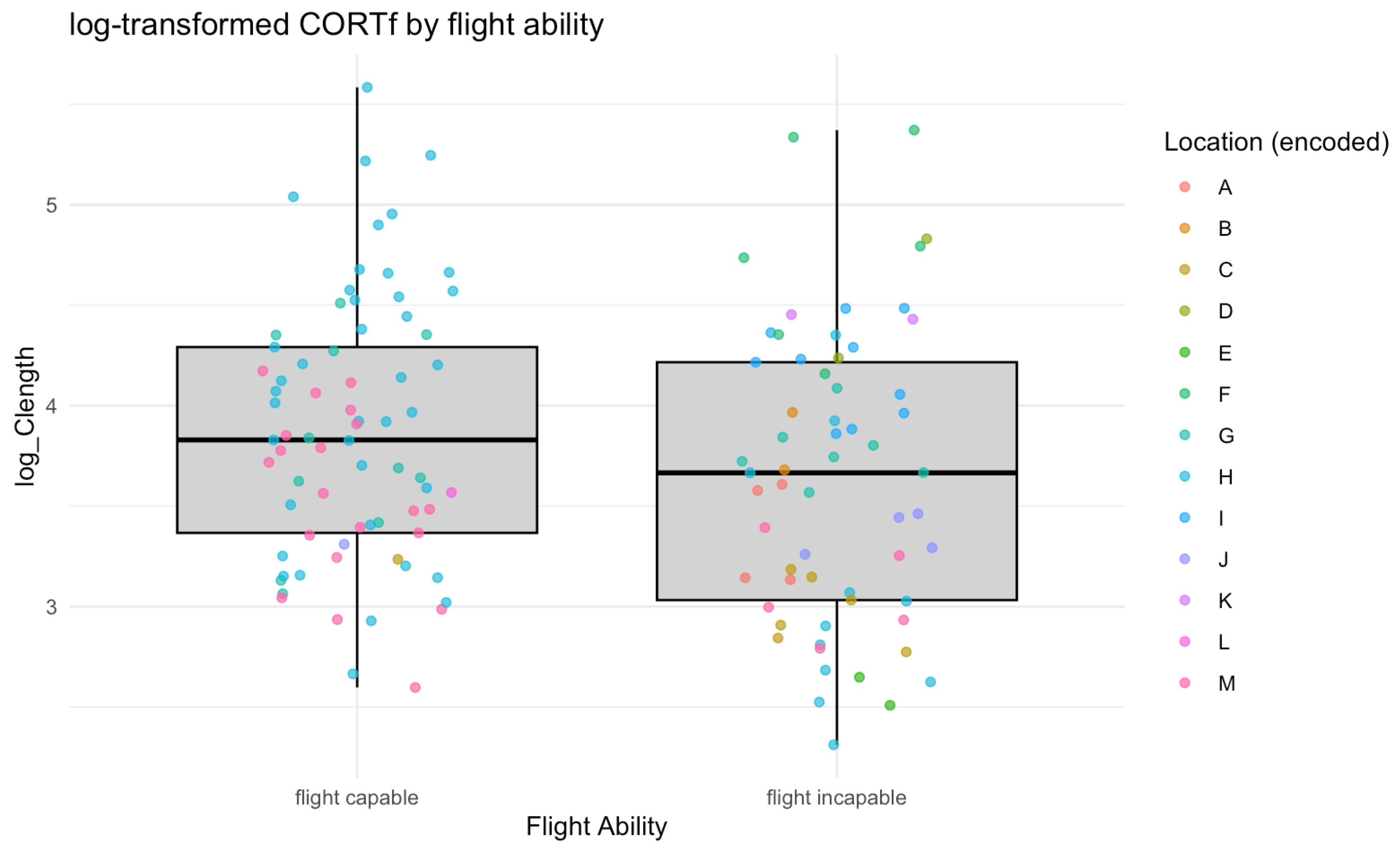
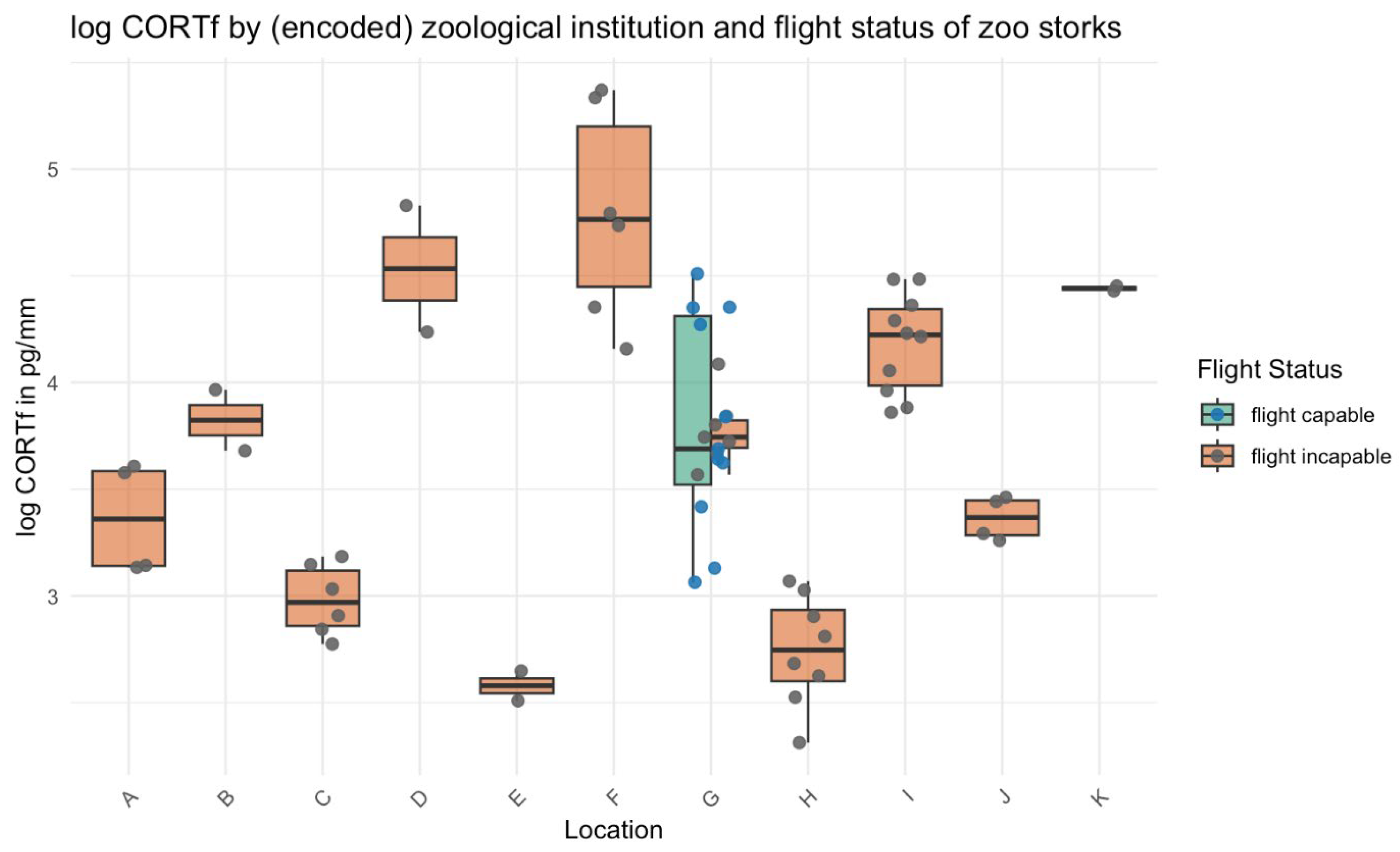
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dollinger, P.; Pagel, T.; Baumgartner, K.; Encke, D.; Engel, H.; Filz, A. Flugunfähigmachen von Vögeln–Für und Wider. Der Zoologische Garten Zeitschrift für die Gesamte Tiergärtnerei 2013, 82, 293–339. [Google Scholar] [CrossRef]
- Haase, G.; Baumgartner, K.; von Fersen, L.; Merle, R.; Wiegard, M.; Will, H.; Reese, L.; Tallo-Parra, O.; Carbajal, A.; Lopez-Bejar, M.; et al. Feather Corticosterone Measurements and Behavioral Observations in the Great White Pelican (Pelecanus onocrotalus) Living under Different Flight Restraint Conditions in German Zoos. Animals 2021, 11, 2522. [Google Scholar] [CrossRef] [PubMed]
- Reese, L.; Baumgartner, K.; von Fersen, L.; Merle, R.; Ladwig-Wiegard, M.; Will, H.; Haase, G.; Tallo-Parra, O.; Carbajal, A.; Lopez-Bejar, M.; et al. Feather Corticosterone Measurements of Greater Flamingos Living under Different Forms of Flight Restraint. Animals 2020, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.; Reitemeier, S.; Gottschalk, J.; Haense, M.; Schmidt, V.; Steinbach-Sobiraj, K.; Krautwald-Junghanns, M.-E.; Einspanier, A. Endocrinologic studies of male psittacine birds for the evaluation of their reproductive status. Tierarztl. Prax. Ausg. K Kleintiere/Heimtiere 2011, 39, 249–257. [Google Scholar]
- Tyson, E. For an End to Pinioning: The Case Against the Legal Mutilation of Birds in Captivity. J. Anim. Ethics 2014, 4, 1–4. [Google Scholar] [CrossRef]
- Hesterman, H.; Gregory, N.G.; Boardman, W.S.J. Deflighting Procedures and Their Welfare Implications in Captive Birds. Anim. Welf. 2001, 10, 405–419. [Google Scholar] [CrossRef]
- Rose, P.; Freeman, M.; Hickey, I.; Kelly, R.; Greenwell, P. Considering What Animals “Need to Do” in Enclosure Design: Questions on Bird Flight and Aviaries. Birds 2024, 5, 586–603. [Google Scholar] [CrossRef]
- Bennett, R.A.; Baumgartner, K. Avian deflighting techniques. In Fowler’s Zoo and Wild Animal Medicine; Elsevier: Amsterdam, The Netherlands, 2015; Volume 8, pp. 650–660. [Google Scholar]
- Stellungnahme der TVT (Tierärztliche Vereinigung für Tierschutz e.V.) Arbeitskreis 7 (Zoo und Zirkus) zum Flugunfähigmachen von Vögeln. 2015. Available online: https://www.tierschutz-tvt.de/alle-merkblaetter-und-stellungnahmen/?no_cache=1&download=TVT-Stellungn._Flugunf%C3%A4higmachen_von_V%C3%B6geln__Mai_2015_.pdf&did=175 (accessed on 23 April 2022).
- Council Directive 1999/22/EC. Council Directive of the European Union Relating to the Keeping of Wild Animals in Zoos. Official Journal L 094, 9 April 1999; 24–26. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=uriserv:OJ.L_.1999.094.01.0024.01.ENG (accessed on 1 July 2022).
- Beckmann, M.; Thal, D. Flugunfähigkeitsbewirkende Behandlungen von Zoovögeln–Rechtliche Rahmenbedingungen des Tier- und Naturschutzrechts. Nat. Recht 2017, 39, 154–163. [Google Scholar] [CrossRef]
- Reese, L.; Ladwig-Wiegard, M.; von Fersen, L.; Haase, G.; Will, H.; Merle, R.; Encke, D.; Maegdefrau, H.; Baumgartner, K.; Thöne-Reineke, C. Deflighting zoo birds and its welfare considerations. Anim. Welf. 2020, 29, 69–80. [Google Scholar] [CrossRef]
- Verbraucherschutz, B.d.J.u.f. Tierschutzgesetz in der Fassung der Bekanntmachung vom 18. Mai 2006 (BGBl. I S. 1206, 1313), zuletzt geändert durch Artikel 1 des Gesetzes vom 17. Dezember 2018 (BGBl. I S. 2586). Available online: https://www.gesetze-im-internet.de/tierschg/BJNR012770972.html (accessed on 15 March 2025).
- Maisack, C.; Schmidt, T. Zum Flugunfähigmachen von Vögeln in Zoos und privaten Geflügelhaltungen. Nat. Recht 2017, 39, 734–741. [Google Scholar] [CrossRef]
- Peng, S.J.-L.; Chang, F.-C.; Sheng-Ting, J.; Fei, A.C.-Y. Welfare assessment of flight-restrained captive birds: Effects of inhibition of locomotion. Wētchasān Sattawaphāet 2013, 43, 235–241. [Google Scholar] [CrossRef]
- Hill, S.P.; Broom, D.M. Measuring zoo animal welfare: Theory and practice. Zoo Biol. 2009, 28, 531–544. [Google Scholar] [CrossRef]
- Rault, J.-L.; Bateson, M.; Boissy, A.; Forkman, B.; Grinde, B.; Gygax, L.; Harfeld, J.L.; Hintze, S.; Keeling, L.J.; Kostal, L.; et al. A consensus on the definition of positive animal welfare. Biol. Lett. 2025, 21, 20240382. [Google Scholar] [CrossRef]
- Ward, S.J.; Sherwen, S.; Clark, F.E. Advances in Applied Zoo Animal Welfare Science. J. Appl. Anim. Welf. Sci. 2018, 21 (Suppl. S1), 23–33. [Google Scholar] [CrossRef]
- Ganswindt, A.; Brown, J.L.; Freeman, E.W.; Kouba, A.J.; Penfold, L.M.; Santymire, R.M.; Vick, M.M.; Wielebnowski, N.; Willis, E.L.; Milnes, M.R. International Society for Wildlife Endocrinology: The future of endocrine measures for reproductive science, animal welfare and conservation biology. Biol. Lett. 2012, 8, 695–697. [Google Scholar] [CrossRef]
- Paul, E.S.; Browne, W.; Mendl, M.T.; Caplen, G.; Trevarthen, A.; Held, S.; Nicol, C.J. Assessing animal welfare: A triangulation of preference, judgement bias and other candidate welfare indicators. Anim. Behav. 2022, 186, 151–177. [Google Scholar] [CrossRef]
- Veasey, J.S.; Waran, N.K.; Young, R.J. On Comparing the Behaviour of Zoo Housed Animals with Wild Conspecifics as a Welfare Indicator. Anim. Welf. 1996, 5, 13–24. [Google Scholar] [CrossRef]
- Hughes, B.; Duncan, I. The notion of ethological ‘need’, models of motivation and animal welfare. Anim. Behav. 1988, 36, 1696–1707. [Google Scholar] [CrossRef]
- Walkup, K.R. Applied Primatology: Species-Specific Behavior of Captive Japanese Macaques (Macaca fuscata) Under Varying Zoo Conditions and in the Wild; Iowa State University Digital Repository: Ames, IA, USA, 2004. [Google Scholar]
- Johns, D.W.; Marchant, T.A.; Fairhurst, G.D.; Speakman, J.R.; Clark, R.G.; Williams, T. Biomarker of burden: Feather corticosterone reflects energetic expenditure and allostatic overload in captive waterfowl. Funct. Ecol. 2017, 32, 345–357. [Google Scholar] [CrossRef]
- Dickens, M.J.; Romero, L.M. A consensus endocrine profile for chronically stressed wild animals does not exist. Gen. Comp. Endocrinol. 2013, 191, 177–189. [Google Scholar] [CrossRef]
- Cockrem, J.F. Individual variation in glucocorticoid stress responses in animals. Gen. Comp. Endocrinol. 2013, 181, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, J.; Bartolomucci, A.; Buwalda, B.; de Boer, S.; Flügge, G.; Korte, S.; Meerlo, P.; Murison, R.; Olivier, B.; Palanza, P.; et al. Stress revisited: A critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011, 35, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef] [PubMed]
- Voit, M.; Baumgartner, K.; von Fersen, L.; Merle, R.; Reese, L.; Wiegard, M.; Will, H.; Tallo-Parra, O.; Carbajal, A.; Lopez-Bejar, M.; et al. Comparison of Two Different Feather Sampling Methods to Measure Corticosterone in Wild Greater Flamingos (Phoenicopterus roseus) and Wild Mallards (Anas platyrhynchos). Animals 2021, 11, 2796. [Google Scholar] [CrossRef]
- Torres-Medina, F.; Cabezas, S.; Marchant, T.A.; Wikelski, M.; Romero, L.M.; Hau, M.; Carrete, M.; Tella, J.L.; Blas, J. Corticosterone implants make stress hyporesponsive birds. J. Exp. Biol. 2018, 221, jeb173864. [Google Scholar] [CrossRef]
- Blas, J. Chapter 33-Stress in Birds, 6th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 769–810. [Google Scholar]
- Baos, R.; Blas, J.; Bortolotti, G.R.; Marchant, T.A.; Hiraldo, F. Adrenocortical response to stress and thyroid hormone status in free-living nestling white storks (Ciconia ciconia) exposed to heavy metal and arsenic contamination. Environ. Health Perspect. 2006, 114, 1497–1501. [Google Scholar] [CrossRef]
- Dufty, A.M. Stress Responsiveness in Nestlings: A Comparison of Two Sampling Techniques. Auk 2008, 125, 225–229. [Google Scholar] [CrossRef][Green Version]
- Möstl, E.; Rettenbacher, S.; Palme, R. Measurement of Corticosterone Metabolites in Birds’ Droppings: An Analytical Approach. Ann. N. Y. Acad. Sci. 2005, 1046, 17–34. [Google Scholar] [CrossRef]
- Russell, W.M.; Burch, R.L. The Principles of Humane Experimental Technique, 1st ed.; Methuen: London, UK, 1959. [Google Scholar]
- Voit, M.; Merle, R.; Baumgartner, K.; von Fersen, L.; Reese, L.; Ladwig-Wiegard, M.; Will, H.; Tallo-Parra, O.; Carbajal, A.; Lopez-Bejar, M.; et al. Validation of an Alternative Feather Sampling Method to Measure Corticosterone. Animals 2020, 10, 2054. [Google Scholar] [CrossRef]
- Bartels, T.; Berk, J.; Cramer, K.; Kanitz, E.; Otten, W. Research Note: It’s not just stress—Fecal contamination of plumage may affect feather corticosterone concentration. Poult. Sci. 2021, 100, 101494. [Google Scholar] [CrossRef]
- Bortolotti, G.R.; Marchant, T.; Blas, J.; Cabezas, S. Tracking stress: Localisation, deposition and stability of corticosterone in feathers. J. Exp. Biol. 2009, 212, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.A.; Lattin, C.R.; Romero, L.M.; Dearborn, D.C. Feather coloration in museum specimens is related to feather corticosterone. Behav. Ecol. Sociobiol. 2012, 67, 341–348. [Google Scholar] [CrossRef]
- Bloesch, M.D.; Sutter, E. Die Mauser der Schwungfedern beim Weißstorch (Ciconia ciconia). Der Ornithol. Beob. 1977, 74, 161–188. [Google Scholar]
- Snyder, N.F.R.; Johnson, E.V.; Clendenen, D.A. Primary Molt of California Condors. Condor 1987, 89, 468–485. [Google Scholar] [CrossRef]
- Tryjanowski, P.; Grzywaczewski, G.; Zbyryt, A. Foraging of White Stork Ciconia ciconia in Forests–The Heritage of an Ancient Behaviour? Pol. J. Ecol. 2018, 66, 250–256. [Google Scholar] [CrossRef]
- Klausen, B. A mixed-species exhibit for African water birds (including pelicans, flamingos, spoonbills and storks) at Odense Zoo, Denmark: Breeding success, animal welfare and education. Int. Zoo Yearb. 2013, 48, 61–68. [Google Scholar] [CrossRef]
- Bialas, J.T.; Dylewski, Ł.; Dylik, A.; Janiszewski, T.; Kaługa, I.; Królak, T.; Kruszyk, R.; Pawlukojć, K.; Pestka, Z.; Polakowski, M.; et al. Impact of land cover and landfills on the breeding effect and nest occupancy of the white stork in Poland. Sci. Rep. 2021, 11, 7279. [Google Scholar] [CrossRef]
- Blas, J.; Baos, R.; Bortolotti, G.R.; Marchant, T.A.; Hiraldo, F. Age-related variation in the adrenocortical response to stress in nestling white storks (Ciconia ciconia) supports the developmental hypothesis. Gen. Comp. Endocrinol. 2006, 148, 172–180. [Google Scholar] [CrossRef]
- Rotics, S.; Kaatz, M.; Resheff, Y.S.; Turjeman, S.F.; Zurell, D.; Sapir, N.; Eggers, U.; Flack, A.; Fiedler, W.; Jeltsch, F.; et al. The challenges of the first migration: Movement and behaviour of juvenile vs. adult white storks with insights regarding juvenile mortality. J. Anim. Ecol. 2016, 85, 938–947. [Google Scholar] [CrossRef]
- Nagy, M.; Couzin, I.D.; Fiedler, W.; Wikelski, M.; Flack, A. Synchronization, coordination and collective sensing during thermalling flight of freely migrating white storks. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170011. [Google Scholar] [CrossRef]
- Flack, A.; Fiedler, W.; Blas, J.; Pokrovsky, I.; Kaatz, M.; Mitropolsky, M.; Aghababyan, K.; Fakriadis, I.; Makrigianni, E.; Jerzak, L.; et al. Costs of migratory decisions: A comparison across eight white stork populations. Sci. Adv. 2016, 2, e1500931. [Google Scholar] [CrossRef] [PubMed]
- Arslangundogdu, Z.; Dalyan, C.; Bacak, E.; Yardim, U.; Gezgin, C.; Beskardes, V. Spring migration of the White Stork, Ciconia ciconia, and the Black Stork, Ciconia nigra, over the Bosphorus (Aves: Ciconiidae). Zool. Middle East 2011, 53, 7–13. [Google Scholar] [CrossRef]
- Arslangündoğdu, Z.; Bacak, E.; Beşkardeş, V.; Dalyan, C.; Smith, L.; Payne, M.R.; Yardım, Ü. Autumn migration of the White Stork, Ciconia ciconia, and the Black Stork, C. nigra, over the Bosphorus (Aves: Ciconiidae). Zool. Middle East 2017, 63, 103–108. [Google Scholar] [CrossRef]
- Fulin, M.; Jerzak, L.; Sparks, T.H.; Tryjanowski, P. Relationship between arrival date, hatching date and breeding success of the white stork (Ciconia ciconia) in Slovakia. Biologia 2009, 64, 361–364. [Google Scholar] [CrossRef][Green Version]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef]
- Carbajal, A.; Tallo-Parra, O.; Sabes-Alsina, M.; Mular, I.; Lopez-Bejar, M. Feather corticosterone evaluated by ELISA in broilers: A potential tool to evaluate broiler welfare. Poult. Sci. 2014, 93, 2884–2886. [Google Scholar] [CrossRef]
- Monclús, L.; Carbajal, A.; Tallo-Parra, O.; Sabés-Alsina, M.; Darwich, L.; Molina-López, R.A.; Lopez-Bejar, M. Relationship between feather corticosterone and subsequent health status and survival in wild Eurasian Sparrowhawk. J. Ornithol. 2017, 158, 773–783. [Google Scholar] [CrossRef]
- Bortolotti, G.R.; Marchant, T.A.; Blas, J.; German, T. Corticosterone in Feathers Is a Long-Term, Integrated Measure of Avian Stress Physiology. Funct. Ecol. 2008, 22, 494–500. [Google Scholar] [CrossRef]
- Romero, L.M.; Fairhurst, G.D. Measuring corticosterone in feathers: Strengths, limitations, and suggestions for the future. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 202, 112–122. [Google Scholar] [CrossRef]
- Karaer, M.C.; Čebulj-Kadunc, N.; Snoj, T. Stress in wildlife: Comparison of the stress response among domestic, captive, and free-ranging animals. Front. Vet.-Sci. 2023, 10, 1167016. [Google Scholar] [CrossRef]
- Rangel-Negrín, A.; Alfaro, J.L.; Valdez, R.A.; Romano, M.C.; Serio-Silva, J.C. Stress in Yucatan spider monkeys: Effects of environmental conditions on fecal cortisol levels in wild and captive populations. Anim. Conserv. 2009, 12, 496–502. [Google Scholar] [CrossRef]
- Rich, E.L.; Romero, L.M. Exposure to chronic stress downregulates corticosterone responses to acute stressors. Am. J. Physiol. Integr. Comp. Physiol. 2005, 288, R1628–R1636. [Google Scholar] [CrossRef] [PubMed]
- Feenders, G.; Klaus, K.; Bateson, M.; Iwaniuk, A. Fear and Exploration in European Starlings (Sturnus vulgaris): A Comparison of Hand-Reared and Wild-Caught Birds. PLoS ONE 2011, 6, e19074. [Google Scholar] [CrossRef]
- Bocheński, M.; Jerzak, L.; Tryjanowski, P.; Sparks, T.H. Behaviour of the white stork Ciconia ciconia: A review. In The White Stork in Poland: Studies in Biology, Ecology and Conservation; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 2006; pp. 301–330. [Google Scholar]
- Pallieres, C.G.D.; Rose, P.E.; Rouco, C. Two’s company, three species is a crowd? A webcam-based study of the behavioural effects of mixed-species groupings in the wild and in the zoo. PLoS ONE 2023, 18, e0284221. [Google Scholar] [CrossRef]
- Leonardi, R.; Buchanan-Smith, H.M.; Dufour, V.; MacDonald, C.; Whiten, A. Living together: Behavior and welfare in single and mixed species groups of capuchin (Cebus apella) and squirrel monkeys (Saimiri sciureus). Am. J. Primatol. 2009, 72, 33–47. [Google Scholar] [CrossRef]
- Pearson, E.L.; Davis, J.M.; Litchfield, C.A. A case study of orangutan and siamang behavior within a mixed-species zoo exhibit. J. Appl. Anim. Welf. Sci. 2010, 13, 330–346. [Google Scholar] [CrossRef]
- Goodale, E.; Sridhar, H.; Sieving, K.E.; Bangal, P.; Colorado, G.B.J.Z.; Farine, D.R.; Heymann, E.W.; Jones, H.H.; Krams, I.; Martínez, A.E.; et al. Mixed company: A framework for understanding the composition and organization of mixed-species animal groups. Biol. Rev. 2020, 95, 889–910. [Google Scholar] [CrossRef]
- Turcu, M.-C.; Paștiu, A.I.; Bel, L.V.; Pusta, D.L. A Comparison of Feathers and Oral Swab Samples as DNA Sources for Molecular Sexing in Companion Birds. Animals 2023, 13, 525. [Google Scholar] [CrossRef]
- Romero, L.M. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 2002, 128, 1–24. [Google Scholar] [CrossRef]
- Edens, F.; Martin, G.; Carter, T. Housing and seasonal influences on commercial caged layers 1. Corticosterone Levels. Eur. Poult. Sci. 1984, 48, 121–125. [Google Scholar] [CrossRef]

| Zoological Institution | All Individuals | Sampled Individuals | Flight Capable Individuals/Nestlings | Flight Incapable Individuals |
|---|---|---|---|---|
| A | 4 | 4 | 0 | 4 |
| B | 2 | 2 | 0 | 2 |
| C 1 | 6 | 6 | x | 6 |
| D | 2 | 2 | 0 | 2 |
| E | 2 | 2 | 0 | 2 |
| F | 6 | 6 | 0 | 6 |
| G 2 | 18 | 7 | 11 | 7 |
| H | 8 | 8 | 0 | 8 |
| I 3 | ±125 | 10 | 100 | 25 |
| J | 4 | 4 | 0 | 4 |
| K | 3 | 2 | 1 | 2 |
| Total Number | 180 | 53 | 112+ x | 68 |
| Behaviour | Definition | Example |
|---|---|---|
| Affiliative Behaviour | Interactions between two or more storks promoting bonding. | Up-down display: bill clattering combined with neck movements touching the back. Allopreening: mutual preening in nests, often initiated by females. |
| Defensive/Aggressive Interactions | Behaviours showing defence or aggression towards others, typically arising from territorial disputes, competition for resources, mate protection, nest defence. | Threat up-down display: neck extension, bill clattering, tail cocking, wing pumping. Hissing: audible hiss combined with defensive displays. Forward stretch display: horizontal posture, retracted neck, pointed bill, aggressive stepping. |
| Foraging Behaviour | Methods of hunting and prey collection. | Lurking: waiting by animal burrows for prey to appear. Collecting prey: slow walking while picking prey from ground/plants. |
| Resting and Roosting | Stationary posture characterized by minimal movement, indicating periods of low activity and energy conservation. | Standing: still on one leg and/or laying beak onto the chest. |
| Locomotion Activity Score | Scale: 0 = resting/roosting, 1 = slow walking, 2 = walking, 3 = fast walking, 4 = accelerating, 5 = running/fluttering. | |
| Fluttering | Wing flutters or attempts to fly, characterized by short, rapid wing movements. | Lift-off Attempt: abrupt, short wing flaps signalling an initial attempt to fly but not resulting in complete airborne movement. |
| Influencing Factor | Estimate logCORTf in pg/mm | p-Value | 95%—Confidence Intervall |
|---|---|---|---|
| location L (intercept) | 3.568 | <0.001 * | 2.404; 4.731 |
| location A | −0.202 | 0.759 | −1.503; 1.098 |
| location B | 0.256 | 0.723 | −1.169; 1.680 |
| location C | −0.550 | 0.383 | −1.793; 0.694 |
| location D | 0.966 | 0.182 | −0.459; 2.391 |
| location E | −0.989 | 0.172 | −2.414; 0.435 |
| location F | 1.224 | 0.056 | −0.033; 2.480 |
| location G | 0.228 | 0.706 | −0.967; 1.423 |
| location H | 0.295 | 0.621 | −0.880; 1.469 |
| location I | 0.615 | 0.320 | −0.605; 1.835 |
| location J | −0.214 | 0.740 | −1.488; 1.060 |
| location K | 0.874 | 0.227 | −0.551; 2.299 |
| location M | −0.120 | 0.841 | −1.306; 1.066 |
| rehabilitation category 1 (intercept) | 3.888 | <0.001 * | 3.707; 4.069 |
| rehabilitation category 2 | −0.675 | 0.161 | −1.622; 0.273 |
| rehabilitation category 3 | −0.544 | 0.001 * | −0.872; −0.215 |
| flight status (capable; intercept) | 3.864 | <0.001 | 3.706; 4.022 |
| flight status (incapable) | −0.227 | 0.058 | −0.461; 0.007 |
| study group (reha bird; intercept) | 3.821 | <0.001 | 3.657; 3.984 |
| study group (zoo bird) | −0.150 | 0.234 | −0.399; 0.098 |
| study group (zoo nestling) | −0.012 | 0.956 | −0.455; 0.431 |
| Influencing Factor | Estimate logCORTf in pg/mm | p-Value | 95%—Confidence Intervall |
|---|---|---|---|
| Intercept | 3.568 | <0.001 * | 2.772; 4.363 |
| flight status (incapable) | −0.077 | 0.743 | −0.409; 0.366 |
| rehabilitation category 2 | −1.000 | <0.001 * | −1.719; −0.555 |
| rehabilitation category 3 | −0.328 | 0.073 | −0.758; −0.154 |
| study group (zoo bird) | −1.065 | <0.001 * | −1.547; −0.710 |
| study group (zoo nestling) | −1.109 | <0.01 * | −1.664; −0.572 |
| location A | −0.097 | 0.883 | −1.180; 0.986 |
| location B | 0.360 | 0.607 | −0.793; 1.514 |
| location C | −0.460 | 0.450 | −1.462; 0.542 |
| location D | 1.071 | 0.129 | −0.083; 2.224 |
| location E | −0.884 | 0.209 | −2.038; 0.269 |
| location F | 1.329 | <0.05 * | 0.271; 23.87 |
| location G | 0.313 | 0.916 | −0.738; 1.364 |
| location H | 0.647 | 0.624 | −0.027; 1.592 |
| location I | 0.720 | 0.190 | −0.318; 1.758 |
| location J | −0.130 | 0.255 | −1.132; 0.871 |
| location K | 0.979 | 0.831 | −0.175; 2.132 |
| location M | −0.052 | 0.915 | −1.029; 0.925 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liermann, F.; Baumgartner, K.; Simon, R.; Will, H.; von Fersen, L.; Merle, R.; Thöne-Reineke, C. Effects of Flight Restraint and Housing Conditions on Feather Corticosterone in White Storks Under Human Care. Animals 2025, 15, 1878. https://doi.org/10.3390/ani15131878
Liermann F, Baumgartner K, Simon R, Will H, von Fersen L, Merle R, Thöne-Reineke C. Effects of Flight Restraint and Housing Conditions on Feather Corticosterone in White Storks Under Human Care. Animals. 2025; 15(13):1878. https://doi.org/10.3390/ani15131878
Chicago/Turabian StyleLiermann, Frederike, Katrin Baumgartner, Ralph Simon, Hermann Will, Lorenzo von Fersen, Roswitha Merle, and Christa Thöne-Reineke. 2025. "Effects of Flight Restraint and Housing Conditions on Feather Corticosterone in White Storks Under Human Care" Animals 15, no. 13: 1878. https://doi.org/10.3390/ani15131878
APA StyleLiermann, F., Baumgartner, K., Simon, R., Will, H., von Fersen, L., Merle, R., & Thöne-Reineke, C. (2025). Effects of Flight Restraint and Housing Conditions on Feather Corticosterone in White Storks Under Human Care. Animals, 15(13), 1878. https://doi.org/10.3390/ani15131878





