Lameness and Hoof Disorders in Sheep and Goats from Small Ruminant Farms in Selangor, Malaysia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Farms and Animals
2.3. Farm Characteristics
2.3.1. Locomotion Scoring
2.3.2. Animal Characteristics
2.3.3. Hoof Examination
2.3.4. Claw Lesions
2.4. Data Analysis
3. Results
3.1. Descriptive Findings
3.2. Animal-Level Characteristics
3.3. Prevalence of Lameness
3.4. Prevalence of Claw Lesions
3.5. Factors Associated with Lameness and Claw Lesions in Sheep and Goat Farms
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaler, J.; Medley, G.F.; Grogono-Thomas, R.; Wellington, E.M.H.; Calvo-Bado, L.A.; Wassink, G.J.; King, E.M.; Moore, L.J.; Russell, C.; Green, L.E. Factors associated with changes of state of foot conformation and lameness in a flock of sheep. Prev. Vet. Med. 2010, 97, 237–244. [Google Scholar] [CrossRef]
- Gelasakis, A.I.; Kalogianni, A.I.; Bossis, I. Aetiology, risk factors, diagnosis and control of foot-related lameness in dairy sheep. Animals 2019, 9, 509. [Google Scholar] [CrossRef]
- Chesterton, R.; Chesterton, S.; Laven, R. Lesions found at foot trimming of dairy goats: Baseline data for comparing lesions and locomotion scoring. Vet. J. 2022, 290, 105927. [Google Scholar] [CrossRef] [PubMed]
- Jaques, N.; Turner, S.A.; Vallée, E.; Heuer, C.; Lopez-Villalobos, N. The Effect of Lameness on Milk Production of Dairy Goats. Animals 2023, 13, 1728. [Google Scholar] [CrossRef]
- Ibishi, L.; Musliu, A.; Mehmedi, B.; Rexhepi, A.; Youngs, C.R.; Behluli, B. Economic losses due to clinical lameness in Kosovo dairy cattle. Vet. Stanica 2021, 53, 295–304. [Google Scholar] [CrossRef]
- Groenevelt, M.; Anzuino, K.; Smith, S.E.; Lee, M.D.; Grogono-Thomas, R. A case report of lameness in two dairy goat herds; a suspected combination of nutritional factors concurrent with treponeme infection. BMC Res. Notes 2015, 8, 791. [Google Scholar] [CrossRef] [PubMed]
- Hempstead, M.N.; Lindquist, T.M.; Shearer, J.K.; Shearer, L.C.; Cave, V.M.; Plummer, P.J. Welfare Assessment of 30 Dairy Goat Farms in the Midwestern United States. Front. Vet. Sci. 2021, 8, 646715. [Google Scholar] [CrossRef]
- Strobel, H. Klauenpflege Schaf Und Ziege [Claw Trimming Sheep and Goat], 2nd ed.; Eugen Ulmer KG: Stuttgart, Germany, 2014. [Google Scholar]
- Best, C.M.; Roden, J.; Phillips, K.; Pyatt, A.Z.; Behnke, M.C. new insight into the prevalence and risk factors for three distinct hoof conformation traits in UK commercial sheep flocks. Vet. Sci. 2021, 8, 176. [Google Scholar] [CrossRef]
- Lewis, K.E.; Green, M.J.; Witt, J.; Green, L.E. Multiple model triangulation to identify factors associated with lameness in British sheep flocks. Prev. Vet. Med. 2021, 93, 105395. [Google Scholar] [CrossRef]
- Aguiar, G.M.N.; Simões, S.V.D.; Silva, T.R.; Assis, A.C.O.; Medeiros, J.M.A.; Garino, F., Jr.; Riet-Correa, F. Foot rot and other foot diseases of goat and sheep in the semiarid region of northeastern Brazil. Pesqui. Vet. Bras. 2011, 31, 879–884. [Google Scholar] [CrossRef]
- Winter, J.R.; Kaler, J.; Ferguson, E.; KillBride, A.L.; Green, L.E. Changes in prevalence of, and risk factors for, lameness in random samples of English sheep flocks: 2004–2013. Prev. Vet. Med. 2015, 122, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Anzuino, K.; Bell, N.J.; Bazeley, K.J.; Nicol, C.J. Assessment of welfare on 24 commercial UK dairy goat farms based on direct observations. Vet. Rec. 2010, 167, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Jaques, N.; Turner, S.; Vallée, É.; Heuer, C.; Deeming, L.; López-Villalobos, N. Prevalence and incidence rate of clinical lameness in three New Zealand dairy goat farms. N. Z. J. Agric. Res. 2023, 67, 419–433. [Google Scholar] [CrossRef]
- Sadiq, M.B.; Ramanoon, S.Z.; Mansor, R.; Syed-Hussain, S.S.; Shaik Mossadeq, W.M. Prevalence of lameness, claw lesions, and associated risk factors in dairy farms in Selangor, Malaysia. Trop. Anim. Health Prod. 2017, 49, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.B.; Ramanoon, S.Z.; Mossadeq, W.M.S.; Mansor, R.; Syed-Hussain, S.S. Cow- and herd-level factors associated with lameness in dairy farms in Peninsular Malaysia. Prev. Vet. Med. 2020, 184, 105163. [Google Scholar] [CrossRef]
- Bitrus, A.A.; Abba, Y.; Jesse, F.F.A.; Yi, L.M.; Teoh, R.; Sadiq, M.A.; Chung, E.L.T.; Lila, M.A.M.; Haron, A.W. Clinical management of foot rot in goats: A case report of lameness. J. Adv. Vet. Anim. Res. 2017, 4, 110–116. [Google Scholar] [CrossRef]
- Jesse, F.F.A.; Bitrus, A.A.; Abba, Y.; Raju, V.N.; Hambali, I.U.; Peter, I.D.; Haron, A.W.; Lila, M.A.M.; Norsidin, J.M. Seroprevalence of small ruminant caprine arthritis encephalitis lentivirus among goats from selected small ruminant farms in Selangor, Malaysia. Vet. World 2018, 11, 172–176. [Google Scholar] [CrossRef]
- Jensen, K.C.; Oehm, A.W.; Campe, A.; Stock, A.; Woudstra, S.; Feist, M.; Müller, K.E.; Hoedemaker, M.; Merle, R. German farmers’ awareness of lameness in their dairy herds. Front. Vet. Sci. 2022, 9, 866791. [Google Scholar] [CrossRef]
- Sadiq, M.B.; Ramanoon, S.Z.; Shaik Mossadeq, W.M.; Mansor, R.; Syed-Hussain, S.S. Dairy farmers perception of and actions in relation to lameness management. Animals 2019, 9, 270. [Google Scholar] [CrossRef]
- Sadiq, M.B.; Ramanoon, S.Z.; Mansor, R.; Syed-Hussain, S.S.; Mossadeq, W.M.S. Dairy farmers’ knowledge, awareness and practices regarding bovine lameness in Malaysian dairy farms. Trop. Anim. Health Prod. 2024, 56, 45. [Google Scholar] [CrossRef]
- Thrusfield, M.; Christley, R.; Brown, H.; Diggle, P.J.; French, N.; Howe, K.; Kelly, L.; O’Connor, A.; Sargeant, J.; Wood, H. Veterinary Epidemiology, 3rd ed.; Blackwell Publishing Company: Hoboken, NJ, USA, 2005. [Google Scholar]
- Christodoulopoulos, G. Foot lameness in dairy goats. Res. Vet. Sci. 2009, 86, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Quirk, T.J. Excel 2016 for Social Science Statistics: A Guide to Solving Practical Problems, 1st ed.; Springer: Heidelberg, Germany, 2016. [Google Scholar]
- Deeming, L.; Beausoleil, N.J.; Stafford, K.J.; Webster, J.S.; Zobel, G. Technical note: The development of a reliable 5-point gait scoring system for use in dairy goats. J. Dairy Sci. 2018, 101, 4491–4497. [Google Scholar] [CrossRef]
- Ngwa, A.; Dawson, L.; Puchała, R.; Detweiler, G.; Merkel, R.; Tovar-Luna, I.; Sahlu, T.; Ferrell, C.L.; Goetsch, A. Urea space and body condition score to predict body composition of meat goats. Small Rumin. Res. 2007, 73, 27–36. [Google Scholar] [CrossRef]
- Zeder, M.A.; Lapham, H.A. Assessing the reliability of criteria used to identify postcranial bones in sheep, Ovis, and goats, Capra. J. Archaeol. Sci. 2010, 37, 2887–2905. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Dhungyel, O.P.; de Silva, K.; Dhand, N.K.; Whittngton, R.J. An objective method for assessment of foot conformation in sheep. Small Rumin. Res. 2018, 167, 22–28. [Google Scholar] [CrossRef]
- Hill, N.P.; Murphy, P.E.; Nelson, A.J.; Mouttotou, N.; Green, L.E.; Morgan, K.L. Lameness and foot lesions in adult British dairy goats. Vet. Rec. 1997, 141, 412–416. [Google Scholar] [CrossRef]
- Lottner, S. Felduntersuchung Zur Bekämpfung Der Moderhinke Bei Schafen Mittels Vakzinen Und Genetischer Marker [Field Study for Fighting Foot Rot in Sheep by Means of Vaccines and Genetic Markers]. Ph.D. Thesis, University of Veterinary Medicine Hannover, Hannover, Germany, 2006. [Google Scholar]
- Heck, R.H.; Thomas, S.L. An Introduction to Multilevel Modeling Techniques: Mlm and Sem Approaches, 4th ed.; Routledge: London, UK, 2020. [Google Scholar]
- Moschovas, M.; Kalogianni, A.I.; Simitzis, P.; Pavlatos, G.; Petrouleas, S.; Bossis, I.; Gelasakis, A.I. A Cross-Sectional Epizootiological Study and Risk Assessment of Foot-Related Lesions and Lameness in Intensive Dairy Sheep Farms. Animals 2021, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, M.M.; Bukar, M.M.; Zira, A.B. Occurrence of small ruminant lameness in Maiduguri and its environs. Sokoto J. Vet. Sci. 2021, 6, 1–4. [Google Scholar]
- Grenho Ajuda, I.; Vieira, A.; Stilwell, G. Are there differences in dairy goats claws’ temperature, before and after trimming? In Proceedings of the IEEE International Symposium on Medical Measurements and Applications, Lisbon, Portugal, 11–12 June 2014. [Google Scholar]
- Gessese, A.T.; Ayele, A.; Kinde, M.Z.; Asmare, A. Prevalence of lameness in dairy cows and associated risk factors at Hawassa town dairy farms, Ethiopia. Vet. Med. Int. 2024, 2024, 2732333. [Google Scholar] [CrossRef]
- Green, L.E.; George, T.R.N. Assessment of current knowledge of footrot in sheep with particular reference to Dichelobacter nodosus and implications for elimination or control strategies for sheep in Great Britain. Vet. J. 2008, 175, 173–180. [Google Scholar] [CrossRef]
- Sailer, L.M.; Holinger, M.; Burla, J.-B.; Wechsler, B.; Zanolari, P.; Friedli, K. Influence of Housing and Management on Claw Health in Swiss Dairy Goats. Animals 2021, 11, 1873. [Google Scholar] [CrossRef] [PubMed]
- Chapinal, N.; de Pasille, A.M.; Rushen, J. Weight distribution and gait in dairy cattle are affected by milking and late pregnancy. J. Dairy Sci. 2009, 92, 581–588. [Google Scholar] [CrossRef]
- Sogstad, Å.M.; Fjeldaas, T.; Østerås, O. Association of claw disorders with claw horn colour in Norwegian red cattle—A cross-sectional study of 2607 cows from 112 herds. Acta Vet. Scand. 2011, 53, 59. [Google Scholar] [CrossRef]
- Hepburn, N.L.; Kinninmonth, L.; Galbraith, H. Pigmentation, Impression Hardness and the Presence of Melanosomes in Bovine Claw Tissue. J. Agric. Sci. 2007, 145, 283–290. [Google Scholar] [CrossRef]
- Lewis, K.; Green, M.; Clifton, R.; Monaghan, E.; Prosser, N.; Nabb, E.; Green, L. Footbathing and foot trimming, and no quarantine: Risks for high prevalence of lameness in a random sample of 264 sheep flocks in England, 2022. Animals 2024, 14, 2066. [Google Scholar] [CrossRef] [PubMed]
- Best, C.M.; Roden, J.; Pyatt, A.Z.; Behnke, M.; Phillips, K. Uptake of the lameness Five-Point Plan and its association with farmer-reported lameness prevalence: A cross-sectional study of 532 UK sheep farmers. Prev. Vet. Med. 2020, 181, 105064. [Google Scholar] [CrossRef]
- Ibrahim, A.; Mahmoud, U.T.; Khalil, N.S.A.; Hussein, H.A.; Ali, M.M. A pilot study on surgical trimming impact on severely overgrown claws in sheep: Behavioral, physiological, and ruminal function aspects. J. Vet. Behav. 2018, 23, 66–75. [Google Scholar] [CrossRef]
- Sadiq, M.B.; Ramanoon, S.Z.; Shaik Mossadeq, W.M.; Mansor, R.; Syed-Hussain, S.S. A modified functional hoof trimming technique reduces the risk of lameness and hoof lesion prevalence in housed dairy cattle. Prev. Vet. Med. 2021, 195, 105463. [Google Scholar] [CrossRef]
- Sadiq, M.B.; Ramanoon, S.Z.; Shaik Mossadeq, W.M.; Mansor, R.; Syed-Hussain, S.S. Treatment protocols for claw horn lesions and their impact on lameness recovery, pain sensitivity, and lesion severity in moderately lame primiparous dairy cows. Front. Vet. Sci. 2022, 9, 1060520. [Google Scholar] [CrossRef]
- Zanolari, P.; Dürr, S.; Jores, J.; Steiner, A.; Kuhnert, P. Ovine footrot: A review of current knowledge. Vet. J. 2021, 271, 105647. [Google Scholar] [CrossRef]
- Deeming, L.; Beausoleil, N.J.; Stafford, K.J.; Webster, J.R.; Cox, N.R.; Zobel, G. Evaluating the long-term conformation and hoof growth effects of starting hoof trimming at 5 months of age in New Zealand dairy goats. J. Dairy Sci. 2023, 106, 1065–1077. [Google Scholar] [CrossRef] [PubMed]

| Management System | Herd Size | Feeding | Housing and Flooring | Pasture Access | Exercise Area | Deworming Routine | Vaccination Routine | Hoof Trimming | Footbath | |
|---|---|---|---|---|---|---|---|---|---|---|
| Goat farms | ||||||||||
| Farm 1 | Intensive | >50 | Forage, pellet | Wooden Raised | No | No | No | None | None | None |
| Farm 2 | Semi-intensive | <50 | Soy, pellet, palm leaves | Wooden Raised | Yes | Yes | No | None | None | None |
| Farm 3 | Intensive | >50 | Napier, pellet | Wooden and concrete floor | No | No | Yes | None | None | None |
| Farm 4 | Intensive | <50 | Forage, pellet | Wooden Raised | No | No | No | None | None | None |
| Farm 5 | Semi-intensive | >50 | Forage, Pellet, Silage, PKC | Wooden Raised | Yes | Yes | Yes | None | None | None |
| Farm 6 | Semi-intensive | >50 | Forage, Pellet, Silage, PKC | Wooden Raised | Yes | Yes | Yes | None | None | None |
| Sheep farms | ||||||||||
| Farm 1 | Intensive | 150 | Forage, Pellet, Silage | Wooden Raised | No | No | Yes | Yes | Twice/year | No |
| Farm 2 | Semi-intensive | 200 | Soy, pellet, palm leaves | Wooden Raised | Yes | No | Yes | Yes | Twice/year | Yes |
| Farm 3 | Intensive | 110 | Forage, Pellet, Silage, PKC | Wooden raised | No | No | Yes | Yes | Once/three months | Yes |
| Farm 4 | Semi-intensive | 200 | Forage, Pellet, Silage, PKC | Wooden raised | Yes | No | Yes | Yes | No | No |
| Characteristics | Sheep | Goat | |||
|---|---|---|---|---|---|
| Frequency | % | Frequency | % | ||
| Age | Age | ||||
| ≤2 years | 41 | 32.5 | ≤2 years | 62 | 62.0 |
| >2 years | 85 | 67.5 | >2 years | 38 | 38.0 |
| Mean age (SD) | Mean age (SD) | 2.35 (0.58) | |||
| Breed | Breed | ||||
| Damara | 49 | 38.8 | Katjang | 51 | 51.0 |
| Others | 77 | 61.2 | Exotic | 49 | 49.0 |
| Gender | Gender | ||||
| Male | 9 | 7.1 | Male | 46 | 46.0 |
| Female | 117 | 92.9 | Female | 54 | 54.0 |
| Hock condition | Hock condition | ||||
| Normal | 72 | 57.1 | Normal | 85 | 85.0 |
| Mild hair loss | 50 | 39.6 | Mild hair loss | 10 | 10.0 |
| Severy injury | 4 | 3.3 | Severy injury | 5 | 5.0 |
| BCS | BCS | ||||
| <3 | 64 | 50.7 | <3 | 45 | 45.0 |
| ≥3 | 62 | 49.2 | ≥3 | 55 | 55.0 |
| Mean BCS (SD) | 3.0 | Mean BCS (SD) | 2.75 (0.50) | ||
| Pregnancy status * | Pregnancy status * | ||||
| Pregnant | 14 | 11.1 | Pregnant | 20 | 20.0 |
| Not Pregnant | 103 | 88.9 | Not Pregnant | 34 | 34.0 |
| Number of Lame | Number of Non-Lame | Total | Prevalence (%) | 95% Confidence Interval (%) | |
|---|---|---|---|---|---|
| Sheep | |||||
| Farm 1 | 12 | 26 | 38 | 31.58 | 18.03–48.79 |
| Farm 2 | 18 | 14 | 32 | 56.25 | 37.88–73.17 |
| Farm 3 | 8 | 22 | 30 | 26.67 | 12.98–46.18 |
| Farm 4 | 16 | 10 | 26 | 61.54 | 40.57–79.09 |
| Overall | 54 | 72 | 126 | 42.86 | 34.19–51.98 |
| Goat | |||||
| Farm 1 | 4 | 20 | 24 | 20.8 | 14.62–34.05 |
| Farm 2 | 3 | 9 | 12 | 25.0 | 15.21–36.45 |
| Farm 3 | 8 | 17 | 25 | 30.8 | 20.41–42.44 |
| Farm 4 | 1 | 12 | 13 | 7.7 | 2.56–14.24 |
| Farm 5 | 7 | 19 | 26 | 26.9 | 12.31–38.02 |
| Overall | 23 | 77 | 100 | 23.0 | 16.32–38.41 |
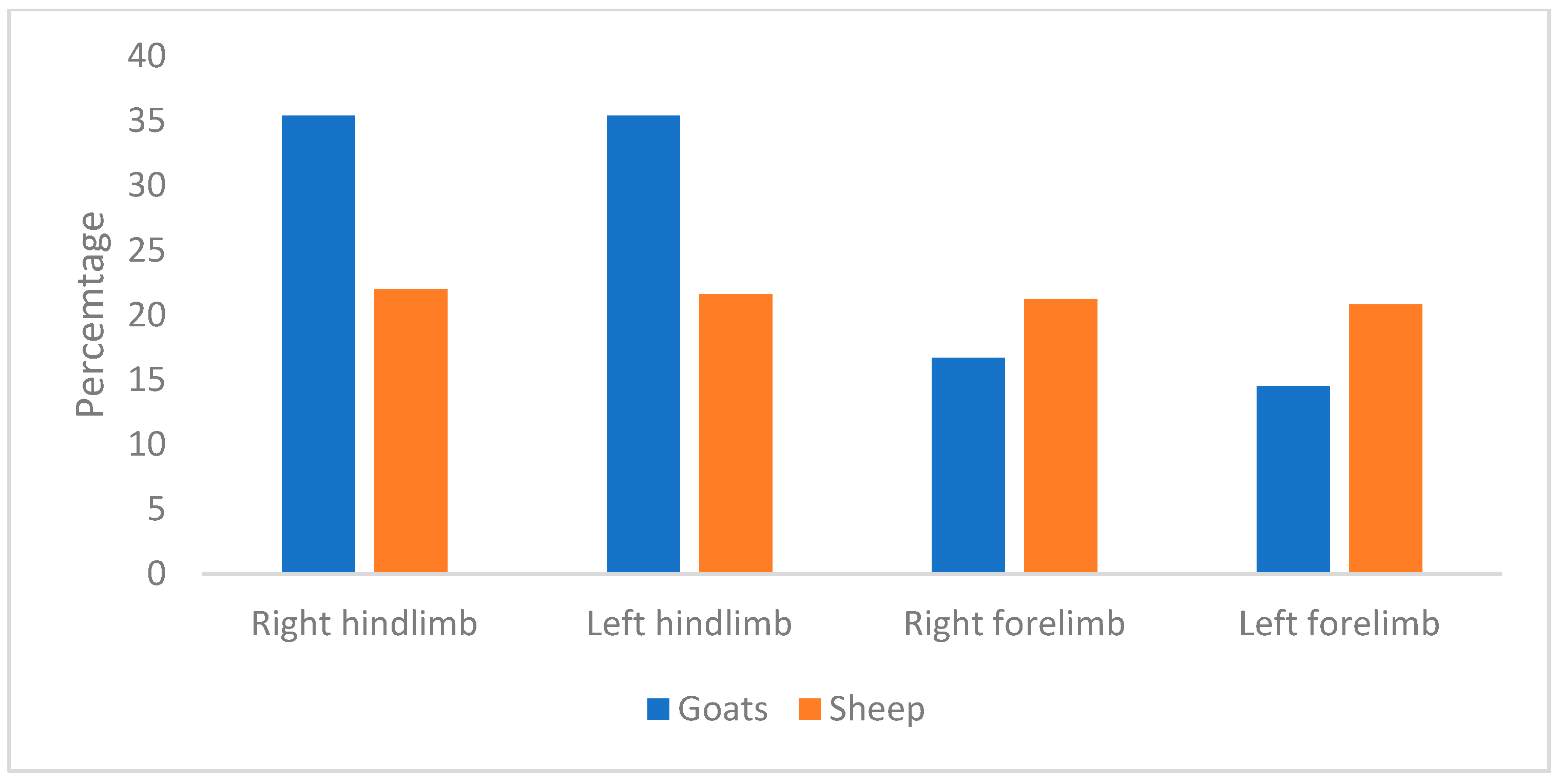
| Sheep | Goat | |||
|---|---|---|---|---|
| Affected limb | Lame | % | Lame | % |
| Left Forelimb | 2 | 4.0 | 2 | 8.7 |
| Left Hindlimb | 28 | 52.0 | 11 | 47.8 |
| Right Forelimb | 4 | 8.0 | 3 | 13.0 |
| Right Hindlimb | 20 | 38.0 | 7 | 30.4 |
| Total | 54 | 126 | 24 | 100 |
| Types of Hoof Conditions | Total | Prevalence (95% CI) | ||||
|---|---|---|---|---|---|---|
| Farm 1 | Farm 2 | Farm 3 | Farm 4 | |||
| Overgrowth | 38 | 22 | 29 | 25 | 114 | 90.48 (83.62, 94.77) |
| WLD/Shelly hoof | 16 | 6 | 10 | 18 | 50 | 39.68 (31.19, 48.80) |
| Sole bruise | 13 | 0 | 0 | 0 | 13 | 10.32 (5.83, 17.33) |
| Normal hooves | 0 | 9 | 1 | 1 | 11 | 8.73 (4.65, 15.44) |
| No. of animals with hoof conditions | 38 | 23 | 29 | 25 | 115 | 91.27 (84.56, 95.35) |
| Total no. of animals | 38 | 32 | 30 | 26 | 126 | |
| Prevalence of by farm (95% CI) | 100.0 (88.5, 100) | 71.9 (53.3, 85.6) | 96.7 (80.95, 99.8) | 96.2 (78.4, 99.8) | 91.3 (84.6, 95.4) | |
| Types of Hoof Conditions | Number of Animals | Total | Prevalence (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Farm 1 (n = 20) | Farm 2 (n = 20) | Farm 3 (n = 20) | Farm 4 (n = 20) | Farm 5 (n = 20) | |||
| Overgrowth | 9 | 3 | 7 | 3 | 6 | 28 | 28.02 (12.01–38.2) |
| WLD/Shelly hoof | 4 | 1 | 3 | 2 | 2 | 12 | 12.01 (5.10–18.42) |
| Sole bruise | 2 | 1 | 2 | 0 | 1 | 6 | 6.12 (2.82–14.04) |
| Wall fissure/crack | 2 | 0 | 2 | 0 | 1 | 5 | 5.32 (1.84–11.08) |
| No. of animals with hoof conditions | 13 | 3 | 14 | 4 | 9 | 43 | 43 (21.41–58.05) |
| Prevalence of by farm (95% CI) | 62.8 (44.71–86.20) | 16.7 (9.48–34.01) | 69.2 (41.54–83.22) | 19.2 (8.42–30.12) | 46.2 (24.82–68.22) | ||
| Variables | Normal | Present | Prevalence (%) | 95% CI | p-Value | Odds Ratio (95% CI) |
|---|---|---|---|---|---|---|
| Pregnancy status | ||||||
| Pregnant | 11 | 3 | 78.5 | 48.8–94.3 | 0.026 | 5.02 (1.21–20.72) |
| Not pregnant | 39 | 64 | 37.8 | 28.6–47.10 | Ref | |
| Management system | ||||||
| Semi-intensive | 34 | 24 | 58.6 | 44.9–71.1 | <0.027 | 3.09 (1.14–8.38) |
| Intensive | 20 | 48 | 29.4 | 27.2–51.1 | Ref | |
| Hoof trimming | ||||||
| Yes | 38 | 62 | 38.0 | 28.6–48.2 | 0.71 | 1.23 (0.39–3.84) |
| No | 16 | 10 | 61.5 | 40.7–79.0 | Ref | |
| Presence of Footbath | ||||||
| Yes | 8 | 22 | 26.6 | 12.9–46.1 | 0.77 | 0.85 (0.28–2.50) |
| No | 46 | 50 | 47.9 | 37.7–58.3 | Ref |
| Variables | Normal | Present | Prevalence (%) | p-Value | OR (95% CI) |
|---|---|---|---|---|---|
| Breed | |||||
| Damara | 10 | 39 | 79.5 | 0.04 | Ref |
| Others | 1 | 76 | 98.7 | 12.92 (1.06–160.29) | |
| Management system | |||||
| Semi-intensive | 8 | 24 | 75.0 | 0.21 | 0.13 (0.006–3.02) |
| Intensive | 26 | 68 | 70.8 | Ref | |
| Stage of production-based feeding | |||||
| Yes | 2 | 94 | 97.9 | 0.573 | 0.46 (0.033–6.56) |
| No | 9 | 21 | 70.0 | Ref |
| Variables | B | SE | Wald | p-Value | Odds Ratio (95% CI) |
|---|---|---|---|---|---|
| Breed | |||||
| Katjang | 1.93 | 0.77 | 8.43 | 0.03 | 2.45 (I.14–6.30) |
| Exotic | Ref | ||||
| Overgrown hooves | |||||
| Present | 2.13 | 0.66 | 10.42 | 0.001 | 8.43 (2.31–30.76) |
| Absent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, F.D.B.; Mohd Roslan, S.N.B.; Lit Kai, J.T.; Ahmad Tajuddin, A.b.; Ramanoon, S.Z.; Othman, A.H.; Sadiq, M.B. Lameness and Hoof Disorders in Sheep and Goats from Small Ruminant Farms in Selangor, Malaysia. Animals 2025, 15, 1858. https://doi.org/10.3390/ani15131858
Rashid FDB, Mohd Roslan SNB, Lit Kai JT, Ahmad Tajuddin Ab, Ramanoon SZ, Othman AH, Sadiq MB. Lameness and Hoof Disorders in Sheep and Goats from Small Ruminant Farms in Selangor, Malaysia. Animals. 2025; 15(13):1858. https://doi.org/10.3390/ani15131858
Chicago/Turabian StyleRashid, Fatini Dayana Binti, Siti Nabilah Binti Mohd Roslan, Jacky Tan Lit Kai, Afida binti Ahmad Tajuddin, Siti Zubaidah Ramanoon, Azalea Hani Othman, and Mohammed Babatunde Sadiq. 2025. "Lameness and Hoof Disorders in Sheep and Goats from Small Ruminant Farms in Selangor, Malaysia" Animals 15, no. 13: 1858. https://doi.org/10.3390/ani15131858
APA StyleRashid, F. D. B., Mohd Roslan, S. N. B., Lit Kai, J. T., Ahmad Tajuddin, A. b., Ramanoon, S. Z., Othman, A. H., & Sadiq, M. B. (2025). Lameness and Hoof Disorders in Sheep and Goats from Small Ruminant Farms in Selangor, Malaysia. Animals, 15(13), 1858. https://doi.org/10.3390/ani15131858





