Large-Scale Embryo Transfer Operation in Dromedary Camels: Retrospective Analysis of the Association Between Key Clinical Factors and the 2-Month Pregnancy Rate
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Camels and Housing
2.2. Ovarian Super-Stimulation Protocol
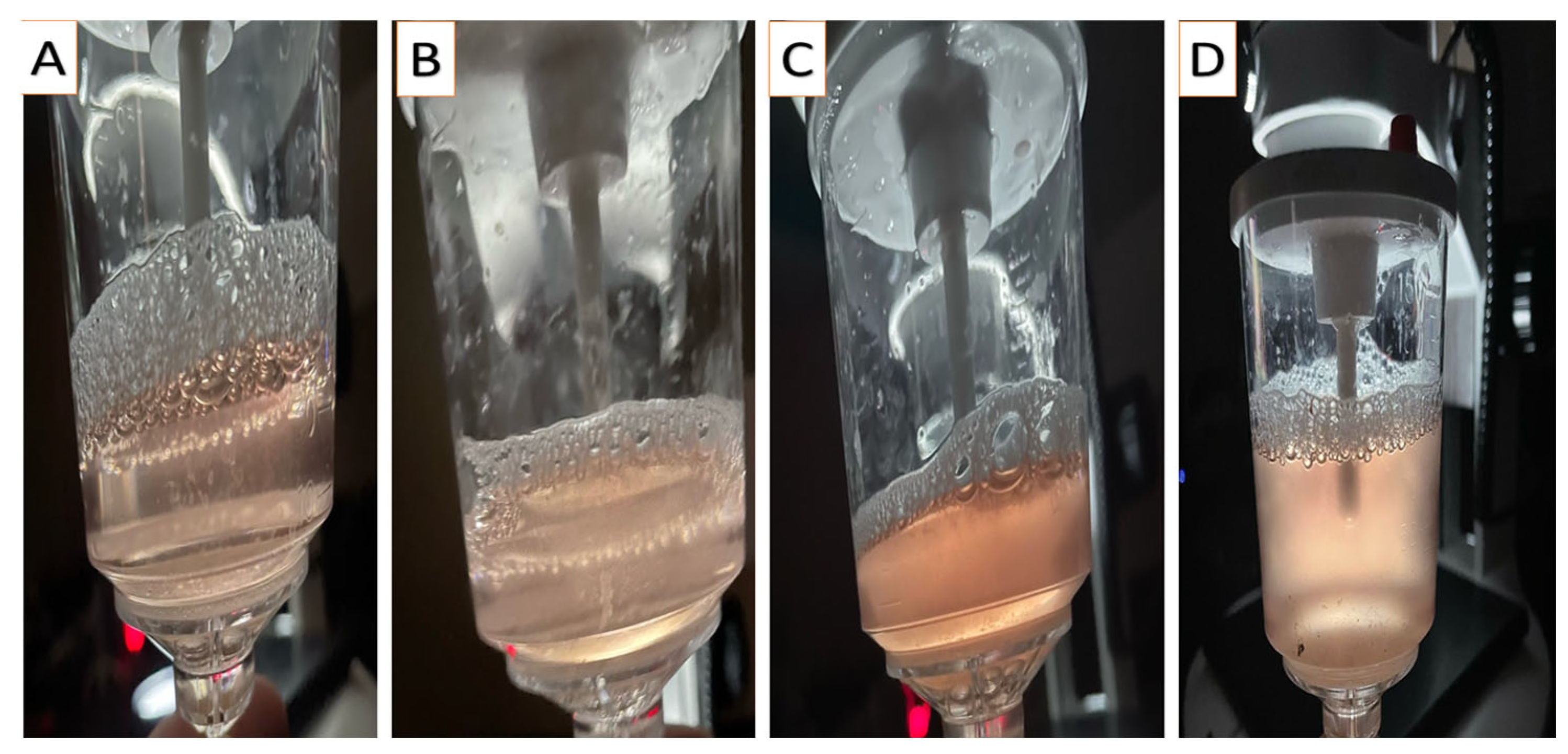
2.3. Embryo Recovery
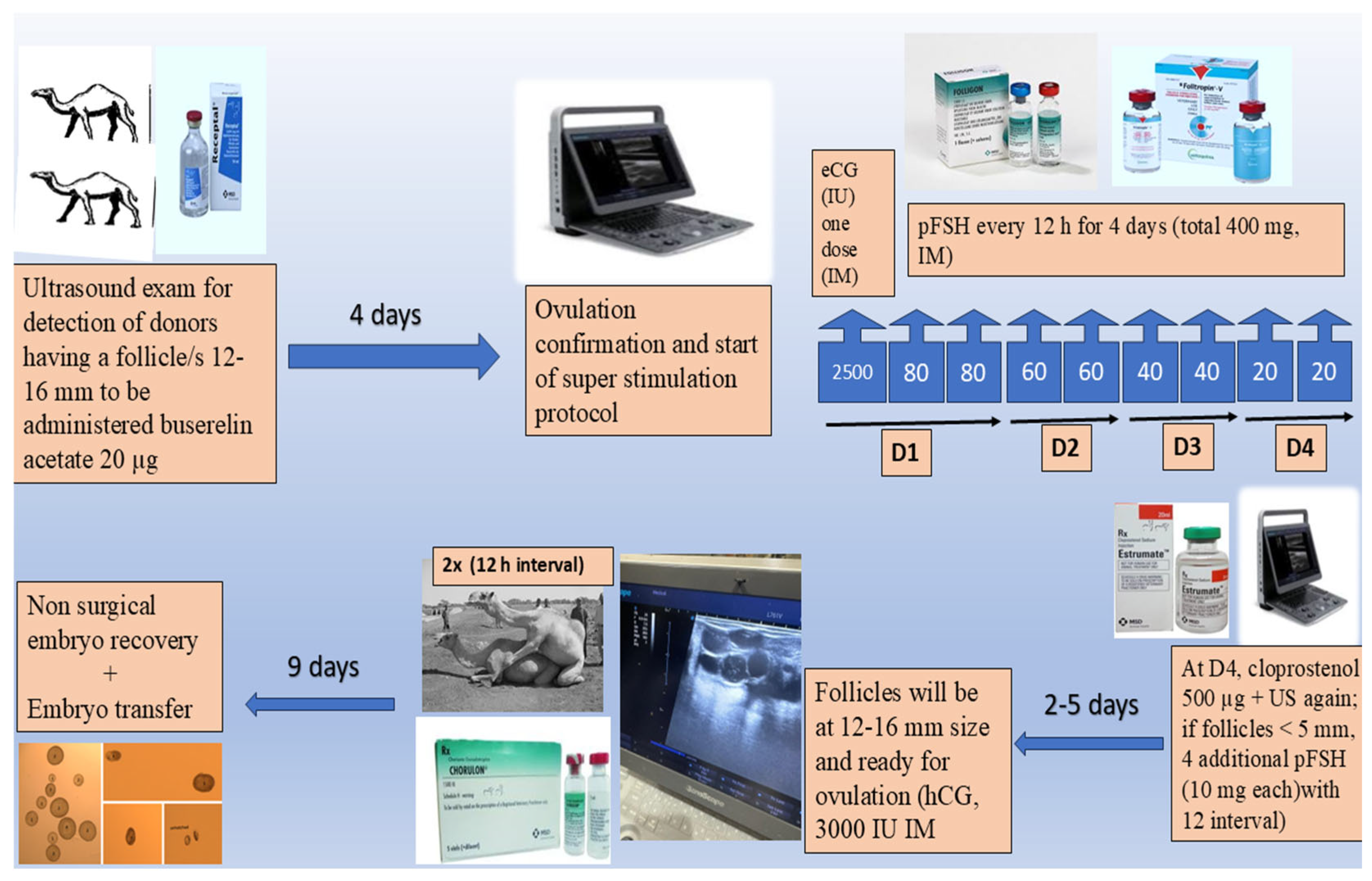
2.4. Embryo Transfer
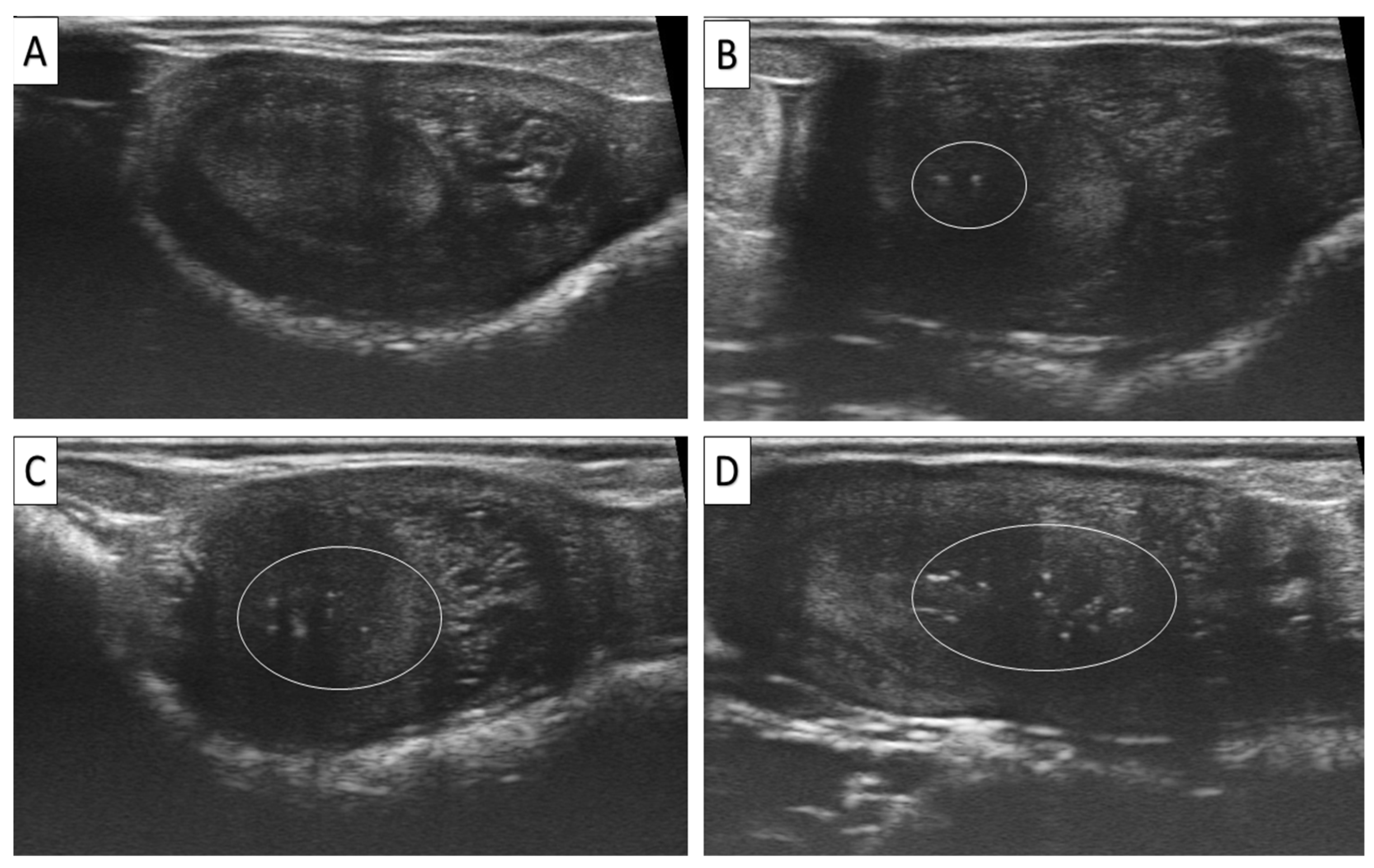
2.5. Assessment of EMs and Uterine Tone
2.6. Pregnancy Diagnosis
2.7. Statistical Analysis
3. Result
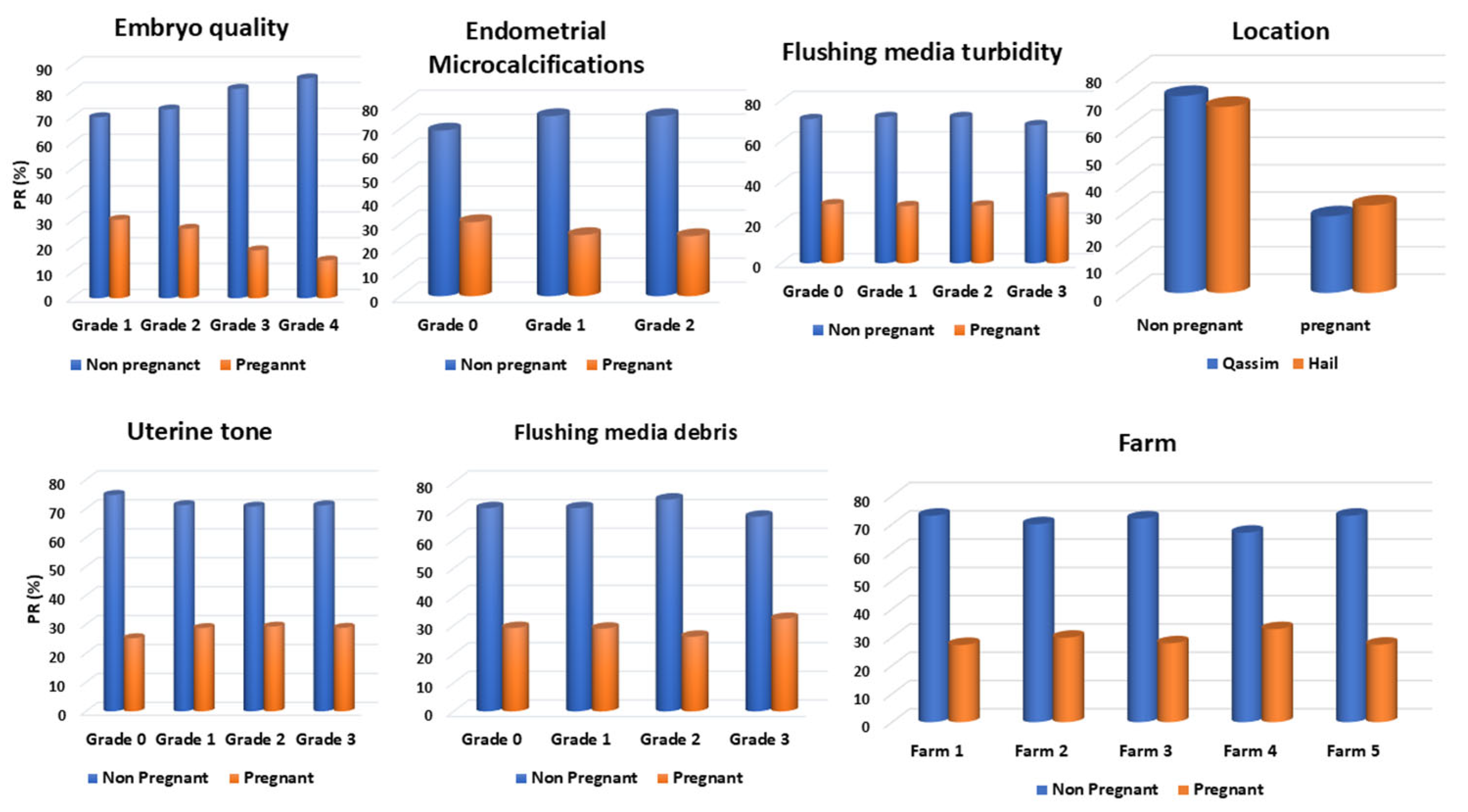
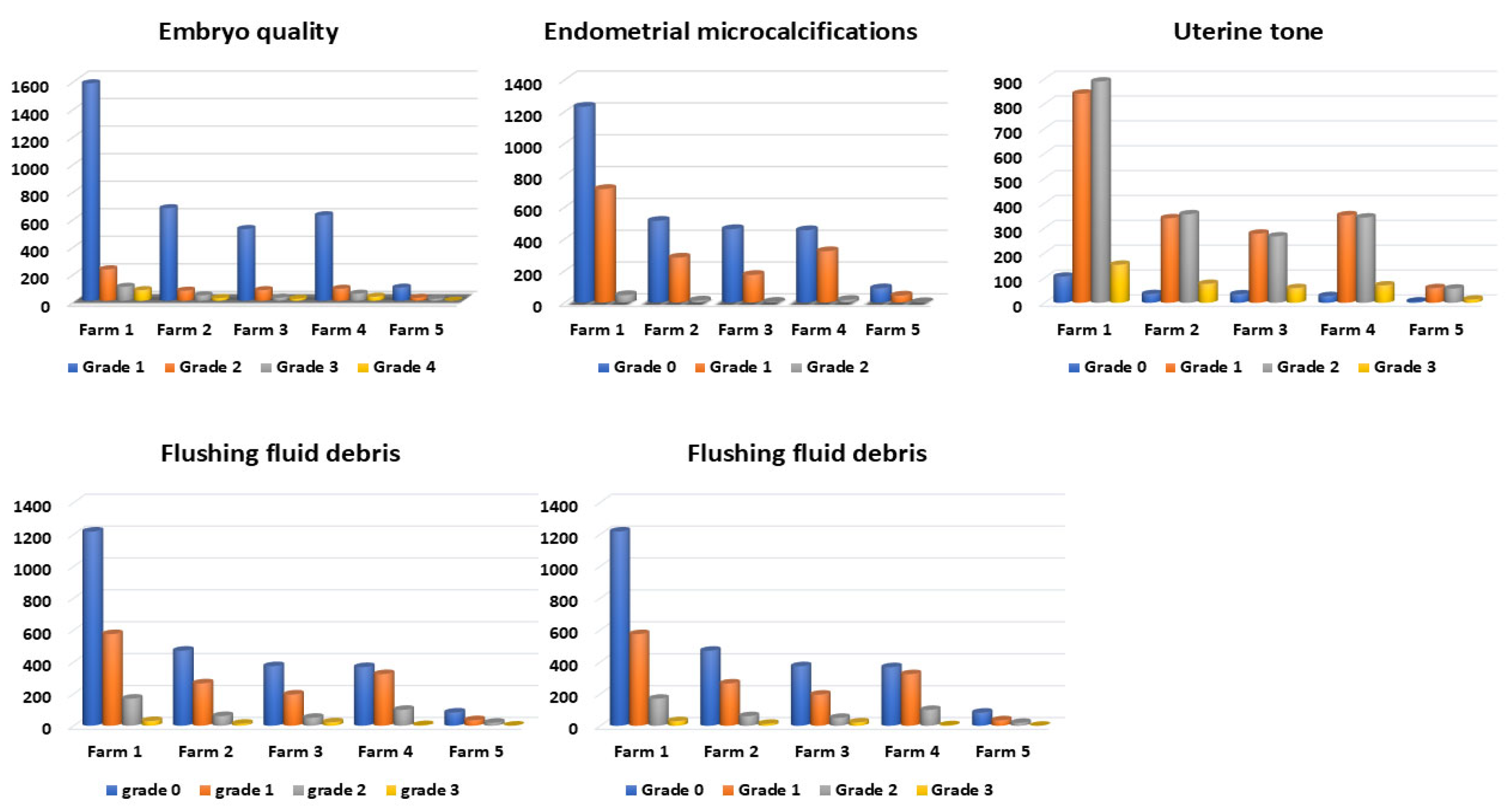
3.1. Effect of Uterine Factors (EMs and Tone) on PR
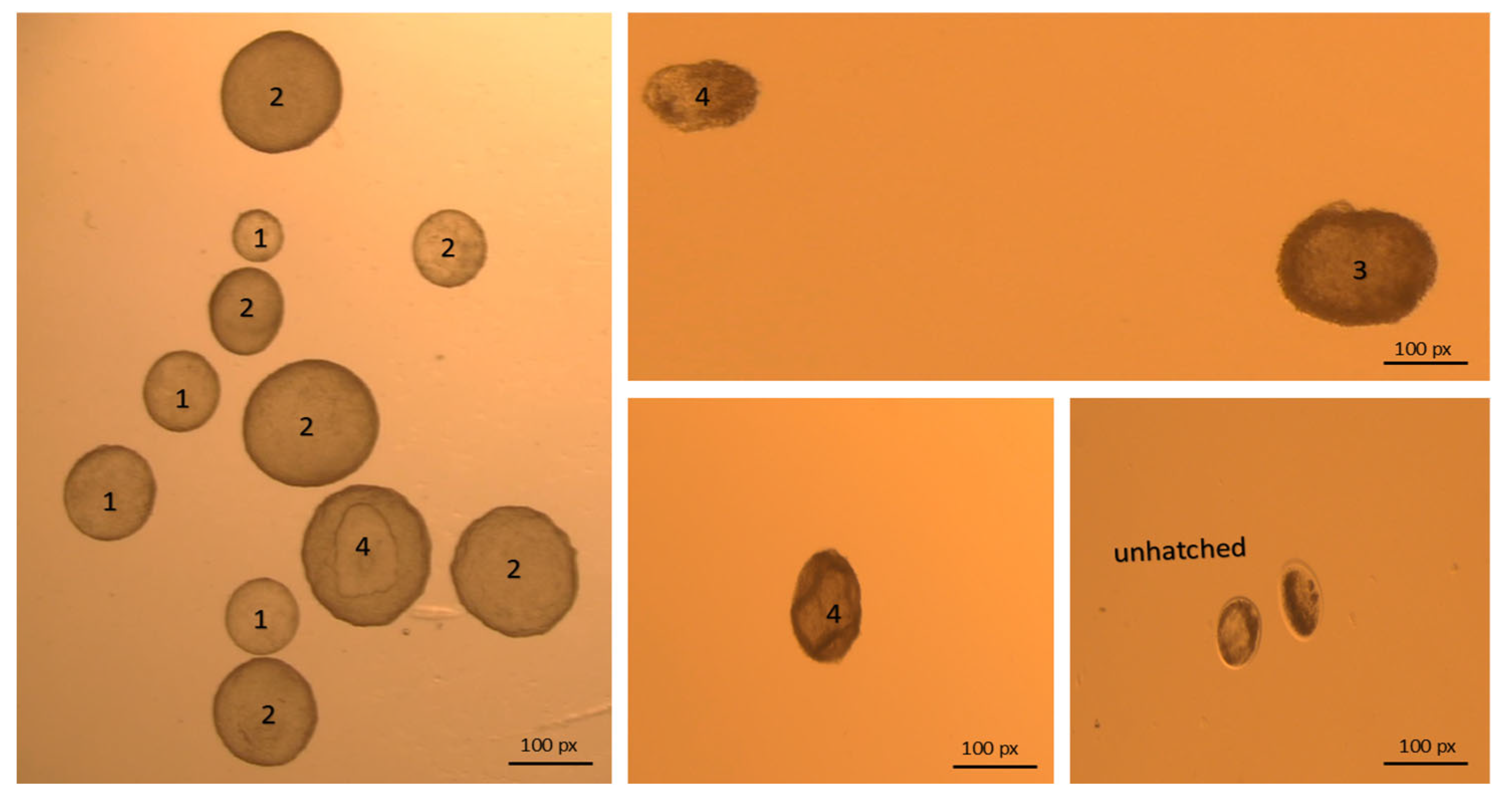
3.2. Effect of Embryo Quality on PR
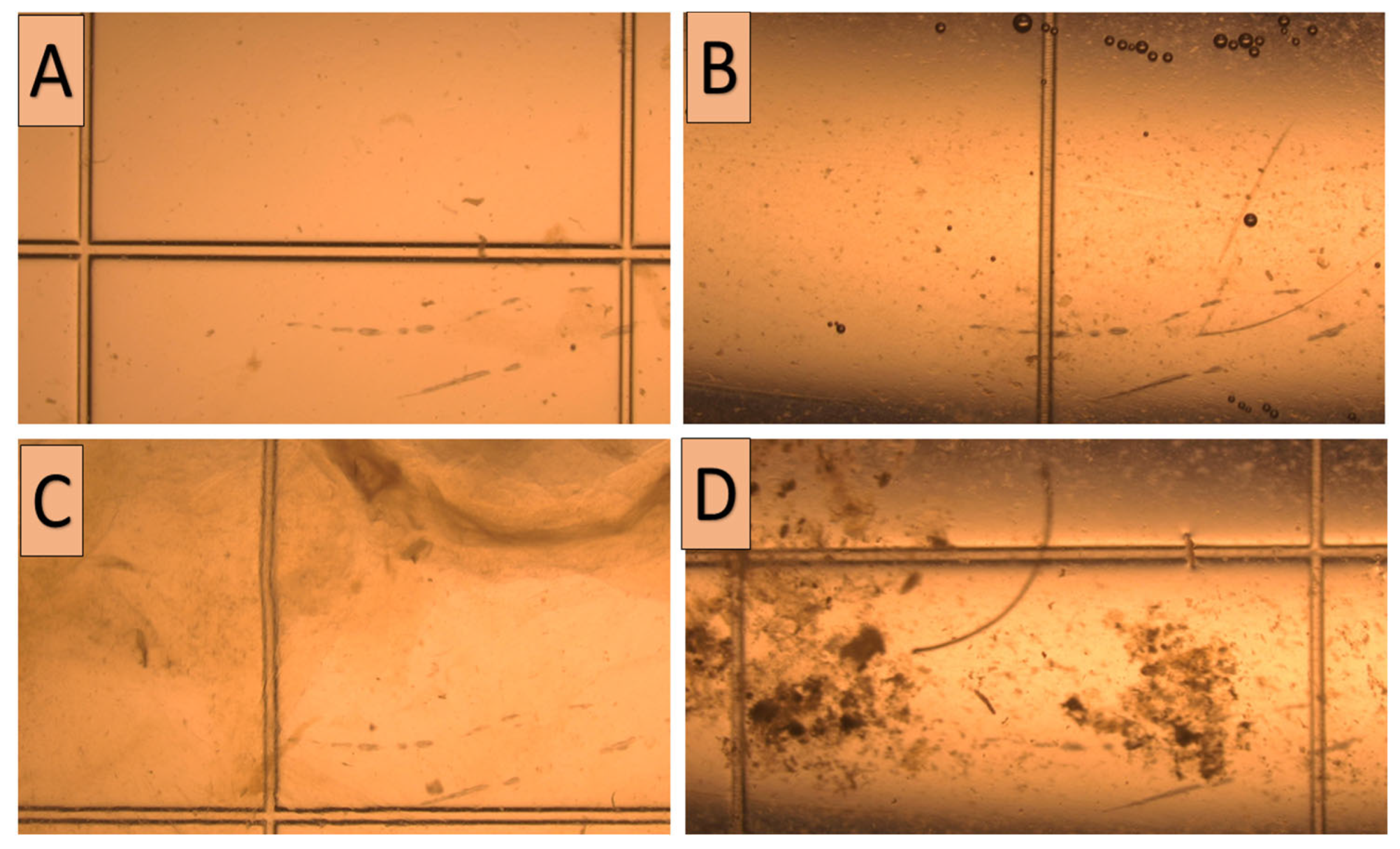
3.3. Effect of Flushing Fluid Debris and Turbidity on Embryo Quality and PR
| Examined Factor | Classification Categories | Frequencies (%) | Pregnancy Rate (%) | Confidence Interval (95%) | Odds Ratio | p-Value | |
|---|---|---|---|---|---|---|---|
| 1 | Uterine tone | Total | NS | ||||
| Grade 0 | 206 (4.7) | 52/206 (25.24) | Indicator | ||||
| Grade 1 | 1870 (42.9) | 538/1870 (28.77) | 0.840–1.645 | 1.176 | NS | ||
| Grade 2 | 1913 (43.9) | 560/1913 (29.27) | 0.843–1.656 | 1.182 | NS | ||
| Grade 3 | 107 (8.5) | 107/371 (28.84) | 0.709–1.576 | 1.057 | NS | ||
| 2 | Endometrial Microcalcifications | Total | <0.01 | ||||
| Grade 0 | 2747 (63) | 847/2747 (30.83) | Indicator | ||||
| Grade 1 | 1533 (35.2) | 390/1533 (25.44) | 0.667–0.885 | 0.768 | <0.01 | ||
| Grade 2 | 80 (1.8) | 20/80 (25.00) | 0.449–1.255 | 0.751 | <0.05 | ||
| 3 | Flushing fluid turbidity | Total | NS | ||||
| Grade 0 | 2829 (64.9) | 822/2829 (29.05) | Indicator | ||||
| Grade 1 | 1116 (25.6) | 314/1116 (28.13) | 0.834–1.137 | 0.974 | NS | ||
| Grade 2 | 338 (7.8) | 96/338 (28.40) | 0.782–1.314 | 1.014 | NS | ||
| Grade 3 | 77 (1.8) | 25/77 (32.46) | 0.697–1.966 | 1.170 | NS | ||
| 4 | Flushing fluid debris | Total | NS | ||||
| Grade 0 | 2509 (57.5) | 731/2509 (29.13) | Indicator | ||||
| Grade 1 | 1391 (31.9) | 402/1391 (28.90) | 0.874–1.172 | 1.012 | NS | ||
| Grade 2 | 395 (9.1) | 103/395 (26.07) | 0.673–1.113 | 0.865 | NS | ||
| Grade 3 | 65 (1.5) | 21/65 (32.30) | 0.627–1.921 | 1.097 | NS | ||
| 5 | Embryo quality | Total | <0.01 | ||||
| Grade 1 | 3493 (80.1) | 1061/3493 (30.37) | Indicator | ||||
| Grade 2 | 491 (11.3) | 132/491(26.88) | 0.684–1.046 | 0.846 | NS | ||
| Grade 3 | 232 (5.3) | 43/232 (18.53) | 0.381–0.752 | 0.535 | <0.01 | ||
| Grade 4 | 144 (3.3) | 21/144 (14.58) | 0.236–0.604 | 0.378 | <0.01 | ||
| 6 | Farm | Total | <0.05 | ||||
| Farm 1 | 1989 (45.6) | 543/1989 (27.30) | Indicator | ||||
| Farm 2 | 807 (18.5) | 241/807 (29.86) | 0.947–1.358 | 1.134 | NS | ||
| Farm 3 | 638 (14.6) | 178/638 (27.89) | 0.844–1.258 | 1.030 | NS | ||
| Farm 4 | 793 (18.2) | 261/793 (32.91) | 1.095–1.564 | 1.309 | <0.05 | ||
| Farm 5 | 135 (3.1) | 37/135 (27.40) | 0.659–1.451 | 0.978 | NS | ||
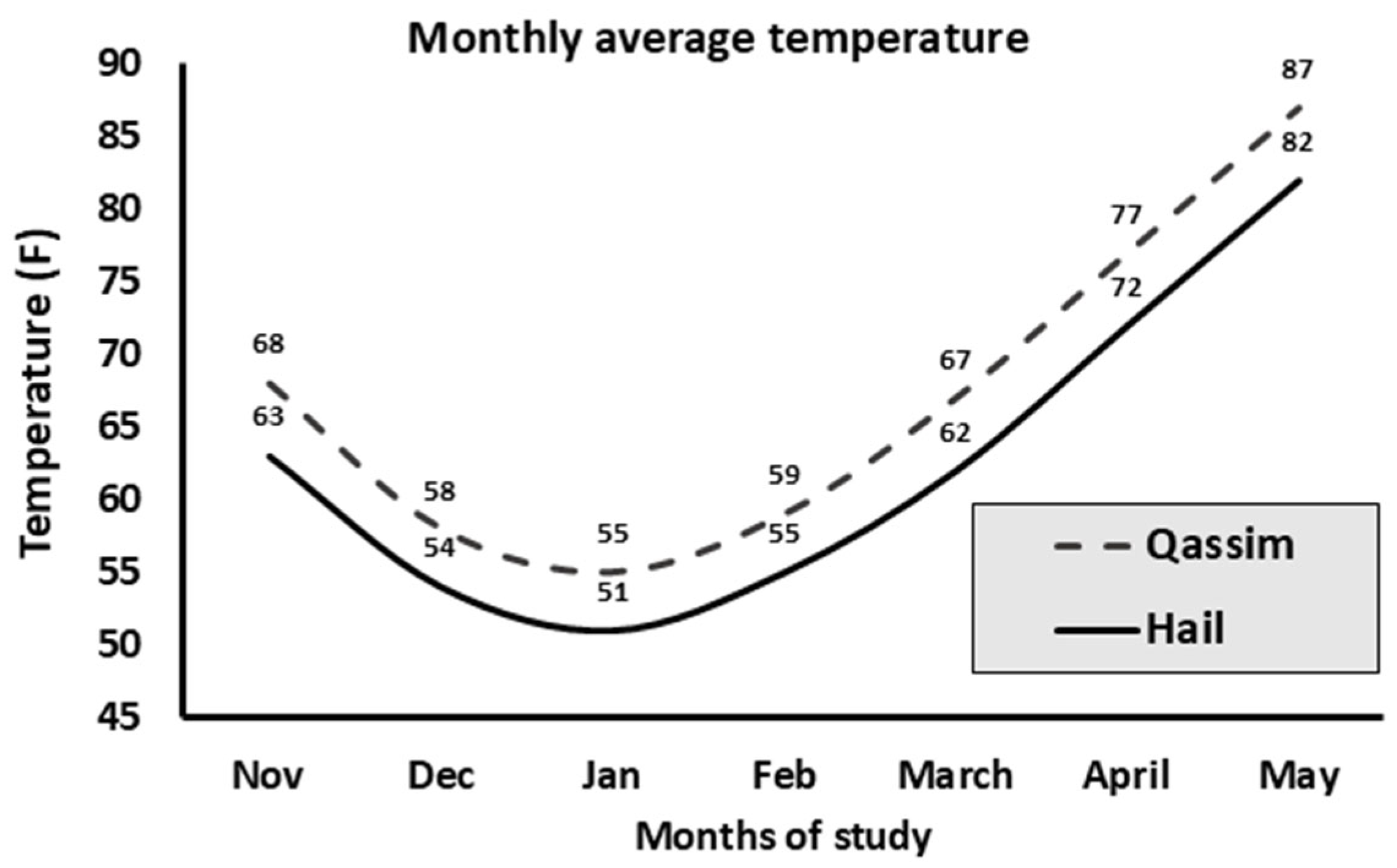
| 7 | Location | Qassim region | 3432 (78.7) | 959/3432 (27.94) | Indicator | ||
| Hail region | 928 (21.3) | 298/928 (32.11) | 1.056–1.469 | 1.246 | <0.01 | ||
| 8 | Temperature | High | 0.978–1.018 | 0.998 | NS | ||
| Low | 0.985–1.036 | 1.010 | NS | ||||
| Embryo Quality | Debris | Turbidity | |||
|---|---|---|---|---|---|
| Embryo quality | Correlation Coefficient | 1.000 | 0.058 ** | 0.033 * | |
| Significance | . | <0.01 | <0.05 | ||
| Debris | Correlation Coefficient | 0.058 ** | 1.000 | 0.248 ** | |
| Significance | 0.000 | . | <0.01 | ||
| Turbidity | Correlation Coefficient | 0.033 * | 0.248 ** | 1.000 | |
| Significance | 0.031 | <0.01 | . | ||
3.4. Location Effect on PR
3.5. Farm Effect on PR
3.6. Temperature Effect on PR
3.7. Effect of Interaction Between All Examined Factors on PR

4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skidmore, J.A. Reproductive physiology in female old world camelids. Anim. Reprod. Sci. 2011, 124, 148–154. [Google Scholar] [CrossRef]
- Tibary, A.; Anouassi, A. Theriogenology in Camelidae: Anatomy, Physiology, Pathology and Artificial Breeding; Actes, Ed.; Institut Agronomique et Vétérinaire Hassan II: Rabat, Morocco, 1997. [Google Scholar]
- Skidmore, J.A.; Billah, M.; Allen, W. Investigation of factors affecting pregnancy rate after embryo transfer in the dromedary camel. Reprod. Fertil. Dev. 2002, 14, 109–116. [Google Scholar] [CrossRef]
- Anouassi, A.; Tibary, A. Development of a large commercial camel embryo transfer program: 20 years of scientific research. Anim. Reprod. Sci. 2013, 136, 211–221. [Google Scholar] [CrossRef]
- Karen, A.; Mansour, N. Factors affecting pregnancy rates and pregnancy losses after embryo transfer in dromedary camels. Anim. Reprod. Sci. 2020, 221, 106580. [Google Scholar] [CrossRef]
- Karen, A.; Abd-Elfattah, A.; Nasef, M.; Rahman, R.U.; Ihsan, M.B.; Muthukumaran, S. Factors affecting outcomes of embryo transfer in dromedary camels: A retrospective study. Reprod. Domest. Anim. 2021, 57, 402–417. [Google Scholar] [CrossRef]
- Abd-Elfattah, A.; Agag, M.; Nasef, M.; Muthukumaran, S.; El-Raey, M.; El-Khawaga, A.; Karen, A. Preservation of dromedary camel embryos at 4° C for up to 5 days: Factors affecting the pregnancy and pregnancy loss rates. Theriogenology 2020, 143, 44–49. [Google Scholar] [CrossRef]
- Vaughan, J.; Mihm, M.; Wittek, T. Factors influencing embryo transfer success in alpacas—A retrospective study. Anim. Reprod. Sci. 2013, 136, 194–204. [Google Scholar] [CrossRef]
- Pérez-Marín, C.C.; Vizuete, G.; Borge, C.; Galisteo, J.J. Cytological and bacteriological sampling from filters used for embryo recovery to evaluate the uterine status of donor mares. Acta Vet. Hung. 2018, 66, 462–473. [Google Scholar] [CrossRef]
- Mulligan, B.P.; Skidmore, J.A. A comparison of culture and cooling for the short term preservation of in vivo derived dromedary camel embryos of varying morphological quality. Theriogenology 2023, 210, 28–33. [Google Scholar] [CrossRef]
- Gilbert, O.R. The effects of endometritis on the establishment of pregnancy in cattle. Reprod. Fertil. Dev. 2012, 24, 252–257. [Google Scholar] [CrossRef]
- Sumar, J.B. Embryo transfer in domestic South American camelids. Anim. Reprod. Sci. 2013, 136, 170–177. [Google Scholar] [CrossRef]
- Feyles, V.; Moyana, T.N.; Pierson, R.A. Recurrent pregnancy loss associated with endometrial hyperechoic areas (endometrial calcifications): A case report and review of the literature. Clin. Exp. Obstet. Gyn. 2000, 27, 5–8. [Google Scholar]
- Walter, I.; Helmreich, M.; Handler, J.; Aurich, C. Mineralised deposits in the uterine glands of mares with chronic endometrial degeneration. Vet. Rec. 2003, 153, 708–710. [Google Scholar]
- Truskinovsky, A.M.; Gerscovich, E.O.; Duffield, C.R.; Vogt, P.J. Endometrial Microcalcifications Detected by Ultrasonography: Clinical Associations, Histopathology, and Potential Etiology. Int. J. Gyn. Pathol. 2007, 27, 61–67. [Google Scholar] [CrossRef]
- Bonafos, L.D.; Carnevale, E.M.; Smith, C.A.; Ginther, O.J. Development of uterine tone in nonbred and pregnant mares. Theriogenology 1994, 42, 1247–1255. [Google Scholar] [CrossRef]
- Porter, D.G.; Berham, H.R. Prostaglandin-induced myometrial activity inhibited by progesterone. Nature 1971, 232, 627–628. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Mckenna, D.; Weston, P.G.; Whitmore, H.L.; Gustafsson, B.K. Uterine motility in the cow during the estrous cycle. I. Spontaneous activity. Theriogenology 1987, 27, 337–347. [Google Scholar] [CrossRef]
- Guimarães, C.R.; Seber, M.F.; Neto, J.D.; Fernandes, C.A.; Rodrigues, É.P.; Torres, B.A.; Moreira Viana, J.H.; Palhão, M.P. Uterine tone: A ne-447 glected criterion for the selection of bovine embryo transfer recipients. Reprod. Domest. Anim. 2024, 59, e14671. [Google Scholar] [CrossRef]
- Fernandes, C.A. Inovulações não cirúrgicas e taxa de gestação de receptoras de embrião. Arq. Bras. Med. Veterinária Zootec. 1999, 51, 263–266. [Google Scholar] [CrossRef]
- Tinson, A.; Sambyal, R.; McCallum, C. Factors affecting embryo recovery in dromedary camels: Review of results over the last 20 yrs. In Proceedings of the ICAR Satellite Meeting on Camelid Reproduction, Vancouver, BC, Canada, 3–5 August 2012; pp. 121–126. [Google Scholar]
- Pereira, M.C.; Vaz, M.M.; Miranda, S.P.; Araújo, S.R.; Menezes, D.B.; das Chagas Medeiros, F. Uterine cavity calcifications: A report of 7 cases and a systematic literature review. J. Minim. Invasive Gyn. 2014, 213, 346–352. [Google Scholar] [CrossRef]
- AbdullGaffar, B.; AlMulla, A. Endometrial calcifications. Int. J. Surg. Pathol. 2020, 28, 590–599. [Google Scholar] [CrossRef]
- Herzog, K.; Brockhan-Lüdemann, M.; Kaske, M.; Beindorff, N.; Paul, V.; Niemann, H.; Bollwein, H. Luteal blood flow is a more appropriate indicator for luteal function during the bovine estrous cycle than luteal size. Theriogenology 2010, 73, 691–697. [Google Scholar] [CrossRef]
- Lüttgenau, J.; Bollwein, H. Evaluation of bovine luteal blood flow by using color Doppler ultrasonography. Reprod. Biol. 2014, 14, 103–109. [Google Scholar] [CrossRef]
- Mann, G.E.; Lamming, G.E. Relationship between maternal endocrine environment, early embryo development and inhibition of the luteolytic mechanism in cows. Reproduction 2009, 121, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Butler, W.R. Nutritional interactions with reproductive performance in dairy cattle. Anim. Reprod. Sci. 2000, 60–61, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, A.R.; Dalley, D.; Borman, J.M.; Macmillan, K.L.; Schwarzenberger, F. Progesterone clearance rate in lactating dairy cows with two levels of dry matter and metabolisable energy intakes. Anim. Reprod. Sci. 2002, 72, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, J.; Allen, W.; Heap, R. Oestrogen synthesis by the peri-implantation conceptus of the one-humped camel 459 (Camelus dromedarius). J. Reprod. Fertil. Suppl. 1994, 101, 363–367. [Google Scholar] [CrossRef]
- Ararooti, T.; Niasari-Naslaji, A.; Asadi-Moghaddam, B.; Razavi, K.; Panahi, F. Superovulatory response following FSH, eCG-FSH and hMG and pregnancy rates following transfer of hatched blastocyst embryos with different diameter and shape in dromedary camel. Theriogenology 2018, 106, 149–156. [Google Scholar] [CrossRef]
- McKinnon, A.O.; Tinson, A.H.; Nation, G. Embryo transfer in dromedary camels. Theriogenology 1994, 41, 145–150. [Google Scholar] [CrossRef]
- Ferraz, P.A.; Burnley, C.; Karanja, J.; Viera-Neto, A.; Santos, J.E.P.; Chebel, R.C.; Galvão, K.N. Factors affecting the success of a large embryo transfer program in Holstein cattle in a commercial herd in the southeast region of the United States. Theriogenology 2016, 86, 1834–1841. [Google Scholar] [CrossRef]
- Dobson, H.; Tebble, J.E.; Smith, R.F.; Ward, W.R. Is stress really all that important? Theriogenology 2001, 55, 65–73. [Google Scholar] [CrossRef]
- Cooke, R.F.; Bohnert, D.W.; Cappellozza, B.I.; Mueller, C.J.; Delcurto, T. Effects of temperament and acclimation to handling on reproductive performance of Bos taurus beef females. J. Anim. Sci. 2012, 90, 3547–3555. [Google Scholar] [CrossRef]
- Hansen, P.J. Effects of heat stress on mammalian reproduction. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 3341–3350. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Ryu, G.; Kim, D.; Baek, H.U.; Kim, J.; Lee, K.; Kim, S.; Kim, S.; Dang, C.-G.; et al. A retrospective analysis of conception per embryo transfer in dairy cattle in South Korea. Theriogenology 2024, 226, 363–368. [Google Scholar] [CrossRef]
- Chebel, R.C.; Dem’etrio, D.G.B.; Metzger, J. Factors affecting success of embryo collection and transfer in large dairy herds. Theriogenology 2008, 69, 98–106. [Google Scholar] [CrossRef]
- Putney, D.J.; Drost, M.; Thatcher, W.W. Embryonic development in superovulated dairy cattle exposed to elevated ambient temperatures between Days 1 to 7 post insemination. Theriogenology 1988, 30, 195–209. [Google Scholar] [CrossRef]
- Ambrose, J.D.; Drost, M.; Monson, R.L.; Rutledge, J.J.; Leibfried-Rutledge, M.L.; Thatcher, M.J.; Kassa, T.; Binelli, M.; Hansen, P.J.; Chenoweth, P.J.; et al. Efficacy of timed embryo transfer with fresh and frozen in vitro produced embryos to increase pregnancy rates in, heat-stressed dairy cattle. J. Dairy Sci. 1999, 82, 2369–2376. [Google Scholar] [CrossRef]
- Lees, A.M.; Sejian, V.; Wallage, A.L.; Steel, C.C.; Mader, T.L.; Lees, J.C.; Gaughan, J.B. The impact of heat load on cattle. Animals 2019, 9, 322. [Google Scholar] [CrossRef]
- Fesseha, H.; Desta, W. Dromedary camel and its adaptation mechanisms to desert environment: A review. Int J. Zool. Stu. 2020, 5, 23–28. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, T.K.; Ismail, S.T.; El-Sherbiny, H.R. Large-Scale Embryo Transfer Operation in Dromedary Camels: Retrospective Analysis of the Association Between Key Clinical Factors and the 2-Month Pregnancy Rate. Animals 2025, 15, 1859. https://doi.org/10.3390/ani15131859
Osman TK, Ismail ST, El-Sherbiny HR. Large-Scale Embryo Transfer Operation in Dromedary Camels: Retrospective Analysis of the Association Between Key Clinical Factors and the 2-Month Pregnancy Rate. Animals. 2025; 15(13):1859. https://doi.org/10.3390/ani15131859
Chicago/Turabian StyleOsman, Taher Kamal, Sayed Taha Ismail, and Hossam R. El-Sherbiny. 2025. "Large-Scale Embryo Transfer Operation in Dromedary Camels: Retrospective Analysis of the Association Between Key Clinical Factors and the 2-Month Pregnancy Rate" Animals 15, no. 13: 1859. https://doi.org/10.3390/ani15131859
APA StyleOsman, T. K., Ismail, S. T., & El-Sherbiny, H. R. (2025). Large-Scale Embryo Transfer Operation in Dromedary Camels: Retrospective Analysis of the Association Between Key Clinical Factors and the 2-Month Pregnancy Rate. Animals, 15(13), 1859. https://doi.org/10.3390/ani15131859






