Structural and Functional Responses of Small Mammal Communities to Land Abandonment in a Region of High Biodiversity
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
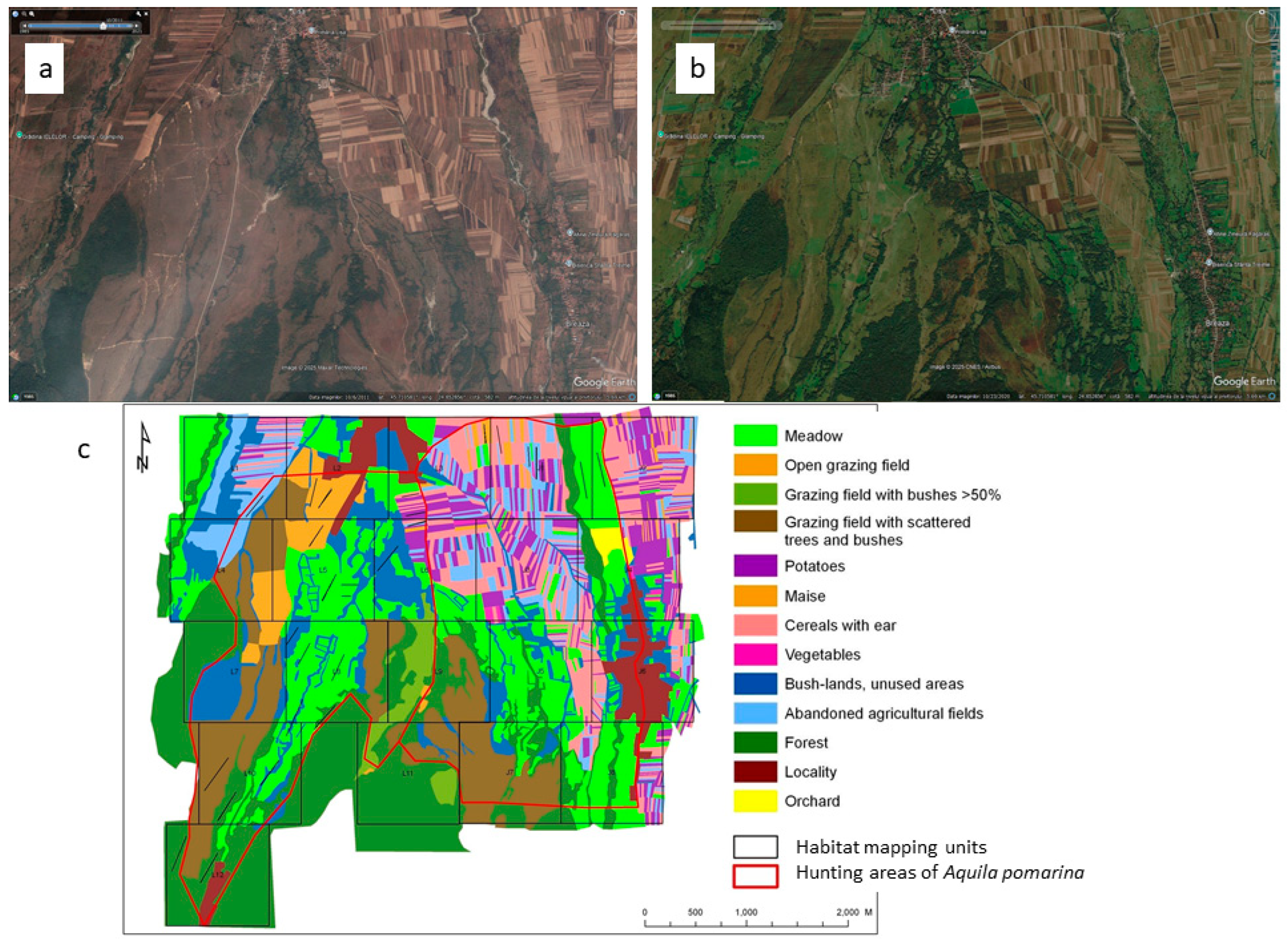
2.1. Study Areas
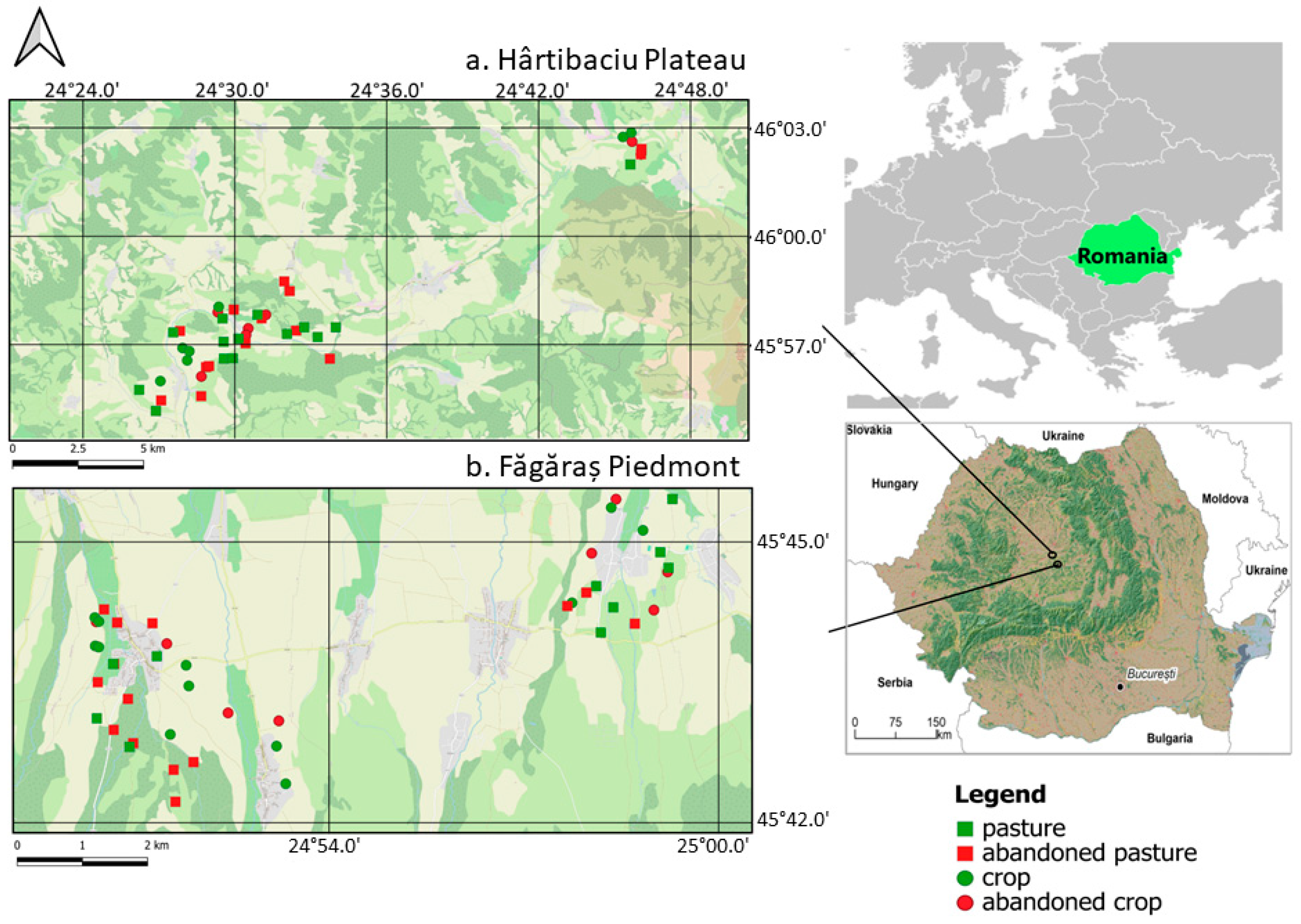
2.2. Small Mammal Trapping
2.3. Functional Traits
2.4. Data Analysis
3. Results
3.1. Small Mammal Species Composition, Richness and Diversity
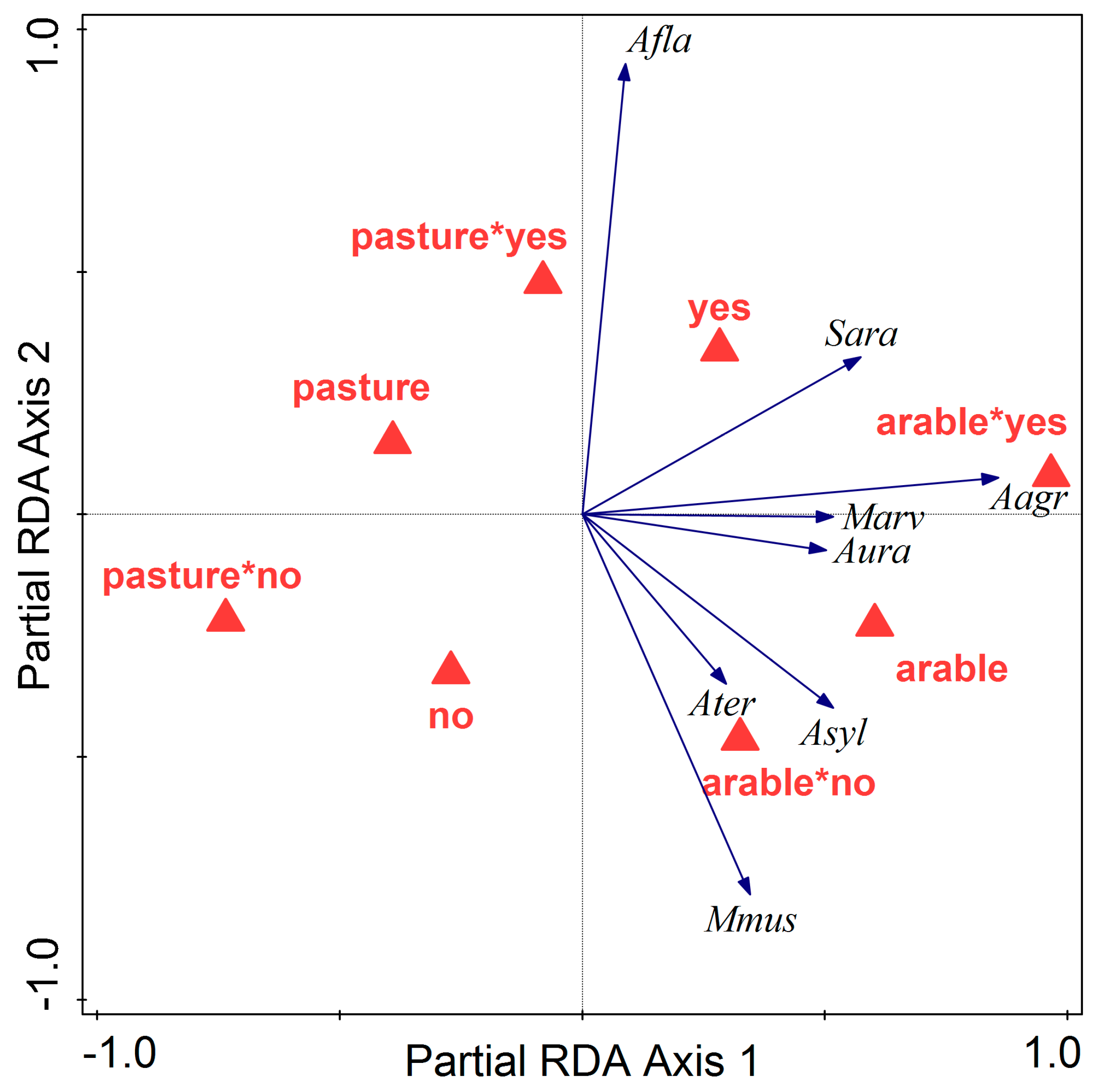
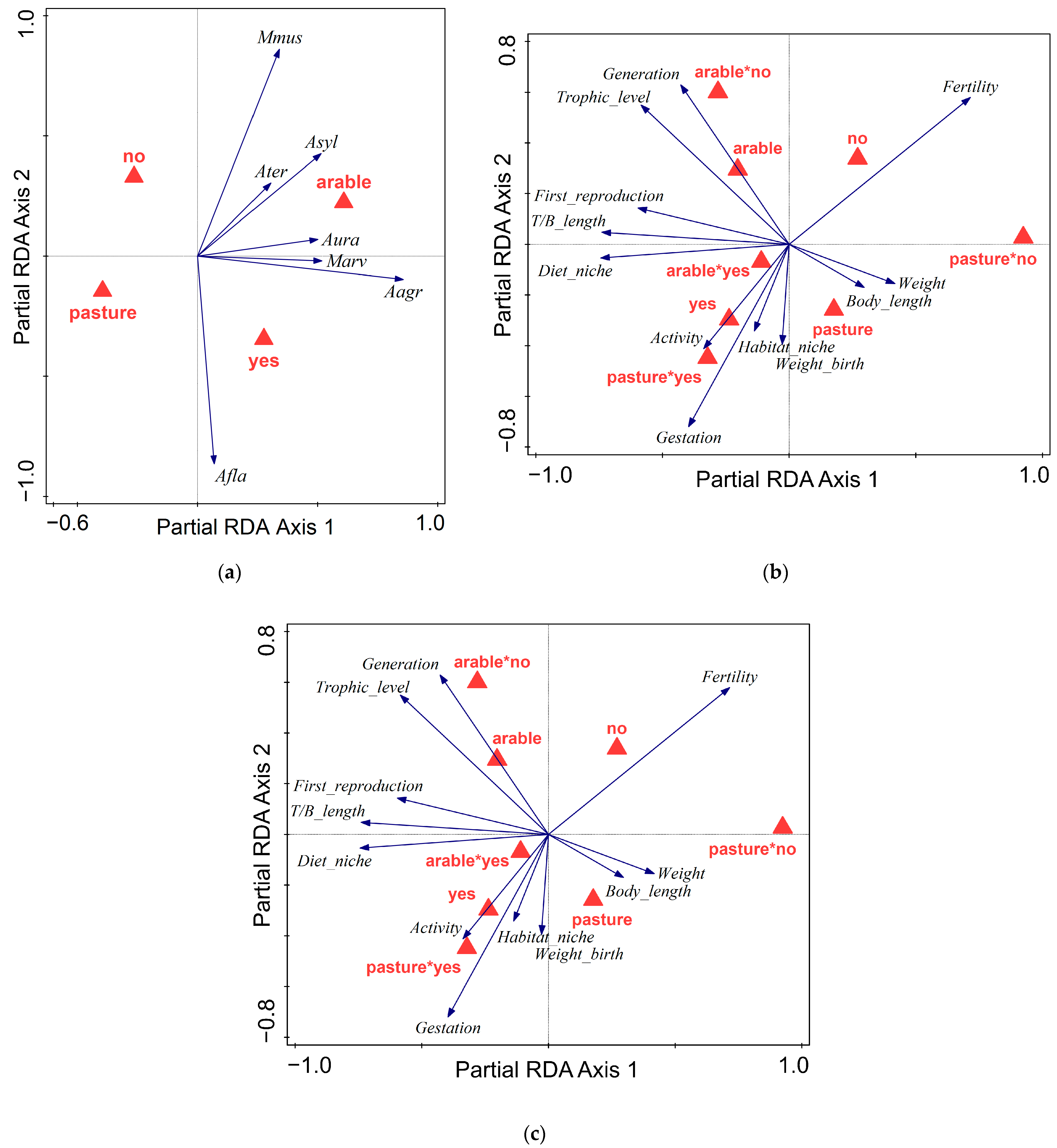
3.2. Influence of Land Abandonment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAP | Common agricultural policy |
| GLMM | Generalised linear mixed model |
| RDA | Redundancy analysis |
| CWM | Community weighted mean |
Appendix A
| Coefficients | Estimate | Std. Error | Test Statistic | p |
|---|---|---|---|---|
| abundance | z | |||
| intercept | 1.785 | 0.373 | ||
| pasture | −2.299 | 0.327 | −7.029 | <0.001 |
| abandonment | 0.281 | 0.3 | 0.935 | 0.349 |
| pasture * abandonment | 1.515 | 0.432 | 3.502 | <0.001 |
| marginal (and conditional) R2 | 0.47 (0.738) | |||
| species richness | t | |||
| intercept | 2.442 | 0.225 | ||
| pasture | −1.675 | 0.255 | −6.572 | <0.001 |
| abandonment | 0.976 | 0.249 | 3.918 | <0.001 |
| R2 (adjusted) | 0.36 | |||
| Chao | t | |||
| intercept | 2.62 | 0.267 | ||
| pasture | −1.862 | 0.303 | −6.149 | <0.001 |
| abandonment | 1.159 | 0.296 | 3.917 | <0.001 |
| R2 (adjusted) | 0.336 | |||
| H | t | |||
| intercept | 0.594 | 0.069 | ||
| pasture | −0.479 | 0.078 | −6.12 | <0.001 |
| abandonment | 0.271 | 0.076 | 3.546 | <0.001 |
| R2 (adjusted) | 0.324 | |||
| Rao | t | |||
| intercept | 0.187 | 0.022 | ||
| pasture | −0.152 | 0.025 | −6.141 | <0.001 |
| abandonment | 0.109 | 0.024 | 4.504 | <0.001 |
| R2 (adjusted) | 0.356 | |||
| Country | Region | Individuals (Species) | Surveyed Habitats | Trapping | Reference |
|---|---|---|---|---|---|
| Romania | S Transylvania, Hârtibaciu Plateau and Făgăraș Piedmont | 991 (16) | corn, wheat and potato crops, fallow fields, pastures, abandoned pastures | 6707 (live traps) | present study |
| Romania | S Transylvania, Hârtibaciu Plateau | 136 (13) | mix of agricultural (hayfields, corn and potato crops) and non-agricultural (forests, clearcut, reedbed) habitats | 418 (live traps) | [41] |
| Romania | Central Transylvanian Plain | 200 (8) | sessile oak-hornbeam woodland, meadow, pasture, pasture with shrubs, oak woodland, forest edge | 1200 (live traps) | [42] |
| Romania | S Transylvania, Râul Negru River Basin | 192 (9) | cemetery, churchyard, river bank, crops | 1654 (live traps) | [18] |
| Slovakia | Nitra, SW Slovakia | 125 (5) | wheat, peas, and grass mixture crops in the conditions of ecological farming and barley, rapeseed, and maize crops | 32,850 (pitfall traps) | [6] |
| Poland | north-eastern Poland, near Łuknajno Biosphere Reserve | not mentioned (12) | mosaic of agricultural and forest habitats comprising mixed forest, lakeshore alder forest, wheat, oat, rye, maize, and potato crops, which were abandoned during the second half of the study period | not mentioned (live-traps in a 30-year study) | [20] |
| Czech Republic | S Moravian Pannonian lowland | 892 (9) | alfalfa, spring barley, maize, winter rape, sunflower, and winter wheat fields, steppe-type set-aside and old abandoned orchards in a mixed agricultural landscape | 35,900 (snap-traps) | [10] |
| Czech Republic | S Moravia | 5536 (14) | wheat, barley, maize, sugar beet, alfalfa, vegetables, tobacco, potatoes, sunflowers, windbreaks, small forests, fallow land, and vineyards. | 51,480 (snap-traps) | [23] |
| Germany | S Lower Saxony | 410 (11) | organic and conventional winter wheat field plots, covering a gradient of landscape complexity with arable land varying between 94% and 41% | 3960 (live traps) | [44] |
| Germany | NE Germany, Brandenburg | 455 (7) | Cereal fields, oilseed rape fields, grassland, forest, field margins, kettle holes | 3840 (live traps) | [67] |
| Switzerland | C Switzerland, Wauwil | 349 (6) | low-intensity meadows, wild-flower strips, and herbaceous strips, artificial grassland and autumn-sown wheat | 5400 (live traps) | [22] |
| Italy | NE Italy, Friuli Venezia Giulia region | 668 (4) | three study areas differentiated along a gradient of agricultural intensification | 4630 (live traps) | [8] |
| France | Brittany, south of the Mont Saint-Michel Bay | 1782 (8) | hedges, woods, and meadows | 19,584 (live traps) | [3] |
| France | Brittany, Mont Saint-Michel Bay | >1523 (10) | agricultural edge habitats, such as dykes and hedgerows in an intensively cultivated landscape on a reclaimed area (polders) | 9180 (live traps) | [21] |
| France | Brittany, south of the Mont-Saint-Michel Bay | 1379 (8) | 24 plots located in hedgerows | 11,424 (live traps) | [2] |
| Ukraine | Slobozhanshchyna (eastern Ukraine) | 380 (12) | ravine–gully systems, agroecosystems, field-protecting forest strips, riparian vegetation along artificial reservoirs and streams, dry meadows, and pastures | 4900 (live trap) | [46] |
| Russia | central part of European Russia: in the vicinity of the Bakanovo village, the Staritsa Region of Tver Oblast’ | 1869 (10) | abandoned crops and pasture | not explicit (18-year study using live traps) | [57] |
| Russia | southeast Tver Oblast on the right-hand bank of the Volga River within the Smolensk–Moscow Province | 4774 (16) | meadows, tillage and fallow land of various ages | not explicit (12-year study using live traps) | [40] |
| Republic of Moldova and Romania | central Moldova Bacău county | Bacău—11 species, Moldova—9 species | agroecosystems | not explicit | [68] |
| Republic of Moldova | central Moldova | 9395 (13) | different types of biotopes, varying in their degree of heterogeneity and human impact | not explicit | [47] |
References
- Green, R.E.; Cornell, S.J.; Scharlemann, J.P.W.; Balmford, A. Farming and the Fate of Wild Nature. Science 2005, 307, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Michel, N.; Burel, F.; Legendre, P.; Butet, A. Role of Habitat and Landscape in Structuring Small Mammal Assemblages in Hedgerow Networks of Contrasted Farming Landscapes in Brittany, France. Landsc. Ecol. 2007, 22, 1241–1253. [Google Scholar] [CrossRef]
- Butet, A.; Michel, N.; Rantier, Y.; Comor, V.; Hubert-Moy, L.; Nabucet, J.; Delettre, Y. Responses of Common Buzzard (Buteo buteo) and Eurasian Kestrel (Falco tinnunculus) to Land Use Changes in Agricultural Landscapes of Western France. Agric. Ecosyst. Environ. 2010, 138, 152–159. [Google Scholar] [CrossRef]
- van Zanten, B.T.; Verburg, P.H.; Espinosa, M.; Gomez-y-Paloma, S.; Galimberti, G.; Kantelhardt, J.; Kapfer, M.; Lefebvre, M.; Manrique, R.; Piorr, A.; et al. European Agricultural Landscapes, Common Agricultural Policy and Ecosystem Services: A Review. Agron. Sustain. Dev. 2014, 34, 309–325. [Google Scholar] [CrossRef]
- Overmars, K.P.; Helming, J.; van Zeijts, H.; Jansson, T.; Terluin, I. A Modelling Approach for the Assessment of the Effects of Common Agricultural Policy Measures on Farmland Biodiversity in the EU27. J. Environ. Manag. 2013, 126, 132–141. [Google Scholar] [CrossRef]
- Langraf, V.; Petrovičová, K.; Schlarmannová, J.; Brygadyrenko, V. The Spatial Structure of Small Mammals (Eulipotyphla, Rodentia) in Ecological and Conventional Farming Conditions. Agrology 2022, 5, 49–54. [Google Scholar]
- Duelli, P.; Obrist, M.K. Regional Biodiversity in an Agricultural Landscape: The Contribution of Seminatural Habitat Islands. Basic Appl. Ecol. 2003, 4, 129–138. [Google Scholar] [CrossRef]
- Gentili, S.; Sigura, M.; Bonesi, L. Decreased Small Mammals Species Diversity and Increased Population Abundance along a Gradient of Agricultural Intensification. Hystrix It. J. Mamm. 2014, 25, 39–44. [Google Scholar] [CrossRef]
- Nadeem, F.; Nawaz, A.; Farooq, M. Crop Rotations, Fallowing, and Associated Environmental Benefits. In Oxford Research Encyclopedia of Environmental Science; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Janova, E.; Heroldova, M. Response of Small Mammals to Variable Agricultural Landscapes in Central Europe. Mamm. Biol. 2016, 81, 488–493. [Google Scholar] [CrossRef]
- Tscharntke, T.; Batáry, P.; Dormann, C.F. Set-aside Management: How Do Succession, Sowing Patterns and Landscape Context Affect Biodiversity? Agric. Ecosyst. Environ. 2011, 143, 37–44. [Google Scholar] [CrossRef]
- Donald, P.F.; Pisano, G.; Rayment, M.D.; Pain, D.J. The Common Agricultural Policy, EU Enlargement and the Conservation of Europe’s Farmland Birds. Agric. Ecosyst. Environ. 2002, 89, 167–182. [Google Scholar] [CrossRef]
- Dorresteijn, I.; Loos, J.; Hanspach, J.; Fischer, J. Socioecological Drivers Facilitating Biodiversity Conservation in Traditional Farming Landscapes. Ecosyst. Health Sustain. 2015, 1, 11879002. [Google Scholar] [CrossRef]
- Culbert, P.D.; Dorresteijn, I.; Loos, J.; Clayton, M.K.; Fischer, J.; Kuemmerle, T. Legacy Effects of Past Land Use on Current Biodiversity in a Low-Intensity Farming Landscape in Transylvania (Romania). Landsc. Ecol. 2017, 32, 429–444. [Google Scholar] [CrossRef]
- Fischer, J.; Hartel, T.; Kuemmerle, T. Conservation Policy in Traditional Farming Landscapes. Conserv. Lett. 2012, 5, 167–175. [Google Scholar] [CrossRef]
- Tscharntke, T.; Klein, A.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape Perspectives on Agricultural Intensification and Biodiversity—Ecosystem Service Management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Benedek, A.M.; Sîrbu, I. Responses of Small Mammal Communities to Environment and Agriculture in a Rural Mosaic Landscape. Mamm. Biol. 2018, 90, 55–65. [Google Scholar] [CrossRef]
- Lazăr, A.; Benedek, A.M. Small Mammal (Mammalia, Orders Soricomorpha and Rodentia) Communities in the Lower Black River Basin, Romania. Trav. Du Mus. Natl. Hist. Nat. Grigore Antipa 2019, 62, 125–135. [Google Scholar] [CrossRef]
- Burja, C.; Burja, V. Farm Size and Efficiency of the Production Factors in Romanian Agriculture. Ekon. Polj. 2016, 63, 361–374. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Kozakiewicz, A. Long-Term Dynamics and Biodiversity Changes in Small Mammal Communities in a Mosaic of Agricultural and Forest Habitats. Ann. Zool. Fenn. 2008, 45, 263–269. [Google Scholar] [CrossRef]
- Butet, A.; Paillat, G.; Delettre, Y. Factors Driving Small Rodents Assemblages from Field Boundaries in Agricultural Landscapes of Western France. Landsc. Ecol. 2006, 21, 449–461. [Google Scholar] [CrossRef]
- Aschwanden, J.; Holzgang, O.; Jenni, L. Importance of Ecological Compensation Areas for Small Mammals in Intensively Farmed Areas. Wildl. Biol. 2007, 13, 150–158. [Google Scholar] [CrossRef]
- Heroldová, M.; Bryja, J.; Zejda, J.; Tkadlec, E. Structure and Diversity of Small Mammal Communities in Agriculture Landscape. Agric. Ecosyst. Environ. 2007, 120, 206–210. [Google Scholar] [CrossRef]
- Li, J.; Dirzo, R.; Wang, Y.; Zeng, D.; Liu, J.; Ren, P.; Zhong, L.; Ding, P. Rapid Morphological Change in a Small Mammal Species after Habitat Fragmentation over the Past Half-Century. Divers. Distrib. 2021, 27, 2615–2628. [Google Scholar] [CrossRef]
- Paniccia, C.; Laura Carranza, M.; Frate, L.; Di Febbraro, M.; Rocchini, D.; Loy, A. Distribution and Functional Traits of Small Mammals across the Mediterranean Area: Landscape Composition and Structure Definitively Matter. Ecol. Indic. 2022, 135, 108550. [Google Scholar] [CrossRef]
- Hantak, M.M.; McLean, B.S.; Li, D.; Guralnick, R.P. Mammalian Body Size Is Determined by Interactions between Climate, Urbanization, and Ecological Traits. Commun. Biol. 2021, 4, 972. [Google Scholar] [CrossRef]
- Șoneriu, I.; Grecu, F. Podișul Hîrtibaciului. In Carpații Românești și Depresiunea Transilvaniei; Geografia României; Academia Republicii Socialiste România: Bucharest, Romania, 1987; Volume 3, pp. 578–590. [Google Scholar]
- QGIS Association. QGIS Geographic Information System, Version 3.36.1; Open Source Geospatial Foundation: Chicago, IL, USA, 2025; Available online: https://qgis.org/ (accessed on 10 June 2025).
- Popescu, N.; Dragu, G. Depresiunea Făgărașului. In Carpații Românești și Depresiunea Transilvaniei; Geografia României; Academia Republicii Socialiste România: Bucharest, Romania, 1987; Volume 3, pp. 603–610. [Google Scholar]
- Aulagnier, S.; Haffner, P.; Mitchell-Jones, A.J.; Moutou, F.; Zima, J. Mammals of Europe, North Africa and the Middle East; A. & C. Black, Bloomsbury Publishing: London, UK, 2009; ISBN 978-1-4081-1399-8. [Google Scholar]
- Soria, C.; Pacifici, M.; Di Marco, M.; Beard, S.; Rondinini, C. COMBINE: A Coalesced Mammal Database of Intrinsic and Extrinsic Traits. Ecology 2021, 102, e03344. [Google Scholar] [CrossRef]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO 5, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; ISBN 978-1-107-69440-8. [Google Scholar]
- De Bello, F.; Thuiller, W.; Leps, J.; Choler, P.; Clement, J.C.; Macek, P.; Sebastia, M.T.; Lavorel, S. Partitioning of Functional Diversity Reveals the Scale and Extent of Trait Convergence and Divergence. J. Veg. Sci. 2009, 20, 475–486. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package 2024. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 12 January 2025).
- R Core Development Team. R: The R Project for Statistical Computing, Version 3.6.1. 2019. Available online: https://www.r-project.org/ (accessed on 21 October 2019).
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.10; Microcomputer Power: Ithaca, NY, USA, 2018. [Google Scholar]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: Interpolation and Extrapolation for Species Diversity 2024. Available online: https://rdrr.io/cran/iNEXT/ (accessed on 12 January 2025).
- Nakagawa, S.; Johnson, P.C.D.; Schielzeth, H. The Coefficient of Determination R2 and Intra-Class Correlation Coefficient from Generalized Linear Mixed-Effects Models Revisited and Expanded. J. R. Soc. Interface 2017, 14, 20170213. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference (Version 1.47.5). 2023. Available online: https://www.rdocumentation.org/packages/MuMIn/versions/1.47.5 (accessed on 9 April 2023).
- Shchipanov, N.A.; Kouptsov, A.V.; Kalinin, A.A.; Demidova, T.B.; Oleinichenko, V.Y.; Lyapina, M.G.; Aleksandrov, D.Y.; Raspopova, A.A.; Pavlova, S.V.; Tumasyan, P.A. Small Mammals of the Southeast Tver Oblast. Communication 1. The Fauna and Biotopic Distribution. Contemp. Probl. Ecol. 2010, 3, 587–592. [Google Scholar] [CrossRef]
- Benedek, A.M. Small Mammals (Ordo Insectivora and Ordo Rodentia) from Agnita—Sighişoara Area (Romania). Trasylv. Rev. Syst. Ecol. Res. 2007, 4, 187–198. [Google Scholar]
- Sevianu, E.; Coroiu, I. Small Mammals of Central Transylvania Plain: Species Composition, Diversity and Abundance. Stud. Univ. Babes-Bolyai Biol. 2004, 49, 19–31. [Google Scholar]
- Benedek, A. Comunități de mamifere mici (Ordinele Soricomorpha și Rodentia) din Transilvania; Editura Universităţii “Lucian Blaga” din Sibiu: Sibiu, Romania, 2014; ISBN 978-606-12-0786-2. [Google Scholar]
- Fischer, C.; Thies, C.; Tscharntke, T. Small Mammals in Agricultural Landscapes: Opposing Responses to Farming Practices and Landscape Complexity. Biol. Conserv. 2011, 144, 1130–1136. [Google Scholar] [CrossRef]
- Rodríguez, C.; Peris, S. Habitat Associations of Small Mammals in Farmed Landscapes: Implications for Agri-Environmental Schemes. Anim. Biol. 2007, 57, 301–314. [Google Scholar] [CrossRef]
- Markovska, O. Small Mammals in Natural and Agricultural Lands of Slobozhanshchyna (Eastern Ukraine): Results of a Five-Year-Long Survey. GEO&BIO 2022, 2022, 143–153. [Google Scholar] [CrossRef]
- Sîtnic, V.; Munteanu, A.; Savin, A.; Nistreanu, V.; Larion, A. Structura și diversitatea comunităților de rozătoare sub impactul transformărilor socio-umane și schimbărilor climatice din Republica Moldova. Bul. Acad. Ştiinţe Moldovei. Ştiinţele Vieţii 2018, 336, 137–144. [Google Scholar]
- Torre, I.; Díaz, M.; Martínez-Padilla, J.; Bonal, R.; Viñuela, J.; Fargallo, J.A. Cattle Grazing, Raptor Abundance and Small Mammal Communities in Mediterranean Grasslands. Basic Appl. Ecol. 2007, 8, 565–575. [Google Scholar] [CrossRef]
- Cao, C.; Shuai, L.-Y.; Xin, X.-P.; Liu, Z.-T.; Song, Y.-L.; Zeng, Z.-G. Effects of Cattle Grazing on Small Mammal Communities in the Hulunber Meadow Steppe. PeerJ 2016, 4, e2349. [Google Scholar] [CrossRef] [PubMed]
- Marás, G.A.; Trucco, C.E.; Nuñez-Reguiro, M.M.; Andrade-Díaz, M.S.; Trigo, C.B.; Caruso, M.F.; Derlindati, E.J.; Tálamo, A. Relationships between Livestock Grazing Intensity and Mammal Predator-Prey: A Study Case in Copo National Park in the Dry Chaco Forests. J. Nat. Conserv. 2022, 67, 126186. [Google Scholar] [CrossRef]
- Bock, C.E.; Bock, J.H.; Kenney, W.R.; Hawthorne, V.M. Responses of Birds, Rodents, and Vegetation to Livestock Exclosure in a Semidesert Grassland Site. J. Range Manag. 1984, 37, 239. [Google Scholar] [CrossRef]
- Eschen, R.; Brook, A.J.; Maczey, N.; Bradbury, A.; Mayo, A.; Watts, P.; Buckingham, D.; Wheeler, K.; Peach, W.J. Effects of Reduced Grazing Intensity on Pasture Vegetation and Invertebrates. Agric. Ecosyst. Environ. 2012, 151, 53–60. [Google Scholar] [CrossRef]
- Schmidt, N.M.; Olsen, H.; Leirs, H. Livestock Grazing Intensity Affects Abundance of Common Shrews (Sorex Araneus) in Two Meadows in Denmark. BMC Ecol 2009, 9, 2. [Google Scholar] [CrossRef]
- Schmidt, N.M.; Olsen, H.; Bildsøe, M.; Sluydts, V.; Leirs, H. Effects of Grazing Intensity on Small Mammal Population Ecology in Wet Meadows. Basic Appl. Ecol. 2005, 6, 57–66. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Čepukienė, A.; Balčiauskienė, L. Small Mammal Community Response to Early Meadow–Forest Succession. Ecosyst 2017, 4, 11. [Google Scholar] [CrossRef]
- Neilly, H.; Ward, M.; Cale, P. Converting Rangelands to Reserves: Small Mammal and Reptile Responses 24 Years after Domestic Livestock Grazing Removal. Austral Ecol. 2021, 46, 1112–1124. [Google Scholar] [CrossRef]
- Shchipanov, N.A.; Tumasian, P.A.; Kuptsov, A.V.; Raspopova, A.A.; Kasatkin, M.V.; Kalinin, A.A.; Demidova, T.B.; Pavlova, S.V. Different Threshold Responses in Spontaneously Changing Postagrogenic Forest and Unmanaged Grassland. Shifts in Small Mammal Populations and Communities Triggered by an Extraordinary Drought. Environ. Monit Assess 2025, 197, 234. [Google Scholar] [CrossRef]
- Thomas, S.M.; Soka, G.E.; Mulungu, L.S. Influence of Vegetation Structure, Seasonality, and Soil Properties on Rodent Diversi Community Assemblages in West Mount Kilimanjaro, Tanzania. Ecol. Evol. 2022, 12, e9211. [Google Scholar] [CrossRef]
- Beca, G. Conservation Status of Bioturbator Mammals and Their Potential Role in Restoring Degraded Agricultural Landscapes. Ph.D. Thesis, The University of Western Australia, Perth, Australia, 2021. [Google Scholar]
- Beca, G.; Valentine, L.E.; Galetti, M.; Hobbs, R.J. Ecosystem Roles and Conservation Status of Bioturbator Mammals. Mamm. Rev. 2022, 52, 192–207. [Google Scholar] [CrossRef]
- Collins, S.L.; Uno, G.E. Seed Predation, Seed Dispersal, and Disturbance in Grasslands: A Comment. Am. Nat. 1985, 125, 866–872. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, Y.; Zhang, P.; Zhang, H.; Baskin, C.C.; Zhang, T.; Chen, Y.; Hu, G.; Yang, X.; Mao, H.; et al. Rodents Mediate the Relationship between Seed Rain, Seed Bank, and Plant Community with Increased Grazing Disturbance. Ecol. Appl. 2024, 34, e2984. [Google Scholar] [CrossRef]
- Bricker, M.; Pearson, D.; Maron, J. Small-Mammal Seed Predation Limits the Recruitment and Abundance of Two Perennial Grassland Forbs. Ecology 2010, 91, 85–92. [Google Scholar] [CrossRef]
- Lukyanova, L.E.; Ukhova, N.L.; Ukhova, O.V.; Gorodilova, Y.V. Common Shrew (Sorex Araneus, Eulipotyphla) Population and the Food Supply of Its Habitats in Ecologically Contrasting Environments. Russ. J. Ecol. 2021, 52, 316–328. [Google Scholar] [CrossRef]
- Moro, D.; Gadal, S. Benefits of Habitat Restoration to Small Mammal Diversity and Abundance in a Pastoral Agricultural Landscape in Mid-Wales. Biodivers. Conserv. 2007, 16, 3543–3557. [Google Scholar] [CrossRef]
- Google. Google Earth Pro, Version 5.0. 2009. Available online: https://www.google.com/earth (accessed on 7 March 2020).
- Fischer, C.; Schröder, B. Predicting Spatial and Temporal Habitat Use of Rodents in a Highly Intensive Agricultural Area. Agric. Ecosyst. Environ. 2014, 189, 145–153. [Google Scholar] [CrossRef]
- Nistreanu, V.; Paraschiv, D.; Larion, A. Rodent Species in Agricultural Ecosystems from the Central Part of the Republic of Moldova and Bacău County, Romania. One Health Risk Manag. Special edition November 2023, article 69. Available online: https://journal.ohrm.bba.md/index.php/journal-ohrm-bba-md/article/view/649 (accessed on 10 June 2025).

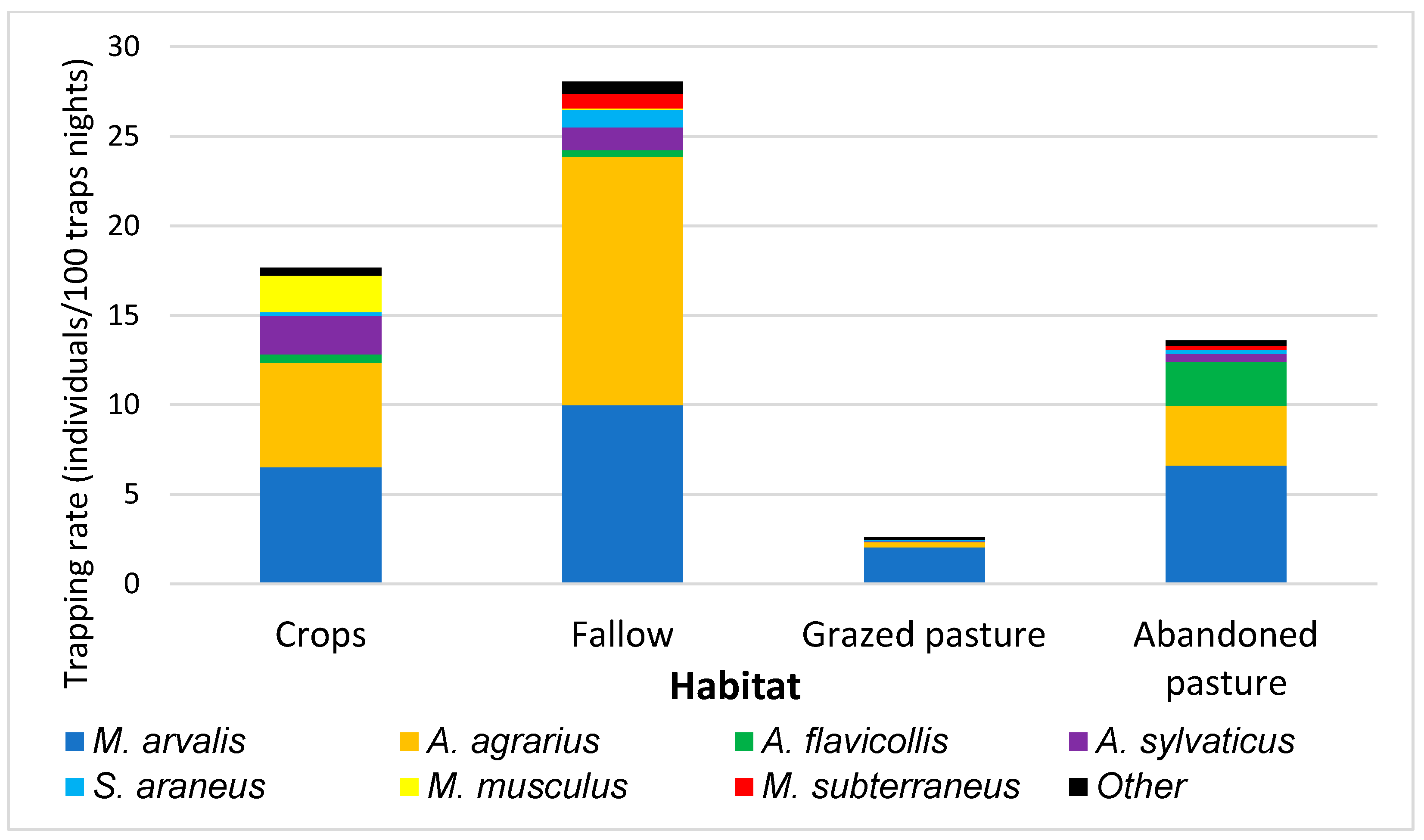
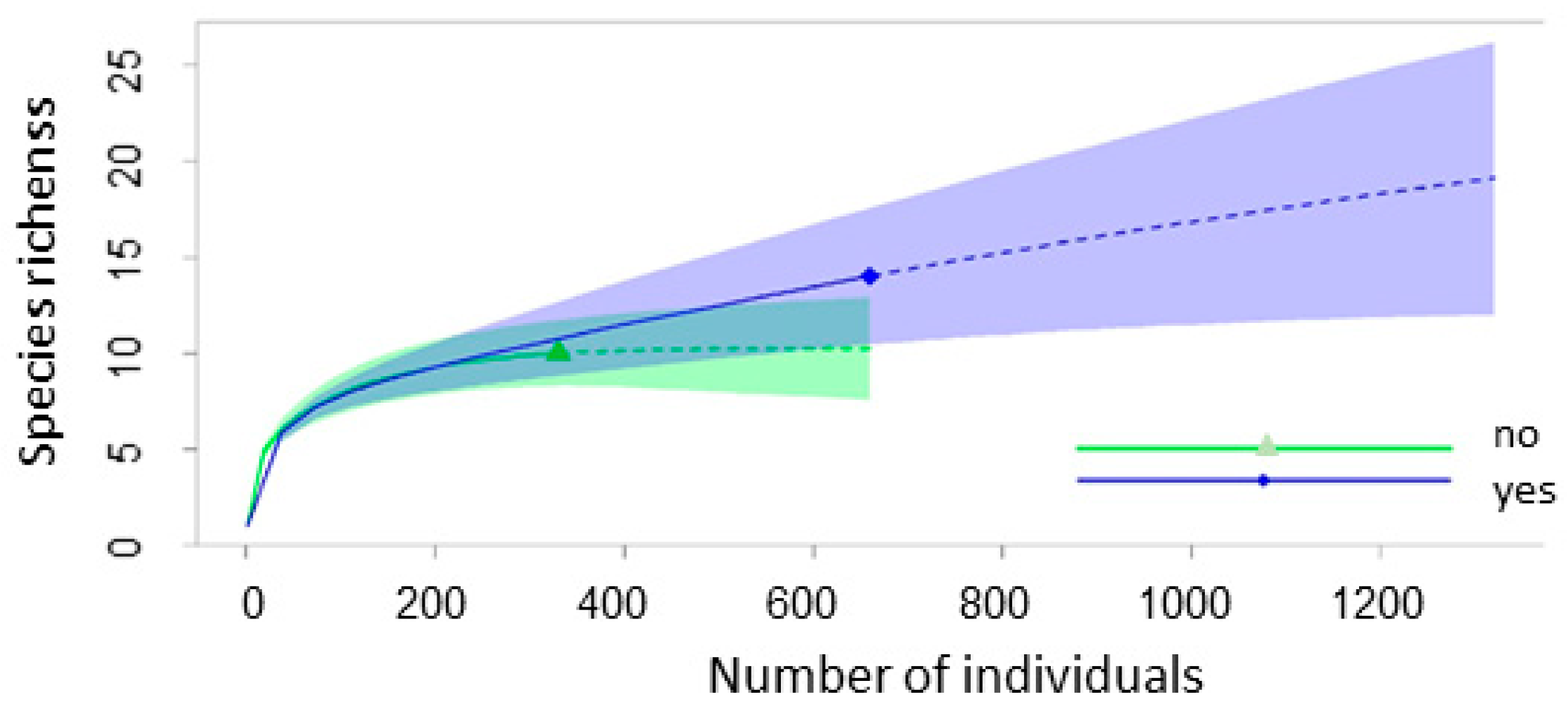
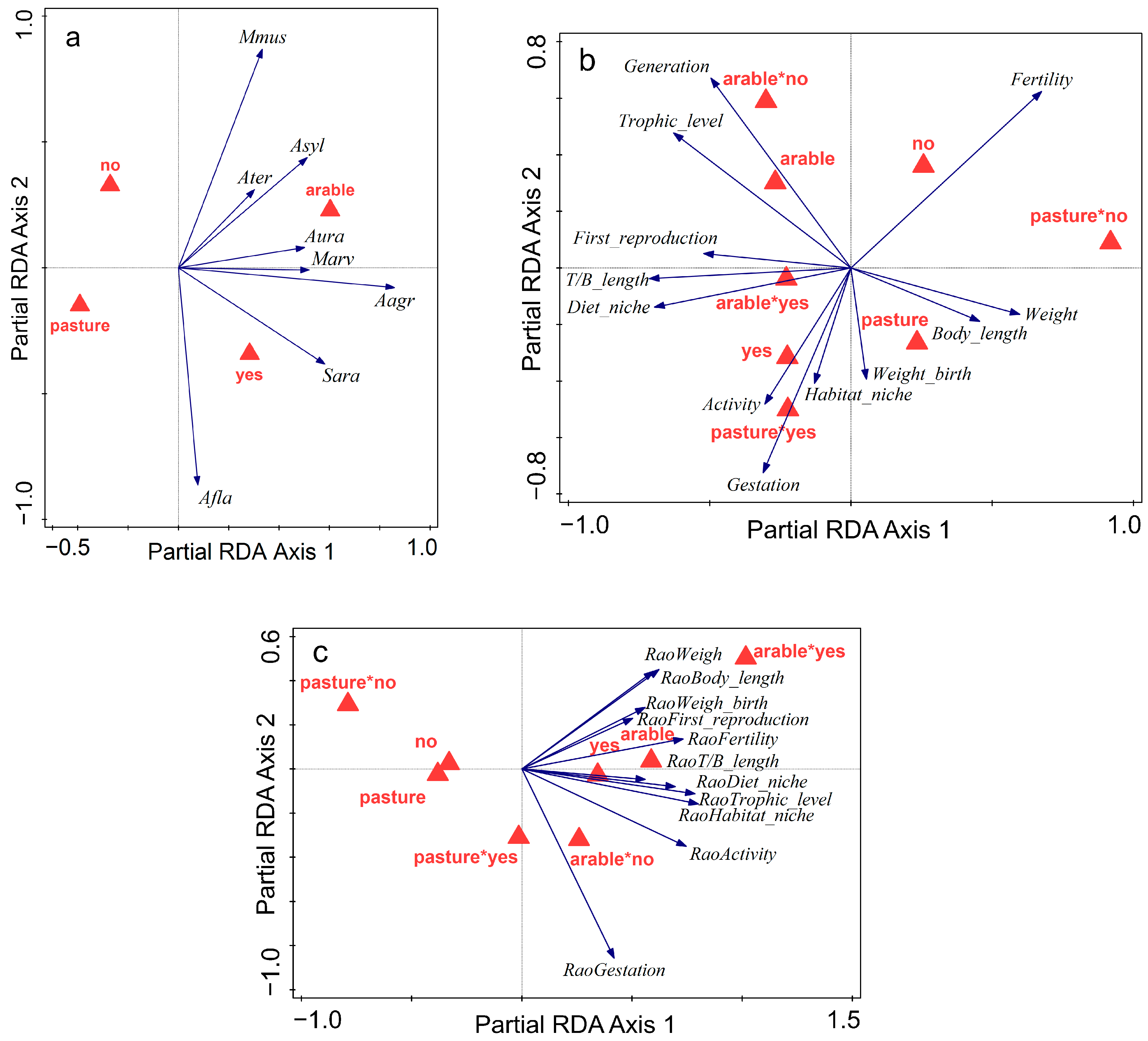
| Season | Crops | Fallow | Grazed Pasture | Abandoned Pasture | Total |
|---|---|---|---|---|---|
| Autumn 2010 | 258 (5) | 412 (6) | 348 (6) | 487 (7) | 1505 (24) |
| Summer 2011 | 737 (9) | 485 (5) | 511 (7) | 643 (11) | 2826 (32) |
| Autumn 2011 | 618 (7) | 436 (5) | 864 (14) | 908 (12) | 2376 (38) |
| Total | 1613 (21) | 1333 (16) | 1723 (27) | 2038 (30) | 6707 (94) |
| Species | Arable Land | Pasture | Total | ||
|---|---|---|---|---|---|
| No | Yes | No | Yes | ||
| Microtus arvalis | 105 | 133 | 35 | 135 | 408 |
| Apodemus agrarius | 94 | 185 | 5 | 68 | 352 |
| Apodemus flavicollis | 8 | 5 | 0 | 59 | 72 |
| Apodemus sylvaticus | 35 | 17 | 1 | 9 | 62 |
| Sorex araneus | 3 | 13 | 1 | 5 | 22 |
| Arvicola terrestris | 1 | 2 | 1 | 0 | 4 |
| Mus musculus | 33 | 1 | 0 | 0 | 34 |
| Apodemus uralensis | 5 | 5 | 0 | 3 | 13 |
| Microtus subterraneus | 0 | 11 | 0 | 4 | 15 |
| Micromys minutus | 0 | 1 | 0 | 0 | 1 |
| Sorex minutus | 0 | 0 | 2 | 0 | 2 |
| Crocidura suaveolens | 1 | 0 | 0 | 0 | 1 |
| Crocidura leucodon | 0 | 0 | 0 | 1 | 1 |
| Clethrionomys glareolus | 0 | 0 | 0 | 1 | 1 |
| Muscardinus avellanarius | 0 | 0 | 0 | 1 | 1 |
| Rattus norvegicus | 0 | 1 | 0 | 0 | 1 |
| Number of species | 9 | 11 | 6 | 10 | 16 |
| Number of species per transect (min-max) | 0–6 | 2–7 | 0–2 | 0–4 | 0–7 |
| Chao (min–max) | 0–6 | 2–8 | 0–3 | 0–8 | 0–8 |
| Shannon (mean value per transect) | 0.709 | 0.881 | 0.111 | 0.466 | |
| Rao (mean per transect) | 0.207 | 0.291 | 0.042 | 0.156 | |
| Number of individuals | 285 | 375 | 45 | 286 | 991 |
| Total abundance (individuals/100 trap-nights) | 17.7 | 28.1 | 2.6 | 14 | 14.8 |
| Coefficients | Estimate | Std. Error | Test Statistic | p |
|---|---|---|---|---|
| abundance | z | |||
| intercept | 1.791 | 0.37 | ||
| pasture | −2.23 | 0.322 | −6.194 | <0.001 |
| abandonment | 0.319 | 0.299 | 1.065 | 0.286 |
| pasture * abandonment | 1.429 | 0.429 | 10.29 | <0.001 |
| marginal (and conditional) R2 | 0.462 (0.733) | |||
| species richness | t | |||
| intercept | 2.611 | 0.248 | ||
| pasture | −1.827 | 0.281 | −6.5 | <0.001 |
| abandonment | 1.211 | 0.274 | 4.407 | <0.001 |
| R2 (adjusted) | 0.371 | |||
| Chao | t | |||
| intercept | 2.929 | 0.307 | ||
| pasture | −2.097 | 0.348 | −6.024 | <0.001 |
| abandonment | 1.412 | 0.34 | 4.152 | <0.001 |
| R2 (adjusted) | 0.337 | |||
| H | t | |||
| intercept | 0.66 | 0.072 | ||
| pasture | −0.512 | 0.082 | −6.225 | <0.001 |
| abandonment | 0.284 | 0.08 | 3.351 | <0.001 |
| R2 (adjusted) | 0.329 | |||
| Rao | t | |||
| intercept | 0.256 | 0.027 | ||
| pasture | −0.202 | 0.031 | −6.441 | <0.001 |
| abandonment | 0.135 | 0.03 | 4.421 | <0.001 |
| R2 (adjusted) | 0.368 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazăr, A.; Sandu, M.A.; Benedek, A.M.; Sîrbu, I. Structural and Functional Responses of Small Mammal Communities to Land Abandonment in a Region of High Biodiversity. Animals 2025, 15, 1857. https://doi.org/10.3390/ani15131857
Lazăr A, Sandu MA, Benedek AM, Sîrbu I. Structural and Functional Responses of Small Mammal Communities to Land Abandonment in a Region of High Biodiversity. Animals. 2025; 15(13):1857. https://doi.org/10.3390/ani15131857
Chicago/Turabian StyleLazăr, Anamaria, Marcela Alexandra Sandu, Ana Maria Benedek, and Ioan Sîrbu. 2025. "Structural and Functional Responses of Small Mammal Communities to Land Abandonment in a Region of High Biodiversity" Animals 15, no. 13: 1857. https://doi.org/10.3390/ani15131857
APA StyleLazăr, A., Sandu, M. A., Benedek, A. M., & Sîrbu, I. (2025). Structural and Functional Responses of Small Mammal Communities to Land Abandonment in a Region of High Biodiversity. Animals, 15(13), 1857. https://doi.org/10.3390/ani15131857










