Oxidative Stress Mediates the Dual Regulatory Effects of Bovine Uterine ECM Remodeling Through the TGF-β1/Smad3 Pathway: Molecular Mechanisms of MMPs and COL-IV Imbalances
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Cell Culture and Treatment
2.3. RNA Isolation and RT-qPCR
2.4. Protein Extraction and Western Blot
2.5. Translation
2.6. Immunohistochemistry (IHC)
2.7. Immunofluorescence (IF)
2.8. ROS Detection
2.9. Statistical Analysis
3. Results
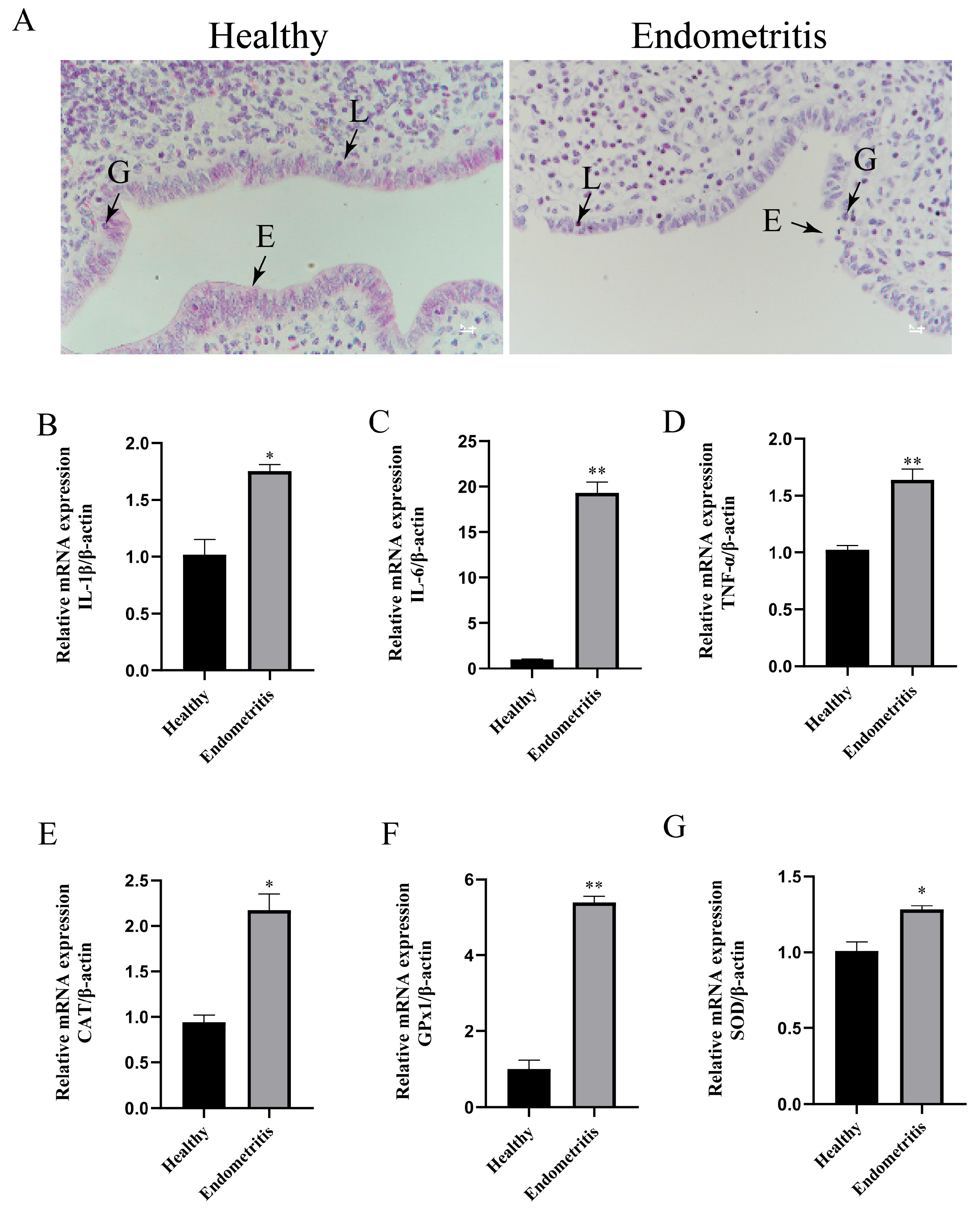
3.1. Detection of Inflammatory and Oxidative-Stress-Related Factors in Bovine Uterine Tissues
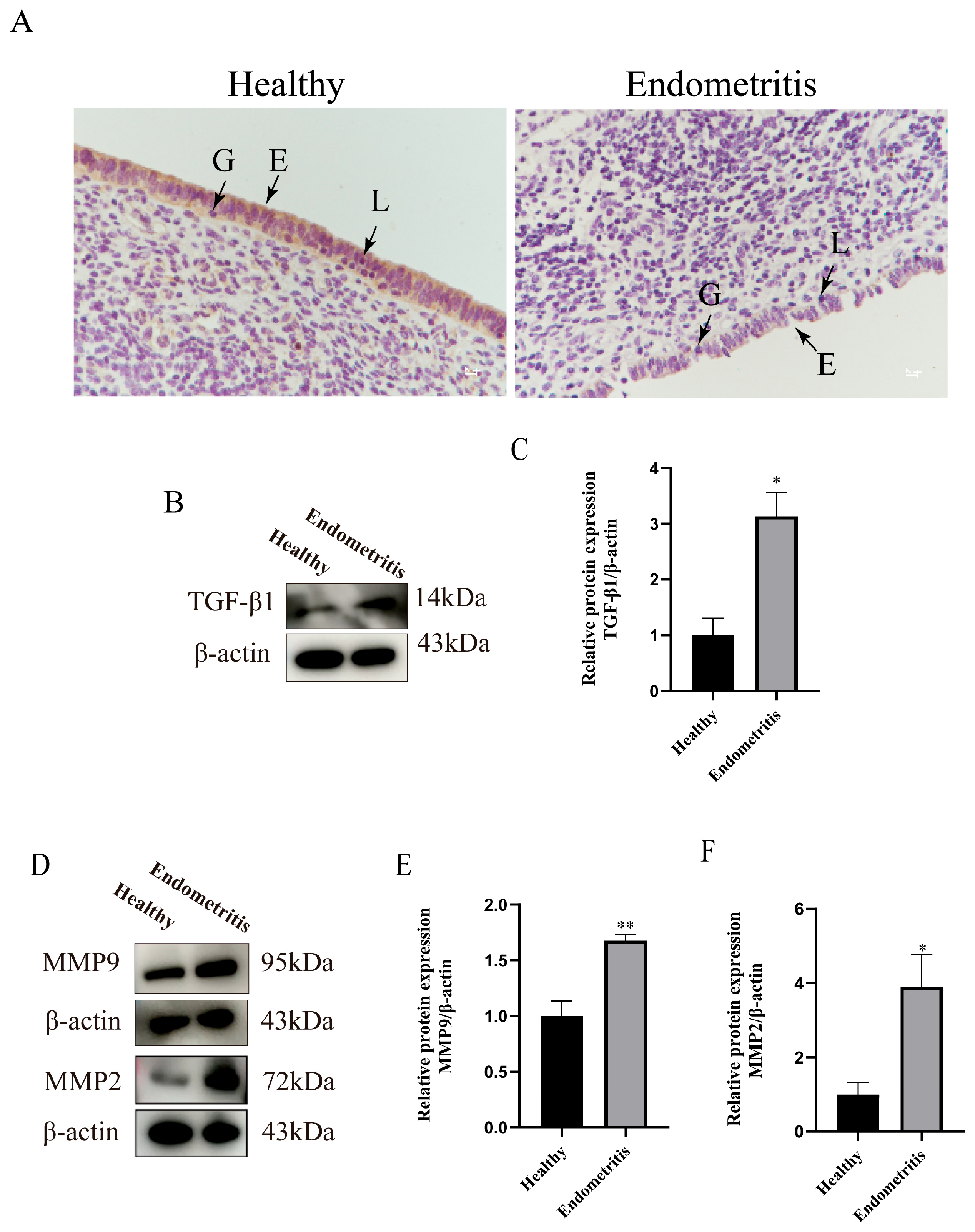
3.2. Detection of TGF-β1/Smad3 Signaling Pathway and ECM-Remodeling-Related Factors in Bovine Uterine Tissues
3.3. H2O2 Activates Inflammation and Oxidative Stress in bEECs
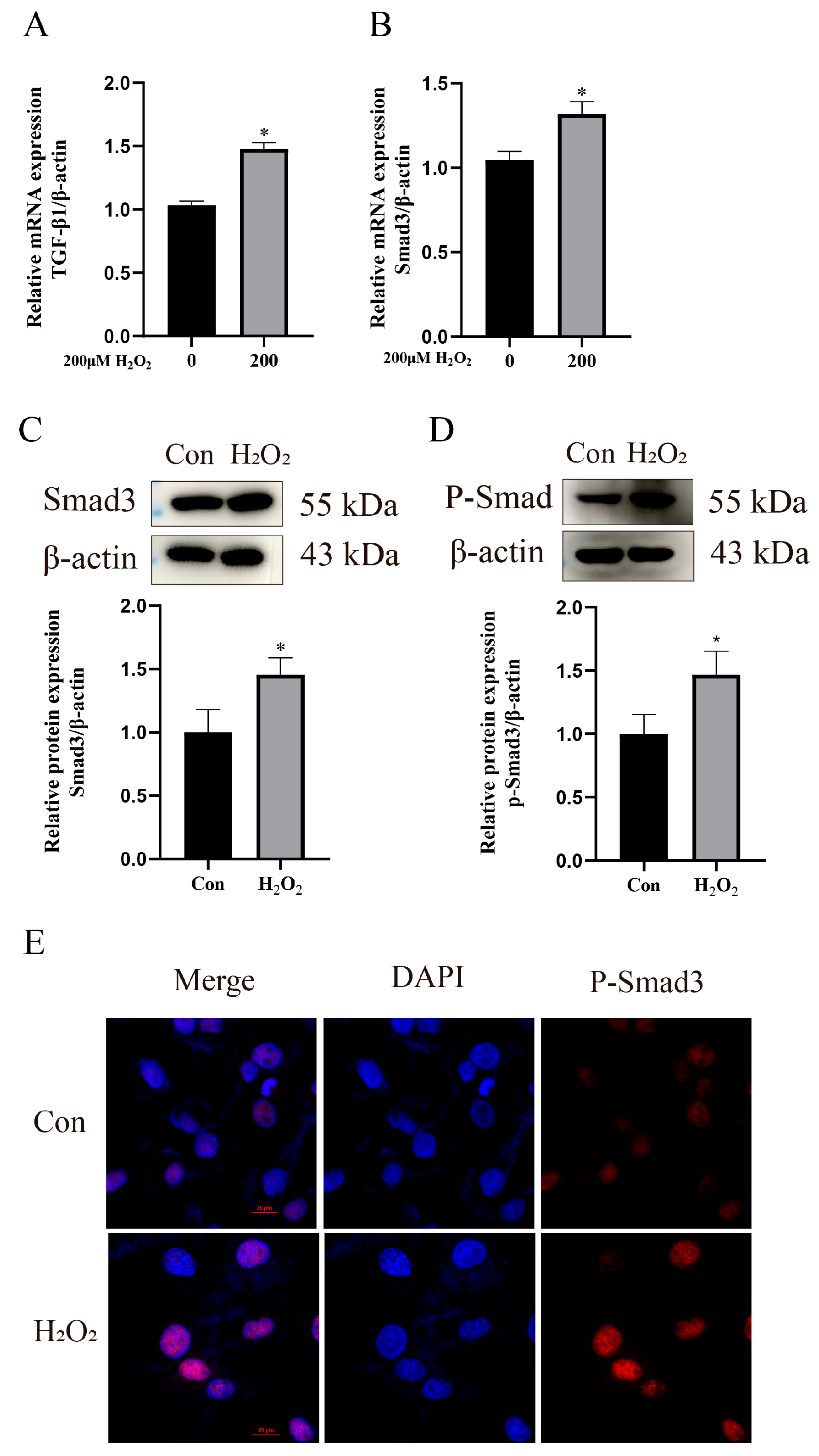
3.4. H2O2 Activates the TGF-β1/Smad3 Pathway to Enhance ECM Remodeling
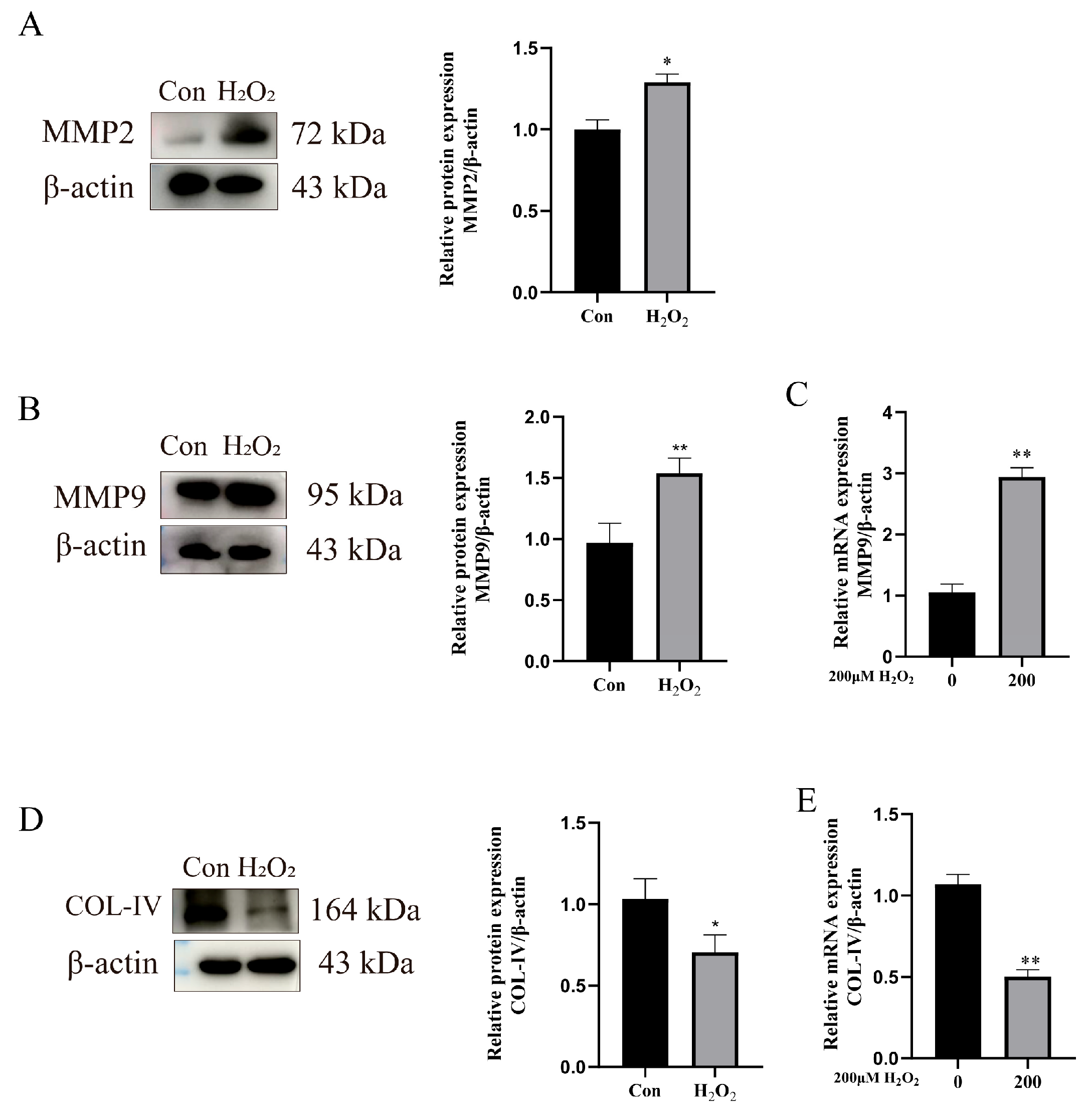
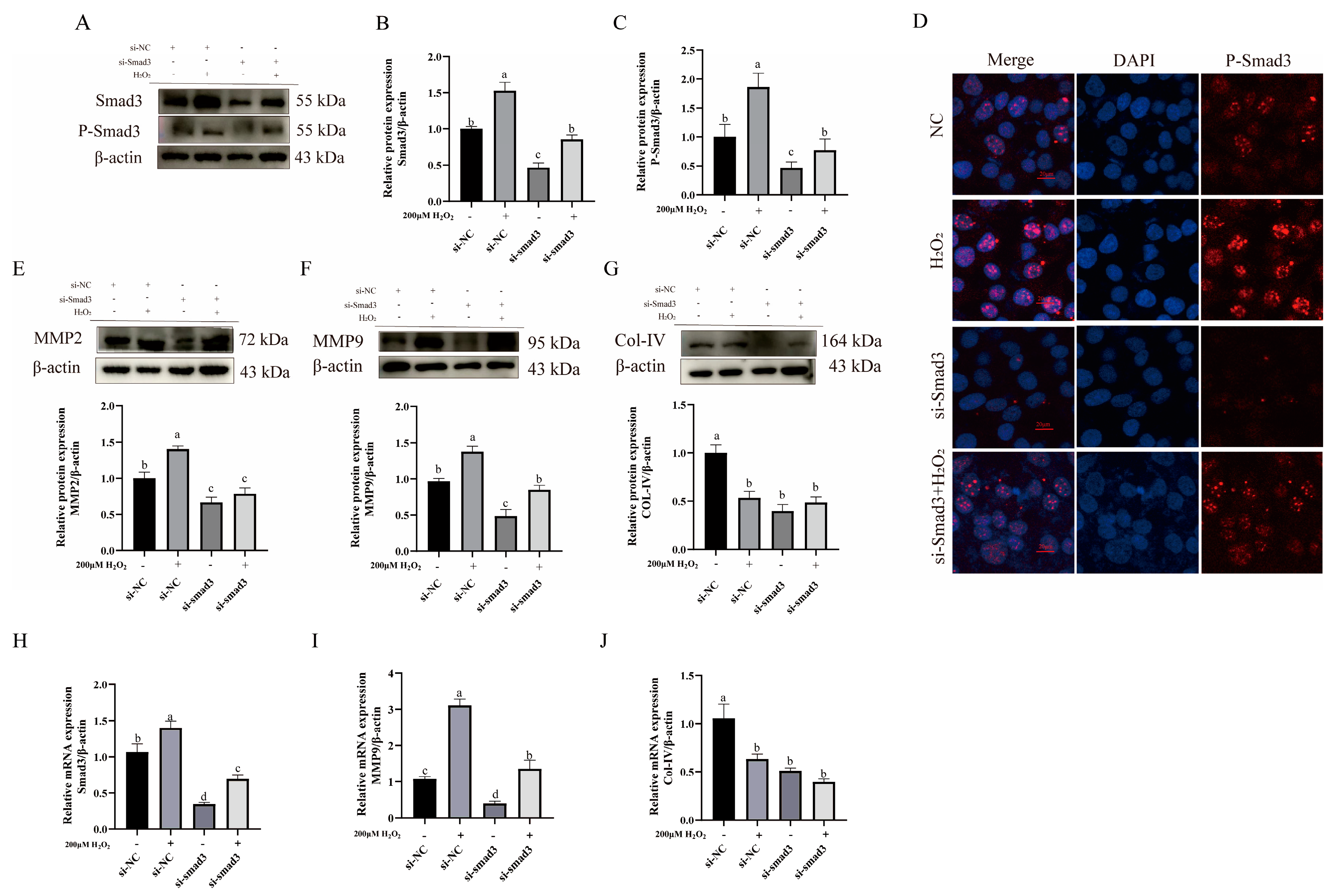
3.5. Impact of TGF-β1/Smad3 Pathway Expression on ECM Remodeling-Related Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Yao, X.; Xie, T.; Chang, Z.; Guo, Y.; Ni, H. Exosome-derived Uterine MiR-218 Isolated from Cows with Endometritis Regulates the Release of Cytokines and Chemokines. Microb. Biotechnol. 2020, 13, 1103–1117. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Augustine, R.A.; Iremonger, K.J.; Brown, C.H. Central Kisspeptin Does Not Affect ERK1/2 or P38 Phosphorylation in Oxytocin Neurons of Late-Pregnant Rats. Int. J. Mol. Sci. 2022, 23, 7729. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, X.; Li, B.; Sun, Y.; Jin, T.; Feng, M.; Ni, Y.; Liu, M. Lactobacillus Rhamnosus GR-1 Alleviates Escherichia Coli-Induced Inflammation via NF-ΚB and MAPKs Signaling in Bovine Endometrial Epithelial Cells. Front. Cell. Infect. Microbiol. 2022, 12, 809674. [Google Scholar] [CrossRef] [PubMed]
- Getahun, A.M.; Hunderra, G.C.; Gebrezihar, T.G.; Boru, B.G.; Desta, N.T.; Ayana, T.D. Comparative Study on Lesions of Reproductive Disorders of Cows and Female Dromedary Camels Slaughtered at Addis Ababa, Adama and Akaki Abattoirs with Bacterial Isolation and Characterization. BMC Vet. Res. 2021, 17, 134. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Barve, K.; Addepalli, V.; Utpat, S.V.; Kulkarni, Y.A. Triphala Churna—A Traditional Formulation in Ayurveda Mitigates Diabetic Neuropathy in Rats. Front. Pharmacol. 2021, 12, 662000. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. Int. J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [CrossRef]
- Jha, A.K.; Yang, W.; Kirn-Safran, C.B.; Farach-Carson, M.C.; Jia, X. Perlecan Domain I-Conjugated, Hyaluronic Acid-Based Hydrogel Particles for Enhanced Chondrogenic Differentiation via BMP-2 Release. Biomaterials 2009, 30, 6964–6975. [Google Scholar] [CrossRef]
- Ullah, I.; Busch, J.F.; Rabien, A.; Ergün, B.; Stamm, C.; Knosalla, C.; Hippenstiel, S.; Reinke, P.; Kurtz, A. Adult Tissue Extracellular Matrix Determines Tissue Specification of Human IPSC-Derived Embryonic Stage Mesodermal Precursor Cells. Adv. Sci. 2020, 7, 1901198. [Google Scholar] [CrossRef]
- Aiello, G.; Rescigno, F.; Meloni, M.; Baron, G.; Aldini, G.; Carini, M.; D’Amato, A. Oxidative Stress Modulation by Carnosine in Scaffold Free Human Dermis Spheroids Model: A Proteomic Study. Int. J. Mol. Sci. 2022, 23, 1468. [Google Scholar] [CrossRef]
- Vanderstichele, S.; Vranckx, J.J. Anti-Fibrotic Effect of Adipose-Derived Stem Cells on Fibrotic Scars. World J. Stem Cells 2022, 14, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.-W.; Wang, J.-M.; Wang, D.; Wu, R.; Ji, Z.-L. Triptolide Exerts Protective Effects against Fibrosis Following Ileocolonic Anastomosis by Mechanisms Involving the MiR-16-1/HSP70 Pathway in IL-10-Deficient Mice. Int. J. Mol. Med. 2017, 40, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-S.; Park, D.-H. The Effect of Korean Red Ginseng on Full-Thickness Skin Wound Healing in Rats. J. Ginseng Res. 2019, 43, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Aman, S.; Li, Y.; Cheng, Y.; Yang, Y.; Lv, L.; Li, B.; Xia, K.; Li, S.; Wu, H. DACH1 Inhibits Breast Cancer Cell Invasion and Metastasis by Down-Regulating the Transcription of Matrix Metalloproteinase 9. Cell Death Discov. 2021, 7, 351. [Google Scholar] [CrossRef]
- Pan, C.; Wang, C.; Zhang, L.; Song, L.; Chen, Y.; Liu, B.; Liu, W.-T.; Hu, L.; Pan, Y. Procyanidins Attenuate Neuropathic Pain by Suppressing Matrix Metalloproteinase-9/2. J. Neuroinflam. 2018, 15, 187. [Google Scholar] [CrossRef]
- Pan, S.; Shah, S.D.; Panettieri, R.A.; Deshpande, D.A. Bnip3 Regulates Airway Smooth Muscle Cell Focal Adhesion and Proliferation. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 317, L758–L767. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Zou, J.; Jia, Y.; Wang, Y.; Li, J.; Wang, C.; Sun, J.; Guo, D.; Wang, F.; et al. The Mechanism Action of German Chamomile (Matricaria recutita L.) in the Treatment of Eczema: Based on Dose–Effect Weight Coefficient Network Pharmacology. Front. Pharmacol. 2021, 12, 706836. [Google Scholar] [CrossRef]
- Rodríguez, T.M.; Saldías, A.; Irigo, M.; Zamora, J.V.; Perone, M.J.; Dewey, R.A. Effect of TGF-Β1 Stimulation on the Secretome of Human Adipose-Derived Mesenchymal Stromal Cells. Stem Cells Transl. Med. 2015, 4, 894–898. [Google Scholar] [CrossRef]
- Yang, L.; Roh, Y.S.; Song, J.; Zhang, B.; Liu, C.; Loomba, R.; Seki, E. TGF-β Signaling in Hepatocytes Participates in Steatohepatitis Through Regulation of Cell Death and Lipid Metabolism. Hepatology 2014, 59, 483–495. [Google Scholar] [CrossRef]
- Fan, P.-C.; Chen, C.-C.; Peng, C.-C.; Chang, C.-H.; Yang, C.-H.; Yang, C.; Chu, L.J.; Chen, Y.-C.; Yang, C.-W.; Chang, Y.-S.; et al. A Circulating MiRNA Signature for Early Diagnosis of Acute Kidney Injury Following Acute Myocardial Infarction. J. Transl. Med. 2019, 17, 139. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Yue, W.; Xu, K.; Cai, W.; Cui, F.; Li, Z.; Wang, W.; He, J. Andrographolide Attenuates Epithelial-Mesenchymal Transition Induced by TGF-Β1 in Alveolar Epithelial Cells. J. Cell Mol. Med. 2020, 24, 10501–10511. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Y.; Li, B.-S.; Yang, Q.; Tang, J.-M.; Min, J.; Hong, S.-S.; Guo, W.-J.; Hong, L. Role of Transforming Growth Factor β-1 in the Pathogenesis of Pelvic Organ Prolapse: A Potential Therapeutic Target. Int. J. Mol. Med. 2017, 40, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Stelcer, E.; Milecka, P.; Komarowska, H.; Jopek, K.; Tyczewska, M.; Szyszka, M.; Lesniczak, M.; Suchorska, W.; Bekova, K.; Szczepaniak, B.; et al. Adropin Stimulates Proliferation and Inhibits Adrenocortical Steroidogenesis in the Human Adrenal Carcinoma (HAC15) Cell Line. Front. Endocrinol. 2020, 11, 561370. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Diao, H.; Zhao, Y.; Xu, H.; Pei, S.; Gao, J.; Wang, J.; Hussain, T.; Zhao, D.; Zhou, X.; et al. Overexpression of Matrix Metalloproteinase-9 in Breast Cancer Cell Lines Remarkably Increases the Cell Malignancy Largely via Activation of Transforming Growth Factor Beta/SMAD Signalling. Cell Prolif. 2019, 52, e12633. [Google Scholar] [CrossRef]
- Chen, S.J.; Yuan, W.; Mori, Y.; Levenson, A.; Trojanowska, M.; Varga, J. Stimulation of Type I Collagen Transcription in Human Skin Fibroblasts by TGF-Beta: Involvement of Smad 3. J. Investig. Dermatol. 1999, 112, 49–57. [Google Scholar] [CrossRef]
- Turner, A.W.; Nikpay, M.; Silva, A.; Lau, P.; Martinuk, A.; Linseman, T.A.; Soubeyrand, S.; McPherson, R. Functional Interaction between COL4A1/COL4A2 and SMAD3 Risk Loci for Coronary Artery Disease. Atherosclerosis 2015, 242, 543–552. [Google Scholar] [CrossRef]
- Song, P.; Liu, C.; Sun, M.; Liu, J.; Lin, P.; Wang, A.; Jin, Y. Oxidative Stress Induces Bovine Endometrial Epithelial Cell Damage through Mitochondria-Dependent Pathways. Animals 2022, 12, 2444. [Google Scholar] [CrossRef]
- Sun, M.; Song, P.; Zhao, Y.; Li, B.; Wang, P.; Cong, Z.; Hua, S. Mechanisms of LPS-Induced Epithelial Mesenchymal Transition in BEECs. Theriogenology 2024, 216, 30–41. [Google Scholar] [CrossRef]
- Grötter, L.G.; Cainelli, S.; Peralta, M.B.; Angeli, E.; Belotti, E.M.; Ortega, H.H.; Rey, F.; Velázquez, M.M.L.; Gareis, N.C. Metalloproteases and Their Inhibitors in the Postpartum Endometrial Remodeling in Dairy Cows: Their Relationship with Days to Conception after Parturition. Vet. Res. Commun. 2024, 49, 53. [Google Scholar] [CrossRef]
- Kan, X.; Hu, G.; Liu, Y.; Xu, P.; Huang, Y.; Cai, X.; Guo, W.; Fu, S.; Liu, J. Mammary Fibrosis Tendency and Mitochondrial Adaptability in Dairy Cows with Mastitis. Metabolites 2022, 12, 1035. [Google Scholar] [CrossRef]
- Yamada, O.; Todoroki, J.; Takahashi, T.; Hashizume, K. The Dynamic Expression of Extracellular Matrix in the Bovine Endometrium at Implantation. J. Vet. Med. Sci. 2002, 64, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.-T.N.; Shynlova, O.; Lye, S.J. Matrix Metalloproteinase Expression in the Rat Myometrium During Pregnancy, Term Labor, and Postpartum. Biol. Reprod. 2016, 95, 24. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Dirandeh, E.; Sayyar, M.A.; Ansari-Pirsaraei, Z.; Deldar, H.; Thatcher, W.W. Peripheral Leucocyte Molecular Indicators of Inflammation and Oxidative Stress Are Altered in Dairy Cows with Embryonic Loss. Sci. Rep. 2021, 11, 12771. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, H.; Liu, Y.; Gibson, S.A.; Yan, Z.; Xu, X.; Gaggar, A.; Li, P.-K.; Li, C.; Wei, S.; et al. Protective Effect of Suppressing STAT3 Activity in LPS-Induced Acute Lung Injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L868–L880. [Google Scholar] [CrossRef]
- Myers, J.N.; Schäffer, M.W.; Korolkova, O.Y.; Williams, A.D.; Gangula, P.R.; M’Koma, A.E. Implications of the Colonic Deposition of Free Hemoglobin-Alpha Chain: A Previously Unknown Tissue by-Product in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2014, 20, 1530–1547. [Google Scholar] [CrossRef]
- Orner, C.A.; Geary, M.B.; Hammert, W.C.; O’Keefe, R.J.; Loiselle, A.E. Low-Dose and Short-Duration Matrix Metalloproteinase 9 Inhibition Does Not Affect Adhesion Formation during Murine Flexor Tendon Healing. Plast. Reconstr. Surg. 2016, 137, 545e–553e. [Google Scholar] [CrossRef]
- Zingaropoli, M.A.; Latronico, T.; Pasculli, P.; Masci, G.M.; Merz, R.; Ciccone, F.; Dominelli, F.; Del Borgo, C.; Lichtner, M.; Iafrate, F.; et al. Tissue Inhibitor of Matrix Metalloproteinases-1 (TIMP-1) and Pulmonary Involvement in COVID-19 Pneumonia. Biomolecules 2023, 13, 1040. [Google Scholar] [CrossRef]
- Pieri, M. Hypothesis as to How a Common Missense Mutation in COL4A3 May Confer Protection against Diabetic Kidney Disease. J. Am. Soc. Nephrol. 2020, 31, 663–664. [Google Scholar] [CrossRef]
- Sun, K.; Tang, S.; Hou, Y.; Xi, L.; Chen, Y.; Yin, J.; Peng, M.; Zhao, M.; Cui, X.; Liu, M. Oxidized ATM-Mediated Glycolysis Enhancement in Breast Cancer-Associated Fibroblasts Contributes to Tumor Invasion through Lactate as Metabolic Coupling. EBioMedicine 2019, 41, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, Y.; Fang, Z.; Qi, W.; Xu, Y. TRIM66 Hastens the Malignant Progression of Non-Small Cell Lung Cancer via Modulating MMP9-Mediated TGF-β/SMAD Pathway. Cytokine 2022, 153, 155831. [Google Scholar] [CrossRef]
- Yang, W.; Ng, F.L.; Chan, K.; Pu, X.; Poston, R.N.; Ren, M.; An, W.; Zhang, R.; Wu, J.; Yan, S.; et al. Coronary-Heart-Disease-Associated Genetic Variant at the COL4A1/COL4A2 Locus Affects COL4A1/COL4A2 Expression, Vascular Cell Survival, Atherosclerotic Plaque Stability and Risk of Myocardial Infarction. PLoS Genet. 2016, 12, e1006127. [Google Scholar] [CrossRef]
- Li, F.; Majd, H.; Weir, M.D.; Arola, D.D.; Xu, H.H.K. Inhibition of Matrix Metalloproteinase Activity in Human Dentin via Novel Antibacterial Monomer. Dent. Mater. 2015, 31, 284–292. [Google Scholar] [CrossRef]
- Cooke, E.J.; Wyseure, T.; Zhou, J.Y.; Gopal, S.; Nasamran, C.A.; Fisch, K.M.; Manon-Jensen, T.; Karsdal, M.A.; Mosnier, L.O.; von Drygalski, A. Mechanisms of Vascular Permeability and Remodeling Associated with Hemarthrosis in Factor VIII-Deficient Mice. J. Thromb. Haemost. 2019, 17, 1815–1826. [Google Scholar] [CrossRef]
- Newby, A.C. Dual Role of Matrix Metalloproteinases (Matrixins) in Intimal Thickening and Atherosclerotic Plaque Rupture. Physiol. Rev. 2005, 85, 1–31. [Google Scholar] [CrossRef]
- Bataller, R.; Brenner, D.A. Liver Fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef]
- Eddy, A.A. Molecular Basis of Renal Fibrosis. Pediatr. Nephrol. 2000, 15, 290–301. [Google Scholar] [CrossRef]
- Qin, G.; Xia, J.; Zhang, Y.; Guo, L.; Chen, R.; Sang, N. Ambient Fine Particulate Matter Exposure Induces Reversible Cardiac Dysfunction and Fibrosis in Juvenile and Older Female Mice. Part. Fibre Toxicol. 2018, 15, 27. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Satooka, H.; Watanabe, S.; Honda, T.; Miyachi, Y.; Watanabe, T.; Verkman, A.S. Aquaporin-3-Mediated Hydrogen Peroxide Transport Is Required for NF-ΚB Signalling in Keratinocytes and Development of Psoriasis. Nat. Commun. 2015, 6, 7454. [Google Scholar] [CrossRef]
- Latronico, T.; Petraglia, T.; Sileo, C.; Bilancia, D.; Rossano, R.; Liuzzi, G.M. Inhibition of MMP-2 and MMP-9 by Dietary Antioxidants in THP-1 Macrophages and Sera from Patients with Breast Cancer. Molecules 2024, 29, 1718. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Maharjan, P.; Jin, M.; Park, T.; Maharjan, A.; Amatya, R.; Yang, J.; Min, K.A.; Shin, M.C. Potential Albumin-Based Antioxidant Nanoformulations for Ocular Protection against Oxidative Stress. Pharmaceutics 2019, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhang, X.; Li, J.; Zhao, D.; Gao, B.; Zhou, H.; Gao, S.; Zhang, L. Silencing TRIP13 Inhibits Cell Growth and Metastasis of Hepatocellular Carcinoma by Activating of TGF-Β1/Smad3. Cancer Cell Int. 2018, 18, 208. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Fang, T.; Zhang, Y.; Zhang, Y.; Gao, J.; Xue, Y. Association of the TGFβ Gene Family with Microenvironmental Features of Gastric Cancer and Prediction of Response to Immunotherapy. Front. Oncol. 2022, 12, 920599. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, Y.; Gong, W.; Gao, X.; Qi, H.; Liu, K.; Qi, J. C-Peptide Prevents SMAD3 Binding to Alpha Promoters to Inhibit Collagen Type IV Synthesis. J. Mol. Endocrinol. 2018, 61, 47–56. [Google Scholar] [CrossRef]

| Reagent | Volume (μL) |
|---|---|
| ChamQ SYBR qPCR Green Master Mix | 10.0 |
| Primer (F + R) (10 μM) | 1.0 |
| cDNA | 4.0 |
| RNase Free dH2O | 5.0 |
| Total | 20.0 |
| Gene Name | ID | Sequence (5′-3′) | Size (bp) |
|---|---|---|---|
| TNF-α | NM_173966 | F: GCTCTTACCGGAACACTTCG R: GGACACCTTGACCTCCTGAA | 238 |
| IL-1β | NM_174093 | F: AACCGAGAAGTGGTGTTCTGC R: TTGGGGTAGACTTTGGGGTCT | 107 |
| IL-6 | NM_173923.2 | F: CTACCTCCAGAACGAGTATG R: CAGCAGGTCAGTGTTTGTGG | 136 |
| IL-8 | NM_173925.2 | F: CATTCCACACCTTTCCACCC R: AGGCAGACCTCGTTTCCATT | 116 |
| CAT | NM_001035386.2 | F: AGAGGAAACGCCTGTGTGAG R: ATGCGGGAGCCATATTCAGG | 115 |
| SOD | NM_174615.2 | F: CTCTACTTGGTTGGGGCGTC R: TCGAAGTGGATGGTGCCTTG | 122 |
| GPx1 | NM_174076.3 | F: AACGTAGCATCGCTCTGAGG R: GATGCCCAAACTGGTTGCAG | 121 |
| TGF-β1 | NM_001007816.1 | F: CCGGAGGACCGAGACCTG R: CGTCAGATACTCCGAGGTGC | 97 |
| Smad3 | XM_005202615.5 | F: AGCATAGCACGGAAGCAGG R: GGTCTTATCCAAAGCGTCTGC | 87 |
| MMP9 | NM_174744.2 | F: CCATCGCGGAGATTAGGAAC R: CAGGCCACTTGCTCTTGACA | 117 |
| COL-Ⅳ | XM_025000171.2 | F: CGTGCCACTACTACGCGAAC R: CCTTGAGTGTGTCGGCAGAG | 93 |
| ACTB (β-Actin) | NM_173979.3 | F: GGCACCCAGCACAATGAAGA R: GCCAATCCACACGGAGTACTT | 67 |
| Gene Name | Sequence (5′-3′) |
|---|---|
| Si-NC | F: UUCUCCGAACGUGUCACGUTT R: ACGUGACACGUUCGGAGAATT |
| Si-Smad3 | F: GAGUUCGCCUUCAACAUGATT R: UCAUGUUGAAGGCGAACUCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.; Wang, Z.; Yang, M.; Zhang, R.; Xue, Z.; Zhou, D.; Wang, A.; Lin, P.; Jin, Y. Oxidative Stress Mediates the Dual Regulatory Effects of Bovine Uterine ECM Remodeling Through the TGF-β1/Smad3 Pathway: Molecular Mechanisms of MMPs and COL-IV Imbalances. Animals 2025, 15, 1847. https://doi.org/10.3390/ani15131847
Tan J, Wang Z, Yang M, Zhang R, Xue Z, Zhou D, Wang A, Lin P, Jin Y. Oxidative Stress Mediates the Dual Regulatory Effects of Bovine Uterine ECM Remodeling Through the TGF-β1/Smad3 Pathway: Molecular Mechanisms of MMPs and COL-IV Imbalances. Animals. 2025; 15(13):1847. https://doi.org/10.3390/ani15131847
Chicago/Turabian StyleTan, Jiamei, Zongjie Wang, Mingmao Yang, Ruihang Zhang, Zhongqiang Xue, Dong Zhou, Aihua Wang, Pengfei Lin, and Yaping Jin. 2025. "Oxidative Stress Mediates the Dual Regulatory Effects of Bovine Uterine ECM Remodeling Through the TGF-β1/Smad3 Pathway: Molecular Mechanisms of MMPs and COL-IV Imbalances" Animals 15, no. 13: 1847. https://doi.org/10.3390/ani15131847
APA StyleTan, J., Wang, Z., Yang, M., Zhang, R., Xue, Z., Zhou, D., Wang, A., Lin, P., & Jin, Y. (2025). Oxidative Stress Mediates the Dual Regulatory Effects of Bovine Uterine ECM Remodeling Through the TGF-β1/Smad3 Pathway: Molecular Mechanisms of MMPs and COL-IV Imbalances. Animals, 15(13), 1847. https://doi.org/10.3390/ani15131847




