Influence of Paratuberculosis Vaccination on the Local Immune Response in Experimentally Infected Calves: An Immunohistochemical Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Tissue Samples and Classification of the Lesions
2.3. Immunohistochemistry
2.4. Evaluation of the Immunolabeling
2.5. Statistical Analysis
3. Results
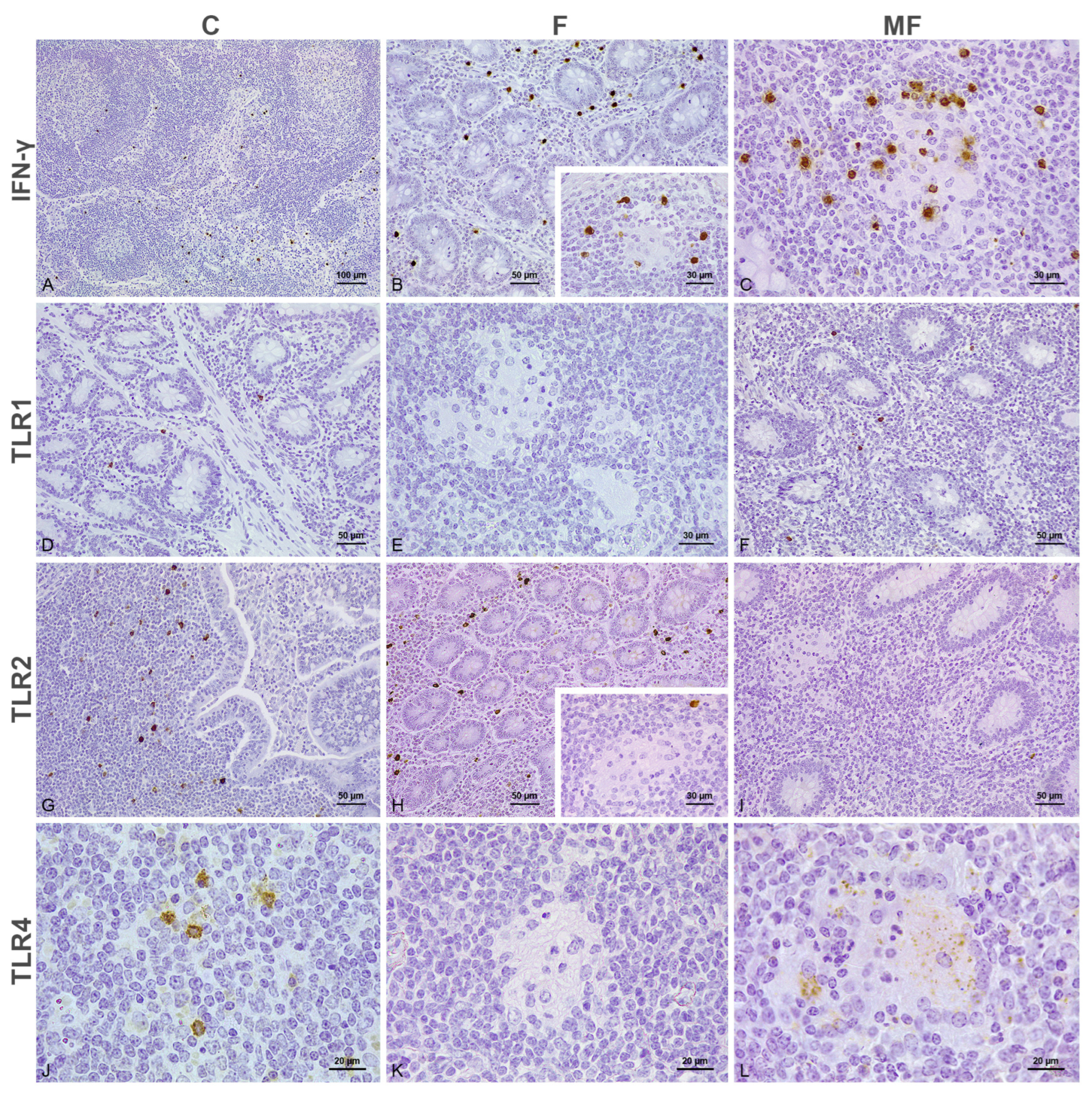
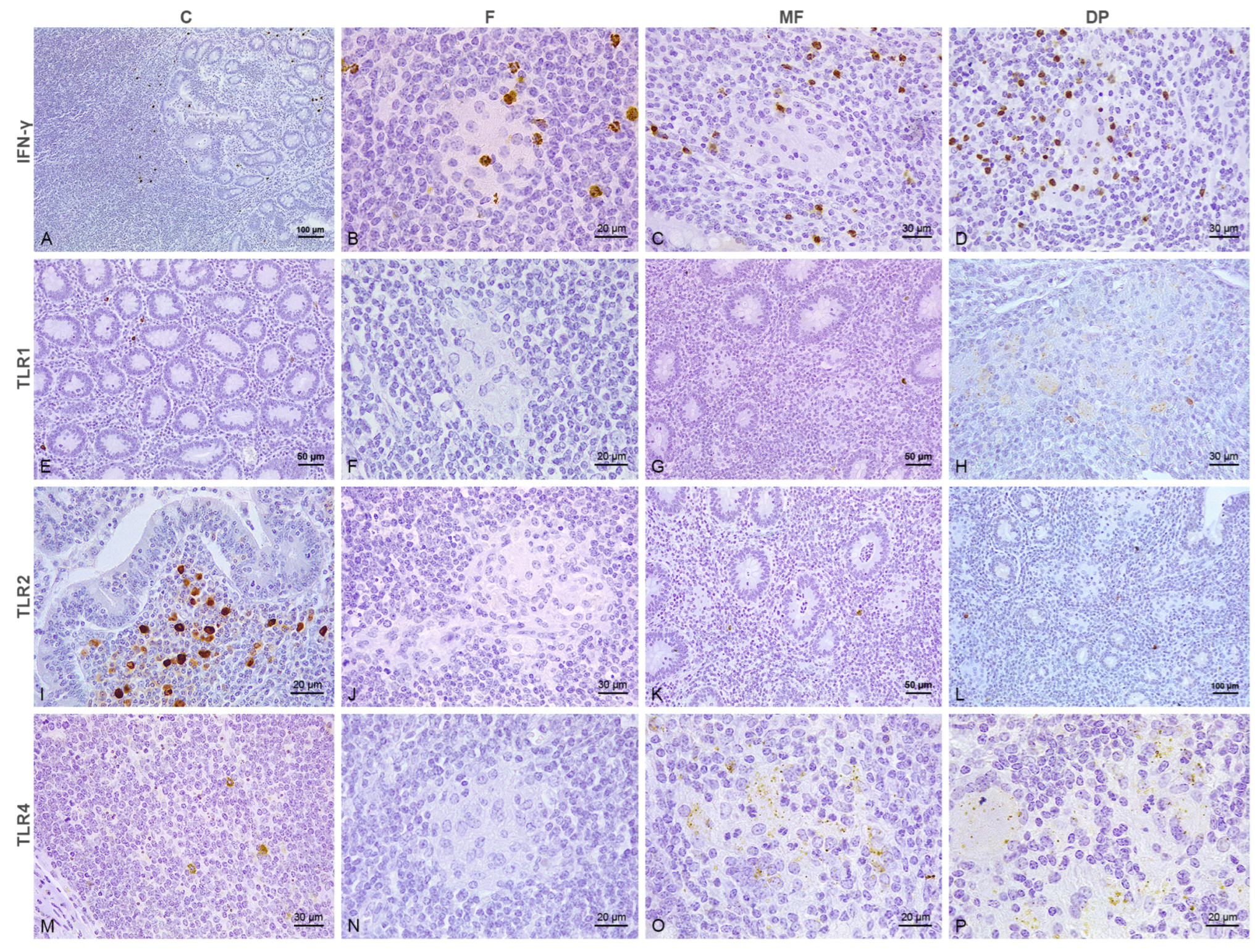
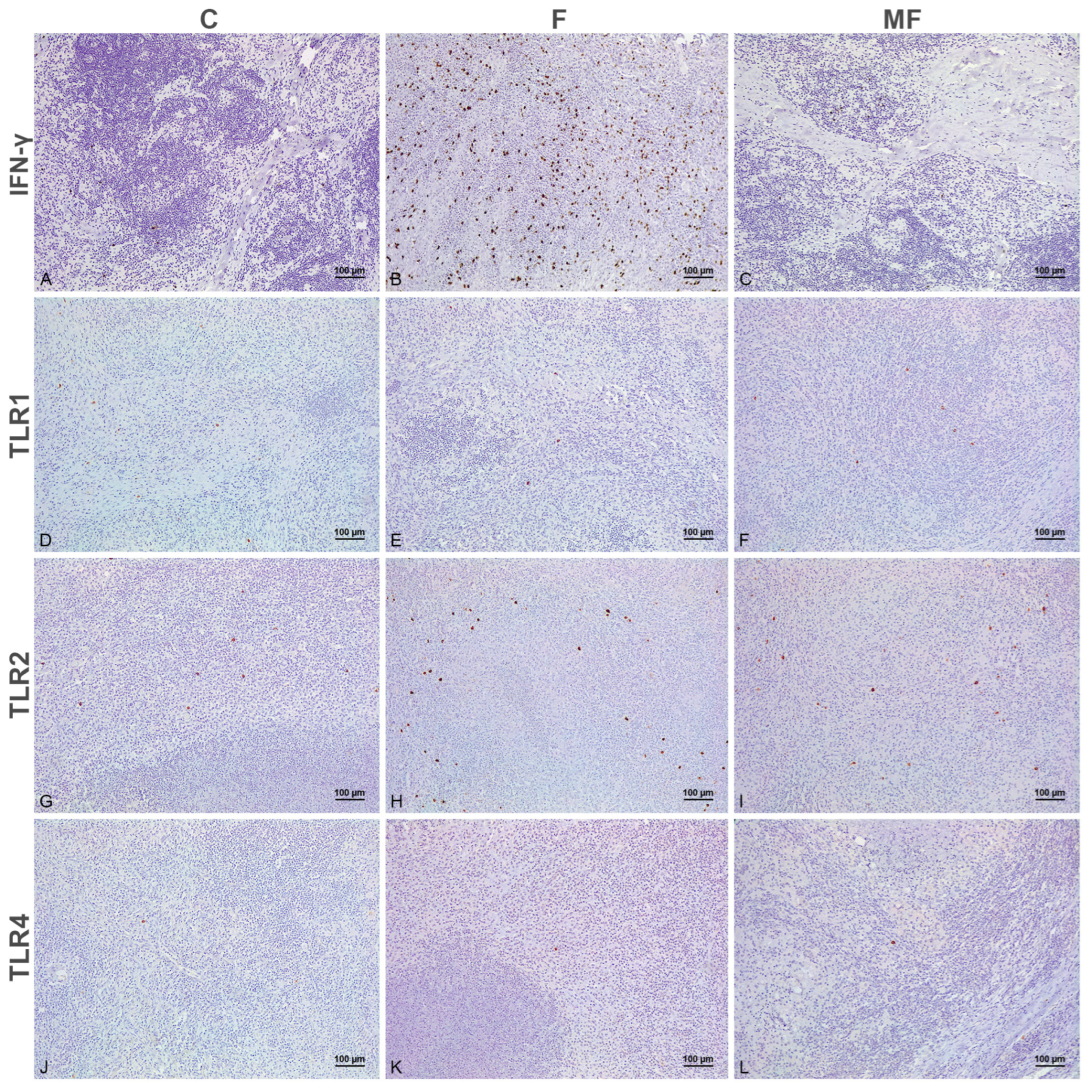
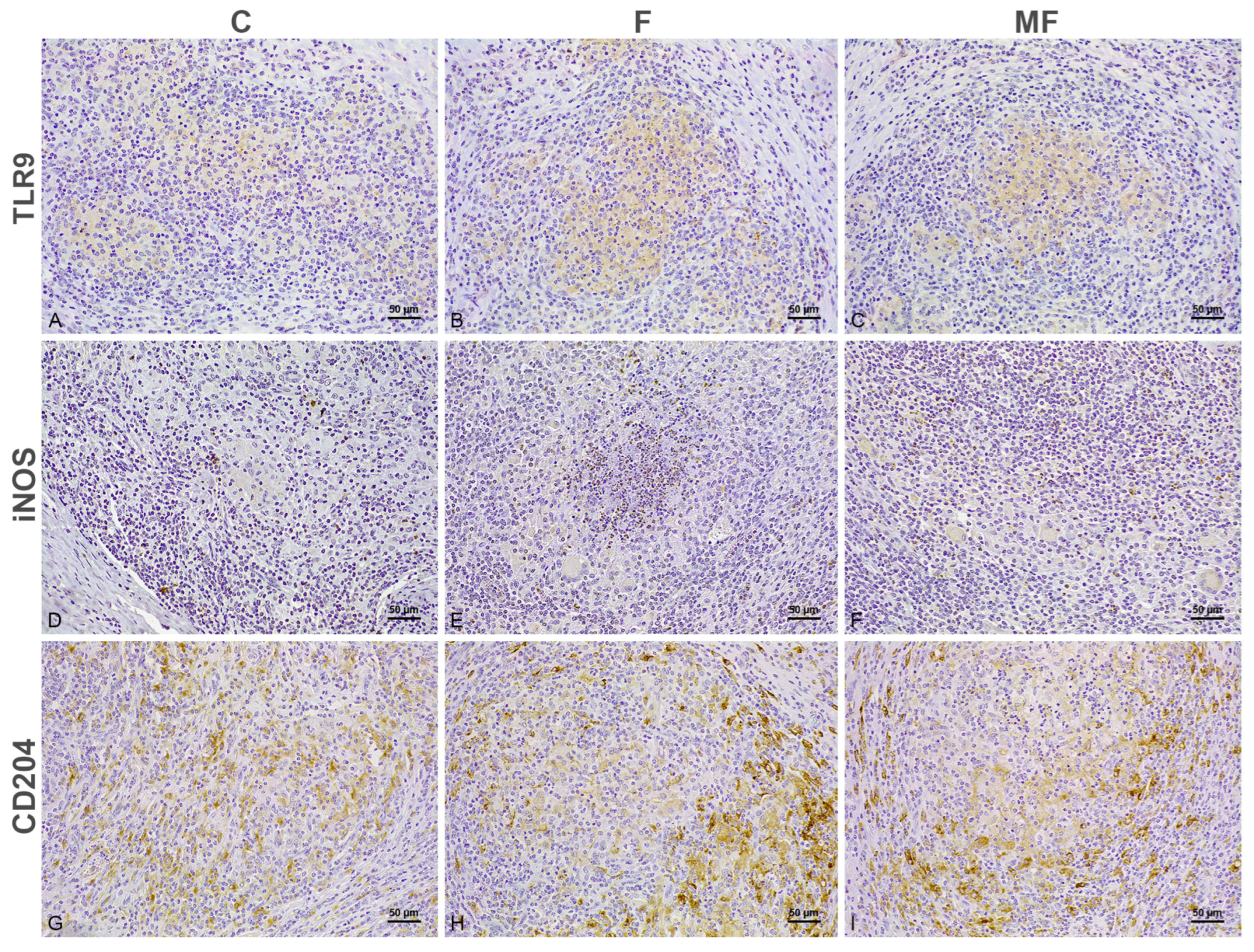
3.1. Distribution of the Immunolabeled Cells
3.2. Number of Immunolabeled Cells and Histological Scoring (H-Score) According to the Type of Lesion and Vaccination Status
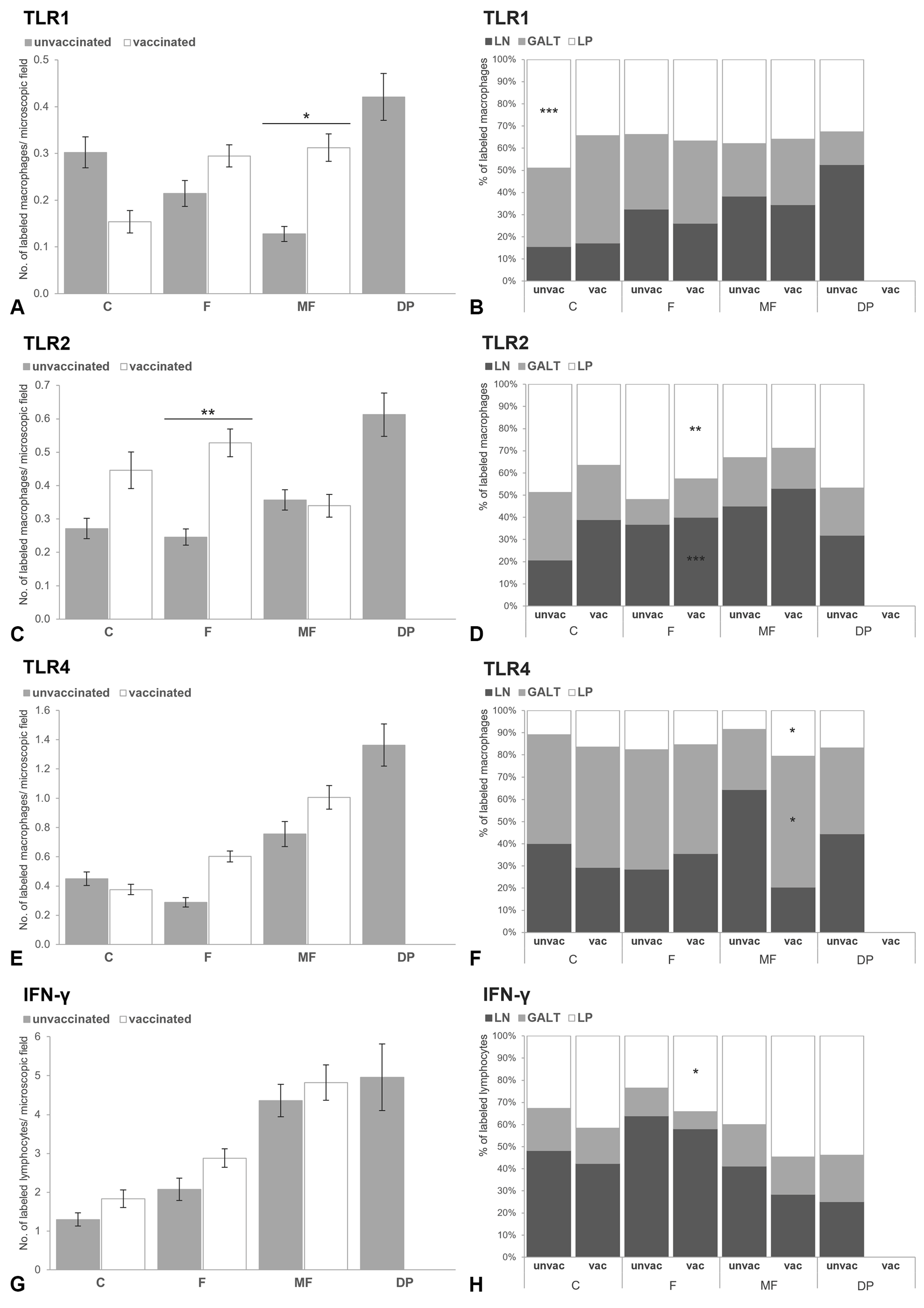
3.2.1. Intestine
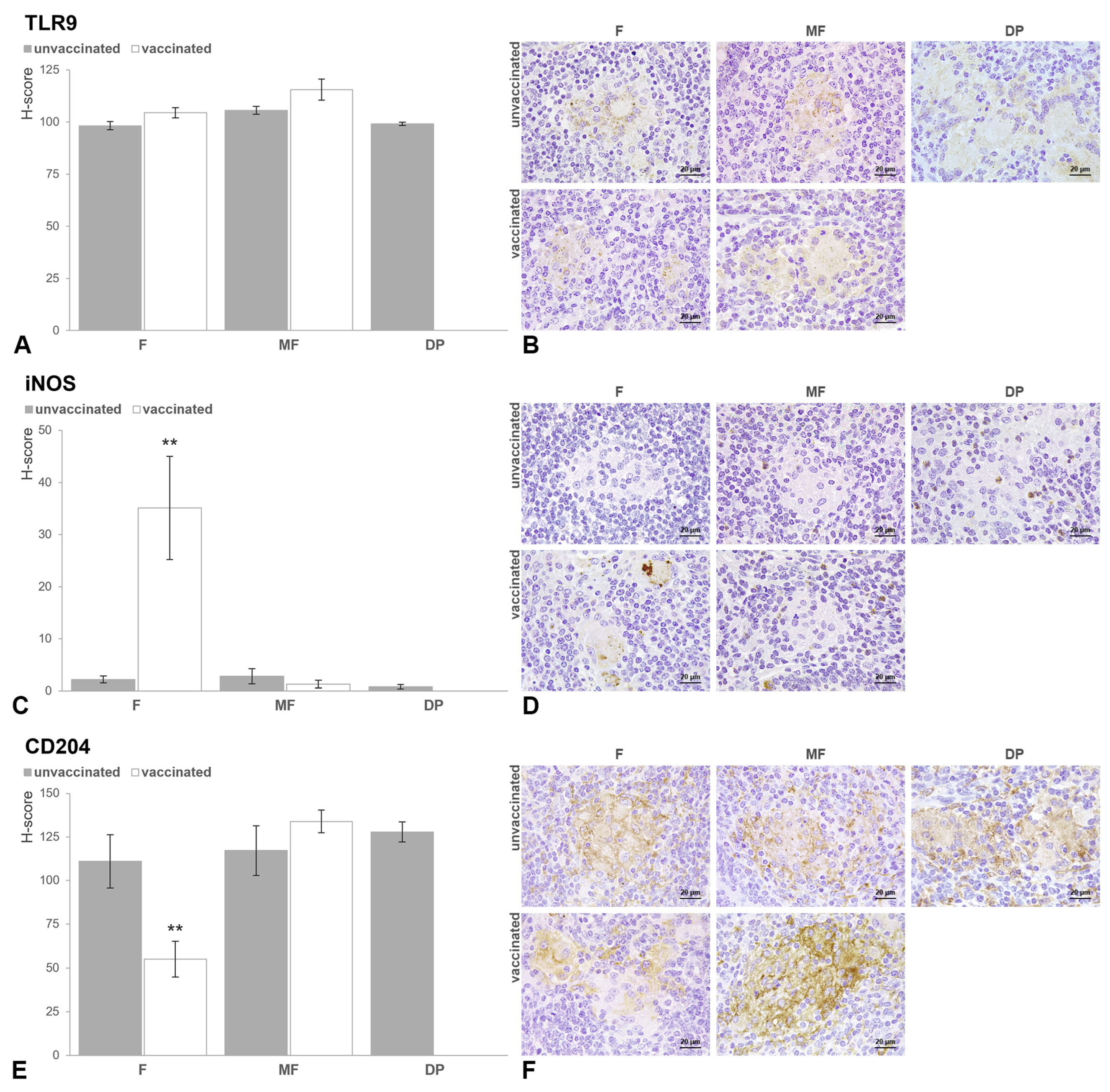
3.2.2. Scapular Lymph Node
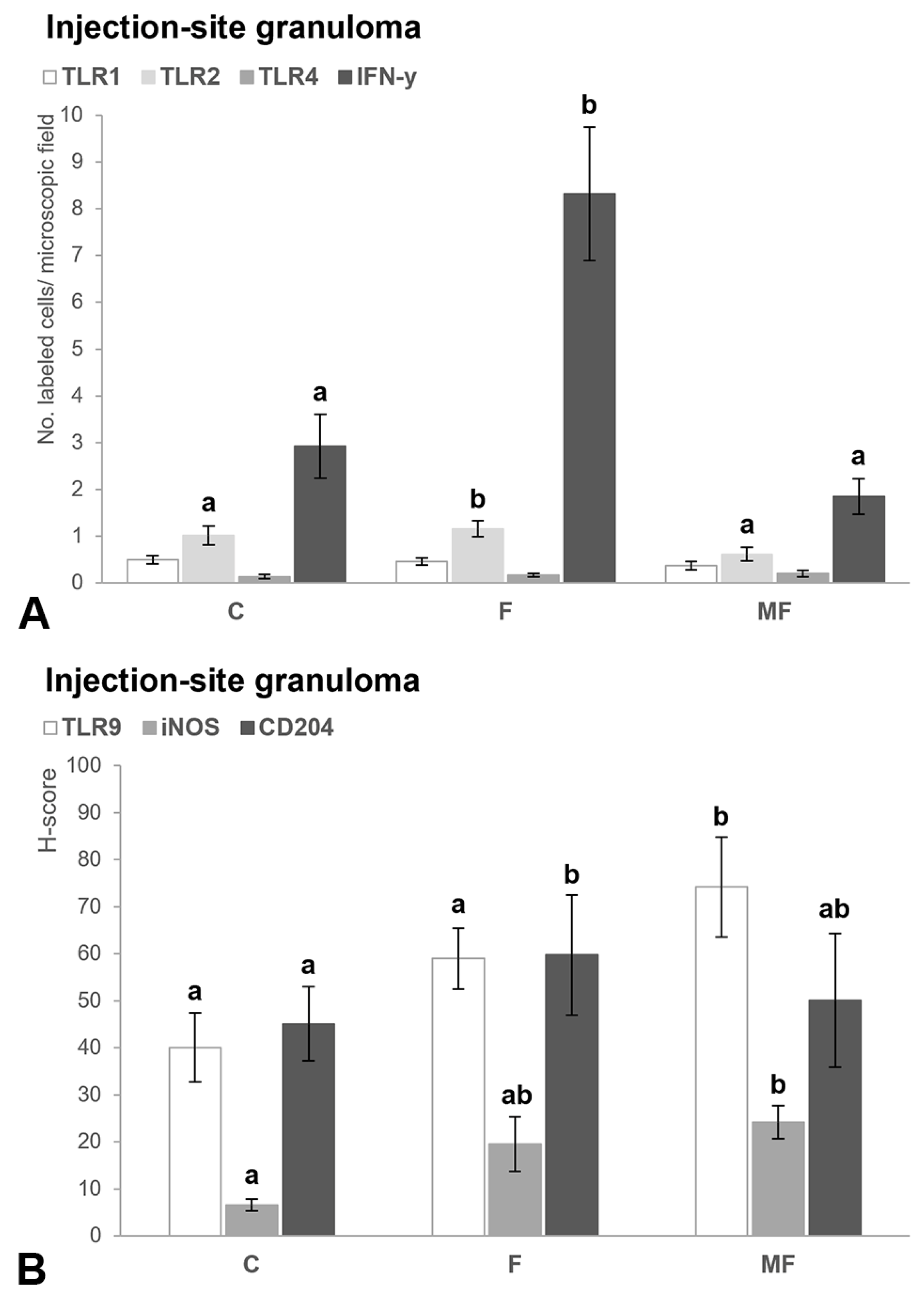
3.2.3. Injection Site—Granuloma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johne, H.; Frothingham, L. Ein Eigenthumlicher Fall von Tuberculose Beim Rind. Dtsch. Z. Tiermed. Vgl. Pathologie 1895, 21, 438–454. [Google Scholar]
- Rasmussen, P.; Barkema, H.W.; Mason, S.; Beaulieu, E.; Hall, D.C. Economic Losses Due to Johne’s Disease (paratuberculosis) in Dairy Cattle. J. Dairy Sci. 2021, 104, 3123–3143. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Hearn, M.; Molina, E.; Geijo, M.; Vazquez, P.; Sevilla, I.A.; Garrido, J.M.; Juste, R.A. Immunization of Adult Dairy Cattle with a New Heat-Killed Vaccine Is Associated with Longer Productive Life Prior to Cows Being Sent to Slaughter with Suspected paratuberculosis. J. Dairy Sci. 2012, 95, 618–629. [Google Scholar] [CrossRef]
- Juste, R.A.; Alonso-Hearn, M.; Molina, E.; Geijo, M.; Vazquez, P.; Sevilla, I.A.; Garrido, J.M. Significant Reduction in Bacterial Shedding and Improvement in Milk Production in Dairy Farms after the Use of a New Inactivated Paratuberculosis Vaccine in a Field Trial. BMC Res. Notes 2009, 2, 233. [Google Scholar] [CrossRef] [PubMed]
- Knust, B.; Patton, E.; Ribeiro-Lima, J.; Bohn, J.J.; Wells, S.J. Evaluation of the Effects of a Killed Whole-Cell Vaccine against Mycobacterium avium Subsp paratuberculosis in 3 Herds of Dairy Cattle with Natural Exposure to the Organism. J. Am. Vet. Med. Assoc. 2013, 242, 663–669. [Google Scholar] [CrossRef]
- Bastida, F.; Juste, R.A. Paratuberculosis Control: A Review with a Focus on Vaccination. J. Immune Based Ther. Vaccines 2011, 9, 8. [Google Scholar] [CrossRef]
- Muñoz, M. Eficacia de Una Vacuna Inactivada Frente a La Paratuberculosis Bovina En Un Modelo Experimental de Terneros y Su Influencia En La Patogenia de La Enfermedad. Ph.D. Thesis, Universidad de León, León, France, 2014. [Google Scholar]
- Shu, D.; Subharat, S.; Wedlock, D.N.; Luo, D.; De Lisle, G.W.; Buddle, B.M. Diverse Cytokine Profile from Mesenteric Lymph Node Cells of Cull Cows Severely Affected with Johne’s Disease. Clin. Vaccine Immunol. 2011, 18, 1467–1476. [Google Scholar] [CrossRef]
- Subharat, S.; Shu, D.; de Lisle, G.W.; Buddle, B.M.; Wedlock, D.N. Altered Patterns of Toll-like Receptor Gene Expression in Cull Cows Infected with Mycobacterium avium Subsp. paratuberculosis. Vet. Immunol. Immunopathol. 2012, 145, 471–478. [Google Scholar] [CrossRef]
- Coussens, P.M.; Verman, N.; Coussens, M.A.; Elftman, M.D.; McNulty, A.M. Cytokine Gene Expression in Peripheral Blood Mononuclear Cells and Tissues of Cattle Infected with Mycobacterium avium Subsp. paratuberculosis: Evidence for an Inherent Proinflammatory Gene Expression Pattern. Infect. Immun. 2004, 72, 1409–1422. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The Role of Pattern-Recognition Receptors in Innate Immunity: Update on Toll-like Receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Kravitz, A.; Pelzer, K.; Sriranganathan, N. The Paratuberculosis Paradigm Examined: A Review of Host Genetic Resistance and Innate Immune Fitness in Mycobacterium avium Subsp. paratuberculosis Infection. Front. Vet. Sci. 2021, 8, 721706. [Google Scholar] [CrossRef] [PubMed]
- Zapico, D.; Espinosa, J.; Criado, M.; Gutiérrez, D.; Ferreras, M.d.C.; Benavides, J.; Pérez, V.; Fernández, M. Immunohistochemical Expression of TLR1, TLR2, TLR4, and TLR9 in the Different Types of Lesions Associated with Bovine Paratuberculosis. Vet. Pathol. 2024, 62, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Bafica, A.; Scanga, C.A.; Feng, C.G.; Leifer, C.; Cheever, A.; Sher, A. TLR9 Regulates Th1 Responses and Cooperates with TLR2 in Mediating Optimal Resistance to Mycobacterium Tuberculosis. J. Exp. Med. 2005, 202, 1715–1724. [Google Scholar] [CrossRef]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-Gamma (IFN-γ): Exploring Its Implications in Infectious Diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Schaible, U.E. Macrophage Defense Mechanisms against Intracellular Bacteria. Immunol. Rev. 2015, 264, 182–203. [Google Scholar] [CrossRef]
- Sweeney, R.W.; Jones, D.E.; Habecker, P.; Scott, P. Interferon-Gamma and Interleukin 4 Gene Expression in Cows Infected with Mycobacterium paratuberculosis. Am. J. Vet. Res. 1998, 59, 842–847. [Google Scholar] [CrossRef]
- Pérez, V.; Tellechea, J.; Corpa, J.M.; Gutiérrez, M.; Marín, J.F.G. Relation between Pathologic Findings and Cellular Immune Responses in Sheep with Naturally Acquired Paratuberculosis. Am. J. Vet. Res. 1999, 60, 123–127. [Google Scholar] [CrossRef]
- Lee, H.; Stabel, J.R.; Kehrli, M.E. Cytokine Gene Expression in Ileal Tissues of Cattle Infected with Mycobacterium Paratuberculosis. Vet. Immunol. Immunopathol. 2001, 82, 73–85. [Google Scholar] [CrossRef]
- Smeed, J.A.; Watkins, C.A.; Rhind, S.M.; Hopkins, J. Differential Cytokine Gene Expression Profiles in the Three Pathological Forms of Sheep Paratuberculosis. BMC Vet. Res. 2007, 3, 18. [Google Scholar] [CrossRef]
- Fernández, M.; Fuertes, M.; Elguezabal, N.; Castaño, P.; Royo, M.; Ferreras, M.C.; Benavides, J.; Pérez, V. Immunohistochemical Expression of Interferon-γ in Different Types of Granulomatous Lesions Associated with Bovine Paratuberculosis. Comp. Immunol. Microbiol. Infect Dis. 2017, 51, 1–8. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage Plasticity, Polarization, and Function in Health and Disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Fernández, M.; Benavides, J.; Castaño, P.; Elguezabal, N.; Fuertes, M.; Royo, M.; Ferreras, M.C.; Pérez, V. Macrophage Subsets Within Granulomatous Intestinal Lesions in Bovine Paratuberculosis. Vet. Pathol. 2017, 54, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Jenvey, C.J.; Shircliff, A.L.; Bannantine, J.P.; Stabel, J.R. Phenotypes of Macrophages Present in the Intestine Are Impacted by Stage of Disease in Cattle Naturally Infected with Mycobacterium avium Subsp. paratuberculosis. PLoS ONE 2019, 14, e0217649. [Google Scholar] [CrossRef] [PubMed]
- Suwanruengsri, M.; Uemura, R.; Kanda, T.; Fuke, N.; Nueangphuet, P.; Pornthummawat, A.; Yasuda, M.; Hirai, T.; Yamaguchi, R. Production of Granulomas in Mycoplasma Bovis Infection Associated with Meningitis-Meningoencephalitis, Endocarditis, and Pneumonia in Cattle. J. Vet. Diagn. Investig. 2022, 34, 68–76. [Google Scholar] [CrossRef]
- Larenas-Muñoz, F.; Hamed, M.G.; Ruedas-Torres, I.; María Sánchez-Carvajal, J.; Domínguez, J.; José Pallarés, F.; Carrasco, L.; Rodríguez-Gómez, I.M.; Gómez-Laguna, J. Macrophage Polarization in Lymph Node Granulomas from Cattle and Pigs Naturally Infected with Mycobacterium Tuberculosis Complex. Vet. Pathol. 2024, 61, 792–802. [Google Scholar] [CrossRef]
- Arrazuria, R.; Ladero, I.; Molina, E.; Fuertes, M.; Juste, R.; Fernández, M.; Pérez, V.; Garrido, J.; Elguezabal, N. Alternative Vaccination Routes against Paratuberculosis Modulate Local Immune Response and Interference with Tuberculosis Diagnosis in Laboratory Animal Models. Vet. Sci. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Pooley, H.B.; Whittington, R.J.; Begg, D.J.; Purdie, A.C.; Plain, K.M.; de Silva, K. Sheep Vaccinated against Paratuberculosis Have Increased Levels of B Cells Infiltrating the Intestinal Tissue. Vet. Immunol. Immunopathol. 2022, 252, 110482. [Google Scholar] [CrossRef]
- González, J.; Geijo, M.V.; García-Pariente, C.; Verna, A.; Corpa, J.M.; Reyes, L.E.; Ferreras, M.C.; Juste, R.A.; García Marín, J.F.; Pérez, V. Histopathological Classification of Lesions Associated with Natural Paratuberculosis Infection in Cattle. J. Comp. Pathol. 2005, 133, 184–196. [Google Scholar] [CrossRef]
- Criado, M.; Benavides, J.; Vallejo, R.; Arteche, N.; Gutiérrez, D.; Ferreras, M.C.; Pérez, V.; Espinosa, J. Local Assessment of WC1 + Γδ T Lymphocyte Subset in the Different Types of Lesions Associated with Bovine Paratuberculosis. Comp. Immunol. Microbiol. Infect Dis. 2020, 69, 101422. [Google Scholar] [CrossRef]
- Detre, S.; Saccani Jotti, G.; Dowsett, M. A “Quickscore” Method for Immunohistochemical Semiquantitation: Validation for Oestrogen Receptor in Breast Carcinomas. J. Clin. Pathol. 1995, 48, 876–878. [Google Scholar] [CrossRef]
- Whittingham, M.J.; Stephens, P.A.; Bradbury, R.B.; Freckleton, R.P. Why Do We Still Use Stepwise Modelling in Ecology and Behaviour? J. Anim. Ecol. 2006, 75, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Zuur, A.F.; Ieno, E.N.; Smith, G.M. Analysing Ecological Data; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. (Eds.) A Practical Information-Theoretic Approach. In Model Selection and Multimodel Inference; Springer: New York, NY, USA, 2002. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A Protocol for Data Exploration to Avoid Common Statistical Problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Corbiere, F.; Guellouz, D.; Tasca, C.; Foures, L.; Dubaux, E.; Foucras, G. Effects of Silirum®-Based Vaccination Programs on Map Fecal Shedding and Serological Response in Seven French Dairy Herds. Animals 2023, 13, 1569. [Google Scholar] [CrossRef] [PubMed]
- Muskens, J.; Van Zijderveld, F.; Eger, A.; Bakker, D. Evaluation of the Long-Term Immune Response in Cattle after Vaccination against Paratuberculosis in Two Dutch Dairy Herds. Vet. Microbiol. 2002, 86, 269–278. [Google Scholar] [CrossRef]
- Muñoz, M.; García-Marín, J.F.; García-Pariente, C.; Reyes, L.E.; Verna, A.; Moreno, O.; Fuertes, M.; Doce, J.; Puentes, E.; Garrido, J.; et al. Efficacy of a Killed Vaccine (SILIRUM) in Calves Challenged with Mycobacterium avium Subsp. paratuberculosis. In Proceedings of the 8th International Colloquium on Paratuberculosis, Kopenhagen, Denmark, 14–18 August 2005; Manning, E., Nielsen, S., Eds.; IAP: Madison, WI, USA, 2006; pp. 208–217. [Google Scholar]
- Sweeney, R.W.; Whitlock, R.H.; Bowersock, T.L.; Cleary, D.L.; Meinert, T.R.; Habecker, P.L.; Pruitt, G.W. Effect of Subcutaneous Administration of a Killed Mycobacterium avium Subsp paratuberculosis Vaccine on Colonization of Tissues Following Oral Exposure to the Organism in Calves. Am. J. Vet. Res. 2009, 70, 493–497. [Google Scholar] [CrossRef]
- Facciuolo, A.; Lee, A.H.; Trimble, M.J.; Rawlyk, N.; Townsend, H.G.G.; Bains, M.; Arsic, N.; Mutharia, L.M.; Potter, A.; Gerdts, V.; et al. A Bovine Enteric Mycobacterium Infection Model to Analyze Parenteral Vaccine-Induced Mucosal Immunity and Accelerate Vaccine Discovery. Front. Immunol. 2020, 11, 586659. [Google Scholar] [CrossRef]
- Vazquez, P.; Garrido, J.M.; Molina, E.; Geijo, M.V.; Gomez, N.; Perez, V.; Sevilla, I.A.; Alonso-Hearn, M.; Cortes, A.; Juste, R.A. Latent Infections Are the Most Frequent Form of Paratuberculosis in Slaughtered Friesian Cattle. Span. J. Agric. Res. 2014, 12, 1049–1060. [Google Scholar] [CrossRef]
- Kasurinen, A.; Hagström, J.; Laitinen, A.; Kokkola, A.; Böckelman, C.; Haglund, C. Evaluation of Toll-like Receptors as Prognostic Biomarkers in Gastric Cancer: High Tissue TLR5 Predicts a Better Outcome. Sci. Rep. 2019, 9, 12553. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Ahn, M.H.; Jung, J.Y.; Kim, J.W.; Suh, C.H.; Kwon, J.E.; Yim, H.; Kim, H.A. Elevated Expression of TLR2 and Its Correlation with Disease Activity and Clinical Manifestations in Adult-Onset Still’s Disease. Sci. Rep. 2022, 12, 10240. [Google Scholar] [CrossRef]
- Ng, L.K.; Rich, A.M.; Hussaini, H.M.; Thomson, W.M.; Fisher, A.L.; Horne, L.S.; Seymour, G.J. Toll-like Receptor 2 Is Present in the Microenvironment of Oral Squamous Cell Carcinoma. Br. J. Cancer 2011, 104, 460–463. [Google Scholar] [CrossRef]
- Ramos-Vara, J.A.; Miller, M.A. When Tissue Antigens and Antibodies Get Along: Revisiting the Technical Aspects of Immunohistochemistry-The Red, Brown, and Blue Technique. Vet. Pathol. 2014, 51, 42–87. [Google Scholar] [CrossRef] [PubMed]
- Leifer, C.A.; Medvedev, A.E. Molecular Mechanisms of Regulation of Toll-like Receptor Signaling. J. Leukoc. Biol. 2016, 100, 927. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 Trafficking and Its Influence on LPS-Induced pro-Inflammatory Signaling. Cell Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.J.; Souza, C.D.; Evanson, O.A.; Sanders, M.; Rutherford, M. Bovine Monocyte TLR2 Receptors Differentially Regulate the Intracellular Fate of Mycobacterium Avium Subsp. Paratuberculosis and Mycobacterium Avium Subsp. Avium. J. Leukoc. Biol. 2008, 83, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Quero, L.; Hanser, E.; Manigold, T.; Tiaden, A.N.; Kyburz, D. TLR2 Stimulation Impairs Anti-Inflammatory Activity of M2-like Macrophages, Generating a Chimeric M1/M2 Phenotype. Arthritis. Res. Ther. 2017, 19, 245. [Google Scholar] [CrossRef]
- Hu, W.; Spaink, H.P. The Role of TLR2 in Infectious Diseases Caused by Mycobacteria: From Cell Biology to Therapeutic Target. Biology 2022, 11, 246. [Google Scholar] [CrossRef]
- Koets, A.; Eda, S.; Sreevatsan, S. The within Host Dynamics of Mycobacterium avium Ssp. paratuberculosis Infection in Cattle: Where Time and Place Matter. Vet. Res. 2015, 46, 61. [Google Scholar] [CrossRef]
- Yamashiro, L.H.; Oliveira, S.C.; Báfica, A. Innate Immune Sensing of Nucleic Acids from Mycobacteria. Microbes. Infect. 2014, 16, 991–997. [Google Scholar] [CrossRef]
- Ito, T.; Schaller, M.; Hogaboam, C.M.; Standiford, T.J.; Chensue, S.W.; Kunkel, S.L. TLR9 Activation Is a Key Event for the Maintenance of a Mycobacterial Antigen-Elicited Pulmonary Granulomatous Response. Eur. J. Immunol. 2007, 37, 2847–2855. [Google Scholar] [CrossRef]
- Mattila, J.T.; Ojo, O.O.; Kepka-Lenhart, D.; Marino, S.; Kim, J.H.; Eum, S.Y.; Via, L.E.; Barry, C.E.; Klein, E.; Kirschner, D.E.; et al. Microenvironments in Tuberculous Granulomas Are Delineated by Distinct Populations of Macrophage Subsets and Expression of Nitric Oxide Synthase and Arginase Isoforms. J. Immunol. 2013, 191, 773–784. [Google Scholar] [CrossRef]
- Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; Flynn, J.L.; Kirschner, D.E. Macrophage Polarization Drives Granuloma Outcome during Mycobacterium Tuberculosis Infection. Infect. Immun. 2015, 83, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Luo, Q.; Guo, Y.; Chen, J.; Xiong, G.; Peng, Y.; Ye, J.; Li, J. Mycobacterium Tuberculosis-Induced Polarization of Human Macrophage Orchestrates the Formation and Development of Tuberculous Granulomas In Vitro. PLoS ONE 2015, 10, e0129744. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, R.J.; Li, Y.; Bell, K.; Doig, K.; Potter, A.; Griebel, P.J.; Kusalik, A.; Napper, S. Mycobacterium avium Subsp. paratuberculosis Inhibits Gamma Interferon-Induced Signaling in Bovine Monocytes: Insights into the Cellular Mechanisms of Johne’s Disease. Infect Immun. 2012, 80, 3039–3048. [Google Scholar] [CrossRef]
- Arsenault, R.J.; Maattanen, P.; Daigle, J.; Potter, A.; Griebel, P.; Napper, S. From Mouth to Macrophage: Mechanisms of Innate Immune Subversion by Mycobacterium avium Subsp. paratuberculosis. Vet. Res. 2014, 45, 54. [Google Scholar] [CrossRef]
- Koets, A.; Rutten, V.; Hoek, A.; Van Mil, F.; Müller, K.; Bakker, D.; Gruys, E.; Van Eden, W. Progressive Bovine Paratuberculosis Is Associated with Local Loss of CD4(+) T Cells, Increased Frequency of Gamma Delta T Cells, and Related Changes in T-Cell Function. Infect. Immun. 2002, 70, 3856–3864. [Google Scholar] [CrossRef]
- Sturge, C.R.; Benson, A.; Raetz, M.; Wilhelm, C.L.; Mirpuri, J.; Vitetta, E.S.; Yarovinsky, F. TLR-Independent Neutrophil-Derived IFN-γ Is Important for Host Resistance to Intracellular Pathogens. Proc. Natl. Acad. Sci. USA 2013, 110, 10711–10716. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.C.; Yamada, M.; Martin, J.R.; Dang, H.; Brickey, W.J.; Bergmeier, W.; Dinauer, M.C.; Doerschuk, C.M. Mechanisms of Interferon-γ Production by Neutrophils and Its Function during Streptococcus Pneumoniae Pneumonia. Am. J. Respir. Cell. Mol. Biol. 2015, 52, 349–364. [Google Scholar] [CrossRef]
- Saini, R.; Singh, S. Inducible Nitric Oxide Synthase: An Asset to Neutrophils. J. Leukoc. Biol. 2019, 105, 49–61. [Google Scholar] [CrossRef]
- Lv, J.; He, X.; Wang, H.; Wang, Z.; Kelly, G.T.; Wang, X.; Chen, Y.; Wang, T.; Qian, Z. TLR4-NOX2 Axis Regulates the Phagocytosis and Killing of Mycobacterium Tuberculosis by Macrophages. BMC Pulm. Med. 2017, 17, 194. [Google Scholar] [CrossRef]
- Chang, E.Y.; Guo, B.; Doyle, S.E.; Cheng, G. Cutting Edge: Involvement of the Type I IFN Production and Signaling Pathway in Lipopolysaccharide-Induced IL-10 Production. J. Immunol. 2007, 178, 6705–6709. [Google Scholar] [CrossRef]
- Hussain, T.; Shah, S.Z.A.; Zhao, D.; Sreevatsan, S.; Zhou, X. The Role of IL-10 in Mycobacterium avium Subsp. paratuberculosis Infection. Cell Commun. Signal 2016, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Mundra, A.; Yegiazaryan, A.; Karsian, H.; Alsaigh, D.; Bonavida, V.; Frame, M.; May, N.; Gargaloyan, A.; Abnousian, A.; Venketaraman, V. Pathogenicity of Type I Interferons in Mycobacterium Tuberculosis. Int. J. Mol. Sci. 2023, 24, 3919. [Google Scholar] [CrossRef] [PubMed]
- Criado, M.; Reyes, L.E.; Marín, J.F.G.; Gutiérrez-Expósito, D.; Zapico, D.; Espinosa, J.; Pérez, V. Adjuvants Influence the Immune Cell Populations Present at the Injection Site Granuloma Induced by Whole-Cell Inactivated Paratuberculosis Vaccines in Sheep. Front. Vet. Sci. 2024, 11, 1284902. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapico, D.; Espinosa, J.; Muñoz, M.; Reyes, L.E.; Benavides, J.; Marín, J.F.G.; Fernández, M. Influence of Paratuberculosis Vaccination on the Local Immune Response in Experimentally Infected Calves: An Immunohistochemical Analysis. Animals 2025, 15, 1841. https://doi.org/10.3390/ani15131841
Zapico D, Espinosa J, Muñoz M, Reyes LE, Benavides J, Marín JFG, Fernández M. Influence of Paratuberculosis Vaccination on the Local Immune Response in Experimentally Infected Calves: An Immunohistochemical Analysis. Animals. 2025; 15(13):1841. https://doi.org/10.3390/ani15131841
Chicago/Turabian StyleZapico, David, José Espinosa, María Muñoz, Luis Ernesto Reyes, Julio Benavides, Juan Francisco García Marín, and Miguel Fernández. 2025. "Influence of Paratuberculosis Vaccination on the Local Immune Response in Experimentally Infected Calves: An Immunohistochemical Analysis" Animals 15, no. 13: 1841. https://doi.org/10.3390/ani15131841
APA StyleZapico, D., Espinosa, J., Muñoz, M., Reyes, L. E., Benavides, J., Marín, J. F. G., & Fernández, M. (2025). Influence of Paratuberculosis Vaccination on the Local Immune Response in Experimentally Infected Calves: An Immunohistochemical Analysis. Animals, 15(13), 1841. https://doi.org/10.3390/ani15131841






