Genetic Diversity and Population Differentiation of Yangtze Finless Porpoise in Poyang Lake
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethical Approval
2.2. Animals and Sample Collection
2.3. Sample DNA Extraction
2.4. PCR Amplification and Sequencing
2.5. Genetic Diversity Analysis
2.6. Genetic Differentiation and Genetic Structure Analysis
3. Results
3.1. Genetic Diversity Analysis
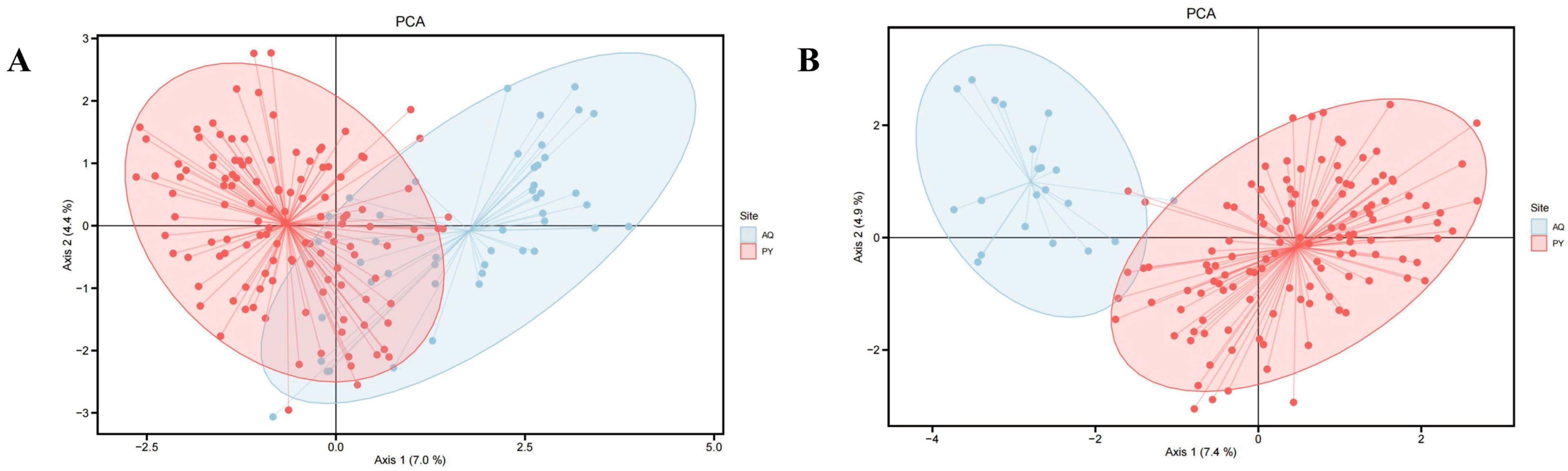
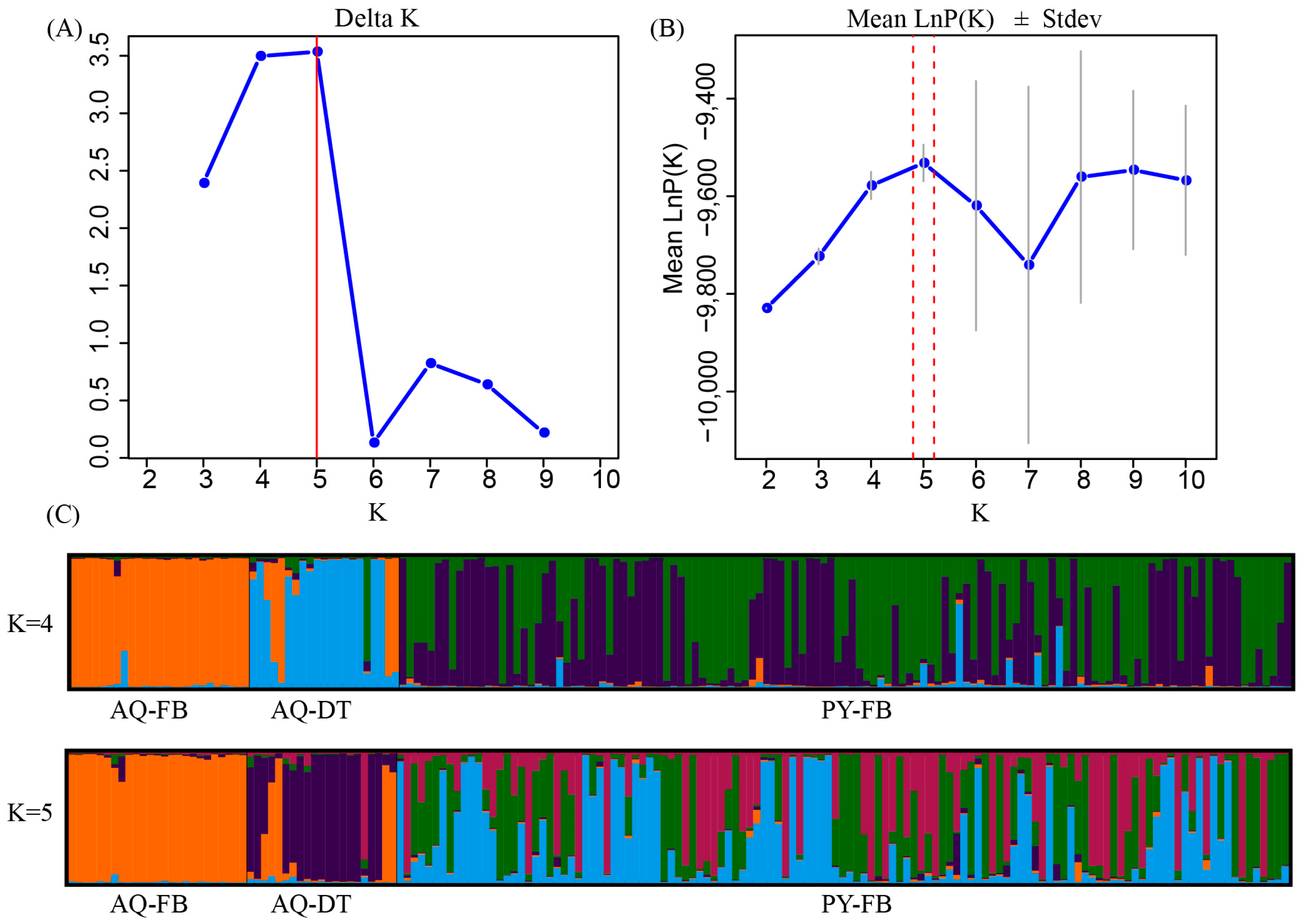
3.2. Genetic Differentiation and Genetic Structure Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, X.; Guang, X.; Sun, D.; Xu, S.; Yang, G. Population genomics of finless porpoises reveal an incipient cetacean species adapted to freshwater. Nat. Commun. 2018, 9, 1276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Liu, R.J.; Zhao, Q.Z.; Zhang, G.C.; Wei, Z.; Wang, X.Q.; Yang, J. The population of finless porpoise in the middle and lower reaches of Yangtze River. Acta Theriol. Sin. 1993, 13, 260–270. [Google Scholar]
- Mei, Z.G.; Hao, Y.J.; Zheng, J.S.; Wang, Z.T.; Wang, K.X.; Wang, D. Population status and conservation outlooks of Yangtze finless porpoise in the Lake Poyang. J. Lack Sci. 2021, 33, 1289–1298. [Google Scholar]
- Wang, D.; Turvey, S.; Zhao, X.; Mei, Z. Neophocaena asiaeorientalis ssp. asiaeorientalis the IUCN Red List of Threatened Species; Version 3.1.; IUCN: Gland, Switzerlandand; Cambridge, UK, 2013. [Google Scholar]
- Mei, Z.G.; Cheng, P.L.; Wang, K.X.; Wei, Q.W.; Bariow, J.; Wang, D. A first step for the Yangtze. Science 2020, 367, 1314. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Mei, Z.G.; Chen, M.; Han, Y.; Zhang, X.Q.; Moore, J.E.; Zhao, X.J.; Hao, Y.J.; Wang, K.X.; Wang, D. Population survey showing hope for population recovery of the critically endangered Yangtze finless porpoise. Biol. Conserv. 2019, 241, 108315. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, D.; Zhang, X.F.; Zhao, Q.Z.; Wang, K.X.; Kuang, X.A. Population size, behavior, movement pattern and protection of Yangtze finless porpoise at Balijiang section of the Yangtze River. Resour. Environ. Yangtze Basin 2002, 11, 427–432. [Google Scholar]
- Xu, P.; Liu, K.; Ying, C.P.; Yin, D.H.; Lin, D.Q.; Zhang, J.L. Progress and prospects on the protection of Yangtze finless porpoises. Acta Hydrobiol. Sin. 2024, 6, 1077–1084. [Google Scholar]
- Liu, X.; Mei, Z.G.; Zhang, J.X.; Sun, J.J.; Zhang, N.N.; Guo, Y.Y.; Wang, K.X.; Hao, Y.J.; Wang, D. Seasonal Yangtze finless porpoise (Neophocaena asiaeorientalis asiaeorientalis) movements in the Poyang Lake, China: Implications on flexible management for aquatic animals in fluctuating freshwater ecosystems. Sci. Total Environ. 2022, 807, 150782. [Google Scholar] [CrossRef]
- Xiao, W.; Zhang, X.F. A preliminary study on the population size of Yangtze finless propoise in Poyang Lake. Chin. Biodivers. 2000, 8, 106–111. [Google Scholar]
- Yu, J.H.; Hu, G.L. Impacts of habitat changes in Poyang Lake on the Yangtze finless porpoise (Neophocaena asiaeorientalis asiaeorientalis). Chin. J. Vet. Med. 2019, 12, 131–134. [Google Scholar]
- Kimura, S.; Akamatsu, T.; Li, S.H.; Dong, L.J.; Wang, K.X.; Wang, D.; Arai, N. Seasonal changes in the local distribution of Yangtze finless porpoises related to fish presence. Mar. Mammal Sci. 2012, 28, 308–324. [Google Scholar] [CrossRef]
- Zhao, X.J.; Wang, D. Abundance and distribution of Yangtze finless porpoise in balijiang section of the Yangtze River. Resour. Environ. Yangtze Basin 2011, 20, 1432–1439. [Google Scholar]
- Chen, M.M.; Zheng, Y.; Hao, Y.J.; Mei, Z.G.; Wang, K.X.; Zhao, Q.Z.; Zheng, J.S.; Wang, D. Parentage-Based Group Composition and Dispersal Pattern Studies of the Yangtze Finless Porpoise Population in Poyang Lake. Int. J. Mol. Sci. 2016, 17, 1268. [Google Scholar] [CrossRef] [PubMed]
- Hohenlohe, P.A.; Funk, W.C.; Rajora, O.P. Population genomics for wildlife conservation and management. Mol. Ecol. 2020, 30, 62–82. [Google Scholar] [CrossRef]
- Ottewell, K.; Byrne, M. Conservation Genetics for Management of Threatened Plant and Animal Species. Diversity 2022, 14, 251. [Google Scholar] [CrossRef]
- Gibbs, S.E.; Salgado Kent, C.P.; Slat, B.; Morales, D.; Fouda, L.; Reisser, J. Cetacean sightings within the Great Pacific Garbage Patch. Mar. Biodivers. 2019, 49, 2021–2027. [Google Scholar] [CrossRef]
- Yong, L.; Zhang, Y.K.; Zhao, L.Y.; Zeng, Q.H.; Lin, L.S.; Gao, M.H.; Cheng, H.; Wang, X.Y. Research advances on the ecology of Sousa chinensis. Biodivers. Sci. 2023, 31, 22670. [Google Scholar] [CrossRef]
- Buss, D.L.; Atmore, L.M.; Zico, M.H.; Goodall-Copestake, W.P.; Brace, S.; Archer, F.I.; Baker, C.S.; Barnes, I.; Carroll, E.L.; Hart, T.; et al. Historical Mitogenomic Diversity and Population Structure of Southern Hemisphere Fin Whales. Genes 2023, 14, 1038. [Google Scholar] [CrossRef]
- Fernando, F.; Susana, C.; Carlos, O. Genetic diversity and population structure of humpback whales (Megaptera novaeangliae) from Ecuador based on mitochondrial DNA analyses. J. Cetacean Res. Manag. 2023, 12, 71–77. [Google Scholar]
- Yang, G.; Liu, S.; Ren, W.H.; Zhou, K.Y.; Wei, F.W. Mitochondrial control region variability of baiji and the Yangtze finless porpoises, two sympatric small cetaceans in the Yangtze River. Acta Theriol. 2003, 48, 469–483. [Google Scholar] [CrossRef]
- Yang, G.; Ren, W.H.; Zhou, K.Y.; Liu, S.; Ji, G.Q.; Yan, J.; Wang, L.M. Population genetic structure of finless porpoises, Neophocaena phocaenoides, in Chinese waters, inferred from mitochondrial control region sequences. Mar. Mammal Sci. 2002, 18, 336–347. [Google Scholar] [CrossRef]
- Zheng, J.S.; Xia, J.H.; He, S.P.; Wang, D. Population genetic structure of the Yangtze finless porpoise (Neophocaena phocaenoides asiaeorientalis): Implications for management and Conservation. Biochem. Genet. 2005, 43, 307–320. [Google Scholar] [CrossRef]
- Mei, Z.G.; Zhang, X.Q.; Huang, S.L.; Zhao, X.J.; Hao, Y.J.; Zhang, L.; Qian, Z.Y.; Zheng, J.S.; Wang, K.X.; Wang, D. The Yangtze finless porpoise: On an accelerating path to extinction? Biol. Conserv. 2014, 172, 117–123. [Google Scholar] [CrossRef]
- Chen, M.M.; Zheng, J.S.; Wu, M.; Ruan, R.; Zhao, Q.Z.; Wang, D. Genetic diversity and population structure of the critically endangered Yangtze finless porpoise (Neophocaena asiaeorientalis asiaeorientalis) as revealed by mitochondrial and microsatellite DNA. Int. J. Mol. Sci. 2014, 15, 11307–11323. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Y.; Chen, M.M.; Wan, X.L.; Tang, B.; Hao, Y.J.; Mei, Z.G.; Fan, F.; Wang, K.X.; Wang, D.; Zheng, J.S. Microsatellite genetic diversity evaluation and development prediction of the Yangtze finless porpoise population in the Poyang Lake. Acta Hydrobiol. Sin. 2023, 47, 1693–1700. [Google Scholar]
- Que, J.L.; Rao, R.C.; Yang, Y.; Min, J.L.; Tian, Z.; Yu, Z.J.; Yu, J.X.; Dai, Y.Y.; Mei, Z.G. Population and distribution characteristics of Yangtze finless porpoise in Jiangxi waters during dry section. Acta Hydrobiol. Sin. 2023, 47, 1701–1708. [Google Scholar]
- Dong, L.J.; Wang, D.; Wang, K.X.; Li, S.H.; Mei, Z.G.; Wang, S.Y.; Akamatsu, T.; Kimura, S. Yangtze finless porpoises along the main channel of Poyang Lake, China: Implications for conservation. Mar. Mammal Sci. 2015, 31, 612–628. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, J.S.; Chen, M.M.; Zhao, Q.Z.; Wang, D. Genetic evaluation and development prognosis on EX Situ conserved Yangtze finless porpoises living in Tian-E-Zhou national natural reserve. Acta Hydrobiol. Sin. 2012, 36, 403–411. [Google Scholar] [CrossRef]
- Zheng, J.S.; Liao, X.L.; Tong, J.G.; Du, H.J.; Milinkovitch, M.C.; Wang, D. Development and characterization of polymorphic microsatellite loci in the endangered Yangtze finless porpoise (Neophocaena phocaenoides asiaeorientalis). Conserv. Genet. 2008, 9, 1007–1009. [Google Scholar] [CrossRef]
- Feng, J.W.; Zheng, J.S.; Zhou, Z.; Lin, G.; Wang, D.; Zheng, B.Y.; Jiang, W.H. Paternity Determination of Captivity-Bred Yangtze finless porpoises Neophocaena phocaenoides asiaeorientalies by Microsatellite genotyping. Prog. Mod. Biomed. 2009, 9, 4015–4020. [Google Scholar]
- Chen, L.; Yang, G. Development of tetranucleotide microsatellite loci for the finless porpoise (Neophocaena phocaenoides). Conserv. Genet. 2008, 9, 1033–1035. [Google Scholar] [CrossRef]
- Rosel, P.E.; France, S.C.; Wang, J.Y.; Kocher, T.D. Genetic structure of harbour porpoise Phocoena phocoena populations in the northwest Atlantic based on mitochondrial and nuclear markers. Mol. Ecol. 1999, 8, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Jeanmougin, F.; Thompson, J.; Gouy, M.; Higgins, D.G.; Gibson, T.J. Multiple sequence alignment with Clustal X. Trends Biochem. Sci. 1998, 23, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP V5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Peakall, R.O.D.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Goudet, J. Fstat (Version 2.9.4), a Program to Estimate and Test Population Genetics Parameters. Updated from Goudet [1995]. 2003. Available online: https://www2.unil.ch/popgen/softwares/fstat.htm (accessed on 13 June 2024).
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol. Bioinform. Online 2005, 1, 47. [Google Scholar] [CrossRef]
- Jombart, T. Adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef]
- Pritchard, J.; Wen, X.; Falush, D. Documentation for STRUCTURE Software; Version 2.3; University of Chicago: Chicago, IL, USA, 2010. [Google Scholar]
- Cui, L.K.; Deng, J.L.; Zhao, L.X.; Hu, Y.H.; Liu, T.L. Genetic diversity and population genetic structure of Setosphaeria turcica from Sorghum in three provinces of China using single nucleotide polymorphism markers. Front. Microbiol. 2022, 13, 853202. [Google Scholar] [CrossRef]
- Balding, D.J. A tutorial on statistical methods for population association studies. Nat. Rev. Genet. 2006, 7, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Kardos, M.; Armstrong, E.E.; Fitzpatrick, S.W.; Hauser, S.; Hedrick, P.W.; Miller, J.M.; Tallmon, D.A.; Funk, W.C. The crucial role of genome-wide genetic variation in conservation. Proc. Natl. Acad. Sci. USA 2021, 118, 1–10. [Google Scholar] [CrossRef]
- Collard, B.C.Y.; Jahufer, M.Z.Z.; Brouwer, J.B.; Pang, E.C.K. An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: The basic concepts. Euphytica 2005, 142, 169–196. [Google Scholar] [CrossRef]
- Crossman, C.A.; Barrett-Lennard, L.G.; Taylor, E.B. Population structure and intergeneric hybridization in harbour porpoises Phocoena phocoena in British Columbia, Canada. Endanger. Species Res. 2014, 26, 1–12. [Google Scholar] [CrossRef]
- Dai, Y.; Sakornwimon, W.; Chantra, R.; Zhao, L.Y.; Wu, F.X.; Aierken, R.; Kittiwattanawong, K.; Wang, X.Y. High genetic differentiation of Indo-Pacific humpback dolphins (Sousa chinensis) along the Asian Coast of the Pacific Ocean. Ecol. Evol. 2022, 12, e8901. [Google Scholar] [CrossRef]
- de Oliveira, V.K.M.; Faria, D.M.; Cunha, H.A.; dos Santos, T.E.C.; Colosio, A.C.; Barbosa, L.A.; Freire, M.C.C.; Farro, A.P.C. Low Genetic Diversity of the Endangered Franciscana (Pontoporia blainvillei) in Its Northernmost, Isolated Population (FMAla, Espirito Santo, Brazil). Front. Mar. Sci. 2020, 7, 608276. [Google Scholar] [CrossRef]
- Chen, M.M.; Zheng, J.S.; Gong, C.; Zhao, Q.Z.; Wang, D. Inbreeding evaluation on the Ex Situ conserved Yangtze finless porpoise population in Tian’ezhou National Natural Reserve. Chin. J. Zool. 2014, 49, 305–316. [Google Scholar]
- Grant, W.; Bowen, B. Shallow population histories in deep evolutionary lineages of marine fishes: Insights from sardines and anchovies and lessons for conservation. J. Hered. 1998, 89, 415–426. [Google Scholar] [CrossRef]
- Yang, G.; Guo, L.; Bruford, M.W.; Wei, F.W.; Zhou, K.Y. Mitochondrial phylogeography and population history of finless porpoises in Sino-Japanese waters. Biol. J. Linn. Soc. 2008, 95, 193–204. [Google Scholar] [CrossRef]
- Ku, J.E.; Choi, S.G. Population Structure of Finless Porpoise (Neophocaena phocaenoides) Discovered off Coastal Waters, Republic of Korea. Genes 2022, 13, 1701. [Google Scholar] [CrossRef]
- Mirimin, L.; Westgate, A.; Rogan, E.; Rosel, P.; Read, A.; Coughlan, J.; Cross, T. Population structure of short-beaked common dolphins (Delphinus delphis) in the North Atlantic Ocean as revealed by mitochondrial and nuclear genetic markers. Mar. Biol. 2009, 156, 821–834. [Google Scholar] [CrossRef]
- Cassens, I.; Van Waerebeek, K.; Best, P.B.; Tzika, A.; Van Helden, A.L.; Crespo, E.A.; Milinkovitch, M.C. Evidence for male dispersal along the coasts but no migration in pelagic waters in dusky dolphins (Lagenorhynchus obscurus). Mol. Ecol. 2005, 14, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Arnason, U.; Gullberg, A. Relationship of baleen whales established by Cytochrome b gene sequence comparison. Nature 1994, 367, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Milinkovitch, M.C. Molecular phylogeny of cetaceans prompts revisions of morphological transformations. Trends Ecol. Evol. 1995, 10, 328–333. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, D.; Turvey, S.T.; Taylor, B.; Akamatsu, T. Distribution patterns of Yangtze finless porpoises in the Yangtze River: Implications for reserve management. Anim. Conserv. 2013, 16, 509–518. [Google Scholar] [CrossRef]
- Wang, C.R.; Suo, W.W.; Jiang, G.M.; Li, H.M.; Liang, Z.Q.; Yang, X.; Yuan, X.P.; Li, H.; Liao, F.C.; Ge, H.Z.; et al. Spatial distribution of the Yangtze finless porpoise and relationship to fish density in East Dongting Lake, China. China Environ. Sci. 2019, 39, 4424–4434. [Google Scholar]
- Wright, S. The genetical structure of population structure by F-statistics with special regard to system of mating. Evolution 1965, 19, 395–420. [Google Scholar] [CrossRef]
- Rivera-León, V.E.; Urbán, J.; Mizroch, S.; Brownell, R.L.; Oosting, T.; Hao, W.S.; Palsboll, P.J.; Bérubé, M. Long-term isolation at a low effective population size greatly reduced genetic diversity in Gulf of California fin whales. Sci. Rep. 2019, 9, 12391. [Google Scholar] [CrossRef]
- Caballero, S.; Hollatz, C.; Rodríguez, S.; Trujillo, F.; Baker, S. Population structure of riverine and coastal dolphins Sotalia fluviatilis and Sotalia guianensis: Patterns of nuclear and mitochondrial diversity and implications for conservation. J. Hered. 2018, 109, 757–770. [Google Scholar] [CrossRef]
- Dai, Y.F.; Chantra, R.; Kittiwattanawong, K.; Zhao, L.Y.; Sakornwimon, W.; Aierken, R.; Wu, F.X.; Wang, X.Y. Genetic structure of the endangered Irrawaddy dolphin (Orcaella brevirostris) in the Gulf of Thailand. Genet. Mol. Biol. 2021, 44, e20200365. [Google Scholar] [CrossRef]
- Cheng, J.; Kao, H.X.; Dong, S.B. Population genetic structure and gene flow of rare and endangered Tetraena mongolica Maxim. revealed by reduced representation sequencing. BMC Plant Biol. 2020, 20, 391. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.G.; Huang, S.L.; Hao, Y.J.; Turvey, S.T.; Gong, W.M.; Wang, D. Accelerating population decline of Yangtze finless porpoise (Neophocaena asiaeorientalis asiaeorientalis). Biol. Conserv. 2012, 153, 192–200. [Google Scholar] [CrossRef]
- Mei, Z.G.; Han, Y.; Turvey, S.T.; Liu, J.J.; Wang, Z.T.; Nabi, G.; Chen, M.; Lei, P.Y.; Hao, Y.J.; Wang, K.X.; et al. Mitigating the effect of shipping on freshwater cetaceans: The case study of the Yangtze finless propoise. Biol. Conserv. 2021, 257, 109132. [Google Scholar] [CrossRef]
- Min, J.L.; Yu, J.X.; Zhang, Y.Y.; Li, D.M.; Que, J.L.; Tian, Z.; Rao, R.C.; Mei, Z.G.; Dai, Y.Y. Distribution risks and protection countermeasures of Yangtze finless porpoise in Poyang Lake during abnormal dry period. Acta Hydrobiol. Sin. 2024, 48, 1642–1650. [Google Scholar]


| Population | PY | AQ | |
|---|---|---|---|
| Year | Number of Fresh Blood Samples | Number of Fresh Blood Samples | Number of Dead Tissue Samples |
| 2017 | 0 | 21 | 5 |
| 2018 | 0 | 0 | 3 |
| 2019 | 0 | 0 | 2 |
| 2021 | 0 | 0 | 7 |
| 2022 | 105 | 0 | 2 |
| 2023 | 20 | 0 | 6 |
| Total | 125 | 21 | 25 |
| Locus | Primer Sequence (5′-3′) | Repeat Motif | Tm (°C) | Modification | Product Range/bp | Reference |
|---|---|---|---|---|---|---|
| YPFSSR5 | F: GAGTGGGGTCAAATCAGGAA | (GT)18 | 60 | 5′ROX | 206–226 | Zhou et al., 2012 [29] |
| R: ATGCCTTTGGCTGCATGTAT | ||||||
| YFPSSR15 | F: TGGAAAGAGGCCTTCAGATG | (GT)22CT(GT)7 | 60 | 5′6-FAM | 177–217 | Zhou et al., 2012 [29] |
| R: TGACAGGTCCAAGAGCCAGT | ||||||
| YFPSSR22 | F: GCTCTCCTTGGCACTTTTCC | (AC)10 | 60 | 5′ROX | 202–216 | Zhou et al., 2012 [29] |
| R: CCTCTCTGCCCAGTTTCCTA | ||||||
| YFPSSR40 | F: ATGAATTCTGTCCCCTGTGC | (AC)16 | 60 | 5′6-FAM | 182–194 | Zhou et al., 2012 [29] |
| R: AGCCCAGTTATCTGGCTTCC | ||||||
| YFPSSR41 | F: TGACACAGGGAATTACTTTCAA | (GT)16 | 60 | 5′ROX | 203–215 | Zhou et al., 2012 [29] |
| R: CCATGACCACGACAATAGCA | ||||||
| YFPSSR51 | F: TTAGTCAGCTCTCCCCATCC | (GT)10 | 60 | 5′TAMRA | 199–209 | Zhou et al., 2012 [29] |
| R: TGCACACTCATACATGTACACACA | ||||||
| YFPSSR73 | F: TCCACCTGAGAAGCAAAACC | (TG)22 | 60 | 5′6-FAM | 168–178 | Zhou et al., 2012 [29] |
| R: GGAACTGGCATTTAGGGTTG | ||||||
| YFP1 | F: TTTGGAAATTGCTAGACTGTGG | (AC)15 | 60 | 5′HEX | 150–164 | Zheng et al., 2008 [30] |
| R: CCTCTTACGCAAGATAAAAGTGG | ||||||
| YFP8 | F: ATACTGGCAACAGCCACTAGGT | (AC)15 | 60 | 5′6-FAM | 188–198 | Zheng et al., 2008 [30] |
| R: CACATTCTTTCCCTTTTTGTCC | ||||||
| YFP42 | F: TCCGTAGGCTTGGTTCTTGTAT | (GT)11(GA)8 | 60 | 5′HEX | 166–184 | Zheng et al., 2008 [30] |
| R: AGGGGACCCTAAGTTTTCAGAG | ||||||
| YFP59 | F: GCACCTGGGTACTGTCCATATT | (CA)15 | 60 | 5′HEX | 148–166 | Zheng et al., 2008 [30] |
| R: TCTTCCAAATACCTGCCTTCAT | ||||||
| YFP69 | F: GAGGACAGGGTGGTATGTTGTT | (GT)14 | 60 | 5′6-FAM | 184–192 | Zheng et al., 2008 [30] |
| R: CATAGTCACCAGTGCATTTCCA | ||||||
| YFPSSR63 | F: ACCTGCCATAGCCCTCTTCT | (GT)18 | 60 | 5′TAMRA | 192–202 | Feng et al., 2009 [31] |
| R: GTTTTGCGTGGAGTCAGACA | ||||||
| YFPSSR71 | F: GAAAAATGGGCTGTGTGGAT | (TG)20 | 60 | 5′TAMRA | 190–200 | Feng et al., 2009 [31] |
| R: TGATTCAGTCACCAGCAACC | ||||||
| YFPSSR75 | F: GTTCATGGTTCCAGGGACTG | (AC)14 | 60 | 5′TAMRA | 190–214 | Feng et al., 2009 [31] |
| R: CTCCCCAAATTCCCTTTTCT | ||||||
| Np404 | F: GGTCAGAACAAGAACACAG | (GATA)3GAT(GATA)9 | 60 | 5′HEX | 160–168 | Chen and Yang 2008 [32] |
| R: CTCCTCCTAATACAGAAATAC | ||||||
| Np409 | F: TGGGAGAGGTATAAGTGGCT | (GATA)3GAT(GATA)9 | 60 | 5′ROX | 205–257 | Chen and Yang 2008 [32] |
| R: TGGATGGGTGGAAGTAGTT | ||||||
| Np428 | F: CCAGAGAATCAGAACCAATAG | (GATA)8(GACA)4 | 60 | 5′HEX | 113–133 | Chen and Yang 2008 [32] |
| R: CCAGAATCACACGAGCCT | ||||||
| Np464 | F: TGGCTGCACTTGCATTGATG | (GAAA)5A2(GAAA)6GA2G(GAAA)5 | 60 | 5′ROX | 259–283 | Chen and Yang 2008 [32] |
| R: CCTAAGAACCCTCTAAATCCA | ||||||
| PPHO130 | F: CAAGCCCTTACACATATG | (CA)25 | 60 | 5′TAMRA | 188–202 | Rosel et al., 1999 [33] |
| R: TATTGAGTAAAAGCAATTTTG |
| Population | Distribution of Haplotypes | Haplotype Diversity | Nucleotide Diversity | |
|---|---|---|---|---|
| Hap1 | Hap2 | Hd | Pi | |
| Poyang (PY) | 76 | 49 | 0.481 ± 0.020 | 0.00078 ± 0.00030 |
| Anqing (AQ1) | 19 | 27 | 0.496 ± 0.029 | 0.00080 ± 0.00031 |
| Anqing (AQ2) | 11 | 14 | 0.513 ± 0.037 | 0.00080 ± 0.00041 |
| PY | AQ | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Locus | Na | Ne | Ho | He | PIC | Fis | HWE | Na | Ne | Ho | He | PIC | Fis | HWE |
| NP404 | 4 | 2.095 | 0.518 | 0.53 | 0.413 | 0.023 | NS | 4 | 2.115 | 0.489 | 0.527 | 0.469 | 0.012 | NS |
| Np409 | 8 | 3.141 | 0.45 | 0.649 | 0.635 | 0.307 | *** | 13 | 5.423 | 0.683 | 0.816 | 0.798 | 0.081 | *** |
| Np428 | 5 | 1.417 | 0.277 | 0.268 | 0.285 | −0.035 | NS | 7 | 1.943 | 0.556 | 0.485 | 0.461 | −0.136 | NS |
| Np464 | 5 | 3.693 | 0.758 | 0.74 | 0.682 | −0.025 | NS | 9 | 5.204 | 0.585 | 0.808 | 0.781 | 0.207 | ** |
| PPHO130 | 7 | 4.817 | 0.693 | 0.79 | 0.763 | 0.123 | * | 9 | 5.478 | 0.833 | 0.817 | 0.797 | −0.085 | NS |
| YFP1 | 6 | 3.424 | 0.782 | 0.714 | 0.667 | −0.096 | NS | 6 | 2.95 | 0.667 | 0.661 | 0.601 | −0.01 | ** |
| YFP8 | 12 | 3.896 | 0.831 | 0.715 | 0.712 | −0.161 | NS | 6 | 4.268 | 0.644 | 0.766 | 0.724 | 0.113 | * |
| YFP42 | 6 | 3.192 | 0.777 | 0.693 | 0.633 | −0.121 | NS | 7 | 3.123 | 0.711 | 0.680 | 0.631 | −0.032 | NS |
| YFP59 | 8 | 5.919 | 0.731 | 0.81 | 0.809 | 0.098 | *** | 10 | 6.202 | 0.778 | 0.839 | 0.819 | 0.041 | ** |
| YFP69 | 4 | 1.649 | 0.34 | 0.342 | 0.367 | 0.004 | NS | 7 | 1.785 | 0.326 | 0.440 | 0.408 | 0.270 | NS |
| YFPSSR15 | 11 | 4.568 | 0.693 | 0.779 | 0.752 | 0.111 | NS | 11 | 4.594 | 0.558 | 0.782 | 0.751 | 0.267 | *** |
| YFPSSR22 | 4 | 2.519 | 0.582 | 0.596 | 0.541 | 0.023 | NS | 6 | 3.625 | 0.636 | 0.724 | 0.677 | 0.105 | ** |
| YFPSSR40 | 5 | 2.938 | 0.588 | 0.662 | 0.619 | 0.112 | NS | 9 | 5.414 | 0.844 | 0.815 | 0.792 | −0.082 | NS |
| YFPSSR41 | 8 | 5.659 | 0.623 | 0.824 | 0.802 | 0.245 | NS | 7 | 5.787 | 0.767 | 0.827 | 0.804 | 0.048 | NS |
| YFPSSR51 | 5 | 2.946 | 0.72 | 0.662 | 0.608 | −0.088 | NS | 6 | 4.029 | 0.614 | 0.752 | 0.713 | 0.133 | * |
| YFPSSR63 | 3 | 2.409 | 0.5 | 0.585 | 0.502 | 0.146 | NS | 6 | 3.018 | 0.364 | 0.669 | 0.606 | 0.392 | *** |
| YFPSSR71 | 8 | 2.245 | 0.595 | 0.544 | 0.525 | −0.094 | NS | 11 | 3.873 | 0.463 | 0.742 | 0.714 | 0.273 | *** |
| YFPSSR73 | 6 | 5.466 | 0.586 | 0.793 | 0.792 | 0.262 | NS | 11 | 5.781 | 0.783 | 0.827 | 0.804 | 0.046 | NS |
| YFPSSR5 | 6 | 3.559 | 0.498 | 0.727 | 0.667 | 0.315 | *** | 11 | 5.055 | 0.682 | 0.802 | 0.773 | 0.135 | *** |
| YFPSSR75 | 10 | 3.173 | 0.654 | 0.683 | 0.659 | 0.044 | NS | 14 | 5.095 | 0.477 | 0.804 | 0.788 | 0.335 | *** |
| Mean | 6.55 | 3.436 | 0.61 | 0.655 | 0.622 | 0.060 | 9 | 4.238 | 0.623 | 0.729 | 0.696 | 0.106 |
| Molecular Marker | mtDNA | SSR |
|---|---|---|
| Before removing the dead samples | 0.059 (3.99) | 0.0628 (3.73) |
| After removing the dead samples | 0.0732 (3.17) | 0.101 (2.23) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Yin, D.; Que, J.; Zhu, X.; Lin, D.; Ying, C.; Yu, J.; Liu, K. Genetic Diversity and Population Differentiation of Yangtze Finless Porpoise in Poyang Lake. Animals 2025, 15, 1838. https://doi.org/10.3390/ani15131838
Zhang H, Yin D, Que J, Zhu X, Lin D, Ying C, Yu J, Liu K. Genetic Diversity and Population Differentiation of Yangtze Finless Porpoise in Poyang Lake. Animals. 2025; 15(13):1838. https://doi.org/10.3390/ani15131838
Chicago/Turabian StyleZhang, Han, Denghua Yin, Jianglong Que, Xiaoyan Zhu, Danqing Lin, Congping Ying, Jinxiang Yu, and Kai Liu. 2025. "Genetic Diversity and Population Differentiation of Yangtze Finless Porpoise in Poyang Lake" Animals 15, no. 13: 1838. https://doi.org/10.3390/ani15131838
APA StyleZhang, H., Yin, D., Que, J., Zhu, X., Lin, D., Ying, C., Yu, J., & Liu, K. (2025). Genetic Diversity and Population Differentiation of Yangtze Finless Porpoise in Poyang Lake. Animals, 15(13), 1838. https://doi.org/10.3390/ani15131838






